Abstract
The desolvation and ionization process of analytes can significantly be improved by enriching the nebulizing gas with a dopant (dopant enriched nitrogen (DEN) gas) in the electrospray source. However, for the analysis of released glycans in negative ion mode, the usage of DEN gas remains largely unexplored. For this purpose, we investigated the effect of different polar protic solvents (methanol, ethanol, and isopropanol) as well as using solely the nebulizing gas or ambient air on the ionization and charge state distribution of released N- and O-glycans. Compared to the standard acetonitrile enriched nitrogen gas, isopropanol showed the highest increase in regards to peak areas. Moreover, it showed large benefits for the identification of glycan structures at high sensitivity as the increased precursor intensities subsequently resulted in higher intensities in tandem MS mode. While similar effects are noted for both neutral and sialylated species, the most significant effect was observed for early eluting glycans where very low acetonitrile concentrations were present in the eluent. The best results in terms of S/N ratios were obtained with methanol, with less effect on the MS/MS signal enhancement. Therefore, the use of this dopant would be particularly beneficial for high sensitivity MS-mode applications. In conclusion, isopropanol enriched DEN gas greatly improves the detection of both N-and O-glycan species and their tandem mass spectra, particularly for the early eluting species whose ionization in the absence of DEN gas is hindered by low organic concentrations.
Introduction
Liquid chromatography coupled to mass spectrometry (MS) has become a leading analytical platform for the identification and (relative) quantification of a wide range of compounds at high sensitivity. For the latter, ionization efficiency is of key importance. Because this is largely regulated by the electrospray ionization (ESI) process at the interface of the LC-MS, optimization of the mobile phase can already significantly improve the ionization of specific analytes.1 With the addition of solvent vapors at the ion source, the ionization efficiency can be further improved.2 To this end, the nebulizing gas (nitrogen; N2) will be enriched with a solvent vapor that is guided around the spray emitter and spray plume to enhance the nebulization and desolvation process.3 This approach is of specific interest for the glycomic field as carbohydrates usually ionize less efficiently than various other analytes due to their hydrophilic nature. Specifically, applications using acetonitrile (MeCN) as a dopant in positive ionization mode revealed a 25-fold increase in sensitivity for the analysis of glycopeptides.4,5
Porous graphitized carbon nanoliquid chromatography (PGC-nanoLC)-MS, where the ESI is operated in negative ion mode, is a very powerful platform for the analysis of N- and O-glycan alditols featuring glycan isomer separation.6−8 However, the usage of a dopant enriched nitrogen (DEN) gas remains largely unexplored for these applications. When compared to positive-ion mode, apart from the often higher ionization efficiency of sialylated and sulfated glycan species, the main benefit of negative mode is its ability for in-depth characterization of linkage isomers as the deprotonated glycan fragments provide not only glycosidic but also cross-ring fragments in tandem MS.9 However, it has been observed that the early eluting species have less ionization efficiency in PGC-LC-MS due to low MeCN concentrations in this elution range.10 This particularly affects early eluting N-linked glycans as well as O-linked glycans such as T and sialylTn antigens. Thereby, these very important tumor antigens might be underrepresented or completely missed in the analysis.
Previous studies have attempted to account for this issue using post column makeup flow (PCMF) for capillary flow PGC-LC, where MeCN and isopropanol (IPA)-based PCMF increased signal response, decreased variation, and improved MS/MS spectral quality compared to reference approach without PCMF.11 A recent study employed the usage of DEN gas for the analysis of N-glycans with capillary zone electrophoresis (CZE) coupled to electrospray ionization mass spectrometry in negative ion mode and observed a significant increase in MS signals.12 However, little is known about the effect of different dopants supplemented directly into the MS source for the PGC-nanoLC analysis of both N- and O-glycans in negative ion mode.
Here, we explore the effect of various DEN gas conditions on the ionization and charge state distribution of glycans in negative ion mode. For this purpose, different dopant solvents (methanol (MeOH), ethanol (EtOH), and IPA) as well as using the nebulizing gas unaccompanied or using solely ambient air were compared to the standard MeCN enriched nitrogen gas. Therefore, the aim of this study was to provide a systematic overview of the different dopant choices based upon the needs and the analytes present in the sample.
Materials and Methods
Chemicals
Glacial acetic acid, 2-propanol (>99.9% Chromasol LC-MS, cat: 34965-2.5L), and potassium hydroxide (KOH) were purchased from Honeywell Fluka. Ethanol (Reag. Ph. Eur, Prod. Nr: 1.00983.1000) and mucin from bovine submaxillary glands (BSM), type I–S was purchased from Merck (Darmstadt, Germany). Acetonitrile (Ultra LC-MS, Art. Nr: 801023802) and methanol (Ultra LC-MS, Art. Nr: 813013802) were purchased from Actu-All Chemicals (Oss, The Netherlands). Peptide N-glycosidase F (PNGase F) was obtained from Roche Diagnostics (Mannheim, Germany). Ammonium bicarbonate (ABC), trifluoroacetic acid (TFA), fetuin from fetal bovine serum (FBS), cation-exchange resin Dowex (50W-X8), hydrochloric acid (HCl), sodium borohydride (NaBH4), dl-dithiothreitol (DTT), and ammonium acetate were obtained from Sigma-Aldrich (Steinheim, Germany). Bulk sorbent Carbograph was obtained from Grace Discovery Sciences (Columbia). Ultrapure water was generated from a Q-Gard 2 system; MultiScreen HTS 96-well plates (hydrophobic Immobilon-P PVDF membrane) and 96-well PP Microplates were obtained from Millipore (Amsterdam, The Netherlands). 96-well PP filter plates were obtained from Orochem Technologies (Naperville, IL). Immunoglobulin G from Human Plasma was purchased from Athens Research & Technology (Georgia, United States). Sialylated fetuin N-linked alditols standard was obtained from Dionex (Thermo Fisher, CA, United States)
N- and O-Glycan Release from Purified Glycoproteins
Twenty micrograms of purified glycoproteins IgG, bovine fetuin, and ten micrograms of bovine submaxillary mucin were blotted onto separate wells of preconditioned hydrophobic Immobilon-P PVDF membrane. Both N- and O-glycans were released from the glycoproteins as described before.13 Detailed information about N- and O-glycan release is provided in Supporting Information 1, S-1. Abundances of glycans per replicate per condition are available in Supporting Information 2, Table S-1. Averaged relative abundances of glycans per condition are listed in Supporting Information 2, Table S-2 (N-glycans) and Table S-3 (O-glycans). Raw data are available online at https://glycopost.glycosmos.org/preview/17497542156058d85d3a5c2.
Measurements with PGC-LC-MS/MS Using Different Dopant Solvents
Analysis was performed using a PGC nanoLC Ultimate 3000 UHPLC system (Dionex/Thermo, Sunnyvale, CA) coupled to an amaZon ETD speed ion trap (Bruker Daltonics, Bremen, Germany). The samples were redissolved in 20 μL of water prior to analysis. The released N-glycans from IgG and fetuin were mixed together in a ratio of 1:1 and 1 μL of the mixture was injected into the system. An estimate of the injected amount is shown in Supporting Information 2, Table S-4. Furthermore, 1 μL of the released O-glycans from BSM was injected for analysis. The glycans were separated on a custom-made PGC column. The LC system was coupled to an ion trap MS using the CaptiveSpray source (Bruker Daltonics) in negative-ionization mode. The drying gas (N2) temperature was set at 280 °C and the flow to 3 L/min. The nebulizer gas pressure was kept at 3 psi (for all conditions except for ambient air). The nanoBooster bottle (Bruker Daltonics) was filled with different dopant solvents, namely MeOH, EtOH, IPA, and MeCN. The measurements were performed by filling the bottle with 800 mL solvent, and the level of the dopant inside the bottle was never below 500 mL. Detailed information about the LC-MS/MS settings is provided in Supporting Information 1, S-1.
Results and Discussion
Effect of Dopant Solvents on the Ionization of N-Glycans
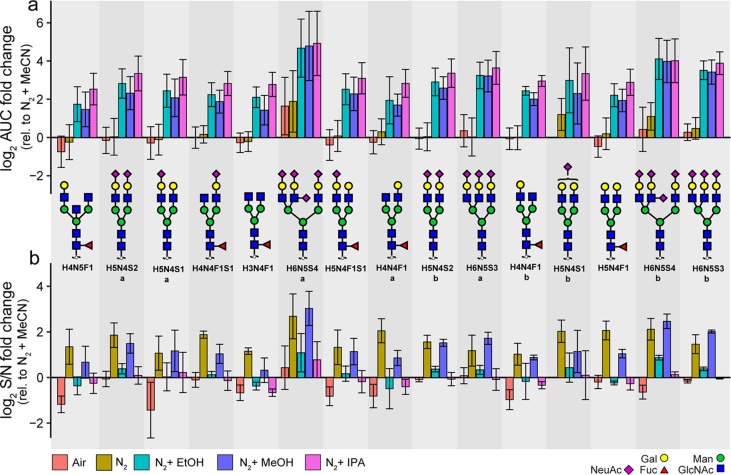
Compared to the standard MeCN enriched nitrogen gas, all of the polar protic solvents tested (IPA, followed by EtOH and MeOH) gave an approximately 8-fold increase in peak areas for the selected 15 most abundant N-glycans released from IgG and bovine fetuin glycoproteins (Figure 1a). Similar effects were detected for the neutral and sialylated glycans. However, significantly higher effect on the peak area is seen for the tetrasialylated early eluting glycan with composition H6N4S4isomer. Our data also show that the increase in peak area comes with a slight decrease in signal-to-noise (S/N) ratios for EtOH and IPA compared to the standard MeCN approach (Figure 1b). From the polar protic solvents, the S/N ratios are the highest when MeOH is used as dopant. The relative abundance remains rather consistent between the different conditions (Supporting Information 1, Figure S-1a). While limited research has been performed about the usage and composition of DEN gas for the analysis of N-glycans in negative mode, previous research showed an increased response of glucose in negative ion mode when MeOH was employed as organic cosolvent compared to MeCN.14 This effect has been attributed to different physical properties of these solvents relevant for ESI, such as surface tension, vapor pressure, and gas phase thermochemistry. Of note, only a single monosaccharide was used to study these effects, in contrast to our study which analyzes the effect of MeOH addition on a broad range of N- and O-glycans. Moreover, a study investigating the effect of polar protic (MeOH and water) and polar aprotic (MeCN and acetone) solvents on ionization of small acidic molecules revealed that the compounds ionized better in polar protic solvents, leading to increased sensitivity and lower limits of detection.15 The effect was consistent in flow injection experiments when pure solvents were used. However, analytes eluting at low organic concentrations showed less pronounced increase in response and sensitivity when MeOH was used as the organic mobile phase in gradient chromatographic separations. Similarly, Henriksen et al. have shown that most of the tested acidic analytes ionize better in negative ion mode when infused in pure MeOH than MeCN, which was attributed to the protic nature of MeOH, a stronger solvation of negative ions and ion pairs results in more deprotonated molecules.16 Intriguingly, they have also observed that the benefits of MeOH on the ionization are absent in aqueous mixtures in LC separations. This might explain why, in a similar investigation for capillary-flow PGC-LC-MS/MS, the authors observed very similar signal intensities for the N- and O-glycans when using MeCN or polar protic solvent (MeOH and IPA) using PCMF.11 This finding emphasizes the advantage of our approach, where there is a clear benefit of an alcohol enriched nebulizing gas even for the aqueous-based separations. On the other hand, compared to the reference MeCN approach, the setup using solely N2 gas or ambient air shows similar results or even a decrease in peak areas for most of the glycans studied. Recently, a study employing DEN gas in CZE coupled to MS in negative ion mode demonstrated that IPA-based DEN gas outperforms MeCN, leading to up to 40-fold increase in peak areas for larger neutral glycans.12
Figure 1.
Effect of different dopant solvents on N-glycans. (A) The fold change of the area under the curve relative to the reference approach (MeCN enriched N2) and (B) fold change of S/N ratios relative to the reference approach (MeCN enriched N2) for the 15 most abundant N-glycans derived from IgG and bovine fetuin (N = 3). The error bars represent the standard deviation from the mean of the technical replicates (N = 3). The glycans are ordered based upon their elution time. H, hexose; N, N-acetylhexosamine; F, deoxyhexose; S, N-acetylneuraminic acid; Sg, N-glycolylneuraminic acid.
An effect on the charge state distribution of glycans was also observed, as illustrated in Figure 2. The polar protic solvents (IPA, MeOH, and EtOH) show the main boosting effect on the doubly charged species of the analytes (Figure 1a, Supporting Information 2, Table S-5), making them the most dominant species per analyte (Figure 2b). Nonetheless, increased peak areas were also observed for the triply charged species compared to the reference approach. Consequently, S/N of the fragment ions were enhanced as well (Supporting Information 2, Table S-6). Interestingly, the triply charged species are relatively more abundant in the approaches that do not use dopant solvents (N2 and ambient air). While the polar protic solvents showed this charge state effect in our DEN gas setup, a similar effect has not been observed in a previous PCMF setup.11 Moreover, another study revealed that IPA-based DEN gas showed an increase in ratios of abundances for triply and doubly charged species compared to conventional CZE-MS.12 The differences between our study and these could be explained by the different solvent compositions during ionization as compared to the post column solvent supplementation in the PCMF study,11 and no gradient elution in the CZE study.12
Figure 2.
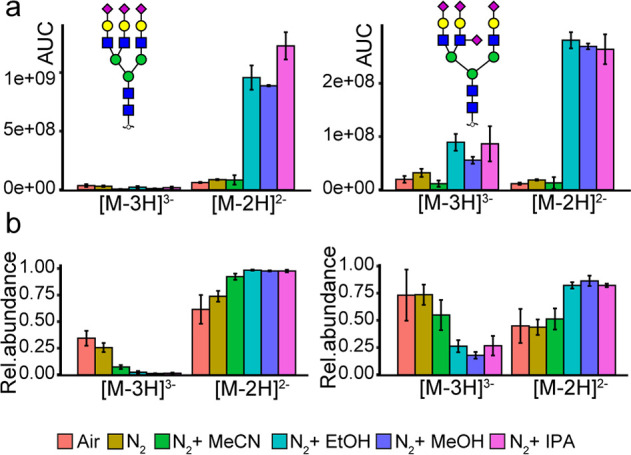
Effect of different dopant solvents on the charge state distribution of N-glycans. (A) Area under the curve and (B) relative abundance of doubly and triply charged species of (1) H6N5S3 and (2) H6N5S4 observed during PGC-LC-MS (N = 3).
Effect of Dopant Solvents on the Ionization of O-Glycans
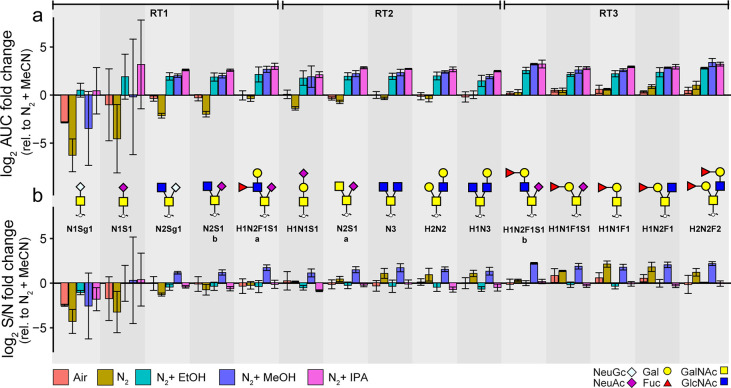
Overall, the O-glycan analysis benefitted a lot from the use of DEN gas. The effects of the dopant solvents were further investigated on a wider range of analyte sizes and elution times. For this purpose, the absolute peak areas of the five most abundant O-glycan species in BSM were investigated over three retention time (RT) windows. Similar to the N-glycans, polar protic solvents (IPA, MeOH, and EtOH) provide approximately a 5-fold increase in peak areas compared to MeCN for all O-glycans in RT windows 2 and 3 (Figure 3a). While similar S/N ratios were obtained for the O-glycans with all dopants (Figure 3b), MeOH shows approximately a 4-fold increase compared to the reference MeCN condition. Therefore, this dopant should be considered for applications that require high sensitivity. While IPA and EtOH provide higher relative areas of early eluting O-glycans, the nonenriched N2 and ambient air showed higher relative areas of later eluting glycans (Supporting Information 1, Figure S-1b). This clearly illustrates that the boosting effect of the dopants is dependent on the elution time of the glycans, as shown in Figure 4. It has been reported previously that early eluting species in the PGC-LC-MS setup ionize less due to the low organic content in the gradient.10 Therefore, it is important to note that the most significant effect of all dopants can be seen on the glycans eluting in the first 10 min of the gradient, e.g., sialyl Tn occupied with an N-acetylneuraminic acid or N-glycolylneuraminic acid. Those glycans elute at very low concentrations of MeCN, and therefore, the ionization of these compounds is expected to be significantly lower compared to the later eluting species.10 It should be noted that the retention of these glycans to the PGC stationary phase is limited; therefore, high CVs in this region are expected. Overall, the peak areas are significantly lower if no dopants are used (solely N2 or ambient air). Therefore, for high-sensitivity detection of these compounds, the use of a dopant setup is advisable. IPA as dopant solvent gives the most pronounced boosting effect, increasing the precursor intensities which is reflected in the quality of the fragmentation spectra as exemplified for the early eluting O-glycans in Supporting Information 1, Figure S-2 and S-3. The MS2 spectra measured with IPA showed many fragments useful for structural determination of the glycan, and in addition, their S/N ratios were significantly improved compared to both MeOH and MeCN (Supporting Information 2, Table S-7). While similar fragments were observed in the spectra with MeOH as a dopant, the improved MS2 data obtained with IPA provided higher confidence in the identification of the glycan isomers due to the higher number and intensity of informative fragment ions.
Figure 3.
Effect of different dopant solvents on O-glycans. (A) The fold change of the area under the curve relative to the reference approach (MeCN enriched N2) and (B) fold change of S/N ratios relative to the reference approach (MeCN enriched N2) for the BSM O-glycans (N = 3) in three retention time windows corresponding to different gradient solvent composition RT1:2–10% MeCN, RT2:10–15% MeCN, RT3: 15–26% MeCN. The error bars represent the standard deviation from the mean of the technical replicates (N = 3). The glycans are ordered based upon their elution time. H, hexose; N, N-acetylhexosamine; F, deoxyhexose; S, N-acetylneuraminic acid; Sg, N-glycolylneuraminic acid.
Figure 4.
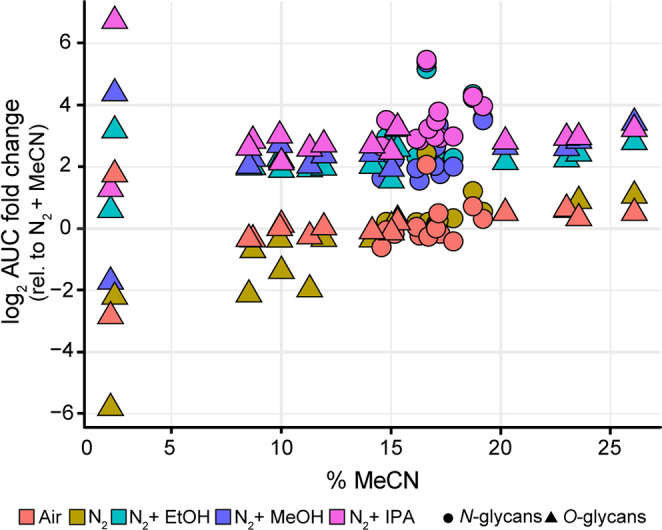
Early eluting glycans show the highest boost with DEN gas. The fold change of the area under the curve with different dopant solvents for both N- and O-glycans as a function of the percent of MeCN in the elution gradient.
While the proposed adaptations give considerable benefit for the analysis of released glycan alditols in negative ionization mode, these benefits may in part depend on the specific electrospray ionization source. In recent years, DEN gas has been successfully adapted to different Bruker electrospray sources and sprayers as well as to other types of mass spectrometers.12,17 Although alcohol-based DEN gas significantly improves the signal for both N- and O-glycans in negative ion mode, analysis of limited sample amounts are still challenging, and further optimization is needed. Moreover, future studies should focus on improving the charging of highly sialylated species to obtain informative MS/MS spectra.
Conclusion
In this study, we demonstrated that the usage of polar protic solvents as dopants such as IPA, EtOH, and MeOH have a beneficial effect on the ionization of both N- and O-glycans in negative ion mode compared to the reference MeCN enriched nitrogen gas. IPA shows the highest effect on peak areas, increasing the precursor intensities, resulting in higher intensities of fragment ions and therefore showing large benefits for the identification of glycan structures. MeOH provides the best results in terms of S/N ratios; therefore, this dopant would be particularly beneficial for high sensitivity MS-mode applications.
Acknowledgments
This work was supported by the European Commission’s Horizon 2020 program “GlyCoCan” project, Grant 676421.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.1c00023.
Supporting Information 1: S1, materials and methods; Figure S1, effect of different dopant solvents on the relative areas of different glycans; Figure S2, effect of different dopant solvents on the fragment spectrum intensities of early eluting O-glycans (PDF)
Supporting Information 2: Table S-1, abundances of N-glycans per replicate; Table S-2, relative abundances of N-glycans per condition; Table S-3, relative abundances of O-glycans per condition; Table S-4, estimation of the injected amount of N-glycans; Table S-5, abundances of N-glycans per charge state; Table S-6, isopropanol enhances fragment ion S/N of triply charged precursors; Table S-7, isopropanol enhances fragment ion S/N of O-glycans (XLSX)
Author Contributions
K.M. performed the experiments. K.M., S.W., T.Z., G.L., and M.W. conceptually designed the work. K.M., G.L., and M.W. wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Mysling S.; Palmisano G.; Hojrup P.; Thaysen-Andersen M. Utilizing Ion-Pairing Hydrophilic Interaction Chromatography Solid Phase Extraction for Efficient Glycopeptide Enrichment in Glycoproteomics. Anal. Chem. 2010, 82 (13), 5598–5609. 10.1021/ac100530w. [DOI] [PubMed] [Google Scholar]
- Nguyen S.; Fenn J. B. Gas-Phase Ions of Solute Species from Charged Droplets of Solutions. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (4), 1111–1117. 10.1073/pnas.0609969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar S.; Ledertheil T.; Hartmer R.; Hagedorn T.; Baessmann C.; Nugent K.. Increasing Peptide Identification Rates for Proteomics Samples by Controlling Peptide Charge States Using CaptiveSpray NanoBooster. Technical Note # TN-4. Bruker Daltonics, 2013.
- Alagesan K.; Kolarich D.. To Enrich or Not to Enrich: Enhancing (Glyco)Peptide Ionization Using the CaptiveSpray NanoBoosterTM. bioRxiv. 2019 10.1101/597922. [DOI] [Google Scholar]
- Kammeijer G. S. M.; Kohler I.; Jansen B. C.; Hensbergen P. J.; Mayboroda O. A.; Falck D.; Wuhrer M. Dopant Enriched Nitrogen Gas Combined with Sheathless Capillary Electrophoresis–Electrospray Ionization-Mass Spectrometry for Improved Sensitivity and Repeatability in Glycopeptide Analysis. Anal. Chem. 2016, 88, 5849. 10.1021/acs.analchem.6b00479. [DOI] [PubMed] [Google Scholar]
- Möginger U.; Grunewald S.; Hennig R.; Kuo C.-W.; Schirmeister F.; Voth H.; Rapp E.; Khoo K.-H.; Seeberger P. H.; Simon J. C.; Kolarich D. Alterations of the Human Skin N- and O-Glycome in Basal Cell Carcinoma and Squamous Cell Carcinoma. Front. Oncol. 2018, 8, 70. 10.3389/fonc.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinneburg H.; Korać P.; Schirmeister F.; Gasparov S.; Seeberger P. H.; Zoldoš V.; Kolarich D. Unlocking Cancer Glycomes from Histopathological Formalin-Fixed and Paraffin-Embedded (FFPE) Tissue Microdissections. Mol. Cell. Proteomics 2017, 16 (4), 524–536. 10.1074/mcp.M116.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsson K. A.; Karlsson N. G.; Hansson G. C. Liquid Chromatography-Electrospray Mass Spectrometry as a Tool for the Analysis of Sulfated Oligosaccharides from Mucin Glycoproteins. J. Chromatogr. A 1999, 854 (1–2), 131–139. 10.1016/S0021-9673(99)00625-1. [DOI] [PubMed] [Google Scholar]
- Harvey D. J. Negative Ion Mass Spectrometry for the Analysis of N -linked Glycans. Mass Spectrom. Rev. 2020, 39 (5–6), 586–679. 10.1002/mas.21622. [DOI] [PubMed] [Google Scholar]
- Pabst M.; Altmann F. Influence of Electrosorption, Solvent, Temperature, and Ion Polarity on the Performance of LC-ESI-MS Using Graphitic Carbon for Acidic Oligosaccharides. Anal. Chem. 2008, 80 (19), 7534–7542. 10.1021/ac801024r. [DOI] [PubMed] [Google Scholar]
- Hinneburg H.; Chatterjee S.; Schirmeister F.; Nguyen-Khuong T.; Packer N. H.; Rapp E.; Thaysen-Andersen M. Post-Column Make-Up Flow (PCMF) Enhances the Performance of Capillary-Flow PGC-LC-MS/MS-Based Glycomics. Anal. Chem. 2019, 91 (7), 4559–4567. 10.1021/acs.analchem.8b05720. [DOI] [PubMed] [Google Scholar]
- Marie A.-L.; Ray S.; Lu S.; Jones J.; Ghiran I.; Ivanov A. R. High-Sensitivity Glycan Profiling of Blood-Derived Immunoglobulin G, Plasma, and Extracellular Vesicle Isolates with Capillary Zone Electrophoresis-Mass Spectrometry. Anal. Chem. 2021, 93, 1991. 10.1021/acs.analchem.0c03102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Madunić K.; Holst S.; Zhang J.; Jin C.; Ten Dijke P.; Karlsson N. G.; Stavenhagen K.; Wuhrer M. Development of a 96-Well Plate Sample Preparation Method for Integrated: N - And O -Glycomics Using Porous Graphitized Carbon Liquid Chromatography-Mass Spectrometry. Mol. Omi. 2020, 16 (4), 355–363. 10.1039/C9MO00180H. [DOI] [PubMed] [Google Scholar]
- Thacker J. B.; Schug K. A. Effects of Solvent Parameters on the Electrospray Ionization Tandem Mass Spectrometry Response of Glucose. Rapid Commun. Mass Spectrom. 2018, 32 (15), 1191–1198. 10.1002/rcm.8155. [DOI] [PubMed] [Google Scholar]
- Huffman B. A.; Poltash M. L.; Hughey C. A. Effect of Polar Protic and Polar Aprotic Solvents on Negative-Ion Electrospray Ionization and Chromatographic Separation of Small Acidic Molecules. Anal. Chem. 2012, 84 (22), 9942–9950. 10.1021/ac302397b. [DOI] [PubMed] [Google Scholar]
- Henriksen T.; Juhler R. K.; Svensmark B.; Cech N. B. The Relative Influences of Acidity and Polarity on Responsiveness of Small Organic Molecules to Analysis with Negative Ion Electrospray Ionization Mass Spectrometry (ESI-MS). J. Am. Soc. Mass Spectrom. 2005, 16 (4), 446–455. 10.1016/j.jasms.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Lageveen-Kammeijer G. S. M.; de Haan N.; Mohaupt P.; Wagt S.; Filius M.; Nouta J.; Falck D.; Wuhrer M. Highly Sensitive CE-ESI-MS Analysis of N-Glycans from Complex Biological Samples. Nat. Commun. 2019, 10 (1), 2137. 10.1038/s41467-019-09910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.