Abstract
The search for suitable strategies to manufacture self-healable nitrile rubber (NBR) composites is the most promising part in the industrial field of polar rubber research. In recent years, some important strategies, specifically, metal–ligand coordination bond formation, ionic bond formation, and dynamic hydrogen bond formation, have been utilized to develop duly self-healable NBR composites. This paper reviews the continuous advancement in the research field related to self-healable NBR composites by considering healing strategies and healing conditions. Special attention is given to understand the healing mechanism in reversibly cross-linked NBR systems. The healing efficiency of a cross-linked NBR network is usually dependent on the definite interaction between functional groups of NBR and a cross-linking agent. Finally, the results obtained from successful studies suggest that self-healing technology has incredible potential to increase the sustainability and lifetime of NBR-based rubber products.
1. Introduction
Currently, one of the most serious issues concerning environmental damage is the unswerving disposal of industrial rubber waste materials into the environment without appropriate reprocessing.1,2 In the present decade, the quantity of waste rubber products has increased day by day due to the unregulated growth of the automotive industry.1,2 In this respect, successful design of self-healable rubber composites for the extension of product lifetime is the most commonly adopted and popular way exercised by rubber scientists.1−14 Self-healing indicates the self-repair of damage in a product with or without the presence of healing materials. The healing ability of rubber compounds in the absence of any externally added healing materials is known as an intrinsic self-healing property. So far, emphatic focus was given by rubber scientists on understanding the self-healing mechanism of intrinsic self-healable rubber composites. As reported by various rubber scientists, intrinsic self-healing occurs via several factors, like hydrogen bonds,3−6 disulfide bonds,7−9 metal–ligand bonding interactions,10 Diels–Alder reactions,11 transesterification reactions,12−14 etc. In some cases, external stimulus, like temperature or pressure, plays a vital role to improve the healing efficiency of self-healable rubber composites. However, it is possible to achieve autonomous self-healing, that is, healing without external stimulus in rubber composites.1 Now, both cross-linking systems and filler structures make a significant contribution to the self-healing efficiency of rubber composites in the presence of a particular filler. On one side, a three-dimensional irreversible covalent cross-linking system has the tendency to prevent the healing of rubber materials. Thus, a reversible cross-linking system is necessary to cause effective self-healing of rubber materials. On the other side, the interfacial interaction between surface functional groups of rubber and filler is another influential aspect regarding the preparation of self-healable filled rubber composites. Thus, rubber researchers have to properly select the cross-linking system and filler material in order to develop a self-healable filled rubber system. In reality, the challenges lying with the design of autonomous self-healable filled rubber composites for industrial engineering are intricate and variegated in nature.
Nitrile rubber (NBR) is an important rubber due to its successful application in oil-resistant materials, such as hoses, seals, gaskets, O-rings, and gloves. In the last 2–3 years, some research groups have reported different interesting mechanistic studies related to the development of self-healable NBR composites. The main aim of the present review is to explore the recent progress and future prospects of self-healable advanced NBR composites.
2. Measurement of Self-Healing Efficiency
For the measurement of self-healing efficiency, initially, dumbbell-shaped test samples were cut into two different parts.10,15 Then, fracture surfaces of rubber samples were placed together to start the healing process. In an alternative way, the broken parts of each tested sample were put into contact under a minimum pressure in order to start the healing process.16 After completion of the healing process, the mechanical properties of the rubber composites were retested. Healing efficiency can be calculated by comparing the tensile strength of the healed sample (Mhealed) with the tensile strength of the original or virgin sample (Moriginal).16 In a simplest way, the healing efficiency can be calculated as follows:16
During the self-healing study, different healing conditions, like temperature and pressure, were optimized for better self-healing efficiency.15
3. Different Strategies for the Preparation of Self-Healable NBR Composites
3.1. Metal–Ligand Coordination Bond Formation
The formation of a metal–ligand coordination bond is the most interesting and simple approach for the development of self-healable NBR material. Zhang et al.10 investigated the self-healing ability of coordination-cross-linked NBR composites using an organometallic compound, namely, cobalt neocaprate, as cross-linking agent. Figure 1 represents the mechanism of the cross-linking reaction between cyano (−CN) groups of NBR chains and cobalt neocaprate to form the cross-linked NBR network. The formation of metal–ligand coordination bonds between −CN groups of NBR and cobalt neocaprate was confirmed from differential scanning calorimetry (DSC), swelling, and stress–strain experiments. The tensile strength of cross-linked NBR samples increased consistently with increasing the amount of cobalt neocaprate, which indicated an effective coordination cross-linking in the NBR network. In the present review, the NBR composite with 30 phr (parts per hundred parts of rubber) cobalt neocaprate is designated as NBR/30-Co. The tensile strength of the NBR/30-Co composite was slightly higher than that of a conventional sulfur-cured NBR composite. As suggested by the authors,10 this type of metal–ligand coordination bond is dynamic and reversible in character. The self-healing property of damaged NBR/cobalt neocaprate composites depends on the destruction and regeneration of the metal–ligand coordination bond under different healing conditions. The authors measured the healing efficiency of the NBR/30-Co sample at different healing temperatures. The damaged NBR/30-Co sample was able to recover its actual tensile strength after healing at 160 °C for 1 h. More interestingly, the damaged NBR/30-Co sample was also capable of recovering its actual tensile strength after healing at 190 °C for only 10 min. It was possible to effectively reduce healing time by increasing the healing temperature for the cobalt neocaprate-based NBR composite, which suggested the enhancement of molecular chain mobility with increasing healing temperature. On the other hand, the conventional sulfur cross-linked NBR composite showed very poor healing efficiency after healing at 190 °C for 10 min.
Figure 1.
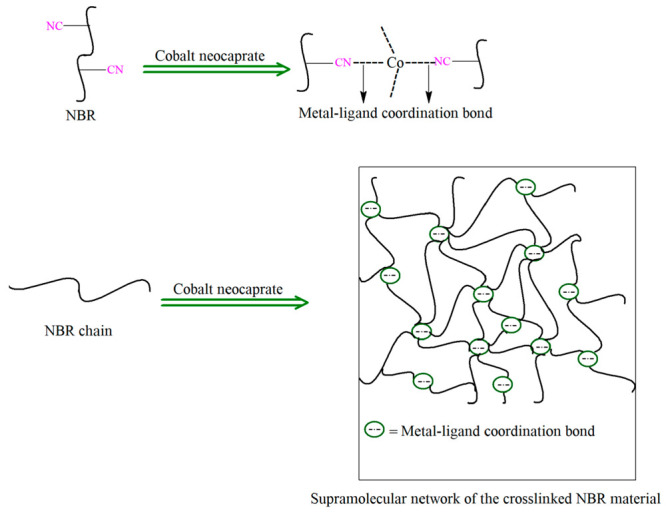
Formation of coordination-cross-linked NBR/cobalt neocaprate composites.10 Adapted with permission from ref (10). Copyright 2019 Springer Nature.
Das et al.15 utilized the combination of 2,6-diaminopyridine (DAP) and metal salts for the design of self-healable carboxylated nitrile rubber (XNBR) composites. In this study, three types of metal salts, namely, cobalt(II) nitrate hexahydrate, nickel(II) nitrate hexahydrate, and zinc(II) nitrate hexahydrate, were used to produce a metal–ligand coordination bond in XNBR composites. Different stepwise reactions for the successful development of metal–ligand coordination bonds in metal-ion-based XNBR/DAP complexes are represented schematically in Figure 2. The formation of coordination bonds between different metal ions and the DAP ligand in XNBR/DAP/metal ion composites was ensured from Fourier transform infrared (FTIR) spectroscopy. As reported by the authors, the XNBR/DAP/cobalt(II) composite showed higher tensile strength as compared to that of either the XNBR/DAP/nickel(II) composite or the XNBR/DAP/zinc(II) composite. An excellent metal–ligand cross-linking reaction was observed between cobalt(II) and DAP, which dramatically increases the mechanical properties of the XNBR/DAP/cobalt(II) composite. On the other hand, XNBR/DAP/zinc(II) showed the lowest tensile strength among the three metal-ion-based XNBR/DAP composites. Thus, the mechanical performances of coordination-cross-linked XNBR composites were strongly dependent on the electronic configuration of the metal ion. Moreover, due to the absence of a metal–ligand cross-linking network, the XNBR/DAP composite without a metal ion showed very poor tensile strength. The healing efficiency of different XNBR/DAP/metal ion composites was varied noticeably with healing temperature and healing time. The healing efficiency of various metal–ligand cross-linked XNBR composites has shown an increasing tendency with increasing healing temperature from room temperature to 80 °C. XNBR/DAP/metal ion complexes showed optimum healing efficiency after healing at 80 °C for 24 h. XNBR/DAP/zinc(II) samples had a healing efficiency better than that of either XNBR/DAP/cobalt(II) or XNBR/DAP/nickel(II) samples under different healing conditions. Again, the self-healing ability of metal-ion-based XNBR/DAP complexes is closely related to the electronic configuration of a particular metal ion. Due to the presence of a completely filled outer d orbital in zinc(II), metal–ligand coordination bonds are very weak and dynamic in the case of the XNBR/DAP/zinc(II) sample. However, due to the presence of an incompletely filled d orbital in metal ions, metal–ligand coordination bonds are strong and less dynamic in the case of XNBR/DAP/cobalt(II) and XNBR/DAP/nickel(II) samples. The dynamic nature of the metal–ligand coordination bond has contributed significantly to facilitate the self-healing performance of XNBR/DAP/metal ion composites. Thus, the XNBR/DAP/zinc(II) composite was able to achieve 100% self-healing efficiency after healing at 80 °C for 24 h. Under same healing condition (at 80 °C for 24 h), the XNBR/DAP/cobalt(II) composite was able to achieve 88% self-healing efficiency. Thus, only the XNBR/DAP/cobalt(II) composite exhibited both excellent tensile strength and rationally good self-healing ability.
Figure 2.
Formation of coordination-cross-linked XNBR/DAP/M2+ composites.15 Adapted with permission from (15). Copyright 2020 BME-PT Hungary.
3.2. Ionic Bond Formation
The formation of ionic clusters is another straightforward strategy for the creation of self-healable XNBR composites. The general concept regarding the formation of an ionically cross-linked rubber network via ionic interactions is represented in Figure 3. Utrera-Barrios et al.16 reported a pioneering way for the development of an ionically cross-linked XNBR compound using zinc oxide (ZnO) as cross-linking agent. As claimed by the authors, the ionic interaction between the Zn2+ group present in ZnO and the COO– group of XNBR was the key factor for the preparation of adequately cross-linked XNBR material.16,17 The formation of effectual ionic cross-linking in XNBR/ZnO compounds was confirmed from a rheometric study. The optimum value of maximum torque was obtained for the XNBR compound with 6 phr ZnO (XNBR/6ZnO). Healing properties of different broken samples were measured after healing at 100 °C for 10 min. The XNBR/6ZnO sample showed very poor healing efficiency. In order to improve healing efficiency, surface-modified ground tire rubber (MGTR) was added to the XNBR/6ZnO compound. In this study, the surface of ground tire rubber (GTR) was modified with poly(acrylic acid) through a free radical polymerization reaction.18Figure 4 represents the formation of the ionically cross-linked XNBR/6ZnO/MGTR compound via interaction between ionic groups. This type of ionic interaction is dynamic in nature, which can make the cross-linked XNBR into a self-healable material. The formulations and properties of different ionic XNBR composites are given in Table 1. The mechanical properties of filled rubber composites are closely related to the nature of interfaces between the rubber matrix and filler.19 As shown in Table 1, the mechanical properties and cross-link densities of MGTR filled XNBR/6ZnO composites were lower than those properties of unfilled XNBR/6ZnO composites. This result was attributed to the shielding effect of the oxide present in the structure of MGTR, which considerably reduces the possibility of the ionic cross-link formation in the XNBR/6ZnO/MGTR network.20 Interestingly, the healing efficiencies of XNBR/6ZnO/MGTR compounds were far better than those of XNBR/6ZnO compounds. In a more advanced way, Utrera-Barrios et al.16 confirmed that the healing efficiency of ionically cross-linked XNBR compounds is dependent on two important factors, namely, cross-link density and molecular mobility. However, cross-link density had an opposite effect to that of molecular mobility in controlling the overall self-healing performance of XNBR materials. Actually, increment in the cross-link density indicates lowering of the molecular mobility in a cross-linked rubber network. More importantly, the molecular mobility can facilitate the regeneration process of ionic bonds in damaged XNBR compounds after the healing operation. Again, the self-healing behavior of ionically cross-linked XNBR systems depends on destruction and regeneration of ionic bonds between Zn2+ and carboxyl groups. The XNBR/6ZnO compound showed an excellent cross-link density value, which causes reduction in the molecular mobility in the rubber network, that is, decrease in the healing efficiency of the XNBR/6ZnO sample. On the other hand, XNBR/6ZnO/MGTR composites showed much lower cross-link density as compared to that of the XNBR/6ZnO sample. As a result, healing efficiencies were more satisfactory for XNBR/6ZnO/MGTR composites than for XNBR/6ZnO composites. Also, surface modification played a vital role in the healing ability of XNBR/6ZnO/MGTR composites. It was found that the healing efficiency of the XNBR/6ZnO compound remained unaffected due to the addition of GTR. As explained by the authors,16 MGTR had a large amount of carboxylic acid groups in its structure. These carboxylic acid groups of MGTR participated predominantly to reproduce the ionic bonds in damaged XNBR/6ZnO/MGTR compounds. Among the various XNBR compounds, the XNBR sample with 6 phr ZnO and 6 phr MGTR (XNBR/6ZnO/6MGTR) showed the best healing efficiency under the same healing conditions.
Figure 3.
Different steps for the formation of an ionically cross-linked rubber network.
Figure 4.
Formation of ionically cross-linked XNBR/ZnO/MGTR composites via ionic interaction.16 Adapted with permission from ref (16). Copyright 2020 Elsevier.
Table 1. Mechanical and Healing Properties of Different XNBR Compounds16.
| formulations | amount of ZnO (phr) | amount of MGTR (phr) | amount of GTR (phr) | tensile strength (MPa) | cross-link density ×104 (mol/cm3) | healing efficiency (Mhealed/Moriginal) ×100 |
|---|---|---|---|---|---|---|
| XNBR/6ZnO | 6 | 20 | 8.6 | ∼15 | ||
| XNBR/6ZnO/5MGTR | 6 | 5 | 12.2 | 5.9 | ∼65 | |
| XNBR/6ZnO/6MGTR | 6 | 6 | 10.4 | 6.0 | ∼70 | |
| XNBR/6ZnO/8MGTR | 6 | 8 | 16 | 6.5 | ∼25 | |
| XNBR/6ZnO/6GTR | 6 | 6 | 19 | 5.6 | ∼10 |
3.3. Dynamic Hydrogen Bond Formation
Liu et al.21 suggested proper use of dynamic hydrogen bonding for the preparation of self-healable XNBR composites. The authors added polyethylenimine-modified cellulose nanocrystals (PEI/CNC) to the XNBR latex in order to develop a supramolecular rubber network. In this study, the key point was the ionic hydrogen bonding interaction between amine groups of PEI/CNC and carboxyl groups of XNBR. The authors conducted FTIR analysis to confirm the presence of ionic hydrogen bonding in a cross-linked XNBR system. Due to this innovative interaction, the ionically cross-linked XNBR/PEI/CNC composite showed tensile strength considerably higher than that of the sulfur cross-linked XNBR/CNC composite. Three cutting/healing operations were performed to check the healing efficiency of the XNBR/PEI/CNC composite. After the first cutting/healing operation, the XNBR/PEI/CNC composite was able to regain 83% of its actual tensile strength. Even after the third cutting/healing process, the ionically cross-linked XNBR/PEI/CNC composite was able to recover 58% of its original tensile strength. This result has been explained in view of the successful reformation of ionic dynamic hydrogen bonding after the healing process of XNBR/PEI/CNC composites.
4. Conclusions and Future Perspectives
In the present decade, the discovery of self-healable and environmentally sound NBR composites is a new-fangled field in rubber technology. The unique surface functional groups of NBR and XNBR offer sufficient scope to develop properly self-healable materials. In this review, we revealed some attractive ways regarding the formation of self-healable NBR and XNBR composites via dynamic metal–ligand coordination and ionic bonding. In the case of the metal–ligand coordination bond, the electronic configuration of metal can control the healing property of XNBR composites. Cobalt neocaprate (30 phr) can be used to prepare NBR composites with 100% healing efficiency after healing at 190 °C for 10 min. On the other side, the combination of DAP/zinc(II) salt has proven to be an ideal cross-linking agent to prepare XNBR composites with 100% healing efficiency after healing at 80 °C for 24 h. Moreoever, MGTR and PEI/CNC are interesting cross-linking materials for the preparation of XNBR composites with good healing efficiency and excellent mechanical properties. Indeed, the standardization of an optimum healing condition for a particular cross-linked NBR system is great importance from an industrial standpoint.
However, the research based on the preparation and characterization of self-healable NBR compounds is still in the preliminary stage. There are two major limitations related to the application of self-healable NBR compounds in the industrial area of rubber technology. These major limitations are presented below point by point.
-
(a)
Until now, researchers have found information about the laboratory-scale production of self-healable NBR composites. However, the suitability of large-scale application of self-healable NBR composites in different rubber-related industries is a questionable matter.
-
(b)
Most of the researchers have tried to develop self-healable NBR composites for unfilled rubber systems. However, the addition of different reinforcing fillers, such as carbon black and silica, is the mandatory part to obtain the desired mechanical properties required for the commercial application of NBR. Thus, it is necessary to establish some strategies for the development of self-healable filled NBR composites. It will be quite a tough challenge to achieve the proper balance between mechanical properties and healing efficiency of filled NBR composites.
Acknowledgments
K.R. would like to acknowledge a senior postdoctoral fellowship supported by Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University for fellowship assistance.
Biographies

Dr. Kumarjyoti Roy received his Ph.D. in 2016 from University of Kalyani, India. Presently, he is working as a senior postdoctoral researcher in the Department of Materials Science, Chulalongkorn University, Thailand. His research interest is based on the development of industrial and environmentally friendly rubber composites.

Dr. Subhas Chandra Debnath received his Ph.D. in 1995 from Indian Association for the Cultivation of Science (IACS), Kolkata, India. He has 3 years postdoctoral research experience including 2 years in The Netherlands and 1 year in Germany. Presently, he is working as an associate professor in the Department of Chemistry, University of Kalyani, India. His research interest is based on the applications of different accelerators and fillers in rubber chemistry and technology.

Mr. Aphiwat Pongwisuthiruchte received his master’s degree (2018) in polymer science at Chulalongkorn University, Thailand. Presently, he is working as a Ph.D. researcher under the supervision of Professor Pranut Potiyaraj at Chulalongkorn University, Thailand. His research interest is based on 3D printing and shape memory of silicone acrylate polymer.

Professor Pranut Potiyaraj received his B.Sc. in Materials Science from Chulalongkorn University, Thailand, in 1994 before accomplishing his Ph.D. in Textiles from The University of Manchester (formerly UMIST), UK, in 2000. His research interests include polymer and rubber composites for advanced applications, bioplastics, 3D printing, and technical textiles.
The authors declare no competing financial interest.
References
- Utrera-Barrios S.; Hernández Santana M.; Verdejo R.; López-Manchado M. A. Design of Rubber Composites with Autonomous Self-Healing Capability. ACS Omega 2020, 5, 1902–1910. 10.1021/acsomega.9b03516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Morera J.; Hernández Santana M.; Verdejo R.; López-Manchado M. A. Giving a Second Opportunity to Tire Waste: An Alternative Path for the Development of Sustainable Self-Healing Styrene–Butadiene Rubber Compounds Overcoming the Magic Triangle of Tires. Polymers 2019, 11, 2122. 10.3390/polym11122122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. A.; Sartore L.; Bignotti F.; Di Landro L. Autonomic Self-Healing in Epoxidized Natural Rubber. ACS Appl. Mater. Interfaces 2013, 5, 1494–1502. 10.1021/am303015e. [DOI] [PubMed] [Google Scholar]
- Cao L.; Yuan D.; Xu C.; Chen Y. Biobased, self-healable, high strength rubber with tunicate cellulose nanocrystals. Nanoscale 2017, 9, 15696–15706. 10.1039/C7NR05011A. [DOI] [PubMed] [Google Scholar]
- Guadagno L.; Vertuccio L.; Naddeo C.; Calabrese E.; Barra G.; Raimondo M.; Sorrentino A.; Binder W. H.; Michael P.; Rana S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Composites, Part B 2019, 157, 1–13. 10.1016/j.compositesb.2018.08.082. [DOI] [Google Scholar]
- Nie J.; Mou W.; Ding J.; Chen Y. Bio-based epoxidized natural rubber/chitin nanocrystals composites: Self-healing and enhanced mechanical properties. Composites, Part B 2019, 172, 152–160. 10.1016/j.compositesb.2019.04.035. [DOI] [Google Scholar]
- Hernández M.; Grande A. M.; Dierkes W.; Bijleveld J.; Van Der Zwaag S.; García S. J. Turning vulcanized natural rubber into a self-healing polymer: Effect of the disulfide/polysulfide ratio. ACS Sustainable Chem. Eng. 2016, 4, 5776–5784. 10.1021/acssuschemeng.6b01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Lu X.; Wang W. A tough polyurethane elastomer with self-healing ability. Mater. Des. 2017, 127, 30–36. 10.1016/j.matdes.2017.04.015. [DOI] [Google Scholar]
- Hernández Santana M.; Huete M.; Lameda P.; Araujo J.; Verdejo R.; López-Manchado M. A. Design of a new generation of sustainable SBR compounds with good trade-off between mechanical properties and self-healing ability. Eur. Polym. J. 2018, 106, 273–283. 10.1016/j.eurpolymj.2018.07.040. [DOI] [Google Scholar]
- Zhang Z. F.; Liu X. T.; Yang K.; Zhao S. G. Design of Coordination-Crosslinked Nitrile Rubber with Self-Healing and Reprocessing Ability. Macromol. Res. 2019, 27, 803–810. 10.1007/s13233-019-7110-8. [DOI] [Google Scholar]
- Tanasi P.; Hernández Santana M.; Carretero-González J.; Verdejo R.; López-Manchado M. A. Thermo-reversible crosslinked natural rubber: A Diels-Alder route for reuse and self-healing properties in elastomers. Polymer 2019, 175, 15–24. 10.1016/j.polymer.2019.04.059. [DOI] [Google Scholar]
- Qiu M.; Wu S.; Tang Z.; Guo B. Exchangeable interfacial crosslinks towards mechanically robust elastomer/carbon nanotubes vitrimers. Compos. Sci. Technol. 2018, 165, 24–30. 10.1016/j.compscitech.2018.06.004. [DOI] [Google Scholar]
- Xu C.; Cui R.; Fu L.; Lin B. Recyclable and heat-healable epoxidized natural rubber/bentonite composites. Compos. Sci. Technol. 2018, 167, 421–430. 10.1016/j.compscitech.2018.08.027. [DOI] [Google Scholar]
- Cao L.; Fan J.; Huang J.; Chen Y. A robust and stretchable cross-linked rubber network with recyclable and self-healable capabilities based on dynamic covalent bonds. J. Mater. Chem. A 2019, 7, 4922–4933. 10.1039/C8TA11587G. [DOI] [Google Scholar]
- Das M.; Pal S.; Naskar K. Exploring various metal-ligand coordination bond formation in elastomers: Mechanical performance and self-healing behaviour. eXPRESS Polym. Lett. 2020, 14, 860–880. 10.3144/expresspolymlett.2020.71. [DOI] [Google Scholar]
- Utrera-Barrios S.; Araujo-Morera J.; Pulido de Los Reyes L.; Verdugo Manzanares R.; Verdejo R.; Lopez-Manchado M. A.; Hernandez Santana M. An effective and sustainable approach for achieving self-healing in nitrile rubber. Eur. Polym. J. 2020, 139, 110032. 10.1016/j.eurpolymj.2020.110032. [DOI] [Google Scholar]
- Xu C.; Huang X.; Li C.; Chen Y.; Lin B.; Liang X. Design of “Zn2+ Salt-Bondings” Cross-Linked Carboxylated Styrene Butadiene Rubber with Reprocessing and Recycling Ability via Rearrangements of Ionic Cross-Linkings. ACS Sustainable Chem. Eng. 2016, 4, 6981–6990. 10.1021/acssuschemeng.6b01897. [DOI] [Google Scholar]
- Yagneswaran S.; Storer W. J.; Tomar N.; Chaur M. N.; Echegoyen L.; Smith D. W. Surface-grafting of ground rubber tire by poly acrylic acid via self-initiated free radical polymerization and composites with epoxy thereof. Polym. Compos. 2013, 34, 769–777. 10.1002/pc.22484. [DOI] [Google Scholar]
- Roy K.; Debnath S. C.; Potiyaraj P. A critical review on the utilization of various reinforcement modifiers in filled rubber composites. J. Elastomers Plast. 2020, 52, 167–193. 10.1177/0095244319835869. [DOI] [Google Scholar]
- Formela K.; Haponiuk J. T. Curing characteristics, mechanical properties and morphology of butyl rubber filled with ground tire rubber (GTR). Iran. Polym. J. 2014, 23, 185–194. 10.1007/s13726-013-0214-7. [DOI] [Google Scholar]
- Liu X.; Lu C.; Wu X.; Zhang X. Self-healing strain sensors based on nanostructured supramolecular conductive elastomers. J. Mater. Chem. A 2017, 5, 9824–9832. 10.1039/C7TA02416A. [DOI] [Google Scholar]






