Abstract
Alzheimer’s disease is the most common type of neurodegenerative disease in the world. Genetic evidence strongly suggests that aberrant generation, aggregation, and/or clearance of neurotoxic amyloid-β peptides (Aβ) triggers the disease. Aβ accumulates at the points of contact of neurons in ordered cords and fibrils, forming the so-called senile plaques. Aβ isoforms of different lengths are found in healthy human brains regardless of age and appear to play a role in signaling pathways in the brain and to have neuroprotective properties at low concentrations. In recent years, different substances have been developed targeting Aβ production, aggregation, interaction with other molecules, and clearance, including peptide-based drugs. Aβ is a product of sequential cleavage of the membrane glycoprotein APP (amyloid precursor protein) by β- and γ-secretases. A number of familial mutations causing an early onset of the disease have been identified in the APP, especially in its transmembrane domain. The mutations are reported to influence the production, oligomerization, and conformational behavior of Aβ peptides. This review highlights the results of structural studies of the main proteins involved in Alzheimer’s disease pathogenesis and the molecular mechanisms by which perspective therapeutic substances can affect Aβ production and nucleation.
Keywords: Alzheimer’s disease, amyloid precursor protein, amyloid-β peptide, structural–dynamical properties, toxic oligomerization, molecular mechanism, neuronal membrane, protein–protein and protein–lipid interactions, bioactive peptides for therapy and diagnosis
1. Introduction
Alzheimer’s disease (AD) predominantly affects the elderly and progressively worsens with age, currently accounting for 60–70% of cases of dementia worldwide. With the ongoing increase in life expectancy, the number of people aged over 65 is predicted to triple by 2050, making this debilitating ailment not only a serious challenge to the healthcare system but also a complex social and economic issue [1]. Clinical manifestations of AD are attributed to selective degeneration of neurons in the regions of the cerebral cortex responsible for cognitive perception and memory. The origin, pathogenesis, and treatment options have been in the focus of multidisciplinary studies over the last decades; however, the pharmaceutical treatment strategies available to date have only a limited effect in slowing down the disease progression rather than restoring the impaired cognitive functions. Based on the clinical evidence, the disease symptoms are associated with the formation of aggregates of two proteins: extraneuronal senile plaques and intraneuronal neurofibrillary tangles (NFTs) [2,3] consisting of oligomerized amyloid-β peptides (Aβ) and phosphorylated tau-proteins, respectively. Besides that, multiple correlations of AD initiation and/or development with various lipid metabolism abnormalities have been found [4,5].
Three major hypotheses were historically proposed in relation to causal factors of AD development [6]. The earliest of the three was the so-called cholinergic hypothesis associating the disease with impaired synthesis of acetylcholine neuromediator. However, based on clinical studies, all the treatment strategies relying on this hypothesis proved to be mostly symptomatic and incapable of curing the disease to complete recovery of patients [7]. Another hypothesis associated the disease development with aberrations in the folding of tau proteins, whose normal function is to stabilize tubulin microtubules playing diverse roles in regulation of neuron morphology through cytoskeleton formation and intraneuronal cargo trafficking. Tau protein affinity to microtubules is regulated by its phosphorylation, and hyperphosphorylation causes the protein to aggregate, forming large fibrils and neurofibrillary tangles destabilizing the system of microtubules, impairing intracellular trafficking, and ultimately causing neuron death [8]. Nevertheless, the more recent genetic evidence strongly suggests that the disease is triggered by abnormal generation and/or clearance of the neurotoxic Aβ produced via sequential extramembrane and intramembrane cleavage of a transmembrane (TM) amyloid precursor protein (APP) by α-, β-, and γ-secretases [3,9]. Accordingly, the “amyloid hypothesis” attributing the main cause of the disease to aberrant production, clearance, and/or conformational distribution of Aβ and especially its oligomeric forms [10,11] has become predominant. The supporting genetic evidence includes the fact that the familial mutations associated with the early onset of AD are mostly localized in the genes encoding APP or the secretases involved in its proteolysis [3,12,13]. Circumstantial supporting evidence for this hypothesis comes from the studies of patients afflicted with Down syndrome who have an extra copy of chromosome 21 [14] and, among other abnormalities, produce 1.5 times as much APP as other people. By the age of 40, almost 100% of people with Down syndrome who die have the changes in the brain commonly associated with AD. A significant role of apolipoprotein E (ApoE) in the disease progression is also consistent with the amyloid hypothesis. Indeed, the presence of the E4 allele of the ApoE gene correlated with amyloid overexpression and accumulation is known to be among the most significant contributors to AD development, simultaneously increasing its risk and causing earlier onset, along with rare familial mutations [2,4]. Although several reasons may underlie the effect of specific apoE isoforms on AD pathogenesis, convincing evidence suggests that the physical interaction of apoE with Aβ plays an important role. APP and its derivatives, including Aβ isoforms, soluble ectodomain, and C-terminal fragments, definitely play diverse, although not fully known and completely understood, roles in neuronal tissue function and homeostasis. Different Aβ isoforms normally occur in the healthy human brain regardless of age and are apparently essential for brain function, participating in synaptic signal transduction, neuroplasticity, and inflammatory response [15,16,17,18,19].
This review highlights the results of recent structural studies of the major currently known factors of AD pathogenesis, as well as the molecular mechanisms by which perspective therapeutic substances affect Aβ production and oligomerization.
2. Diverse Biological Function of the Amyloid Precursor Protein
Amyloid precursor protein (APP), expressed in the cells of virtually all animal tissues and having a pronounced evolutionary conservatism, shares structural and functional similarities with type I membrane-embedded receptors [20]. It was hypothesized to act as a cholesterol receptor in neuronal membrane rafts [21] and to participate in intracellular signaling [20], regulation of neuronal iron metabolism [22], and inflammatory processes [17]. APP gene consists of 20 exons, and its alternative splicing results in the generation of over 10 different isoforms in different tissues, APP695 being predominant in the neuronal tissue. Pronounced evolutionary conservatism of the APP genes implies that the corresponding proteins play vitally important biological functions. Indeed, knockout of APL-1 (APP homolog) gene in C. elegans, commonly used as a model organism, results in a lethal phenotype [23]. Expression of a truncated APL-1 form without the cytoplasmic domain, however, is sufficient for survival [24]. In D. melanogaster, knockout of APPL (APP-like protein) gene is not lethal but causes aberrant synapse morphology and abnormal behavioral patterns [25].
In vertebrates, the APP gene family includes two APP homologs besides the APP itself, APLP1 and APLP2, with partly overlapping functions and a variety of biologically active products resulting from complex proteolytic processing. For example, APP knockout mice proved to be viable and fertile, though having multiple abnormalities including reduced body weight [26]; elevated concentrations of copper [27], cholesterol, and sphingolipids [28] in brain tissues; and multiple neurological impairments. These impairments included decreased size of the frontal lobe commissures and corpus callosum agenesis, corroborating the hypothesis of APP involvement in neurite outgrowth and axonal pathway formation [29]. Interestingly, in this case, all the abnormalities are abolished by the expression of an abbreviated soluble form of APP, sAPPα, corresponding to a product of APP proteolysis by α-secretase [30], implying that this processing pathway is essential for the normal development and function of the neural system. Experiments with knockout of individual APP homologs or their combinations proved partial functional overlap between the homologs and their importance for the formation of intercellular contacts and interactions, as well as neurotrophic and neuroprotective functions [31,32]. The role of APP in intercellular interactions is further corroborated by structural studies. In particular, APP ectodomains of the same cell or two neighboring cells were shown to be capable of forming heparin-mediated cis- and trans-dimers [33]. Besides, APP shares a common proteolytic cleavage mechanism with type I receptors of the Notch family [34], which supports the hypothesis of APP involvement in interactions with neighboring cells and extracellular factors. Likewise, certain interactions of APP with intracellular and plasma membrane components also have functional implications. For example, its ectodomain interacts with membrane-associated prion protein PrP [20,35], whereas its C-terminal segment participates in the cytoplasmic signaling cascades. APP was shown to directly interact with cholesterol molecules in cellular membranes [34,36,37], with multiple lines of evidence implying that such interactions are biologically relevant. These include clinical evidence of cholesterol metabolism abnormalities in AD patients, results of biophysical experiments indicative of competition between cholesterol binding and APP dimerization, and preferential localization of APP and γ-secretases in cholesterol-enriched lipid rafts [38,39,40].
3. Multidomain Structure of the Amyloid Precursor Protein
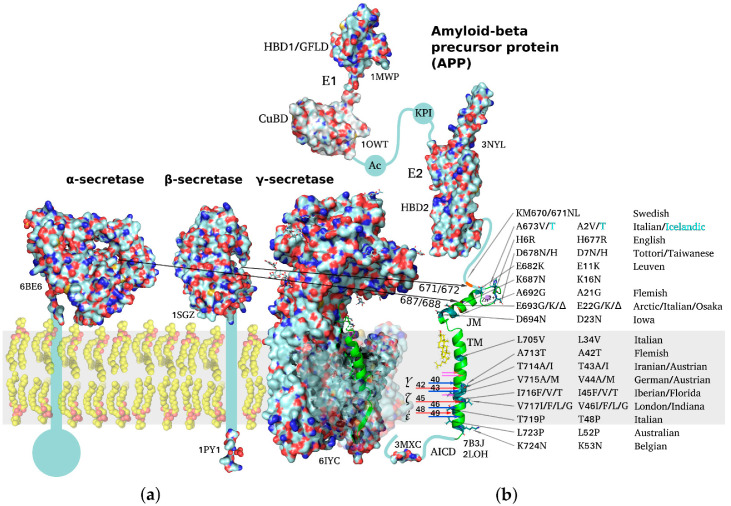
APP is a classical type I multidomain protein with a single TM span, consisting of intracellular, extracellular, and TM parts, capable of lateral dimerization in the plasma membrane and adopting several alternative functional conformations. The following structural domains can be distinguished in unprocessed APP molecules: E1 subunit composed of a heparin-binding domain along with a cysteine-rich growth factor-like domain (HBD1/GFLD) and a copper/zinc-binding domain (CuBD), an acidic region (Ac), a Kunitz-type protease inhibitor domain (KPI, not present in APP695), E2 subunit with a second heparin-binding domain (HBD2), a random coil juxtamembrane (JM) region and TM domain (including the Aβ sequence), and an intracellular C-terminal domain (AICD) (Figure 1) [20,41,42]. APP dimerization is known to be induced by the N-terminal E1 region, the connecting loop between the HBD1/GFLD and CuBD formed by disulfide bridges being essential for stabilization of the homodimeric state, while JM and TM regions also participate in homodimerization [20,43,44]. Due to the relatively large size (~750 a.a. residues) of the protein, its ability to dimerize in a membrane environment, and domain mobility with respect to each other, no high-resolution structures of the full-size APP have been obtained to date, spatial structures of the domains having been resolved separately via different structural methods.
Figure 1.
Schematic representation of structures of amyloid precursor protein and α-, β-, and γ-secretases responsible for Aβ production. PDB accession number is indicated for each molecule throughout the figure. (a) Molecular surfaces of the structural elements of α-, β-, and γ-secretases. Single-span transmembrane (TM) domains of α- and β-secretases are shown as bars where high-resolution structure is unavailable. (b) Full-length APP structure based on individually resolved structures of its parts: flexible intracellular C-terminal domain (AICD), TM domain, connected through a flexible extracellular juxtamembrane (JM) region (containing Aβ metal-binding domain) to an ectodomain consisting of (i) E1 subunit including a cysteine-rich growth factor-like domain (HBD1/GFLD) and a copper/zinc-binding domain (CuBD), an acidic region (Ac), a Kunitz-type protease inhibitor domain (KPI), and (ii) E2 subunit with a second heparin-binding domain (HBD2). Resolved domain structures are shown as ribbon diagrams, and unstructured flexible connecting loops are shown as solid lines. The familial mutations attributed to increased risk or earlier age of AD development are shown in black on the TM and JM segments. A673T mutation decreasing APP proteolysis by β-secretases is highlighted in cyan. Sites of cleavage by α-, β-, and γ-secretases are indicated by arrows color-coded to distinguish between two alternative cleavage cascades generating Aβ1–42 and Aβ1–40 peptides (48 > 45 > 42 vs. 49 > 46 > 43 > 40). Cholesterol molecule interacting with the N-terminal part of APP TM helix is shown. The inset demonstrating the helical APP TM domain (in green) with a C-terminal turn (3 a.a. residues) unfolded into a β-strand is shown in the γ-secretase active center.
Besides performing apparently essential biological functions in its unprocessed form, APP also serves as a predecessor for multiple biologically active products generated through different cleavage pathways. For example, cleavage by α-secretase yields soluble sAPPα, an important neurotrophic factor enhancing proliferation of neuronal stem cells and modulating synaptic plasticity [45,46,47]. A similar β-secretase cleavage product sAPPβ devoid of the Aβ metal-binding domain has no neuroprotective functions of the sAPPα, but was shown to interact with DR6 receptor (“death receptor 6” of the TNFα family), inducing caspase 6 activation and axon pruning. Although no high-resolution structure of the entire ectodomain is available so far, sAPPα structure was resolved by small-angle X-ray scattering [48,49,50] in combination with molecular modeling, fitting the known spatial structures of GFLD, CuBD, KPI, and E2 domains into the low-resolution structure of the APP ectodomain [48,51].
APP TM domain contains the sequences of the β-amyloid peptides, excessive accumulation of which is correlated with advanced AD stages. Moreover, most of the familial mutations associated with early onset of the disease are situated within the TM domain or the immediately adjacent JM regions. The TM domain proper includes residues 700–723 forming a hydrophobic span; however, it is often binned together with the JM region 686–699, which also participates in interactions with the membrane, and with 672–685 metal-binding domain [52,53]. This membrane-associated domain was extensively investigated by NMR spectroscopy methods, including high-resolution and solid-state techniques [54,55,56]. Labile conformation of the metal-binding domain was shown to be modulated by intra- and interprotein Zn2+ binding. The JM region can form a flexible amphiphilic helix submerging under the membrane surface or a β-strand depending on the environment, and this transition can underlie nucleation of amyloid aggregates. A flexible loop connects the JM region to the TM domain, which includes a kink region (following Gly708/Gly709 repeat) responsible for the sensitivity of the TM domain conformation to the membrane environment or point mutations [21,54,56,57,58,59,60]. High-resolution dimeric structure of the α-helical APP TM segment corresponding to Aβ15–55 was first obtained in [43] in DPC micelles used as a membrane environment model. However, the possibility of alternative dimerization of the TM domain was suggested by a number of studies, e.g., in lipid bilayers [61,62]. The presence of two or more dimerization motifs in the TM segment is common for type I receptors, often enabling transition between different functional states of the receptor, e.g., between the dormant and ligand-activated states. Evidence is available linking APP TM and/or extracellular domain dimerization with the choice of proteolytic cleavage pathway [63]. The familial mutations associated with early AD onset localized within the TM or JM region were shown to be capable of altering APP recognition and cleavage by secretases and/or affecting oligomerization and toxicity of mature Aβ species at the expense of global or local conformational rearrangements and intermolecular interactions of varying degree of specificity [58,60,64,65,66,67,68].
Like many intracellular domains of diverse TM proteins, the intracellular AICD domain composed of about 50 residues does not have a stable ordered structure, as demonstrated by solution NMR spectroscopy [69]. However, as is the case with many intrinsically disordered proteins (IDPs), the measured backbone NMR chemical shifts are indicative of transient secondary structure elements, which can serve as precursors of the structure assumed by the domain upon interaction with intracellular ligands or certain components of the cytoplasmic membrane leaflet [70,71]. Overall, AICD is responsible for the intracellular stage of signal transduction and APP trafficking towards the plasma membrane. For example, after being cleaved by γ-secretase, AICD in complex with Fe65 protein is transported into the nucleus where it regulates expression of several microRNA species, with AICD overexpression inhibiting differentiation of stem cells into neurons, mostly through increased expression of mir-663 [72]. More recently, several G-protein-dependent pathways were also hypothesized to be relevant to APP biological function [73].
4. Proteolytical Processing of the Amyloid Precursor Protein
The canonic APP proteolytic processing pathways consist of sequential extramembrane and intramembrane cleavage first by α- or β-secretases and then by γ-secretases (Figure 1), yielding diverse Aβ isoforms depending on the exact cleavage pattern [3,20,74]. Initially, APP can be cleaved by a membrane-bound β-secretase, aspartyl protease BACE (β-site APP-cleaving enzyme), removing a large APP ectodomain and yielding a membrane-bound 99 amino acid C-terminal fragment (CTF). Thereafter, this C99 or APP CTFβ fragment undergoes further sequential cleavage by a large membrane protein complex known as γ-secretase. This intramembrane aspartyl protease complex starts processing C99 in the TM domain at the ε-site, thereby releasing the APP intracellular domain (AICD) from the membrane into the cytosol, initiating an intracellular signaling cascade. A spectrum of Aβ isoforms is generated by downstream γ-secretase cleavages at the ζ- and γ-sites, truncating the APP TM domain until sufficiently short, predominantly 38 to 42 residues long, and Aβ isoforms are released from the membrane into the extraneuronal space or into the lumen of secretory pathway organelles. Normally the major ultimate cleavage product is Aβ1–40, the shorter Aβ1–38 and the longer Aβ1–42 being present as minor components. Onset of AD, however, is associated with an overall increase in production of all Aβ isoforms and a shift of distribution between the isoforms towards a higher fraction of Aβ1–42 that is more hydrophobic and fibrillogenic than Aβ1–40. All Aβ isoforms are capable of associating into cytotoxic oligomers interacting with neuronal membranes and membrane receptors; further aggregation results in the formation of fibrils depositing as the senile plaques observed at the late stages of AD progression. Generation of Aβ is competitively inhibited by an alternative extramembrane cleavage of APP mediated by membrane-bound α-secretase, a metalloprotease of the ADAM (A Disintegrin And Metalloproteinase) family, which removes the N-terminal metal-binding region of Aβ and generates a shorter APP CTFα, C83, further processed by γ-secretase into non-pathogenic P3 peptides.
Competition between α- and β-secretase processing of APP at the initial stage defines the subsequent cleavage cascade and spectrum of products, which suggests a potential AD treatment strategy based on β-secretase inhibition and/or α-secretase upregulation [75,76]. The α-secretase belongs to ADAM metalloproteases, type I transmembrane proteins having modular domain structure. The substrates of extracellular ADAM metalloproteases include growth factors, cytokines, chemokines, receptors, and adhesion factors; thus, the metalloproteases play important roles in intracellular adhesion and signal transduction [77]. ADAM9 and ADAM17 species can process APP; however, the dominant pathway leading to p3 and sAPPα production involves ADAM10 [78,79], the substrates of which, besides APP, include Notch receptor, Eph receptors, HER receptors, TNF-α, and other membrane proteins. Several mutations associated with late onset of AD were found to be localized in ADAM10 prodomain facilitating proper folding of its ectodomain [80]. The mechanisms underlying the metalloprotease specificity are being intensively investigated, the structure of the full-size ectodomain of human ADAM10 having been recently resolved with the aid of X-ray diffraction technique [75].
In neuronal tissues, APP cleavage in the β-site is predominantly performed by BACE1 species of β-secretase, its activity increasing with age or in case of AD development [81]. Its close homolog BACE 2 was also recently implicated in AD pathogenesis [82]. Preferentially expressed in neurons, BACE1 is known to be necessary for normal axon myelinization [83], presumably in relation to its activity towards Neuregulin 1 (Nrg1) substrate [84]. Other BACE1 substrates performing essential biological functions include Notch Jag1 [85]; Navβ2 regulatory subunit of voltage-gated sodium channels [86]; KCNE1 and KCNE2, regulating voltage-gated potassium channels [87]; and Sez6L protein, associated with seizure activity [76]. BACE-1 is a type 1 integral membrane glycoprotein with a bulky ectodomain, a single TM span, and a small intracellular fragment [88]. Interaction of BACE1 with lipid bilayer is critically important for APP processing, BACE1 with deleted TM segment being inactive towards APP in vivo, BACE1 structure provides important clues for understanding the pathogenicity of the “Swedish” familial mutation APP KM670/671NL occurring in the immediate vicinity of the cleavage β-site and causing enhanced interaction of the substrate with β-secretase [45]. Another APP mutation, A673T in the immediate vicinity of the cleavage β-site, was also described, decreasing APP proteolysis by β-secretases and the likelihood of spontaneous AD development [89]. BACE1 TM domain is also distinguished by a unique trimerization motif capable of interacting with copper ions [46]. The correlation between enhanced BACE1 activity and AD development prompted a number of attempts to find safe clinically effective inhibitors of the enzyme. Over 400 spatial structures of BACE1 complexes with diverse inhibitors have been obtained, some of them currently undergoing clinical trials.
Initial APP cleavage by α- or β-secretases enables subsequent γ-secretase processing, removing the steric constraints and providing access to the γ-secretase cleavage site in the membrane interior [90,91]. Having an apparently limited specificity, γ-secretase is known to process dozens of proteins [92], including Notch receptors [34] and APP. The low specificity of the γ-secretase complex is corroborated by a small degree of homology between different substrates and the presence of amino acid residues with diverse physicochemical properties in their cleavage sites. The γ-secretase processing frequently occurs in the cholesterol-enriched endosomal membranes at low pH values [5,21,88,93,94].
The γ-secretase complex is a multidomain enzyme composed of four transmembrane proteins: presenilin (PS, in the form of PS1 or PS2 protein), nicastrin (NCT), anterior pharynx-defective 1 (Aph1), and presenilin enhancer 2 (PEN2) [95]. The diversity of γ-secretase substrates and their involvement in a broad range of cellular processes, along with the fact that the majority of familial mutations associated with AD development affect PSEN1 and PSEN2 subunits [96], stimulate their extensive investigation. The mechanisms of APP mutations affecting ε-, ζ-, and γ- cleavage sites are also apparently based on induced changes in the γ-secretase sequential cleavage process. Sequential removal by γ-secretase of 3–4 a.a. residue-long segments (one turn of a TM domain helix) from the substrate C-terminal side results in the generation of a pool of APP processing products of different lengths [74,97] (Figure 1). The exact mechanism of substrate recognition by γ-secretase has not been fully described, but it appears to involve considerable conformational rearrangements of both the enzyme and the substrate [98]. In order to enable cleavage by a γ-secretase, the C-terminal helical parts of Aβ precursors (APP TM domain fragments) need to unfold into extended conformation and submerge into the membrane to the depth corresponding to the location of γ-secretase active center situated near the cytoplasmic leaflet within accessibility of water molecules. Accordingly, changes in the local membrane environment or mutations affecting the accessibility of C-termini of Aβ precursors to the active center can alter the cleavage rate and the likelihoods of initiation of alternative cleavage cascades (e.g., stepwise reduction of the resulting Aβ-peptide length as 48 > 45 > 42 vs. 49 > 46 > 43 > 40). Consequently, the Aβ1–42/Aβ1–40 ratio can increase due to the alternative cleavage pathway (starting from the C99 ε-site position ε48 rather than ε49) becoming more preferable.
Despite the extensive efforts, due to the large size and conformational mobility of the γ-secretase complex, its structure was only recently resolved with the advancement of protein engineering and cryo-EM techniques. In [99], baculoviral protein expression in insect cells was used to express and reconstitute mature and functional γ-secretase complex with the degree of glycosylation, which is involved in substrate selection, corresponding to that observed in mammal cells. The structural studies culminated in the determination of the structure of the γ-secretase complex with one of its substrates, APP TM domain, with the resolution of 2.6 Å [100]. In order to maintain the complex stability, cysteine residues were introduced into both sequences by means of Q112C mutation of PS1 and V695C mutation of APP-C83. Additionally, Asp385 catalytic residue in PS1 was mutated to Ala in order to prevent substrate cleavage. Since catalytic activity is also required for presenilin autoproteolysis, it was expressed in the form of N- and C-terminal fragments NTF and CTF. The APP TM domain in the complex closely interacts with five neighboring TM segments of presenilin (TM2, TM3, TM5, TM6, and TM7), including the segments hosting a majority of the known pathogenic mutations. The complex formation is accompanied by the unfolding of the APP helix on the C-terminal side between T719 and V721 and the assembly of an intermolecular β-sheet between V722-K725 APP residues, NTF C-terminus (residues 287–290), and CTF N-terminus (residues 377–381). Thus, the APP C-terminal segment forms a β-strand, combining with two induced presenilin β-strands into the β-sheet and ultimately presenting the peptide bond immediately preceding the β-sheet for γ-secretase catalytic cleavage.
The large number and diversity of vital processes dependent on γ-secretase activity [101] imply that any AD treatment strategy targeting the enzyme itself would most likely entail unacceptable side effects. However, knowledge of the structural and dynamic bases of γ-secretase complex interaction with Aβ precursors can be used to selectively influence Aβ production rate and/or distribution between different cleavage products.
5. Aβ Peptide Functional Activities
APP proteolysis results in the production of over a dozen different Aβ peptide fragments, their lengths ranging from 38 to 53 amino acid residues (based on Uniprot.org data).
Aβ concentrations in cerebrospinal fluid are normally within the range of 4–50 pM, the peptide remaining monomeric up to the concentrations of ~3 uM [102]. Extracellular concentrations reach 200 pM, which is still substantially below the spontaneous oligomerization threshold. The biological roles of this peptide are yet to be fully understood, but a number of studies suggest that rather than being neurotoxic in physiological concentrations, it has neuroprotective and neurotrophic neuroprotective actions in trophic deprived conditions [103,104,105]. Aβ may have an important physiological role in synapse elimination during brain development. Inhibition of endogenous Aβ production by exposure to inhibitors either of β- or γ-secretases in primary neuronal cultures caused neuronal cell death [106]. Multiple lines of evidence also link several Aβ species to neuroplasticity and memory formation functions. Thus, high-affinity binding between Aβ1–42 peptides and α7-nicotinic acetylcholine receptor (α7-nAChR), a Ca2+-permeable ion channel expressed in the hippocampal and cortical tissues, either inhibits or activates α7-nAChR signaling in a concentration-dependent manner [104]. At normal concentrations (picomolar range), Aβ peptides positively regulate presynaptic release at hippocampal synapses and facilitate learning by activating α7-nAChRs, whereas when the level of Aβ is low or high (nanomolar range), Aβ peptides either cause deficits in presynaptic function or abolish learning via interactions with α7-nAChRs. In [16], Aβ at concentrations of 0.1–1 uM (considerably exceeding the physiologically normal levels) was shown to have neuroprotective properties, suppressing NDMA-mediated excitotoxicity in neuron cultures, activating IGF-1 and insulin receptors, and causing activation of the PI-3-K signaling pathway. Prion protein PrP, normally mediating multiple trans-membrane signaling processes associated with hematopoietic stem cell replication and neuronal differentiation and performing neuroprotective functions, also has a high affinity for Aβ oligomers. Recent investigations indicated and suggest that this interaction between Aβ oligomers and PrPC may impact synaptic plasticity functions and may play an important role in the pathogenesis of AD. There is a connection between AD and PrPC levels, and its deficiency confers resistance to the synaptic toxicity of oligomeric Aβ in mice and in vitro in hippocampal slice cultures [107].
Aβ apparently plays multiple roles in immune function. Indeed, several new lines of evidence indicate that Aβ may function within the innate immune system as an antimicrobial peptide (AMP), an ancient class of peptides with potent and broad-spectrum antimicrobial activity. In vivo, the peptide was shown to perform protective functions, ameliorating the course of the diseases caused by Salmonella typhimurium in mice and Candida albicans infection in C. elegans worms expressing human peptide [19]. Aβ appears to be an essential part of normal inflammatory processes. The scavenger receptor for advanced glycation end products (RAGE), a transmembrane protein belonging to the immunoglobulin superfamily, specifically binds monomeric and oligomeric Aβ, causing activation of a complex downstream signaling cascade mostly associated with inflammatory processes and oxidative stress induction [108]. Aβ interactions are not limited to plasma membrane surface receptors, its effects of mitochondria having especially pronounced consequences. Both Aβ and tau proteins act synergistically to induce mitochondrial dysfunction, this effect being aggravated in aged mice in the presence of plaques and tangles [109].
Thus, Aβ peptides have multiple and diverse functional roles related to neuronal cell homeostasis in healthy organisms and factor into the development of several seemingly unrelated pathological processes, any of which can potentially advance to develop clinical symptoms known as Alzheimer’s disease. Such a diversity and complex interrelation of Aβ normal functions and roles in abnormal processes make it highly unlikely that an AD treatment strategy targeting any of its interactions individually can be effective, suggesting a focus on affecting Aβ production cascades and using its structure and dynamic properties to modulate its interactions with various targets and counteragents.
6. Structural Properties of Aβ Monomers and Aggregates
Although amyloid fibrillary plaques are a known hallmark of AD, they are not necessarily the main cause of neurodegeneration. Aβ coexists in a number of various forms, each of them potentially having both beneficial and deleterious effects depending on the current state of diverse targets and cell systems they interact with. These forms include soluble monomers of different lengths of the residual hydrophobic TM span, dimers, oligomers, protofibrils, and ultimately the fibrils depositing to form plaques.
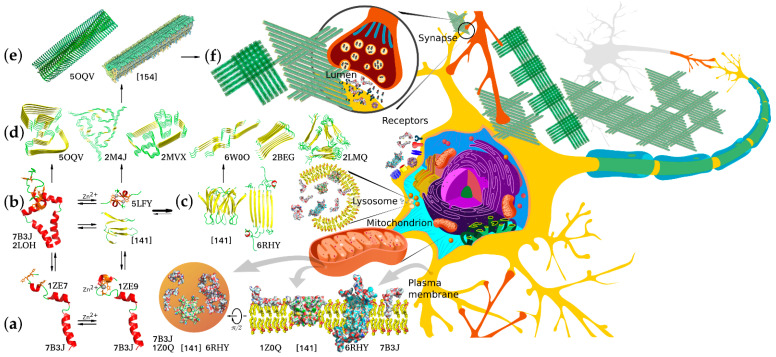
Knowing the biophysical bases of transitions between these forms is crucial for understanding the detrimental effects of abnormal Aβ accumulation and finding ways to preclude or ameliorate these effects. Soluble monomeric products of APP proteolysis can adopt several alternative conformations depending on their length, hydrophobic properties, and local environment. The amino acid residues corresponding to the APP JM region can form a flexible amphiphilic helix with a propensity to submerge under the membrane surface or a β-strand, and this transition can underlie the nucleation of amyloid aggregates. The peptide dimerization and formation of higher oligomers can also occur through the metal-binding domain and be modulated by intra- and interprotein Zn2+ binding, or, in the case of longer Aβ species, through the residual TM parts within the membrane. Accordingly, the APP products of different lengths differ in dimerization modes and constants, propensity for further aggregation, and solubility of the resultant aggregates. Thus, monomers and minor oligomers can either be incorporated into the membrane, potentially causing its permeabilization [110,111], or further aggregate into larger protofibrils and mature fibrils constituting the major component of amyloid plaques. Importantly, different forms of Aβ can participate in aggregation, combinatorically increasing the diversity of aggregates and their properties (Figure 2).
Figure 2.
Structural diversity of Aβ aggregation states, aggregation pathways, and examples of biologically relevant interaction targets. Corresponding PDB accession numbers are indicated alongside the diagrams. (a) Free and cation-bound monomeric Aβ peptides (the structure combined from solution NMR structures of folded Aβ1–16 metal-binding domain and Aβ17–42 fragment) are capable of dimerizing via different dimerization interfaces situated in the TM, JM, and metal-binding site regions using protein–protein and protein–cation interactions involving α-helix, β-strand, and random coil structures. (b) Alternative dimers nucleate aggregation into neurotoxic intermediate oligomers that can interact with different target proteins and lipid membranes of neurons. (c) Two known configurations of predominantly β-structured minor oligomers capable of forming pore-like proteolipid aggregates and prone to further aggregation into protofibrillar structures. (d) Diverse structural motifs that can constitute amyloid fibril core structures capable of further aggregating into filaments and fibrillary deposits. (e) Two possible alternative fibril structures with biaxial and triaxial symmetry depositing to form macroscopic aggregates (f) constituting senile plaques, a known hallmark of AD. All the diverse Aβ aggregation forms appear to interact with multiple alternative targets, thus mediating normal physiological functions or pathological processes. The interactions are known to occur in multiple morphological units and organelles, including plasma and synaptic membranes and inter- and intracellular components.
Determination of the monomeric Aβ spatial structure is complicated by its oligomerization occurring at the concentrations needed for the application of NMR spectroscopy methods. This issue can be circumvented by using water/hexafluoroisopropanol and water/trifluoroethanol mixtures as solvents. At water contents below 80%, the peptide structure consists of two α-helices connected with a short loop [112], similarly to the structure of the APP TM domain [54]. An increase in water fraction results in the peptide transition to β-conformation followed by oligomerization [113].
Structural studies of dimeric Aβ are complicated by the same oligomerization issue, further aggravated by the fact that the dimers serve as nucleation centers for oligomerization and fibrillation [114]. Two generic types of Aβ are known to date: an α-helical structure similar to the conformation of TM domain in membrane-mimicking environments [115] observed in relatively unpolar solvents, such as hexafluoroisopropanol and trifluoroethanol mixtures, and a β-strand structure roughly representing a single link of a β-amyloid fibril. The spatial structure of Aβ dimer in solution in β-conformation obtained in [116] through 1700 ns simulation yielded an estimate of the dimerization energy at about 5 kcal/mol, which is consistent with the strong tendency of Aβ to aggregate in solution.
Presently, Aβ neurotoxicity is widely believed to be primarily associated with its oligomeric forms [117,118,119], with oligomer toxicity exceeding that of both the monomers and the mature fibrils [117]. Based on the outcomes of multiple independent studies, medium-sized oligomers produce the most significant detrimental effects, which decrease at higher molecular weights of the complex. There is no universally agreed upon predominant mechanism of Aβ oligomer toxicity to date, with several alternative hypotheses supported by the accumulated array of experimental evidence. The amphiphilic nature of the peptide suggests direct interactions with cellular membranes as one of the possible mechanisms, and this possibility is being widely explored. Exposure to oligomeric Aβ was shown to cause an increase in calcium flow across the membrane [120] inhibited by submillimolar concentrations of Zn2+ and Cu2+ ions [121]. According to some reports, the channel-like structures formed by Aβ oligomers [122,123,124] can influence the electrical potential of the neurons [125], and short-term memory impairments induced by Aβ oligomers can be caused by an increase in the local concentration of Zn2+ in the presynaptic region [126]. Electrophysiological measurements revealed that Aβ1–42 peptides can form at least three different types of ion channels differing in permeability, whereas Aβ1–40 peptides form no ion channels [127]. The ability of Aβ to form membrane pores was experimentally observed in a number of studies [123,127,128], while molecular modeling predicted the ability of Aβ to create transmembrane channels in interaction with cholesterol [129]. Aβ and its oligomers are also capable of specific interactions with certain lipid membrane components, such as cholesterol and gangliosides. As is often the case, such interactions have bilateral effects—the specific lipids in the neighborhood induce changes in Aβ oligomerization processes and behavior of oligomers, while Aβ interactions modulate local lipid membrane physicochemical properties, affecting through them functioning of membrane-associated protein machinery, both individually and within proteolipid platforms [130,131,132]. As reported in [133], trodusquemine, a naturally occurring aminosterol belonging to a family of compounds able to displace proteins from membranes, both accelerates Aβ1–42 aggregation and inhibits its toxicity, possibly through an increased rate of conversion of oligomers into less toxic mature fibrils.
Another Aβ feature undoubtedly associated with its neurotoxic properties is its ability to chelate transition metal ions, including Al3+, Fe3+, Zn2+, and Cu2+. The metalloproteins resulting from interaction with iron and copper catalyze redox reactions and generate reactive oxygen species (ROS), thus initiating oxidative stress, whereas zinc does not support this mechanism and even inhibits the generation of ROS [134]. It has also been shown that the addition of monomerized Aβ peptide even at high micromolar concentrations causes only an insignificant increase in the ROS generation in the culture of human neuroblastoma cells cultivated under physiological conditions of 5% CO2 (as in the body), and it attenuates radiation-induced ROS [135]. Besides that, metal cation coordination was shown to play a role in the initial stages of Aβ oligomerization and to facilitate its adsorption on lipid membrane surfaces through the formation of salt bridges with negatively charged lipid headgroups. The metal-binding moieties of Aβ are also likely to participate in the formation of channel-like structures with cation selectivity. These mechanisms appear to be responsible for neurotoxicity exhibited by Aβ species in comparison with P3 peptides devoid of the metal-binding segment.
Investigation of the structures and properties of Aβ oligomeric forms requires extensive care and efforts needed for obtaining monodisperse samples of individual oligomeric forms with sufficient stability for the application of structural methods. Several models of the formation of stable oligomers, membrane pores, and channels have been developed with the aid of molecular modeling, atomic force microscopy, electron microscopy, and electrophysiological and other methods. Among them is a model of Aβ1–42 hexamer forming a β-barrel and further assembling into a 36-membered aggregate capable of spanning the membrane and permeabilizing it for ions, including Ca2+ [136]. The oligomeric state of Aβ peptides is essentially transient, at least in vitro, the characteristic lifetimes ranging from several hours to several days [137,138]. Therefore, special experimental techniques are employed to stabilize the oligomers in the structural studies. For example, in the case of Aβ17–36 peptide, additional stabilization of the kink required for interaction of its N- and C-terminal parts with a disulfide bridge allowed resolving its dodecamer structure by X-ray diffraction method (PDB: 5HOY) [139]. Aβ17–36 dodecamer is composed of four trimeric modules and can assemble into transmembrane pores with the luminal radius of ~1 nm and the external diameter of ~11 nm. It takes five dodecamers to shape the annular pore structure. The oligomers used in this study for crystallization bind with A11 antibodies known to interact with Aβ oligomers, including 56 kDa dodecamers, but not with fibrillar Aβ [140]. This corroborates the validity of the model and obtained results.
The inherent instability of protofibrils converting into fibrils greatly complicates their structural investigations. To circumvent this problem, Aβ1–42cc peptide was engineered to be incapable of forming mature fibrils due to a disulfide bond locking it in fibril-incompatible conformation, which does not prevent protofibril formation [141]. Conformation and packing of such protofibrils were investigated by solid-state NMR spectroscopy, revealing the formation of hexameric barrel-like oligomers within the protofibril with residues 16 to 42 of Aβ1–42cc participating in intermonomeric contacts. The core of the oligomers consisted of all residues of the Aβ central and C-terminal hydrophobic regions, with hairpin loops extended from the core. The model accounts for why Aβ1–42 forms oligomers and protofibrils more easily than Aβ1–40. Protofibrils formed by Aβ1–42cc are indistinguishable from wild-type Ab42 protofibrils with respect to many properties: size and morphology as observed by electron microscopy and atomic force microscopy, binding of conformation-specific antibodies and the ANS dye, circular dichroism and infrared spectra, the ability to induce apoptosis in neuroblastoma cell lines, and the ability to attenuate spontaneous synaptic activity in primary neurons [141,142].
Solution NMR studies using DPC as a membrane-mimicking environment revealed an entirely different structure of the oligomeric subunit formed by Aβ1–42 (PDB: 6RHY) [143]. In these conditions, peptides formed tetramers consisting of structurally distinct subunits. The first subunit type comprises a β-hairpin made of two β-strands—β1 and β2, G9 through Ala21 and Gly29 through Val40, respectively. In the subunits of the second type, Leu17 through Phe20 residues form a short α-helix, whereas Gly29-Ile41 make up another β-strand, β3. The tetramer core thus consists of six β-strands linked with only two β-turns, along with two short and two long (including an α-helical segment) flexible N-terminal sequences. Additional experiments were performed with the use of a mixture of Aβ1–42 and Aβ17–42 devoid of the segment forming the α-helix in type two subunit and incapable of folding into the β-hairpin of the first type. With the aid of molecular modeling and SEC/IM-MS, the resultant tetramers were found to be able to spontaneously form β-sandwich octamers [143]. Molecular modeling augmented with electrophysiological measurements demonstrated the ability of these oligomers to form conductive pores in lipid bilayers. Molecular dynamics simulations [144] allowed mapping possible transitions between several predominant geometrically diverse conformation states of tetrameric Aβ, having substantially lower β-structure content compared to fibrillary Aβ.
Aβ aggregation into fibrillary structures depositing in the form of senile plaques has not been directly linked to any of major AD symptoms and may be even viewed as a relatively safe disposal pathway for excessively accumulated Aβ. The fibrillation rates are strongly dependent upon Aβ concentration, and Aβ1–42 can nucleate rapid aggregation of slow-aggregating solutions of other isoforms [145,146]. Extremely high mechanical and thermodynamic stability of mature fibrils in comparison with oligomers deduced from multiple simulations and obtained experimentally [147,148,149] indirectly confirms this notion. Structural information about the fibrils was first obtained by solution and solid-state NMR methods [150,151,152,153,154] augmented by electron microscopy. Several distinct structural patterns were identified with different global folds characterized by uni-, bi-, or triaxial symmetry, sharing a common β-strand–β-turn–β-strand motif, as well as stabilizing intra- and intermolecular contacts between parallel or antiparallel β-strands. In some structures, the residues constituting Aβ metal-binding domain were disordered; in others, they were elements of common β-structure. A similar structure was obtained by solid-state NMR when biological material from the brains of patients with clinically confirmed AD diagnosis was used as seeds for growing the fibrils [152]. This corroborates the biological relevance of the information obtained in other structural studies, taking into account the differences associated with the use of diverse Aβ species, initial oligomeric states, and polymerization conditions [153].
Cryo-electron microscopy (cryo-EM) (4.0 Å) structure of Aβ1–42 fibril composed of two intertwined protofilaments was recently obtained by cryo-electron microscopy (cryo-EM) complemented by solid-state nuclear magnetic resonance (NMR) experiments (PDB: 6RHY) [155]. The backbone and nearly all sidechains were clearly resolved, including the entire N-terminal metal-binding domain, which is a part of the cross-β structure, resulting in an overall “LS”-shaped topology of individual non-planar dimeric subunits of the fibrils. The dimerization interface protects the hydrophobic C-termini from the solvent. The regular helical symmetry has direct implications for the mechanism of fibril elongation and results in distinct binding sites for monomeric Aβ, including contacts across different subunit layers. The unique staggering of the non-planar subunits results in markedly different fibril ends, termed “groove” and “ridge”, leading to different binding pathways on the fibril ends, which has implications for fibril growth.
Thus, the development of the methods of investigation of biomolecule spatial structure allowed obtaining a detailed description of the molecular mechanisms of production of β-amyloid peptides, including the sequence of the events occurring during sequential proteolysis of APP protein. A number of factors contributing to AD development identified based on the accumulated structural information can be used to guide future development of effective compounds for the early diagnosis and effective treatment of AD.
7. Protein-Protein Interactions Targeting Pathological Traits of Aβ Forms and Aggregates
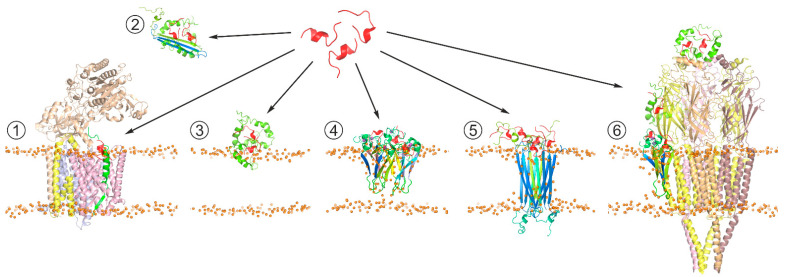
Although Aβ has not been positively proved to be the main causative agent of AD, Aβ production or aggregation is often targeted in therapy development, and hence a lot of AD therapeutic research has been focused on Aβ and its life cycle [156,157,158,159,160]. Inhibition or modulation of APP secretase cleavage as AD therapy appears extremely difficult to safely achieve due to the relatively low specificity of secretases themselves and the necessity of Aβ production for multiple normal neuronal processes. In the light of the growing evidence of intermediate Aβ oligomers being the primary neurotoxic agents, the compounds able to affect Aβ aggregation are being increasingly intensively explored. The accumulated results of structural investigations indicate that Aβ aggregation is essentially driven or modulated by protein-protein and protein-lipid interactions, along with metal cation chelation. Among these, protein-protein interaction appears to be the most promising target for achieving the required selectivity, including discrimination between different Aβ aggregation forms and conformations (Figure 3). From a practical standpoint, short peptides possess several unique attractive properties compared to other small molecule drugs or to biological macromolecules, including a larger adoptable interaction interface with higher affinity and specificity than in small molecules, lower immunogenicity, lower manufacturing cost, ease of delivery to destination tissues, and greater access to chemical diversity than in antibodies [161,162]. In the search for peptides (or peptide-derived compounds) that inhibit Aβ aggregation, at least three general approaches have been taken.
Figure 3.
Schematic representation of the generic strategy for search of peptide-based therapeutic agents affecting Aβ production pathways and/or suppressing neurotoxic effects of Aβ oligomers. The therapeutic agent (shown in red) can (1) act at the stage of APP processing through interaction with Aβ precursors (in green) processed by γ-secretase complex (PDB: 6IYC), modifying production of mature Aβ isoforms; (2) bind soluble Aβ aggregates, suppressing their toxicity; (3) interfere with interactions of toxic Aβ aggregates with membrane surface, inhibiting their conversion into β-structured membrane-bound oligomers; (4) modify toxic properties of membrane-incorporated Aβ oligomers associated with membrane permeabilization [140]; (5) target pore-like transmembrane structures formed by Aβ oligomers (PDB: 6RHY), e.g., inhibiting transmembrane transport of cations; and (6) prevent Aβ from inducing abnormal functioning of soluble and membrane-associated proteins, e.g., nicotinic acetylcholine receptor (PDB: 2BG9), which can be inhibited by diverse Aβ oligomers in different manners.
In one approach, peptides are designed to interact with Aβ through self-complementary sequences, with the most common targets currently including Aβ16–20 KLVFF and Aβ31–34 IIGL sequences situated in the key amyloidogenic APP JM region and the N-terminal half of the APP TM segment. Numerous applications of variations of this approach have been published, intended to perturb toxic Aβ oligomer formation [163]. Alternatively, the search for anti-Aβ peptides can be based on using proteins that are known to be natural Aβ binders as templates, including full-length naturally occurring neuroprotectors and fragments of receptors capable of recognizing Aβ. Specifically, a cyclic peptide corresponding to the Aβ binding domain of the transthyretin, a stable homotetrameric transport protein circulating in blood and cerebrospinal fluid, has been shown to be neuroprotective against Aβ toxicity in vitro and suppress Aβ aggregation [164,165]. One more example of such decoy peptides could be represented by a peptide corresponding to the 60–76 sequence from the V-domain of the receptor for advanced glycation end products (RAGE) [166,167]. Its therapeutic efficacy is likely to be related to its competition with the RAGE V-domain for binding to Aβ and involvement in Aβ clearance in the brain. Likewise, the HAEE tetrapeptide corresponding to residues 35–38 of nAChR located in the extracellular part of α4 subunit can prevent nAChR interaction with Aβ by binding to the Aβ11–14 metal-binding region. Thus, it is suggested as a potential therapeutic for the treatment of α4β2 nAChR-dependent cholinergic dysfunction in AD [168]. Another strategy is based on the screening of peptide libraries for binding to full-length Aβ, often using phage display or mirror-image phage display techniques [163]. Library screening requires little or no prior knowledge of desired sequence or structural features. A unique advantage of the mirror phage display process is its ability to identify peptides solely consisting of d-enantiomeric amino acid residues, which makes them highly resistant to proteases, with a consequential dramatic increase in their in vivo lifetime. Additionally, d-enantiomeric peptides can be absorbed systemically after oral administration. The immunogenicity of d-enantiomeric peptides is reported to be reduced in comparison to l-enantiomeric peptides [169]. Such peptides were shown to bind the amyloid form of Aβ and label amyloid plaques in AD brain slices; they can also serve as carrier proteins for plaque treatment and in vivo imaging. Many of the peptides derived to date belong to a broad class of β-sheet breakers, aiming to interfere with the Aβ fibrilization process. An interesting alternative is illustrated by a highly specific d-enantiomeric ligand for Aβ identified through screening of a randomized library of over a billion dodecamer peptides, referred to as D3 [170,171,172]. This peptide, along with a number of its more recently obtained derivatives, is capable of transient protein-protein interactions with certain Aβ forms in an IDP/IDP (intrinsically disordered peptide) manner [173], which allows adapting to various potentially toxic oligomeric forms of Aβ, disfavoring formation of intermolecular contacts between Aβ monomers.
The recent progress in the investigation of structural properties of APP and its derivatives, their aggregated forms, and their complexes with relevant biological and pharmaceutical molecules has notably advanced our understanding of the molecular bases of pathogenic processes associated with AD and has drawn a clearer demarcation line between them and the normal functions of APP processing products. Besides suggesting new classes of potential therapeutic and diagnostic agents, this information is also crucial for identifying potential traps and pitfalls associated with undesirable interference with essential normal functions that would make the designed pharmaceuticals unsafe to use.
Acknowledgments
The authors express their sincere thanks to K.A. Beirit for helpful discussions.
Author Contributions
The manuscript was written through author contributions as follows: Section 2, Section 3, Section 4, Section 5, Section 6 and Section 7 by A.S.U. and K.V.P.; Section 1, Section 4, Section 5, Section 6 and Section 7 by A.V.K., I.S.O., A.S.A. and E.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Russian Science Foundation grants #19-15-00427 (preparation of Section 2, Section 3 and Section 4) and #20-64-46027 (preparation of Section 1, Section 4, Section 5, Section 6 and Section 7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association 2016 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. J. Alzheimers Assoc. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Braak H., Braak E. Alzheimer’s Disease: Striatal Amyloid Deposits and Neurofibrillary Changes. J. Neuropathol. Exp. Neurol. 1990;49:215–224. doi: 10.1097/00005072-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe D.J., Hardy J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm M.O.W., Mett J., Grimm H.S., Hartmann T. APP Function and Lipids: A Bidirectional Link. Front. Mol. Neurosci. 2017;10:63. doi: 10.3389/fnmol.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew H., Solomon V.A., Fonteh A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020;11:598. doi: 10.3389/fphys.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maltsev A.V., Bystryak S., Galzitskaya O.V. The Role of β-Amyloid Peptide in Neurodegenerative Diseases. Ageing Res. Rev. 2011;10:440–452. doi: 10.1016/j.arr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Cummings J.L., Kaufer D. Neuropsychiatric Aspects of Alzheimer’s Disease: The Cholinergic Hypothesis Revisited. Neurology. 1996;47:876–883. doi: 10.1212/WNL.47.4.876. [DOI] [PubMed] [Google Scholar]
- 8.Chun W., Johnson G.V.W. The Role of Tau Phosphorylation and Cleavage in Neuronal Cell Death. Front. Biosci. J. Virtual Libr. 2007;12:733–756. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]
- 9.Yang G., Zhou R., Zhou Q., Guo X., Yan C., Ke M., Lei J., Shi Y. Structural Basis of Notch Recognition by Human γ-Secretase. Nature. 2019;565:192–197. doi: 10.1038/s41586-018-0813-8. [DOI] [PubMed] [Google Scholar]
- 10.Hardy J., Allsop D. Amyloid Deposition as the Central Event in the Aetiology of Alzheimer’s Disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-V. [DOI] [PubMed] [Google Scholar]
- 11.Mudher A., Lovestone S. Alzheimer’s Disease—Do Tauists and Baptists Finally Shake Hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/S0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 12.Chávez-Gutiérrez L., Szaruga M. Mechanisms of Neurodegeneration—Insights from Familial Alzheimer’s Disease. Semin. Cell Dev. Biol. 2020;105:75–85. doi: 10.1016/j.semcdb.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Szaruga M., Munteanu B., Lismont S., Veugelen S., Horré K., Mercken M., Saido T.C., Ryan N.S., De Vos T., Savvides S.N., et al. Alzheimer’s-Causing Mutations Shift Aβ Length by Destabilizing γ-Secretase-Aβn Interactions. Cell. 2017;170:443–456.e14. doi: 10.1016/j.cell.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Lott I.T., Head E. Alzheimer Disease and Down Syndrome: Factors in Pathogenesis. Neurobiol. Aging. 2005;26:383–389. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Selkoe D.J. A Mechanistic Hypothesis for the Impairment of Synaptic Plasticity by Soluble Aβ Oligomers from Alzheimer’s Brain. J. Neurochem. 2020;154:583–597. doi: 10.1111/jnc.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuffrida M.L., Caraci F., Pignataro B., Cataldo S., De Bona P., Bruno V., Molinaro G., Pappalardo G., Messina A., Palmigiano A., et al. Beta-Amyloid Monomers Are Neuroprotective. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soscia S.J., Kirby J.E., Washicosky K.J., Tucker S.M., Ingelsson M., Hyman B., Burton M.A., Goldstein L.E., Duong S., Tanzi R.E., et al. The Alzheimer’s Disease-Associated Amyloid Beta-Protein Is an Antimicrobial Peptide. PLoS ONE. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar D.K.V., Choi S.H., Washicosky K.J., Eimer W.A., Tucker S., Ghofrani J., Lefkowitz A., McColl G., Goldstein L.E., Tanzi R.E., et al. Amyloid-β Peptide Protects against Microbial Infection in Mouse and Worm Models of Alzheimer’s Disease. Sci. Transl. Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosztyla M.L., Brothers H.M., Robinson S.R. Alzheimer’s Amyloid-β Is an Antimicrobial Peptide: A Review of the Evidence. J. Alzheimers Dis. JAD. 2018;62:1495–1506. doi: 10.3233/JAD-171133. [DOI] [PubMed] [Google Scholar]
- 20.Deyts C., Thinakaran G., Parent A.T. APP Receptor? To Be or Not To Be. Trends Pharmacol. Sci. 2016;37:390–411. doi: 10.1016/j.tips.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beel A.J., Mobley C.K., Kim H.J., Tian F., Hadziselimovic A., Jap B., Prestegard J.H., Sanders C.R. Structural Studies of the Transmembrane C-Terminal Domain of the Amyloid Precursor Protein (APP): Does APP Function as a Cholesterol Sensor? Biochemistry. 2008;47:9428–9446. doi: 10.1021/bi800993c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duce J.A., Tsatsanis A., Cater M.A., James S.A., Robb E., Wikhe K., Leong S.L., Perez K., Johanssen T., Greenough M.A., et al. Iron-Export Ferroxidase Activity of β-Amyloid Precursor Protein Is Inhibited by Zinc in Alzheimer’s Disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miklós I., Zádori Z. Positive Evolutionary Selection of an HD Motif on Alzheimer Precursor Protein Orthologues Suggests a Functional Role. PLoS Comput. Biol. 2012;8:e1002356. doi: 10.1371/journal.pcbi.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiese M., Antebi A., Zheng H. Intracellular Trafficking and Synaptic Function of APL-1 in Caenorhabditis Elegans. PLoS ONE. 2010;5:e12790. doi: 10.1371/journal.pone.0012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poeck B., Strauss R., Kretzschmar D. Analysis of Amyloid Precursor Protein Function in Drosophila Melanogaster. Exp. Brain Res. 2012;217:413–421. doi: 10.1007/s00221-011-2860-3. [DOI] [PubMed] [Google Scholar]
- 26.Anliker B., Müller U. The Functions of Mammalian Amyloid Precursor Protein and Related Amyloid Precursor-like Proteins. Neurodegener. Dis. 2006;3:239–246. doi: 10.1159/000095262. [DOI] [PubMed] [Google Scholar]
- 27.White A.R., Reyes R., Mercer J.F., Camakaris J., Zheng H., Bush A.I., Multhaup G., Beyreuther K., Masters C.L., Cappai R. Copper Levels Are Increased in the Cerebral Cortex and Liver of APP and APLP2 Knockout Mice. Brain Res. 1999;842:439–444. doi: 10.1016/S0006-8993(99)01861-2. [DOI] [PubMed] [Google Scholar]
- 28.Grimm M.O.W., Grimm H.S., Pätzold A.J., Zinser E.G., Halonen R., Duering M., Tschäpe J.A., De Strooper B., Müller U., Shen J., et al. Regulation of Cholesterol and Sphingomyelin Metabolism by Amyloid-Beta and Presenilin. Nat. Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 29.Muller U.C., Zheng H. Physiological Functions of APP Family Proteins. Cold Spring Harb. Perspect. Med. 2012;2:a006288. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ring S., Weyer S.W., Kilian S.B., Waldron E., Pietrzik C.U., Filippov M.A., Herms J., Buchholz C., Eckman C.B., Korte M., et al. The Secreted -Amyloid Precursor Protein Ectodomain APPs Is Sufficient to Rescue the Anatomical, Behavioral, and Electrophysiological Abnormalities of APP-Deficient Mice. J. Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herms J., Anliker B., Heber S., Ring S., Fuhrmann M., Kretzschmar H., Sisodia S., Muller U. Cortical Dysplasia Resembling Human Type 2 Lissencephaly in Mice Lacking All Three APP Family Members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selkoe D.J. Cell Biology of the Amyloid Beta-Protein Precursor and the Mechanism of Alzheimer’s Disease. Annu. Rev. Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- 33.Gralle M., Ferreira S.T. Structure and Functions of the Human Amyloid Precursor Protein: The Whole Is More than the Sum of Its Parts. Prog. Neurobiol. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Shih I.-M., Wang T.-L. Notch Signaling, Gamma-Secretase Inhibitors, and Cancer Therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 35.Kaiser D.M., Acharya M., Leighton P.L.A., Wang H., Daude N., Wohlgemuth S., Shi B., Allison W.T. Amyloid Beta Precursor Protein and Prion Protein Have a Conserved Interaction Affecting Cell Adhesion and CNS Development. PLoS ONE. 2012;7:e51305. doi: 10.1371/journal.pone.0051305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh A.K., Brindisi M., Tang J. Developing β-Secretase Inhibitors for Treatment of Alzheimer’s Disease. J. Neurochem. 2012;120:71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamayev R., Matsuda S., Arancio O., D’Adamio L. β- but Not γ-Secretase Proteolysis of APP Causes Synaptic and Memory Deficits in a Mouse Model of Dementia: SAPPβ/β-CTF and Not Aβ Cause Memory Deficits. EMBO Mol. Med. 2012;4:171–179. doi: 10.1002/emmm.201100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epand R.M. Cholesterol and the Interaction of Proteins with Membrane Domains. Prog. Lipid Res. 2006;45:279–294. doi: 10.1016/j.plipres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Marenchino M., Williamson P.T.F., Murri S., Zandomeneghi G., Wunderli-Allenspach H., Meier B.H., Krämer S.D. Dynamics and Cleavability at the Alpha-Cleavage Site of APP(684-726) in Different Lipid Environments. Biophys. J. 2008;95:1460–1473. doi: 10.1529/biophysj.108.129726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter L., Munter L.-M., Ness J., Hildebrand P.W., Dasari M., Unterreitmeier S., Bulic B., Beyermann M., Gust R., Reif B. Amyloid Beta 42 Peptide (Aβ42)-Lowering Compounds Directly Bind to Aβ and Interfere with Amyloid Precursor Protein (APP) Transmembrane Dimerization. Proc. Natl. Acad. Sci. USA. 2010;107:14597–14602. doi: 10.1073/pnas.1003026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossjohn J., Cappai R., Feil S.C., Henry A., McKinstry W.J., Galatis D., Hesse L., Multhaup G., Beyreuther K., Masters C.L., et al. Crystal Structure of the N-Terminal, Growth Factor-like Domain of Alzheimer Amyloid Precursor Protein. Nat. Struct. Biol. 1999;6:327–331. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- 42.Barnham K.J., McKinstry W.J., Multhaup G., Galatis D., Morton C.J., Curtain C.C., Williamson N.A., White A.R., Hinds M.G., Norton R.S., et al. Structure of the Alzheimer’s Disease Amyloid Precursor Protein Copper Binding Domain: A REGULATOR OF NEURONAL COPPER HOMEOSTASIS. J. Biol. Chem. 2003;278:17401–17407. doi: 10.1074/jbc.M300629200. [DOI] [PubMed] [Google Scholar]
- 43.Nadezhdin K.D., Bocharova O.V., Bocharov E.V., Arseniev A.S. Dimeric Structure of Transmembrane Domain of Amyloid Precursor Protein in Micellar Environment. FEBS Lett. 2012;586:1687–1692. doi: 10.1016/j.febslet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 44.Istrate A.N., Kozin S.A., Zhokhov S.S., Mantsyzov A.B., Kechko O.I., Pastore A., Makarov A.A., Polshakov V.I. Interplay of Histidine Residues of the Alzheimer’s Disease Aβ Peptide Governs Its Zn-Induced Oligomerization. Sci. Rep. 2016;6:21734. doi: 10.1038/srep21734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin X., Koelsch G., Wu S., Downs D., Dashti A., Tang J. Human Aspartic Protease Memapsin 2 Cleaves the β-Secretase Site of β-Amyloid Precursor Protein. Proc. Natl. Acad. Sci. USA. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bittner H.J., Guixà-González R., Hildebrand P.W. Structural Basis for the Interaction of the Beta-Secretase with Copper. Biochim. Biophys. Acta BBA Biomembr. 2018;1860:1105–1113. doi: 10.1016/j.bbamem.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Li Y., Xu H., Zhang Y. The γ-Secretase Complex: From Structure to Function. Front. Cell. Neurosci. 2014;8 doi: 10.3389/fncel.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gralle M., Oliveira C.L.P., Guerreiro L.H., McKinstry W.J., Galatis D., Masters C.L., Cappai R., Parker M.W., Ramos C.H.I., Torriani I., et al. Solution Conformation and Heparin-Induced Dimerization of the Full-Length Extracellular Domain of the Human Amyloid Precursor Protein. J. Mol. Biol. 2006;357:493–508. doi: 10.1016/j.jmb.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 49.Svergun D.I., Petoukhov M.V., Koch M.H. Determination of Domain Structure of Proteins from X-Ray Solution Scattering. Biophys. J. 2001;80:2946–2953. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konarev P.V., Petoukhov M.V., Svergun D.I. MASSHA—A Graphics System for Rigid-Body Modelling of Macromolecular Complexes against Solution Scattering Data. J. Appl. Crystallogr. 2001;34:527–532. doi: 10.1107/S0021889801006100. [DOI] [Google Scholar]
- 51.Scheidig A.J., Hynes T.R., Pelletier L.A., Wells J.A., Kossiakoff A.A. Crystal Structures of Bovine Chymotrypsin and Trypsin Complexed to the Inhibitor Domain of Alzheimer’s Amyloid β-Protein Precursor (APPI) and Basic Pancreatic Trypsin Inhibitor (BPTI): Engineering of Inhibitors with Altered Specificities. Protein Sci. 1997;6:1806–1824. doi: 10.1002/pro.5560060902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsvetkov P.O., Kulikova A.A., Golovin A.V., Tkachev Y.V., Archakov A.I., Kozin S.A., Makarov A.A. Minimal Zn(2+) Binding Site of Amyloid-β. Biophys. J. 2010;99:L84–L86. doi: 10.1016/j.bpj.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozin S.A., Mezentsev Y.V., Kulikova A.A., Indeykina M.I., Golovin A.V., Ivanov A.S., Tsvetkov P.O., Makarov A.A. Zinc-Induced Dimerization of the Amyloid-β Metal-Binding Domain 1-16 Is Mediated by Residues 11-14. Mol. Biosyst. 2011;7:1053–1055. doi: 10.1039/c0mb00334d. [DOI] [PubMed] [Google Scholar]
- 54.Nadezhdin K.D., Bocharova O.V., Bocharov E.V., Arseniev A.S. Structural and Dynamic Study of the Transmembrane Domain of the Amyloid Precursor Protein. Acta Nat. 2011;3:69–76. doi: 10.32607/20758251-2011-3-1-69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackenzie K.R. Folding and Stability of Alpha-Helical Integral Membrane Proteins. Chem. Rev. 2006;106:1931–1977. doi: 10.1021/cr0404388. [DOI] [PubMed] [Google Scholar]
- 56.Barrett P.J., Song Y., Van Horn W.D., Hustedt E.J., Schafer J.M., Hadziselimovic A., Beel A.J., Sanders C.R. The Amyloid Precursor Protein Has a Flexible Transmembrane Domain and Binds Cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Götz A., Mylonas N., Högel P., Silber M., Heinel H., Menig S., Vogel A., Feyrer H., Huster D., Luy B., et al. Modulating Hinge Flexibility in the APP Transmembrane Domain Alters γ-Secretase Cleavage. Biophys. J. 2019;116:2103–2120. doi: 10.1016/j.bpj.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bocharov E.V., Nadezhdin K.D., Urban A.S., Volynsky P.E., Pavlov K.V., Efremov R.G., Arseniev A.S., Bocharova O.V. Familial L723P Mutation Can Shift the Distribution between the Alternative APP Transmembrane Domain Cleavage Cascades by Local Unfolding of the Ε-Cleavage Site Suggesting a Straightforward Mechanism of Alzheimer’s Disease Pathogenesis. ACS Chem. Biol. 2019;14:1573–1582. doi: 10.1021/acschembio.9b00309. [DOI] [PubMed] [Google Scholar]
- 59.Itkin A., Salnikov E.S., Aisenbrey C., Raya J., Glattard E., Raussens V., Ruysschaert J.-M., Bechinger B. Structural Characterization of the Amyloid Precursor Protein Transmembrane Domain and Its γ-Cleavage Site. ACS Omega. 2017;2:6525–6534. doi: 10.1021/acsomega.7b00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen W., Gamache E., Rosenman D.J., Xie J., Lopez M.M., Li Y.-M., Wang C. Familial Alzheimer’s Mutations within APPTM Increase Aβ42 Production by Enhancing Accessibility of ε-Cleavage Site. Nat. Commun. 2014;5:3037. doi: 10.1038/ncomms4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato T., Tang T.-C., Reubins G., Fei J.Z., Fujimoto T., Kienlen-Campard P., Constantinescu S.N., Octave J.-N., Aimoto S., Smith S.O. A Helix-to-Coil Transition at the Epsilon-Cut Site in the Transmembrane Dimer of the Amyloid Precursor Protein Is Required for Proteolysis. Proc. Natl. Acad. Sci. USA. 2009;106:1421–1426. doi: 10.1073/pnas.0812261106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dominguez L., Foster L., Straub J.E., Thirumalai D. Impact of Membrane Lipid Composition on the Structure and Stability of the Transmembrane Domain of Amyloid Precursor Protein. Proc. Natl. Acad. Sci. USA. 2016;113:E5281–E5287. doi: 10.1073/pnas.1606482113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eggert S., Midthune B., Cottrell B., Koo E.H. Induced Dimerization of the Amyloid Precursor Protein (APP) Leads to Decreased Amyloid-Beta Protein (Abeta) Production. J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.038646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunter S., Brayne C. Understanding the Roles of Mutations in the Amyloid Precursor Protein in Alzheimer Disease. Mol. Psychiatry. 2018;23:81–93. doi: 10.1038/mp.2017.218. [DOI] [PubMed] [Google Scholar]
- 65.Langosch D., Scharnagl C., Steiner H., Lemberg M.K. Understanding Intramembrane Proteolysis: From Protein Dynamics to Reaction Kinetics. Trends Biochem. Sci. 2015;40:318–327. doi: 10.1016/j.tibs.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Tang T.-C., Hu Y., Kienlen-Campard P., El Haylani L., Decock M., Van Hees J., Fu Z., Octave J.-N., Constantinescu S.N., Smith S.O. Conformational Changes Induced by the A21G Flemish Mutation in the Amyloid Precursor Protein Lead to Increased Aβ Production. Structure. 2014;22:387–396. doi: 10.1016/j.str.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polshakov V.I., Mantsyzov A.B., Kozin S.A., Adzhubei A.A., Zhokhov S.S., van Beek W., Kulikova A.A., Indeykina M.I., Mitkevich V.A., Makarov A.A. A Binuclear Zinc Interaction Fold Discovered in the Homodimer of Alzheimer’s Amyloid-β Fragment with Taiwanese Mutation D7H. Angew. Chem. Int. Ed. Engl. 2017;56:11734–11739. doi: 10.1002/anie.201704615. [DOI] [PubMed] [Google Scholar]
- 68.Götz A., Högel P., Silber M., Chaitoglou I., Luy B., Muhle-Goll C., Scharnagl C., Langosch D. Increased H-Bond Stability Relates to Altered ε-Cleavage Efficiency and Aβ Levels in the I45T Familial Alzheimer’s Disease Mutant of APP. Sci. Rep. 2019;9:5321. doi: 10.1038/s41598-019-41766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramelot T.A., Gentile L.N., Nicholson L.K. Transient Structure of the Amyloid Precursor Protein Cytoplasmic Tail Indicates Preordering of Structure for Binding to Cytosolic Factors. Biochemistry. 2000;39:2714–2725. doi: 10.1021/bi992580m. [DOI] [PubMed] [Google Scholar]
- 70.Radzimanowski J., Simon B., Sattler M., Beyreuther K., Sinning I., Wild K. Structure of the Intracellular Domain of the Amyloid Precursor Protein in Complex with Fe65-PTB2. EMBO Rep. 2008;9:1134–1140. doi: 10.1038/embor.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H., Koshiba S., Hayashi F., Tochio N., Tomizawa T., Kasai T., Yabuki T., Motoda Y., Harada T., Watanabe S., et al. Structure of the C-Terminal Phosphotyrosine Interaction Domain of Fe65L1 Complexed with the Cytoplasmic Tail of Amyloid Precursor Protein Reveals a Novel Peptide Binding Mode. J. Biol. Chem. 2008;283:27165–27178. doi: 10.1074/jbc.M803892200. [DOI] [PubMed] [Google Scholar]
- 72.Shu R., Wong W., Ma Q.H., Yang Z.Z., Zhu H., Liu F.J., Wang P., Ma J., Yan S., Polo J.M., et al. APP Intracellular Domain Acts as a Transcriptional Regulator of MiR-663 Suppressing Neuronal Differentiation. Cell Death Dis. 2015;6:e1651. doi: 10.1038/cddis.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Copenhaver P.F., Kögel D. Role of APP Interactions with Heterotrimeric G Proteins: Physiological Functions and Pathological Consequences. Front. Mol. Neurosci. 2017;10:3. doi: 10.3389/fnmol.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou R., Yang G., Shi Y. Macromolecular Complex in Recognition and Proteolysis of Amyloid Precursor Protein in Alzheimer’s Disease. Curr. Opin. Struct. Biol. 2020;61:1–8. doi: 10.1016/j.sbi.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Seegar T.C.M., Killingsworth L.B., Saha N., Meyer P.A., Patra D., Zimmerman B., Janes P.W., Rubinstein E., Nikolov D.B., Skiniotis G., et al. Structural Basis for Regulated Proteolysis by the α-Secretase ADAM10. Cell. 2017;171:1638–1648.e7. doi: 10.1016/j.cell.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhn P.-H., Koroniak K., Hogl S., Colombo A., Zeitschel U., Willem M., Volbracht C., Schepers U., Imhof A., Hoffmeister A., et al. Secretome Protein Enrichment Identifies Physiological BACE1 Protease Substrates in Neurons. EMBO J. 2012;31:3157–3168. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber S., Saftig P. Ectodomain Shedding and ADAMs in Development. Development. 2012;139:3693–3709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- 78.Asai M., Hattori C., Szabó B., Sasagawa N., Maruyama K., Tanuma S., Ishiura S. Putative Function of ADAM9, ADAM10, and ADAM17 as APP Alpha-Secretase. Biochem. Biophys. Res. Commun. 2003;301:231–235. doi: 10.1016/S0006-291X(02)02999-6. [DOI] [PubMed] [Google Scholar]
- 79.Kuhn P.-H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J.W., Kremmer E., Roßner S., Lichtenthaler S.F. ADAM10 Is the Physiologically Relevant, Constitutive α-Secretase of the Amyloid Precursor Protein in Primary Neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vassar R. ADAM10 Prodomain Mutations Cause Late-Onset Alzheimer’s Disease: Not Just the Latest FAD. Neuron. 2013;80:250–253. doi: 10.1016/j.neuron.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmed R.R., Holler C.J., Webb R.L., Li F., Beckett T.L., Murphy M.P. BACE1 and BACE2 Enzymatic Activities in Alzheimer’s Disease. J. Neurochem. 2010;112:1045–1053. doi: 10.1111/j.1471-4159.2009.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z., Xu Q., Cai F., Liu X., Wu Y., Song W. BACE2, a Conditional β-Secretase, Contributes to Alzheimer’s Disease Pathogenesis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willem M., Garratt A.N., Novak B., Citron M., Kaufmann S., Rittger A., DeStrooper B., Saftig P., Birchmeier C., Haass C. Control of Peripheral Nerve Myelination by the Beta-Secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 84.Hu X., Hicks C.W., He W., Wong P., Macklin W.B., Trapp B.D., Yan R. Bace1 Modulates Myelination in the Central and Peripheral Nervous System. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 85.Hu X., He W., Luo X., Tsubota K.E., Yan R. BACE1 Regulates Hippocampal Astrogenesis via the Jagged1-Notch Pathway. Cell Rep. 2013;4:40–49. doi: 10.1016/j.celrep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim D.Y., Carey B.W., Wang H., Ingano L.A.M., Binshtok A.M., Wertz M.H., Pettingell W.H., He P., Lee V.M.-Y., Woolf C.J., et al. BACE1 Regulates Voltage-Gated Sodium Channels and Neuronal Activity. Nat. Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sachse C.C., Kim Y.H., Agsten M., Huth T., Alzheimer C., Kovacs D.M., Kim D.Y. BACE1 and Presenilin/γ-Secretase Regulate Proteolytic Processing of KCNE1 and 2, Auxiliary Subunits of Voltage-Gated Potassium Channels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013;27:2458–2467. doi: 10.1096/fj.12-214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venugopal C., Demos C.M., Rao K.S.J., Pappolla M.A., Sambamurti K. Beta-Secretase: Structure, Function, and Evolution. CNS Neurol. Disord. Drug Targets. 2008;7:278–294. doi: 10.2174/187152708784936626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jonsson T., Atwal J.K., Steinberg S., Snaedal J., Jonsson P.V., Bjornsson S., Stefansson H., Sulem P., Gudbjartsson D., Maloney J., et al. A Mutation in APP Protects against Alzheimer’s Disease and Age-Related Cognitive Decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 90.Wolfe M.S., Xia W., Ostaszewski B.L., Diehl T.S., Kimberly W.T., Selkoe D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 91.Xia W. Intramembrane Proteolysis by Presenilin and Presenilin-like Proteases. J. Cell Sci. 2003;116:2839–2844. doi: 10.1242/jcs.00651. [DOI] [PubMed] [Google Scholar]
- 92.Haapasalo A., Kovacs D.M. The Many Substrates of Presenilin/γ-Secretase. J. Alzheimers Dis. JAD. 2011;25:3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemming M.L., Elias J.E., Gygi S.P., Selkoe D.J. Identification of Beta-Secretase (BACE1) Substrates Using Quantitative Proteomics. PLoS ONE. 2009;4:e8477. doi: 10.1371/journal.pone.0008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song M.S., Baker G.B., Todd K.G., Kar S. Inhibition of β-Amyloid1-42 Internalization Attenuates Neuronal Death by Stabilizing the Endosomal-Lysosomal System in Rat Cortical Cultured Neurons. Neuroscience. 2011;178:181–188. doi: 10.1016/j.neuroscience.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 95.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin Generate an Active Gamma-Secretase Complex. Neuron. 2003;38:9–12. doi: 10.1016/S0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 96.Shea Y.-F., Chu L.-W., Chan A.O.-K., Ha J., Li Y., Song Y.-Q. A Systematic Review of Familial Alzheimer’s Disease: Differences in Presentation of Clinical Features among Three Mutated Genes and Potential Ethnic Differences. J. Formos. Med. Assoc. 2016;115:67–75. doi: 10.1016/j.jfma.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 97.Wolfe M.S. Substrate Recognition and Processing by γ-Secretase. Biochim. Biophys. Acta Biomembr. 2020;1862:183016. doi: 10.1016/j.bbamem.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolfe M.S. Structure, Mechanism and Inhibition of Gamma-Secretase and Presenilin-like Proteases. Biol. Chem. 2010;391:839–847. doi: 10.1515/bc.2010.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan I., Krishnaswamy S., Sabale M., Groth D., Wijaya L., Morici M., Berger I., Schaffitzel C., Fraser P.E., Martins R.N., et al. Efficient Production of a Mature and Functional Gamma Secretase Protease. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-30788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou R., Yang G., Guo X., Zhou Q., Lei J., Shi Y. Recognition of the Amyloid Precursor Protein by Human γ-Secretase. Science. 2019;363 doi: 10.1126/science.aaw0930. [DOI] [PubMed] [Google Scholar]
- 101.Güner G., Lichtenthaler S.F. The Substrate Repertoire of γ-Secretase/Presenilin. Semin. Cell Dev. Biol. 2020;105:27–42. doi: 10.1016/j.semcdb.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Copani A. The Underexplored Question of β-Amyloid Monomers. Eur. J. Pharmacol. 2017;817:71–75. doi: 10.1016/j.ejphar.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 103.Parihar M.S., Brewer G.J. Amyloid-β as a Modulator of Synaptic Plasticity. J. Alzheimers Dis. JAD. 2010;22:741–763. doi: 10.3233/JAD-2010-101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H., Megill A., He K., Kirkwood A., Lee H.-K. Consequences of Inhibiting Amyloid Precursor Protein Processing Enzymes on Synaptic Function and Plasticity. Neural Plast. 2012;2012:272374. doi: 10.1155/2012/272374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giuffrida M.L., Caraci F., De Bona P., Pappalardo G., Nicoletti F., Rizzarelli E., Copani A. The Monomer State of Beta-Amyloid: Where the Alzheimer’s Disease Protein Meets Physiology. Rev. Neurosci. 2010;21 doi: 10.1515/REVNEURO.2010.21.2.83. [DOI] [PubMed] [Google Scholar]
- 106.Plant L.D., Boyle J.P., Smith I.F., Peers C., Pearson H.A. The Production of Amyloid β Peptide Is a Critical Requirement for the Viability of Central Neurons. J. Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barry A.E., Klyubin I., Mc Donald J.M., Mably A.J., Farrell M.A., Scott M., Walsh D.M., Rowan M.J. Alzheimer’s Disease Brain-Derived Amyloid-β-Mediated Inhibition of LTP in Vivo Is Prevented by Immunotargeting Cellular Prion Protein. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han S.-H., Kim Y.H., Mook-Jung I. RAGE: The Beneficial and Deleterious Effects by Diverse Mechanisms of Actions. Mol. Cells. 2011;31:91–97. doi: 10.1007/s10059-011-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rhein V., Song X., Wiesner A., Ittner L.M., Baysang G., Meier F., Ozmen L., Bluethmann H., Dröse S., Brandt U., et al. Amyloid-Beta and Tau Synergistically Impair the Oxidative Phosphorylation System in Triple Transgenic Alzheimer’s Disease Mice. Proc. Natl. Acad. Sci. USA. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hane F., Leonenko Z. Effect of Metals on Kinetic Pathways of Amyloid-β Aggregation. Biomolecules. 2014;4:101–116. doi: 10.3390/biom4010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caughey B., Lansbury P.T. PROTOFIBRILS, PORES, FIBRILS, AND NEURODEGENERATION: Separating the Responsible Protein Aggregates from The Innocent Bystanders. Annu. Rev. Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 112.Crescenzi O., Tomaselli S., Guerrini R., Salvadori S., D’Ursi A.M., Temussi P.A., Picone D. Solution Structure of the Alzheimer Amyloid Beta-Peptide (1-42) in an Apolar Microenvironment. Similarity with a Virus Fusion Domain. Eur. J. Biochem. 2002;269:5642–5648. doi: 10.1046/j.1432-1033.2002.03271.x. [DOI] [PubMed] [Google Scholar]
- 113.Tomaselli S., Esposito V., Vangone P., van Nuland N.A.J., Bonvin A.M.J.J., Guerrini R., Tancredi T., Temussi P.A., Picone D. The α-to-β Conformational Transition of Alzheimer’s Aβ-(1-42) Peptide in Aqueous Media Is Reversible: A Step by Step Conformational Analysis Suggests the Location of β Conformation Seeding. ChemBioChem. 2006;7:257–267. doi: 10.1002/cbic.200500223. [DOI] [PubMed] [Google Scholar]
- 114.Economou N.J., Giammona M.J., Do T.D., Zheng X., Teplow D.B., Buratto S.K., Bowers M.T. Amyloid β-Protein Assembly and Alzheimer’s Disease: Dodecamers of Aβ42, but Not of Aβ40, Seed Fibril Formation. J. Am. Chem. Soc. 2016;138:1772–1775. doi: 10.1021/jacs.5b11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shigemitsu Y., Iwaya N., Goda N., Matsuzaki M., Tenno T., Narita A., Hoshi M., Hiroaki H. Nuclear Magnetic Resonance Evidence for the Dimer Formation of Beta Amyloid Peptide 1–42 in 1,1,1,3,3,3-Hexafluoro-2-Propanol. Anal. Biochem. 2016;498:59–67. doi: 10.1016/j.ab.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 116.Mehrazma B., Rauk A. Exploring Amyloid-β Dimer Structure Using Molecular Dynamics Simulations. J. Phys. Chem. A. 2019;123:4658–4670. doi: 10.1021/acs.jpca.8b11251. [DOI] [PubMed] [Google Scholar]
- 117.Zhao L.N., Long H.W., Mu Y., Chew L.Y. The Toxicity of Amyloid ß Oligomers. Int. J. Mol. Sci. 2012;13:7303–7327. doi: 10.3390/ijms13067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sengupta U., Nilson A.N., Kayed R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rauk A. Why Is the Amyloid Beta Peptide of Alzheimer’s Disease Neurotoxic? Dalton Trans. 2008:1273–1282. doi: 10.1039/b718601k. [DOI] [PubMed] [Google Scholar]
- 120.Kandel N., Zheng T., Huo Q., Tatulian S.A. Membrane Binding and Pore Formation by a Cytotoxic Fragment of Amyloid β Peptide. J. Phys. Chem. B. 2017;121:10293–10305. doi: 10.1021/acs.jpcb.7b07002. [DOI] [PubMed] [Google Scholar]
- 121.Shirwany N.A., Payette D., Xie J., Guo Q. The Amyloid Beta Ion Channel Hypothesis of Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2007;3:597–612. [PMC free article] [PubMed] [Google Scholar]
- 122.Arispe N., Pollard H.B., Rojas E. Giant Multilevel Cation Channels Formed by Alzheimer Disease Amyloid Beta-Protein [A Beta P-(1-40)] in Bilayer Membranes. Proc. Natl. Acad. Sci. USA. 1993;90:10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Scala C., Yahi N., Boutemeur S., Flores A., Rodriguez L., Chahinian H., Fantini J. Common Molecular Mechanism of Amyloid Pore Formation by Alzheimer’s β-Amyloid Peptide and α-Synuclein. Sci. Rep. 2016;6:28781. doi: 10.1038/srep28781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Venko K., Novič M., Stoka V., Žerovnik E. Prediction of Transmembrane Regions, Cholesterol, and Ganglioside Binding Sites in Amyloid-Forming Proteins Indicate Potential for Amyloid Pore Formation. Front. Mol. Neurosci. 2021;14:619496. doi: 10.3389/fnmol.2021.619496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wärmländer S.K.T.S., Österlund N., Wallin C., Wu J., Luo J., Tiiman A., Jarvet J., Gräslund A. Metal-binding to the Amyloid-β Peptides in the Presence of Biomembranes: Potential Mechanisms of Cell Toxicity. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2019;24:1189–1196. doi: 10.1007/s00775-019-01723-9. [DOI] [PubMed] [Google Scholar]
- 126.Sato Y., Takiguchi M., Tamano H., Takeda A. Extracellular Zn2+-Dependent Amyloid-Β1-42 Neurotoxicity in Alzheimer’s Disease Pathogenesis. Biol. Trace Elem. Res. 2021;199:53–61. doi: 10.1007/s12011-020-02131-w. [DOI] [PubMed] [Google Scholar]
- 127.Bode D.C., Baker M.D., Viles J.H. Ion Channel Formation by Amyloid-Β42 Oligomers but Not Amyloid-Β40 in Cellular Membranes. J. Biol. Chem. 2017;292:1404–1413. doi: 10.1074/jbc.M116.762526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serra-Batiste M., Ninot-Pedrosa M., Puig E., Ciudad S., Gairí M., Carulla N. Preparation of a Well-Defined and Stable β-Barrel Pore-Forming Aβ42 Oligomer. In: Sigurdsson E.M., Calero M., Gasset M., editors. Amyloid Proteins. Volume 1779. Springer; New York, NY, USA: 2018. pp. 13–22. [Google Scholar]
- 129.Di Scala C., Troadec J.-D., Lelièvre C., Garmy N., Fantini J., Chahinian H. Mechanism of Cholesterol-Assisted Oligomeric Channel Formation by a Short Alzheimer β-Amyloid Peptide. J. Neurochem. 2014;128:186–195. doi: 10.1111/jnc.12390. [DOI] [PubMed] [Google Scholar]
- 130.Bocharov E.V., Mineev K.S., Pavlov K.V., Akimov S.A., Kuznetsov A.S., Efremov R.G., Arseniev A.S. Helix-Helix Interactions in Membrane Domains of Bitopic Proteins: Specificity and Role of Lipid Environment. Biochim. Biophys. Acta BBA Biomembr. 2017;1859:561–576. doi: 10.1016/j.bbamem.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 131.Bocharov E.V., Sharonov G.V., Bocharova O.V., Pavlov K.V. Conformational Transitions and Interactions Underlying the Function of Membrane Embedded Receptor Protein Kinases. Biochim. Biophys. Acta BBA Biomembr. 2017;1859:1417–1429. doi: 10.1016/j.bbamem.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 132.Pavlov K.V., Akimov S.A., Batishchev O.V., Chekashkina K.V., Bashkirov P.V., Bocharov E.V. Protein-Lipid Interplay in Vital Biological Functions. In: Catalá A., editor. Protein-Lipid Interactions: Perspectives, Techniques and Challenges. Nova Science Publishers; Harpark, NY, USA: 2018. pp. 133–199. [Google Scholar]
- 133.Limbocker R., Chia S., Ruggeri F.S., Perni M., Cascella R., Heller G.T., Meisl G., Mannini B., Habchi J., Michaels T.C.T., et al. Trodusquemine Enhances Aβ 42 Aggregation but Suppresses Its Toxicity by Displacing Oligomers from Cell Membranes. Nat. Commun. 2019;10:225. doi: 10.1038/s41467-018-07699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith D.G., Cappai R., Barnham K.J. The Redox Chemistry of the Alzheimer’s Disease Amyloid Beta Peptide. Biochim. Biophys. Acta. 2007;1768:1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 135.Džinić T., Dencher N.A. Oxygen Concentration and Oxidative Stress Modulate the Influence of Alzheimer’s Disease Aβ1-42 Peptide on Human Cells. Oxid. Med. Cell. Longev. 2018;2018:7567959. doi: 10.1155/2018/7567959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shafrir Y., Durell S., Arispe N., Guy H.R. Models of Membrane-Bound Alzheimer’s Abeta Peptide Assemblies. Proteins. 2010;78:3473–3487. doi: 10.1002/prot.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu Y., Yin T., Peng Q., Kong L., Li C., Tang D., Yin X. Simultaneous Monitoring of Amyloid-β (Aβ) Oligomers and Fibrils for Effectively Evaluating the Dynamic Process of Aβ Aggregation. ACS Sens. 2019;4:471–478. doi: 10.1021/acssensors.8b01493. [DOI] [PubMed] [Google Scholar]
- 138.Shea D., Hsu C.-C., Bi T.M., Paranjapye N., Childers M.C., Cochran J., Tomberlin C.P., Wang L., Paris D., Zonderman J., et al. α-Sheet Secondary Structure in Amyloid β-Peptide Drives Aggregation and Toxicity in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA. 2019;116:8895–8900. doi: 10.1073/pnas.1820585116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ono K., Yamada M. Low-n Oligomers as Therapeutic Targets of Alzheimer’s Disease. J. Neurochem. 2011;117:19–28. doi: 10.1111/j.1471-4159.2011.07187.x. [DOI] [PubMed] [Google Scholar]
- 140.Kreutzer A.G., Hamza I.L., Spencer R.K., Nowick J.S. X-ray Crystallographic Structures of a Trimer, Dodecamer, and Annular Pore Formed by an Aβ17-36 β-Hairpin. J. Am. Chem. Soc. 2016;138:4634–4642. doi: 10.1021/jacs.6b01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lendel C., Bjerring M., Dubnovitsky A., Kelly R.T., Filippov A., Antzutkin O.N., Nielsen N.C., Härd T. A Hexameric Peptide Barrel as Building Block of Amyloid-β Protofibrils. Angew. Chem. Int. Ed. Engl. 2014;53:12756–12760. doi: 10.1002/anie.201406357. [DOI] [PubMed] [Google Scholar]
- 142.Norlin N., Hellberg M., Filippov A., Sousa A.A., Gröbner G., Leapman R.D., Almqvist N., Antzutkin O.N. Aggregation and Fibril Morphology of the Arctic Mutation of Alzheimer’s Aβ Peptide by CD, TEM, STEM and in Situ AFM. J. Struct. Biol. 2012;180:174–189. doi: 10.1016/j.jsb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ciudad S., Puig E., Botzanowski T., Meigooni M., Arango A.S., Do J., Mayzel M., Bayoumi M., Chaignepain S., Maglia G., et al. Aβ(1-42) Tetramer and Octamer Structures Reveal Edge Conductivity Pores as a Mechanism for Membrane Damage. Nat. Commun. 2020;11:3014. doi: 10.1038/s41467-020-16566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nguyen H.L., Krupa P., Hai N.M., Linh H.Q., Li M.S. Structure and Physicochemical Properties of the Aβ42 Tetramer: Multiscale Molecular Dynamics Simulations. J. Phys. Chem. B. 2019;123:7253–7269. doi: 10.1021/acs.jpcb.9b04208. [DOI] [PubMed] [Google Scholar]
- 145.Snyder S.W., Ladror U.S., Wade W.S., Wang G.T., Barrett L.W., Matayoshi E.D., Huffaker H.J., Krafft G.A., Holzman T.F. Amyloid-beta aggregation: Selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys. J. 1994;67:1216–1228. doi: 10.1016/S0006-3495(94)80591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jarrett J.T., Berger E.P., Lansbury P.T., Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 147.Poma A.B., Chwastyk M., Cieplak M. Elastic moduli of biological fibers in a coarse-grained model: Crystalline cellulose and β-amyloids. Phys. Chem. Chem. Phys. 2017;19:28195–28206. doi: 10.1039/C7CP05269C. [DOI] [PubMed] [Google Scholar]
- 148.Poma A.B., Guzman H.V., Li M.S., Theodorakis P.E. Mechanical and thermodynamic properties of Aβ42, Aβ40, and α-synuclein fibrils: A coarse-grained method to complement experimental studies. Beilstein J. Nanotechnol. 2019;10:500–513. doi: 10.3762/bjnano.10.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ruggeri F.S., Adamcik J., Jeong J.S., Lashuel H.A., Mezzenga R., Dietler G. Influence of the β-sheet content on the mechanical properties of aggregates during amyloid fibrillization. Angew. Chem. Int. Ed. Engl. 2015;54:2462–2466. doi: 10.1002/anie.201409050. [DOI] [PubMed] [Google Scholar]
- 150.Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R. 3D Structure of Alzheimer’s Amyloid-β (1–42) Fibrils. Proc. Natl. Acad. Sci. USA. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paravastu A.K., Leapman R.D., Yau W.-M., Tycko R. Molecular Structural Basis for Polymorphism in Alzheimer’s β-Amyloid Fibrils. Proc. Natl. Acad. Sci. USA. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lu J.-X., Qiang W., Yau W.-M., Schwieters C.D., Meredith S.C., Tycko R. Molecular Structure of β-Amyloid Fibrils in Alzheimer’s Disease Brain Tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kodali R., Williams A.D., Chemuru S., Wetzel R. Aβ(1–40) Forms Five Distinct Amyloid Structures Whose β-Sheet Contents and Fibril Stabilities Are Correlated. J. Mol. Biol. 2010;401:503–517. doi: 10.1016/j.jmb.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goodsell D.S., Dutta S., Zardecki C., Voigt M., Berman H.M., Burley S.K. The RCSB PDB “Molecule of the Month”: Inspiring a Molecular View of Biology. PLoS Biol. 2015;13:e1002140. doi: 10.1371/journal.pbio.1002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gremer L., Schölzel D., Schenk C., Reinartz E., Labahn J., Ravelli R.B.G., Tusche M., Lopez-Iglesias C., Hoyer W., Heise H., et al. Fibril Structure of Amyloid-β(1–42) by Cryo–Electron Microscopy. Science. 2017;358:116–119. doi: 10.1126/science.aao2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schenk D., Basi G.S., Pangalos M.N. Treatment Strategies Targeting Amyloid β-Protein. Cold Springer Harb. Perspect. Med. 2012;2:a006387. doi: 10.1101/cshperspect.a006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen Y., Fu A.K.Y., Ip N.Y. Synaptic Dysfunction in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Pharmacol. Ther. 2019;195:186–198. doi: 10.1016/j.pharmthera.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 158.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s Disease Drug Development Pipeline: 2020. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020;6:e12050. doi: 10.1002/trc2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fantini J., Chahinian H., Yahi N. Progress toward Alzheimer’s Disease Treatment: Leveraging the Achilles’ Heel of Aβ Oligomers? Protein Sci. Publ. Protein Soc. 2020;29:1748–1759. doi: 10.1002/pro.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heller G.T., Aprile F.A., Michaels T.C.T., Limbocker R., Perni M., Ruggeri F.S., Mannini B., Löhr T., Bonomi M., Camilloni C., et al. Small-Molecule Sequestration of Amyloid-β as a Drug Discovery Strategy for Alzheimer’s Disease. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Craik D.J., Fairlie D.P., Liras S., Price D. The Future of Peptide-Based Drugs. Chem. Biol. Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 163.Funke S.A., Willbold D. Peptides for Therapy and Diagnosis of Alzheimer’s Disease. Curr. Pharm. Des. 2012;18:755–767. doi: 10.2174/138161212799277752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X., Zhang X., Ladiwala A.R.A., Du D., Yadav J.K., Tessier P.M., Wright P.E., Kelly J.W., Buxbaum J.N. Mechanisms of Transthyretin Inhibition of β-Amyloid Aggregation in Vitro. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:19423–19433. doi: 10.1523/JNEUROSCI.2561-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stein T.D., Johnson J.A. Lack of Neurodegeneration in Transgenic Mice Overexpressing Mutant Amyloid Precursor Protein Is Associated with Increased Levels of Transthyretin and the Activation of Cell Survival Pathways. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Volpina O.M., Samokhin A.N., Koroev D.O., Nesterova I.V., Volkova T.D., Medvinskaya N.I., Nekrasov P.V., Tatarnikova O.G., Kamynina A.V., Balasanyants S.M., et al. Synthetic Fragment of Receptor for Advanced Glycation End Products Prevents Memory Loss and Protects Brain Neurons in Olfactory Bulbectomized Mice. J. Alzheimers Dis. JAD. 2018;61:1061–1076. doi: 10.3233/JAD-170483. [DOI] [PubMed] [Google Scholar]
- 167.Kamynina A., Esteras N., Koroev D.O., Angelova P.R., Volpina O.M., Abramov A.Y. Activation of RAGE Leads to the Release of Glutamate from Astrocytes and Stimulates Calcium Signal in Neurons. J. Cell. Physiol. 2021 doi: 10.1002/jcp.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barykin E.P., Garifulina A.I., Tolstova A.P., Anashkina A.A., Adzhubei A.A., Mezentsev Y.V., Shelukhina I.V., Kozin S.A., Tsetlin V.I., Makarov A.A. Tetrapeptide Ac-HAEE-NH2 Protects A4β2 NAChR from Inhibition by Aβ. Int. J. Mol. Sci. 2020;21:6272. doi: 10.3390/ijms21176272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Funke S.A., Willbold D. Mirror Image Phage Display—A Method to Generate D-Peptide Ligands for Use in Diagnostic or Therapeutical Applications. Mol. Biosyst. 2009;5:783–786. doi: 10.1039/b904138a. [DOI] [PubMed] [Google Scholar]
- 170.Wiesehan K., Buder K., Linke R.P., Patt S., Stoldt M., Unger E., Schmitt B., Bucci E., Willbold D. Selection of D-Amino-Acid Peptides That Bind to Alzheimer’s Disease Amyloid Peptide Abeta1-42 by Mirror Image Phage Display. Chembiochem. Eur. J. Chem. Biol. 2003;4:748–753. doi: 10.1002/cbic.200300631. [DOI] [PubMed] [Google Scholar]
- 171.Ziehm T., Brener O., van Groen T., Kadish I., Frenzel D., Tusche M., Kutzsche J., Reiß K., Gremer L., Nagel-Steger L., et al. Increase of Positive Net Charge and Conformational Rigidity Enhances the Efficacy of D-Enantiomeric Peptides Designed to Eliminate Cytotoxic Aβ Species. ACS Chem. Neurosci. 2016;7:1088–1096. doi: 10.1021/acschemneuro.6b00047. [DOI] [PubMed] [Google Scholar]
- 172.Willbold D., Kutzsche J. Do We Need Anti-Prion Compounds to Treat Alzheimer’s Disease? Molecules. 2019;24:2237. doi: 10.3390/molecules24122237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uversky V.N. The multifaceted roles of intrinsic disorder in protein complexes. FEBS Lett. 2015;589:2498–2506. doi: 10.1016/j.febslet.2015.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.