Abstract

This study presents, for the first time, the successful application of analyzing a whole gas chromatography (GC) chromatogram by nuclear magnetic resonance (NMR) spectroscopy using a continuous repeatable and stable (n = 280) high-resolution (HR) GC fractionation platform with a 96-well plate. Typically with GC– or liquid chromatography–mass spectrometry analysis, (isomer) standards and/or additional NMR analysis are needed to confirm the identification and/or structure of the analyte of interest. In the case of complex substances (e.g., UVCBs), isomer standards are often unavailable and NMR spectra too complex to achieve this. This proof of concept study shows that a HR GC fractionation collection platform was successfully applied to separate, purify, and enrich isomers in complex substances from a whole GC chromatogram, which would facilitate NMR analysis. As a model substance, a chlorinated paraffin (CP) mixture (>8,000 isomers) was chosen. NMR spectra were obtained from all 96 collected fractions, which provides important information for unravelling their full structure. As a proof of concept, a spectral interpretation of a few NMR spectra was made to assign sub-structures. More research is ongoing for the full characterization of CP isomers using multivariate statistical analysis. For the first time, up to only a few CP isomers per fraction were isolated from a highly complex mixture. These may be further purified and certified as standards, which are urgently needed, and can also be used for persistency, bioaccumulation, or toxicity studies.
Typically with gas chromatography (GC) or liquid chromatography–mass spectrometry (LC–MS) analysis, (isomer) standards are needed to confirm the identification of the analyte of interest. In addition, nuclear magnetic resonance (NMR) spectroscopy is needed to confirm the structure of an isomer. This is challenging with complex substances, such as substances with unknown or variable composition, complex reaction products, and biological materials (UVCBs). For example, isomer standards are often unavailable for UVCBs. In addition, their NMR spectra are too complex to unravel the structure of isomers present.
There are over 16,969 UVCB substances registered by the US EPA.1 An example of UVCB with high production volumes (>2 million tonnes/year annually2) is chlorinated paraffins (CPs), also known as polychlorinated n-alkanes. Accurate data are difficult to obtain due to their extremely complex nature (100,000s of isomers),3 the lack of well-defined structural information (unknown chlorine substitution), and consequently, the lack of suitable (isomer) standards.4 For example, while their chlorine substitution remains unknown, results by two dimensional gas chromatography (GC × GC) show that current commercially available isomer standards have a different structural composition (i.e., chlorine substitution) compared to CPs found in technical mixtures.5 Another example is that even simpler (non-commercially available) single-carbon chain mixtures of CPs (e.g., C14) are still too complex to fully characterize the structure of individual isomers by use of NMR spectroscopy.6 Although Yuan et al.7 recently successfully applied NMR predictor software to present the most likely structural matches of CPs found in single chain mixtures, single-CP isomer NMR spectra are still needed to confirm these modeled results.
GC and/or LC fractionation, also known as preparative GC or LC, is a promising tool which found its way in numerous application fields such as environmental,8,9 pharmaceutical,10 food, flavor, and fragrance industrial sectors.11−14 Although chromatographic fractionation using GC can be superior to LC in terms of separation efficiency and analysis time,15,16 GC fractionation is less commonly applied largely due to its difficult setup. An even more complicated setup and thus less commonly applied is preparative GC in combination with NMR, an analyte purification and enrichment tool to elucidate the structure of organic compounds.14,17,18 Prior attempts and marketed GC fractionation systems required complex systems involving cryotrapping the analyte or trapping the analyte on a sorbent, which only allowed the collection of a small number of fractions.19−21 This is suitable when only a few target compounds are to be analyzed, however unsuitable for complex mixtures such as UVCBs.
Recently, a high-resolution GC fractionation platform was developed22−24 that was able to fractionate on 96–384 well plates and post-column off-line analyze all fractions collected from full GC chromatograms, by having, via a split, post-column parallel flame ionization detection (FID) or MS detection. Due to its high-resolution fractionation, capability of fractionating complete GC chromatograms, and the possibility to increase the yield of each fraction collected by straightforward automated multiple fraction collection on the same well plate (or in vials for fraction collection, which is also possible), this platform has been successfully applied to cell-based bioactivity analysis23 and may be a promising tool for enabling the analysis of whole GC chromatograms by NMR spectroscopy.
This proof of concept study demonstrates the use of the continuous GC fraction collection platform with repeatable and stable fractionations (n = 280) on a 96-well plate to purify and enrich isomers of complex substances. In addition, it shows the possibilities of analyzing a whole GC chromatogram by NMR using this platform to potentially identify or confirm their structures. Two other analytical methods [i.e., comprehensive GC × GC and quadrupole time of flight MS in the negative atmospheric pressure chemical ionization mode (APCI-QToF-MS)] were also applied to further explore the separation abilities (i.e., the purification and enrichment of isomers) of the GC fractionation platform. As a model substance, arguably one of the most complex organochlorine substances, CP–C14 mixture with 41.3% chlorine (Cl) by weight, was chosen.25
Experimental Section
Chemicals
n-Hexane (pesticide residue analysis) was purchased from Fluka (Landsmeer, The Netherlands), chloroform-d, n-octane (>99%) from Sigma-Aldrich (Zwijndrecht, The Netherlands), and acetonitrile (99.8%) and iso-octane (+99%) from Thermo Fisher (Geel, Belgium). The polychlorinated n-alkane mixture tetradecane with an average chlorine content of 41.3% by weight (CP–C14) was manufactured by Quimica del Cinca (Barcelona, Spain) and kindly donated by the Chlorinated Paraffins Industry Association (Washington DC, United States).
Instruments
The GC fraction collector platform has been described in previous work22−24 and a schematic overview is presented in S1. Briefly, a PAL system (CTC Analytics AG, Zwingen, Switzerland) was used for sample injection on an Agilent HP 6890 GC oven, equipped with a split/splitless injection port (Agilent Technologies, Santa Clara, CA, USA) and an Agilent Ultra 2 (5%-phenyl)-methylpolysiloxane (25.0 m × 200 μm × 0.11 μm) column for separation. The column eluate was split toward a FID (Agilent Technologies, Santa Clara, CA, USA) for peak detection and an inverted y-piece where the preheated/vaporized trap solvent was infused. The trap solvent was delivered via a Shimadzu LC-10Ai pump and preheating was facilitated using a modified FID. At the inverted y-piece, eluting compounds mix with the vaporized trap solvent and are directed outside the GC oven via a capillary while the trap solvent condenses at ambient room temperature, enabling fraction collection. The capillary exit was connected to a smart grip unit (Da Vinci Laboratory Solutions, Rotterdam, The Netherlands) that was maneuvered over a 96-well plate for fraction collection. The instrument parameters were controlled with Agilent ChemStation 7.0 software, while the PAL system was controlled with Da Vinci Europe Smart PAL interface version 0.0.9.
The CP–C14 mixture was injected (1 μL) with an injection temperature of 275 °C in the splitless mode with a 2 min split time. The purge flow was set to 50 mL/min for 2.0 min, and after that, the gas saver was switched on. The oven temperature started at 150 °C, increased by 10 °C/min to 250 °C, then 35 °C/min to 320 °C and at 320 °C ended with a holding time of 2 min. The helium carrier gas flow was set to 2.0 mL/min. The FID was set to 275 °C, with a hydrogen flow of 40 mL/min, an air flow of 400 mL/min, and a nitrogen makeup flow of 45 mL/min. The solvent’s flow rate was set to 0.3 mL/min, and was preheated to 250 °C before being introduced to the y-piece. Fraction collection started 1.0 min after injection in 96 700 μL glass inserts located on a 96-well plate (Waters, US) with a 7.0 s interval per fraction and was performed in a serpentine motion: A1–A12 followed by B12–B1 and so forth to H1 (graphical abstract). After every fractionation trap, the solvent was evaporated and discarded. Injection of the CP–C14 mixture was repeated 280 times, with a total run time of 65 h. Two types of solvents were tested for their applicability as the trap solvent: n-hexane and n-octane. For GC analysis, 200 μL of each fraction was transferred to a vial containing 800 μL of n-octane. For NMR analysis, each fraction was gently blown down with nitrogen, re-dissolved in 0.6 mL of chloroform-d and transferred to a NMR glass tube. After NMR analysis, chloroform-d was gently evaporated in air and the tube was refilled with ca. 2.5 mL of pentane, of which 40 μL was taken out, blown down, and re-dissolved in acetonitrile for MS analysis.
NMR spectra were recorded on a Bruker Avance II 500 (Fällanden, Switzerland) at a 1H frequency of 500.20 MHz and equipped with a 5 mm inverse triple resonance (1H, 13C, and 15N) cryoprobe with an active shield Z gradient and a Bruker Avance III HD 600 at a 1H frequency of 600.13 MHz and equipped with a 5 mm inverse triple resonance (1H, 13C, and 15N) cryoprobe with an active shield Z gradient. The residual solvent peak was used as an internal standard (1H: δ 7.26 ppm for chloroform). A standard proton experiment (zg30) was used at 25 °C. Data were collected with 65k time domain points and 20 ppm spectral width. The frequency offset was set to 6.175 ppm. For each 1H NMR spectrum, 1024 transients were collected with a recycle delay of 1 s. Spectra were processed with an exponential multiplication equivalent of 0.3 Hz line broadening.
As comprehensive GC × GC is one of the few approaches known for its ability to separate MCCPs to individual components,26 it is a promising tool to evaluate the fractionation process. All fractions, as well as the CP–C14 mixture itself, were injected on the GC × GC-μECD system. Single blobs in the obtained chromatograms could possibly indicate that fractionation to individual isomers was achieved. Each fraction and the CP–C14 mixture were analyzed using the GC × GC system, consisting of an Agilent HP7890 gas chromatograph (Palo Alto, CA, USA) equipped with μECD of which instrument settings and data analysis are reported in previous work.26
All fractions (and the CP–C14 mixture) were also analyzed on a (APCI-QToF-MS) by direct injection using high-performance liquid chromatography (HPLC). The APCI-QToF-MS method is described elsewhere.27 In brief, the APCI-QToF-MS consisted of a Bruker Compact (Bremen, Germany) and a HPLC system Agilent 1290 infinity on which 5 μL was injected and acetonitrile with 10% dichloromethane (v/v) was used as an eluent with an isocratic flow of 250 μL/min. The 10% dichloromethane solution was used as the dopant to enhance the formation of [M + Cl]− fragments of CPs. For integration, strict isotope pattern criteria were applied. The m/z ratios for CP–C14 applied in this study can be found in Table S1, which are related to the two most abundant [M + Cl]− ions of the CP isotope cluster corresponding to the congener groups (CPs with the same molecular formula), expressed as CmCln, C14Cl3 to C14Cl12.27
Results and Discussion
The continuous high-resolution GC fraction collection platform was applied to analyze a whole GC chromatogram by NMR spectroscopy as a proof-of-concept study. Other analytical methods such as GC × GC and APCI-QToF-MS were applied to investigate the separation possibilities of the platform further and as a tool to understand the NMR data (Figure 1).
Figure 1.
Schematic overview and workflow of the continuous high-resolution GC fractionation platform in combination with NMR spectroscopy. First, the continuous high-resolution GC fractionation platform is applied to purify and enrich isomers of complex substances by multiple (n = 280) repeatable and stable fractionations on a single 96-well plate. The solvent of each fraction is then evaporated, after which the fraction is re-dissolved in chloroform-d for NMR spectroscopy. To evaluate the fractionation process and to understand the NMR data, each fraction is also analyzed by GC × GC and (LC)-APCI-TOF-MS.
High-Resolution GC Fraction Analysis by GC-FID
Because the injection volume of the GC fractionation platform was limited to 1 μL and taking into account the relative low sensitivity of (proton) NMR, some test injections on a similar GC system with different CP concentrations (0.1, 1, and 5 mg/mL in isooctane) were performed to find out the highest concentration that did not compromise the resolution. Although the signal intensity differs, the characteristic peaks are similar between the concentration of 0.1 and 1 mg/mL (Figure S1A,B). However, with a concentration of 5 mg/mL, there was an obvious loss of resolution (Figure S1C). Based on these results, the choice was made to proceed with the 1 mg/mL concentrated solution.
Two types of solvents (n-hexane and n-octane) were tested for their applicability as the trap solvent. Although n-octane has superior trapping efficiency compared to n-hexane, also discussed in previous work,24n-hexane was chosen because of its higher volatility: for the NMR measurements, the trap solvent needs to be blown down to dryness and the collected analytes re-dissolved in chloroform-d. The volatility of n-octane is so low that there is a risk of losing MCCPs during evaporation. Furthermore, when using n-octane, the well plates need to be switched regularly to prevent overflowing, while n-hexane is volatile enough to perform a continuous fractionation without the risk of flooding the glass inserts in the well plate.
To ascertain that no retention time shift occurred during fractionation, which could cause mixing of different fractions, frequent chromatogram comparisons were performed. No retention time shift was observed when using n-hexane as the trap solvent between the chromatograms of the 1st and 280th fractionation (Figure S2), indicating an exact peak matching and that mixing of fractions did not occur during fractionation.
High-Resolution GC Fraction Analysis by GC × GC-μECD
To evaluate the fractionation process, the whole C14 mixture and each fraction were analyzed by GC × GC-μECD. The complexity of the mixture and the power of GC × GC separation are clearly shown in the GC × GC chromatogram of CP–C14 mixture (Figures 2 and 3). Single dots are present on the GC × GC chromatogram, which could indicate single CP isomer separation. The dots elute consecutively as diagonal lines, representing the CP congener groups (i.e., isomers with the same molecular formula, expressed as CmCln in Figure 3A), according to previous work.26 The power of GC × GC separation is especially shown when comparing the GC × GC and the single chromatogram (Figure 2) for the congener groups C14Cl5–7. For example, even with an extremely long oven temperature program (90 min), single GC cannot distinguish C14Cl6 from C14Cl5 and C14Cl7, resulting in overlap on 50–53 and 57–61 min in the single GC chromatogram (black squares in Figure 2B), while GC × GC can, resulting in different blobs vertically above each other around the same time (Figure 2A). This is very interesting as the Cl6 and Cl7 congener groups are usually the most dominant in environmental samples.28 Congener groups C14Cl3 to C14Cl12 were observed, while the hump of peaks around the retention time 20 min (Figure 2A) might indicate C14Cl2 presence. Thus far, it remains uncertain whether C14Cl2 is present in technical mixtures as these do not ionize and/or their response is too low to be detected by the current analytical methods used. To our knowledge, only one study29 discussed the presence of CPs with Cl1–2, stating that these CPs could be present in mixtures with <50% Cl. In general, isomer information (i.e., chlorine configuration alongside the carbon chain length) of the blobs detected by GC × GC-μECD remains unknown and standards are needed for identification and confirmation.
Figure 2.
GC × GC-μECD chromatogram (panel A) and single GC chromatogram (horizontal slice at 3.4 min on the second dimension, panel B) of CP–C14 41.3% Cl mixture of which the black squares illustrate the overlap of the congener group C14Cl6 with C14Cl5 and C14Cl7.
Figure 3.
GC × GC-μECD chromatograms of CP–C14 41.3% Cl mixture (panel A) and three fractions (E6–E7 and E11, fractionation time 438–445 and 473 s, panel B–D), all showing (illustrated by blue dotted lines) that fractions contain separate peaks, indicating that fractions contain different isomers. The two other peaks present in fractions E6 and E7 (Figure S3) were of too low intensity to be shown in the GC × GC-μECD chromatogram.
The first peaks were observed in fractions B5–B3 (fractionation time 200–214 s). This is in line with the peaks observed around 3.5 min in the GC-FID chromatogram (Figure S2). After that, peaks remained below detection limits until C5 (263 s). From fraction C6 at 270 s, peaks were detected, of which examples are shown in Figure 3A–C (fraction E6–E7, 438–445 s). Each consecutive fraction contains a separate peak eluting ca. 45 s later than the peak of the fraction before, illustrated in Figures 3A–C and S3 by the blue dotted lines, which indicates that different isomers are collected in each fraction. Interestingly, a certain pattern was identified in the fractions by inspecting the single chromatograms of the GC × GC-μECD, of which an example of fraction E6 and F11 is shown in Figure 3B,D: each fraction contains two peaks with a 5 min interval on the first column retention time, indicating that two different isomers are present. The two peak combination gradually moves with each consecutive fraction alongside the retention time of the first column to the right maintaining the 5 min interval. Furthermore, the second eluting peak of a fraction is eluting just right after the first eluting peak (ca. 7 s) in eight fractions later, indicating that these are two different isomers. An example is shown in Figure 3B,D, where the second eluting peak in fraction E6 is eluting just right after the first eluting peak in fraction F11, illustrated by the blue dotted line. As these are different isomers, it rejects the argument that something went wrong during fractionation. Rather, the difference in oven temperature program could explain this observation. Although the column separated the isomers during GC fractionation to a good degree (indicated by only having two peaks in one fraction rather than an uncountable number of peaks in a 5 min interval on the GC × GC chromatogram, Figure 3D), it was unable to separate the two remaining isomers due to the relatively fast oven temperature program (15 min). With the rather long oven temperature program of the GC × GC-μECD (90 min), the column (same type as GC fractionation) was able to separate these two peaks. This observation clearly shows how complex CP mixtures are and how many isomers are present. Nonetheless, in fractions E3–E7 (Figure S4), the second peak is of such low intensity that those fractions contain (as good as) isolated isomers, which might be used as standards.
High-Resolution GC Fraction Analysis by APCI-TOF-MS
To further evaluate the fractionation process, the unfractionated mixture and fractions were analyzed by the chlorine-optimized APCI-TOF-MS method. The APCI-TOF-MS method omits chromatographic separation and solely focuses on mass separation by using only a guard column in the LC. How and which CP isomers form [M + Cl]− ions by this method remains unknown. CP congener groups C14Cl3 to C14Cl12 were detected in the unfractionated mixture (Figure 4A), although C14Cl3 (zoomed-in panel Figure 4A) and C14Cl11–12 were in low abundance. The CP congener groups C14Cl2 (m/z 303.1234) and C14Cl>12 remained undetected, indicating that they do not ionize (C14Cl2) or that they are absent in the mixture (C14Cl2 and/or C14Cl>12). The absence of C14Cl>12 congener groups is in line with the GC × GC-μECD results and reasonable, as CP–C14 is a low-chlorinated CP mixture (41.3% Cl). In line with the low chlorination degree, C14Cl5 is the most dominant congener group in the mixture.
Figure 4.
Highlighted chlorine-optimized APCI-TOF-MS spectra of CP–C14 41.3% Cl mixture (panel A), as well as fractions E10 (fractionation time 466 s, panel B) and F11 (494 s, panel C). In the spectra of the CP–C14 41.3% Cl mixture [M + Cl]− of congener groups, expressed as CmCln, C14Cl3–12 can be observed, with m/z values of the most abundant ion per congener group 337.0844, 371.0454, 405.0064, 438.9674, 474.9255, 508.8866, 542.8476, 576.8086, 612.7667, and 646.7277, which are according to previous work.26 In the spectra of fraction E10, [M + Cl]− of congener groups C14Cl4 (m/z value 371.0454) and C14Cl5 (m/z value 405.0064) can be observed, while in F11, C14Cl4 (m/z value 371.0454) can be observed. The full m/z ratio list can be found in Table S1.
In the first 53 fractions (A1–E5, fractionation time 0–431 s), peaks remained below detection limits. This included fractions C6–E5, in which peaks were observed by GC × GC-μECD, which indicates that GC × GC-μECD is more sensitive in detecting CPs than APCI-TOF-MS. Two or more m/z spectral clusters representing different congener groups were identified in most fractions, of which an example is shown in Figure 4B, which is consistent with GC × GC-μECD results. In fractions E9–E12 (459–480 s), F12 (487 s), and F9 (508 s), only one spectral cluster was identified (of which an example is shown in Figure 4C), which could indicate that one isomer or isomers with the same molecular formula (i.e., congener groups) were present in these fractions. To our knowledge, congener group standards of CP–C14 are commercially unavailable. Response factors between congener groups are unknown, let alone for different isomers. For APCI-TOF-MS, these fractions could be suitable as standards.
High-Resolution GC Fraction Analysis by NMR
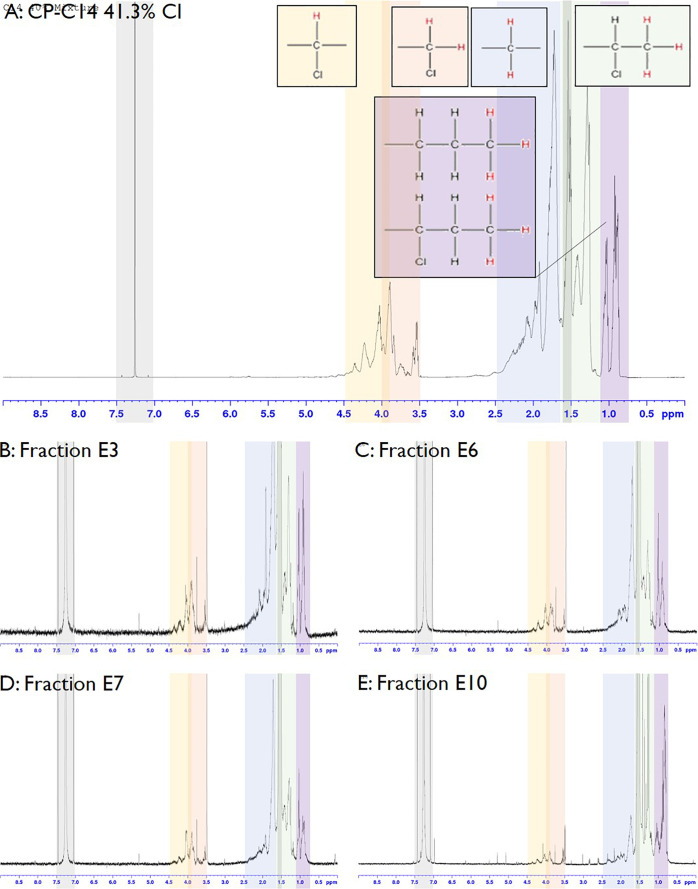
The unfractionated mixture (Figure 4A) and all 96 fractions were analyzed by NMR (Figure S5), of which the mixture and 12 fractions [i.e., A9 (123 s), B4 (207 s), C9 (291 s), D4 (375 s), E3 (4157 s), E6–E7 (438–445 s), E9–E10 (459–466 s), F4 (543 s), G9 (627 s), and H4 (7111 s)] were selected for interpretation.
In the first 49 fractions (A1–E1, 0–403 s), peaks remained below detection limits by spectral interpretation. From fraction E2 and onward, peaks were detected, of which examples are given in Figures 5 and 6. This shows that these fractions were enriched enough to be analyzed by NMR. To the best of our knowledge, this is the first time that a whole GC chromatogram could be analyzed by NMR. Given below are the first steps of structure assignments, by describing what kind of structures are expected and which structures the observed peaks highly likely represent.
Figure 5.
Highlighted 1H NMR spectra of CP–C14 41.3% Cl mixture and fractions (B) E3 (fractionation time 417 s), (C,D) E6–E7 (438–445 s), and (D) E10 (466 s), with highlighted areas of peaks that represent certain fragments of CP–C14 (see panel A), of which the gray-colored areas represent the water peak (1.54 ppm) and chloroform (7–7.5 ppm). Full-sized spectra can be found in S5.
Figure 6.
Highlighted 1H NMR spectra of fractions A9 (fractionation time 123 s), B4 (207 s), C9 (291 s), D4 (365 s), E9 (459 s), F4 (543 s), G9 (627 s), and H4 (711 s). Highlighted areas of peaks represent certain fragments of CP–C14 (see legend), while the gray-colored areas represent the water peak (1.54 ppm) and chloroform (7–7.5 ppm).
A 1H spectrum of 2-chlorobutane was recorded (Figure S6) as a model spectrum to determine the chemical shift of the methyl group adjacent to a chloromethylene group (−CHCl–, green region in Figure S7). Based on the literature6,7,29 and 1H NMR spectra of 2-chlorobutane (Figure S6), we would expect at least some of the following substitution patterns. CPs are made by radical chlorination of paraffinic hydrocarbon mixtures that contain straight-chain saturated hydrocarbons, in which secondary and primary carbons are primarily available for chlorine substitution.29 Therefore, Howard et al.29 suggested that chlorine atoms are probably randomly distributed, up to a point, along the methylene (−CH2−) groups of the carbon chain. The reactivity of the aliphatic hydrogens during free-radical chlorination is dependent on the stability of the intermediate radical species, meaning that tertiary carbons are chlorinated faster than secondary ones, followed by primary ones, which was also found by Yuan et al.7 Geminal chlorination (two chlorines on one carbon) is unfavorable. As the inductive effect of chlorine reduces the reactivity of the neighboring C–H bond, geminal dichlorination is highly unfavorable and does not occur in lower-chlorinated CP mixtures such as the one used in this study (41.3% Cl).7 The same inductive effect also inhibits vicinal substitution (−CHCl–CHCl−) whenever more favorable sites are available. Similarly, terminal chlorination is also less likely to occur in CPs present in the mixture CP–C14 41.3% Cl.7
Following the reasoning above, it is expected to find at least peaks in δ 1.6–2.5 (blue region Figures 5 and 6), which are most likely from the (nonchlorinated) methylene groups, as well as peaks between δ 4.0 and 4.5 (yellow region), representing the chlorinated methylene groups (−CHCl−). These peaks are indeed present in the spectra of the mixture (Figure 5A) and fraction E2 and onward (Figures 5, 6, and S5), while absent in earlier fractions. Interestingly, differences are observed in the number of peaks and peak shapes between the fractions. For example, the peaks in the blue region in fraction F4 (Figure 6F) differ from those in fraction H4 (Figure 6H). A clear upfield shift is visible, indicating that the isomer(s) in fraction H4 contains more chlorinated methylene groups and thus more chlorine atoms. For structure assignment, this warrants future research.
As these fractions are derived from a low-chlorinated CP mixture (41.3%), peaks are also expected between δ 0.8 and 1.2 (purple region Figures 5 and 6), which most likely represent a terminal non-chlorinated methyl (−CH3) group attached to one or more non-chlorinated methylene (−CH2−) groups (see legend in Figure 5), as well as between δ 1.2 and 1.6 (green region), most likely from methyl groups adjacent to chlorinated methylene groups (−CHCl−) as seen in the 1H spectrum of 2-chlorobutane (Figure S6). These peaks are also present in the spectra of the mixture and fractions, again although in different number and peak shapes. The region δ 1.2–1.6 unfortunately partly overlaps with the water peak.
Peaks at 3.5–4.0 (orange region) come from chloromethyl groups (−CH2Cl), representing in this case chlorinated terminal carbons. These are found in higher abundance in the fractions containing higher chlorinated CP isomers (fractions G9 and F4, Figure 6G,H) compared to the rest of the fractions (Figure 6A–F). This is in line with their higher chlorination degree and thus has a higher likelihood of terminal chlorination.7 All in all, the results above indicate that there is variation per well and thus that different isomers are successfully collected according to their properties, during fractionation.
Due to the numerous possibilities and combinations of C–H and C–Cl bonds, it remains a challenge to elucidate the whole structure of individual isomers by spectral interpretation in NMR spectra of even single to a few-CP isomers. For assigning the structure of individual isomers in each fraction, more advanced methods are needed. An option is to use multivariate data analysis with the GC × GC, MS, and NMR data, which falls outside the scope of this proof-of-concept study, but is ongoing research. With this method, the goal is to assess whether there are similarities in NMR shifts (that might stand for certain bonds) in the spectra obtained by NMR analysis between the fractions, using the information on the number of chlorines present in the isomers per fraction, which is obtained by GC × GC and APCI-TOF-MS analysis.
Concentrations in the fractions were too low to perform 13C NMR, correlation spectroscopy, and heteronuclear single quantum coherence experiments with a reasonable resolution and S/N ratio. An option to enable such analyses is to extend the number of fractionations for more enrichment. Fractionation may also be extended to 384 wells in future work depending on the GC resolution and peak widths, although the fractionation might be too scattered to enrich enough material needed for NMR spectroscopy. Other suggestion for follow-up studies is to investigate the possibilities of using larger internal diameter columns and large volume injection to increase the loadability on the GC column to reduce the number of injections. This might be challenging due to the required higher flow rates, which require a new setup of the system and optimization of the backpressures.
Conclusions
A continuous high-resolution GC-fractionation platform was successfully applied to analyze, for the first time, a whole GC chromatogram by NMR spectroscopy. In addition, up to a few CP isomers per fraction from a technical mixture were successfully isolated for the first time.
The application provided 96 NMR spectra of one GC chromatogram containing valuable information of the structure of single to a few CP isomers per fraction. Detailed spectral interpretation of a few NMR spectra was made to assign some of the structures present in the isomers, showing that the isomers in the fractions were enriched enough for NMR spectroscopy. To fully characterize the CP isomers, additional in-depth analysis such as multivariate data analysis is needed, which is outside the scope of this article. CPs are known as one of the most complex (i.e., 10,000 isomers and numerous possibilities and combinations of C–H and C–Cl bonds) and thus challenging mixtures for characterization.7
The next step is to analyze the NMR, GC × GC, and MS data by multivariate data analysis, which is ongoing. The goal is to identify important spectral ranges that could stand for certain sub-structures present in a C14–CP mixture to unravel and confirm the modeled results of the chlorine substitution on CPs in technical mixtures. This can then lay the foundation for assessing quantitative structure–activity relationship predictions. The isolated isomers obtained in this study can be further purified and certified as reference standards, which are urgently needed,4 or used in persistency, bioaccumulation, and toxicity studies.
Acknowledgments
Da Vinci Laboratory Solutions is thanked for providing the GC-fractionation platform and Jaap Schaap from Da Vinci is acknowledged for his advice and support concerning the GC fractionation platform. Quimica del Cinca is acknowledged for synthesizing the CP-14 50% mixture and Andrew Jaques of the Chlorinated Paraffins Industry Association is thanked for arranging the provision of the samples. Dr. Eelco Ruijter is acknowledged for his input and expertise on the interpretation of the NMR Spectra. L.M.v.M. and P.E.G.L. were financially supported by CEFIC LRI (ECO 38, ECO 42). L.M.v.M. was also financially supported by Eurostars (E!113388—CHLOFFIN).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.1c00049.
Schematic overview of the continuous high-resolution GC fraction collection platform; additional information on the m/z spectra of CPs with a carbon chain length of 14 used for the chlorine-optimized APCI-TOF-HRMS analysis; GC-FID chromatograms of CP–C14 41.3% Cl mixture (Section 3); (GC) × GC-μECD chromatogram of CP–C14 41.3% Cl mixture and various fractions; and 1H NMR spectra of CP–C14 41.3% Cl mixture, all 96 fractions, and 2-chlorobutane (PDF)
1H NMR spectra of all 96 fractions (MP4)
The authors declare no competing financial interest.
Supplementary Material
References
- USEPA Non-confidential Toxic Substances Control Act Chemical Substance (TSCA) Inventory. https://www.epa.gov/tsca-inventory/how-access-tsca-inventory#download (Accessed 15 December 2020).
- Van Mourik L. M.; Brandsma S. H.; Yuan B.; Castro M.. Chlorinated Paraffins—State of Science, Insights, Challenges and the Way Forward; SETAC Globe, August 2020 Issue, 2020. [Google Scholar]
- Yuan B.; Muir D.; MacLeod M. Methods for trace analysis of short-, medium-, and long-chain chlorinated paraffins: Critical review and recommendations. Anal. Chim. Acta 2019, 1074, 16–32. 10.1016/j.aca.2019.02.051. [DOI] [PubMed] [Google Scholar]
- Schinkel L.; Bogdal C.; Canonica E.; Cariou R.; Bleiner D.; McNeill K.; Heeb N. V. Analysis of Medium-Chain and Long-Chain Chlorinated Paraffins: The Urgent Need for More Specific Analytical Standards. Environ. Sci. Technol. Lett. 2018, 5, 708–717. 10.1021/acs.estlett.8b00537. [DOI] [Google Scholar]
- Korytár P.; Parera J.; Leonards P. E. G.; Santos F. J.; de Boer J.; Brinkman U. A. T. Characterization of polychlorinated n-alkanes using comprehensive two-dimensional gas chromatography–electron-capture negative ionisation time-of-flight mass spectrometry. J. Chromatogr. A 2005, 1086, 71–82. 10.1016/j.chroma.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sprengel J.; Wiedmaier-Czerny N.; Vetter W. Characterization of single chain length chlorinated paraffin mixtures with nuclear magnetic resonance spectroscopy (NMR). Chemosphere 2019, 228, 762–768. 10.1016/j.chemosphere.2019.04.094. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Lysak D. H.; Soong R.; Haddad A.; Hisatsune A.; Moser A.; Golotvin S.; Argyropoulos D.; Simpson A. J.; Muir D. C. G. Chlorines Are Not Evenly Substituted in Chlorinated Paraffins: A Predicted NMR Pattern Matching Framework for Isomeric Discrimination in Complex Contaminant Mixtures. Environ. Sci. Technol. Lett. 2020, 7, 496–503. 10.1021/acs.estlett.0c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert C.; Schymanski E.; Küster E.; Kühne R.; Schüürmann G.; Brack W. Application of preparative capillary gas chromatography (pcGC), automated structure generation and mutagenicity prediction to improve effect-directed analysis of genotoxicants in a contaminated groundwater. Environ. Sci. Pollut. Res. 2010, 17, 885–897. 10.1007/s11356-009-0286-2. [DOI] [PubMed] [Google Scholar]
- Meinert C.; Moeder M.; Brack W. Fractionation of technical p-nonylphenol with preparative capillary gas chromatography. Chemosphere 2007, 70, 215–223. 10.1016/j.chemosphere.2007.06.055. [DOI] [PubMed] [Google Scholar]
- Codina A.; Ryan R. W.; Joyce R.; Richards D. S. Identification of Multiple Impurities in a Pharmaceutical Matrix Using Preparative Gas Chromatography and Computer-Assisted Structure Elucidation. Anal. Chem. 2010, 82, 9127–9133. 10.1021/ac102151g. [DOI] [PubMed] [Google Scholar]
- Sciarrone D.; Pantò S.; Rotondo A.; Tedone L.; Tranchida P. Q.; Dugo P.; Mondello L. Rapid collection and identification of a novel component from Clausena lansium Skeels leaves by means of three-dimensional preparative gas chromatography and nuclear magnetic resonance/infrared/mass spectrometric analysis. Anal. Chim. Acta 2013, 785, 119–125. 10.1016/j.aca.2013.04.069. [DOI] [PubMed] [Google Scholar]
- Özek G.; Ishmuratova M.; Tabanca N.; Radwan M. M.; Göger F.; Özek T.; Wedge D. E.; Becnel J. J.; Cutler S. J.; Can Başer K. H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012, 35, 650–660. 10.1002/jssc.201100950. [DOI] [PubMed] [Google Scholar]
- Pantò S.; Sciarrone D.; Maimone M.; Ragonese C.; Giofrè S.; Donato P.; Farnetti S.; Mondello L. Performance evaluation of a versatile multidimensional chromatographic preparative system based on three-dimensional gas chromatography and liquid chromatography–two-dimensional gas chromatography for the collection of volatile constituents. J. Chromatogr. A 2015, 1417, 96–103. 10.1016/j.chroma.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Eyres G. T.; Urban S.; Morrison P. D.; Dufour J.-P.; Marriott P. J. Method for Small-Molecule Discovery Based on Microscale-Preparative Multidimensional Gas Chromatography Isolation with Nuclear Magnetic Resonance Spectroscopy. Anal. Chem. 2008, 80, 6293–6299. 10.1021/ac8007847. [DOI] [PubMed] [Google Scholar]
- Kool J.; de Kloe G.; Denker A. D.; van Altena K.; Smoluch M.; van Iperen D.; Nahar T. T.; Limburg R. J.; Niessen W. M. A.; Lingeman H.; Leurs R.; de Esch I. J. P.; Smit A. B.; Irth H. Nanofractionation Spotter Technology for Rapid Contactless and High-Resolution Deposition of LC Eluent for Further Off-Line Analysis. Anal. Chem. 2011, 83, 125–132. 10.1021/ac102001g. [DOI] [PubMed] [Google Scholar]
- Giera M.; Heus F.; Janssen L.; Kool J.; Lingeman H.; Irth H. Microfractionation Revisited: A 1536 Well High Resolution Screening Assay. Anal. Chem. 2009, 81, 5460–5466. 10.1021/ac900622b. [DOI] [PubMed] [Google Scholar]
- Rühle C. P. G.; Niere J.; Morrison P. D.; Jones R. C.; Caradoc-Davies T.; Canty A. J.; Gardiner M. G.; Tolhurst V.-A.; Marriott P. J. Characterization of Tetra-aryl Benzene Isomers by Using Preparative Gas Chromatography with Mass Spectrometry, Nuclear Magnetic Resonance Spectroscopy, and X-ray Crystallographic Methods. Anal. Chem. 2010, 82, 4501–4509. 10.1021/ac100417h. [DOI] [PubMed] [Google Scholar]
- Nojima S.; Kiemle D. J.; Webster F. X.; Apperson C. S.; Schal C. Nanogram-Scale Preparation and NMR Analysis for Mass-Limited Small Volatile Compounds. PLoS One 2011, 6, e18178 10.1371/journal.pone.0018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai N.; Sasamoto K. Selectable one-dimensional or two-dimensional gas chromatography–olfactometry/mass spectrometry with preparative fraction collection for analysis of ultra-trace amounts of odor compounds. J. Chromatogr. A 2011, 1218, 3180–3185. 10.1016/j.chroma.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Mandalakis M.; Gustafsson Ö. Optimization of a preparative capillary gas chromatography–mass spectrometry system for the isolation and harvesting of individual polycyclic aromatic hydrocarbons. J. Chromatogr. A 2003, 996, 163–172. 10.1016/s0021-9673(03)00612-5. [DOI] [PubMed] [Google Scholar]
- Meinert C.; Brack W. Optimisation of trapping parameters in preparative capillary gas chromatography for the application in effect-directed analysis. Chemosphere 2010, 78, 416–422. 10.1016/j.chemosphere.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Pieke E.; Heus F.; Kamstra J. H.; Mladic M.; Velzen M. v.; Kamminga D.; Lamoree M. H.; Hamers T.; Leonards P.; Niessen W. M. A.; Kool J. High-Resolution Fractionation after Gas Chromatography for Effect-Directed Analysis. Anal. Chem. 2013, 85, 8204–8211. 10.1021/ac401384q. [DOI] [PubMed] [Google Scholar]
- Jonker W.; Zwart N.; Stöckl J. B.; de Koning S.; Schaap J.; Lamoree M. H.; Somsen G. W.; Hamers T.; Kool J. Continuous fraction collection of gas chromatographic separations with parallel mass spectrometric detection applied to cell-based bioactivity analysis. Talanta 2017, 168, 162–167. 10.1016/j.talanta.2017.02.067. [DOI] [PubMed] [Google Scholar]
- Jonker W.; Clarijs B.; de Witte S. L.; van Velzen M.; de Koning S.; Schaap J.; Somsen G. W.; Kool J. Gas chromatography fractionation platform featuring parallel flame-ionization detection and continuous high-resolution analyte collection in 384-well plates. J. Chromatogr. A 2016, 1462, 100–106. 10.1016/j.chroma.2016.07.068. [DOI] [PubMed] [Google Scholar]
- Tomy G. T.Analysis of Chlorinated Paraffins in Environmental Matrices: The Ultimate Challenge for the Analytical Chemist. In Chlorinated Paraffins; Boer J., Ed.; Springer Berlin Heidelberg, 2010; Vol. 10, pp 83–106. [Google Scholar]
- van Mourik L. M.; Lava R.; O’Brien J.; Leonards P. E. G.; de Boer J.; Ricci M. The underlying challenges that arise when analysing short-chain chlorinated paraffins in environmental matrices. J. Chromatogr. A 2020, 1610, 460550. 10.1016/j.chroma.2019.460550. [DOI] [PubMed] [Google Scholar]
- Brandsma S. H.; Brits M.; Groenewoud Q. R.; van Velzen M. J. M.; Leonards P. E. G.; de Boer J. Chlorinated Paraffins in Car Tires Recycled to Rubber Granulates and Playground Tiles. Environ. Sci. Technol. 2019, 53, 7595–7603. 10.1021/acs.est.9b01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mourik L. M.; Gaus C.; Leonards P. E. G.; de Boer J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155, 415–428. 10.1016/j.chemosphere.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Howard P. H.; Santadonato J.; Saxena J.. Investigations of Selected Potential Environmental Contaminants: Chlorinated Paraffins; Rep. 68-01-3101; Syracuse University Research Corporation: Syracuse, NY, US, 1975.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.