Abstract
Three-dimensional (3D)-printing techniques such as stereolithography (SLA) are currently gaining momentum for the production of miniaturized analytical devices and molds for soft lithography. However, most commercially available SLA resins inhibit polydimethylsiloxane (PDMS) curing, impeding reliable replication of the 3D-printed structures in this elastomeric material. Here, we report a systematic study, using 16 commercial resins, to identify a fast and straightforward treatment of 3D-printed structures and to support accurate PDMS replication using UV and/or thermal post-curing. In-depth analysis using Raman spectroscopy, nuclear magnetic resonance, and high-resolution mass spectrometry revealed that phosphine oxide-based photo-initiators, leaching out of the 3D-printed structures, are poisoning the Pt-based PDMS catalyst. Yet, upon UV and/or thermal treatments, photo-initiators were both eliminated and recombined into high molecular weight species that were sequestered in the molds.
The introduction of soft lithography has greatly contributed to the expansion of the field of microfluidics into nonspecialized laboratories.1,2 In this technique, structures on a mold are replicated into a transparent, nonfragile, and elastomeric material, polydimethylsiloxane (PDMS), using standard laboratory equipment. PDMS remains the number-one material to produce miniaturized (bio)-analytical devices for molecular separation, biosensing, diagnostic purposes, cell separation, single cell and extracellular vesicle analysis, droplet microfluidics, and for creating mini-organ models in vitro.3
Molds for soft lithography have historically been produced using the standard clean-room processes of photolithography and silicon dry-etching,1 which ensure high replication accuracy and high resolution in the low micrometer range. Yet, these techniques are expensive and time-consuming; and they request access to dedicated facilities and extensive training. Alternative technologies have been explored to produce molds, while addressing these issues. For example, micromachining of aluminum, brass, or poly(methyl methacrylate) plates using micromilling allowed for the creation of nonplanar geometries in about 2 h, with excellent replication of channel down to 10 μm in size.4 Additive approaches have also been proposed using wax printers,5 laser printers,6 fused-deposition modeling,7 or multijet three-dimensional (3D) printers.8 Yet, these techniques all exhibit a lower resolution and often yield rough surfaces, which is far from being ideal for casting. Using stereolithography (SLA), 3D structures are created through the layer-by-layer polymerization of a photosensitive resin in a tank using UV light, for example, at 405 nm, by scanning a laser beam in a plane or projecting images for each plane using digital micromirrors or liquid-crystal display screens.9 The SLA lateral resolution ranges from 50 μm for printers costing less than $1000, down to <5 μm for more advanced setups.10 While SLA has been widely used to produce molds for PDMS casting,11−16 PDMS curing is inhibited in the vicinity of these 3D-printed molds, for nearly all commercially available SLA resins, precluding faithful replication. Alternatively, PDMS strongly adheres to those molds.12,16,17 Therefore, SLA molds must be treated, with, for example, UV post-curing,11−16,18,19 temperature,12,14,17−19 solvents,11,13,16 sonication,11 silanization,12,16,17 or a coating11,20 (for an overview, see Supporting Information Table S1). However, all these treatments have been reported for one specific resin.
The Pt-based catalyst found in PDMS (Sylgard 184) cross-links vinyl-terminated oligomers via a hydrosilylation mechanism.21 Tri-organophosphite, vinyl, maleate, fumarate, and β-alkynol can reversibly or irreversibly inhibit PDMS curing due to their strong affinity for the catalyst or by sequestering it in small droplets.22−24 Moreover, 3D-printed objects release a variety of chemicals in solution, including polyethylene glycols, diethyl-phthalates,25 unreacted monomers,26 and phosphine-oxide photoinitiators,27 which can also inhibit the catalyst. However, to the best of our knowledge, PDMS curing inhibition has never been investigated in the case of 3D-printed molds, and no study has examined the role of post-treatments.
Here, we first developed a generic post-treatment recipe for molds produced from 16 commercial SLA resins. PDMS curing inhibition on those molds was systematically quantified, and the influence of a UV and/or heat treatment was evaluated. Using Raman spectroscopy, nuclear magnetic resonance (NMR), and high-resolution mass spectrometry (HR-MS), we next elucidated this curing inhibition mechanism and examined how the proposed post-treatment remedied to this issue.
Materials and Methods
Experimental Approach for Identifying Post-Treatment Recipes for 16 Commercial Resins
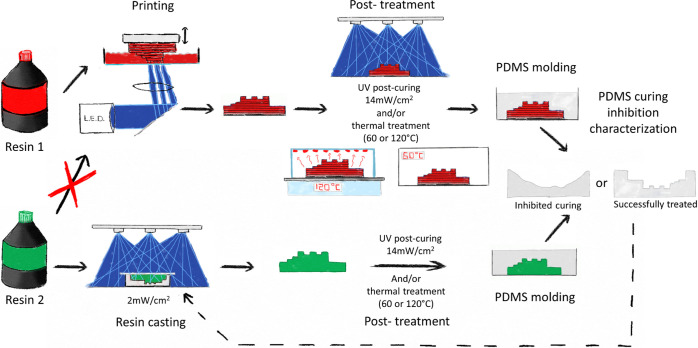
First, 3D-printed molds were treated with UV and heat (60 or 120 °C) before PDMS casting. Curing inhibition was assessed using two test structures through image analysis. To extend the scope of our study to a wider range of resins that could not be processed with our printer, we cast and UV-cured other resins on PDMS molds presenting the same test structures (Figure 1).
Figure 1.
Schematic overview of the experimental approach. A test mold fabricated in resin 1 (FTD deep black or industrial red) was exposed to UV and/or heat, and its PDMS curing inhibition was evaluated (see Figure 2). The resulting PDMS layer was used to cast molds from other resins (resin 2, here) not processable using our printer. These molds were exposed to UV and/or heat, and their PDMS curing inhibition was characterized.
Mold 3D-Printing
Molds were printed using a digital light processing printer (FlashForge Hunter, FlashForge, Jinhua City, China), with a 405 nm light-emitting diode (LED) source, a pixel size of 62.5 × 62.5 μm2, and a step height of 50 μm using industrial red and deep black resins (Fun-To-Do, Alkmaar, The Netherlands). Molds were rinsed with isopropanol before treatment.
Mold Treatment
Printed molds were post-cured in a homemade 405 nm LED UV box for 15 min, 1, 2, or 4 h at 14 mW/cm2 and/or exposed to a thermal treatment at either 60 °C (in an oven) for 1, 2, 4, 8, 24, or 48 h or at 120 °C (on a hotplate covered with a glass dish) for 1, 2, 4, or 8 h.
Quantitative Evaluation of the PDMS Curing Inhibition
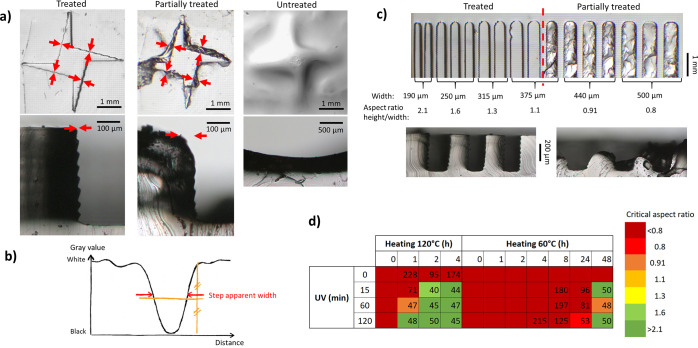
A 1:10 w/w ratio of the curing agent and PDMS pre-polymer (Sylgard 184, Dow Corning, USA) was mixed, degassed under vacuum, and poured on the molds. PDMS was cured for 3 h at 60 °C. Top pictures of the PDMS replicas were captured with a binocular microscope equipped with a camera (Motic sMZ-171, Motic, Xiamen, China) using backlight illumination. PDMS inhibition was quantified using two 400 μm high test structures, a shuriken (Figure 2a), and a comb (Figure 2c), comprising a series of 2 mm long teeth with widths of 190–500 μm (aspect ratio height/width of 2.1–0.8). The width of the transition between the bottom and the top of the shuriken was measured at four locations using ImageJ (NIH, Bethesda, USA), as depicted in Figure 2a. The width at half maximum of the peak corresponding to the transition on a gray-value plot, orthogonal to the wall, was determined (Figure 2b), and these four values were averaged into a so-called “apparent step width” (in μm). From the thinnest perfectly replicated structure in the comb, a “critical aspect ratio” was extracted.
Figure 2.
(a) Microscopy picture of shuriken structures in PDMS for three representative conditions (from left to right; successful, partial replication, and no treatment. Top: top view of PDMS replicates; bottom: cross-sections of PDMS replicates). Red arrows indicate the location of the measurements. (b) Typical gray value profile recorded along the red arrows in (b) to determine the apparent step width. (c) Microscopy pictures of the comb structures in PDMS after successful (left) and partial post-treatment (right), top view (top), and cross-section (bottom). (d) Example of post-treatment screening for FTD industrial red. Numbers correspond to the apparent step width (no value provided in the case of serious PDMS curing inhibition) and the colors to the critical aspect ratio (as detailed in the Experimental Section).
SLA Resin Mold Casting
PDMS replicates from printed molds, after post-treatment, were exposed to plasma (Cuter, Femto Science, Gyeonggi-Do, South Korea) for 40 s at 50 W, 50 kHz, and 0.7 mbar and silanized by vapor deposition of (1H,1H,2H,2H-perfluorooctyl)-trichlorosilane for 20 min in a closed container. 16 resins were cast on these PDMS countermolds: Fun-To-Do industrial black and red, standard black, standard red, and deep black; Formlabs black, clear, flex, and high temperature (Formlabs, Sommerville, USA); DWS GL4000, GM08, DS3000, and DL260 (DWS, Thiene, Italy); EnvisionTec PIC100, R11, and E-Shell 300 (EnvisionTec, Dearborn, USA). Resins were carefully pipetted in the countermolds, which was next covered by a glass slide and exposed to 405 nm UV light (15 s at 2 mW/cm2) on each side, except for the R11, Formlabs Black, and Flex (30 s at 5 mW/cm2). Test molds were removed from the countermolds and exposed to UV on each side (30 s at 5 mW/cm2). Minimal UV doses were employed to limit the interference between casting and post-curing, while supporting successful casting. Cast molds were treated and analyzed using aforementioned protocols.
Mass Loss of the Treated Resins
Tiles of 7.5 × 7.5 × 0.75 mm3 were cast for every resin in PDMS molds, post-cured (2 h at 14 mW/cm2), then heated 4 h at 120 °C, and weighted between each step using a high-precision balance (AS60, Radwag, Radom, Poland) (three tiles per condition).
Sample Preparation for Spectroscopic Analysis
Similar tiles were cast with the industrial red, clear, GL4000 and PIC100 resins and treated using corresponding optimal protocols (see Table 1; UV and thermal treatment at 120 °C for the industrial red and PIC100, only UV for GL4000 and only curing at 120 °C for clear resin).
Table 1. Post-Treatments Established for 3D-Printed Molds as Determined in This Study for 16 Commercial Resinsa.
| recipe 1 |
recipe 2 |
|||||
|---|---|---|---|---|---|---|
| company/provider | resin name | resin characteristics | UV | heating (120 °C) | UV | heating (60 °C) |
| Fun-To-Do | standard black | 1 h | 4 h | X | X | |
| standard red | 2 h | 8 h | X | X | ||
| industrial black | composite | 15 min | 2 h | X | X | |
| industrial red | composite | 15 min | 2 h | 15 min | 48 h | |
| deep black | composite (carbon nanotube) | 15 min | 2 h | 1 h | 48 h | |
| Formlabs | black | 0 h | 1 h | 0 h | 24 h | |
| flex | flexible | 0 h | 2 h | 2 h | 0 h | |
| HT | high temperature | 0 h | 1 h | 0 h | 1 h | |
| clear | transparent | 0 h | 1 h | 0 h | 24 h | |
| Envisiontec | PIC100 | casting resin | 2 h | 4 h | X | X |
| E-shell 300 | transparent, biocompatible | 0 h | 1 h | 0 h | 24 h | |
| R11 | 2 h | 4 h | X | X | ||
| DWS | DL260 | composite with ceramic | 0 h | 1 h | 2 h | 24 h |
| DS3000 | transparent, biocompatible | 0 h | 2 h | 0 h | 24 h | |
| GL4000 | flexible | 1 h | 8 h | 4 h | 0 h | |
| GM08 | flexible, transparent | 1 h | 8 h | 2 h | 24 h | |
“X” stands for the absence of treatment found in the range of tested duration of the two steps.
100 μL of phenylbis(2,4,6-trimethylbenzoyl)-phosphine oxide (BAPO, Sigma-Aldrich, Saint-Louis, USA) and 100 mg of ethyl(2,4,6-trimethylbenzoyl)phenyl phosphinate (TPO-L, IGM resins, Waalwijk, The Netherlands) were treated with UV (1 h at 14 mW/cm2) followed by 2 h at 120 °C on a hotplate covered with a glass dish. Liquids (or gels) condensed on the glass dishes were collected after treatment for analysis.
Raman Spectroscopy
A custom-built Raman upright microscope with an excitation source (Kr + laser Innova 90-K, Coherent Inc., Santa Clara, USA; wavelength 647 nm; power 35 mW) was used for the characterization of the liquid resins; corresponding untreated and treated tiles; corresponding condensed liquids; BAPO and TPO-L untreated, treated with only UV, only heat or both UV then heat; their condensed liquids; methyl methacrylate; and (1,6)-hexanediol di-methacrylate (Sigma-Aldrich). The excitation beam was focused onto the tiles, about 5 to 70 μm below the surface. The liquid samples (40 μL) were placed in a cavity in a borosilicate glass slide. Scattered photons were collected for 100 ms and focused on a 15 μm pinhole at the entrance of a custom-made spectrograph, dispersing them in the range of −40 till +3655 rel·cm–1. Spectral data were recorded with an EMCCD camera (Newton DU-970N-BV, Andor Technology Ltd., Belfast, Northern Ireland), with an average spectral resolution of 2.3 cm–1. By applying a raster scan pattern covering a 30 × 30 μm2 area, 3600 spectra were recorded for each sample. Baseline correction was applied for the treated industrial red and GL4000 resins that gave rise to significant autofluorescence.
Mass Spectroscopy Analysis
Fourier-transform ion cyclotron resonance mass spectra were acquired in a positive ion mode on a SolariX XR instrument with a 9.4 T actively shielded superconducting magnet and a dynamically harmonized cell (Bruker Daltonics, Bremen, Germany) fitted with a nano-electrospray online source. Samples (1 mg/mL in acetone) were introduced in the MS using a syringe pump (Cole-Parmer, USA) at a rate of 10 μL/min, connected to a capillary line (100 μm i.d., 360 μm o.d.), equipped with a SilicaTip needle (10 ± 1 μm, PicoTip Emitter, New Objective, USA), to which a potential of 1.3 kV was applied. The front and back trapping potentials were set at 1.6 V with a skimmer voltage at 10 V, and the ions were accumulated in the hexapole for 0.01 s. Spectra were acquired using broadband detection, 16 M data points, 150 scans accumulation, and a mass range of m/z 144 to 2000.
The instrument was calibrated using sodium trifluoroacetate (0.01 mg/mL in H2O/MeOH 50/50 v/v) with a linear calibration. Spectra were internally calibrated in the Data Analysis software v.5.0 (Bruker Daltonics, Germany) using a list of assigned signals.
Nuclear Magnetic Resonance
NMR spectra were recorded on an AVANCE 500 (Bruker Biospin, France) operating at 202.45 MHz (31P) equipped with a 5 mm triple resonance probe (TXI) at 295 K. 31P spectra were acquired with proton decoupling and reported using indirect referencing. NMR chemical shifts were calibrated using residual solvent (CDCl3, 7.27 ppm). All experiments were run using a software from the Bruker library, and data were processed with Topspin 4.0.
Chemical Inhibition of PDMS
A 10:1 w/w base/curing agent PDMS mixture was supplemented with chemicals or mixtures thereof, all of them at 1% w/v: pure and treated BAPO, pure and treated TPO-L, 2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)-butanone-1 (Irgacure 369), 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (Irgacure 2959), methyl methacrylate (all Sigma-Aldrich), deep black resin, and condensed liquids/gels collected after thermal treatment of either deep black molds, or BAPO and TPO-L. These mixtures were cast on treated 3D-printed deep black molds, and test structures were imaged to quantify curing inhibition.
Results and Discussion
Qualitative and Quantitative Evaluation of PDMS Curing Inhibition
First, 3D-printed test molds in the industrial red resin were employed to evaluate the PDMS curing inhibition and the effects of post-treatments. Without any post-treatment, PDMS on the mold was completely uncured (Figure 2a, right). After 1 h baking at 120 °C, the replication of sharp vertical steps (Figure 2a, center and Figure 2c, right) failed due to either incomplete curing of PDMS or its strong attachment to the mold. After 1 h UV exposure and 1 h thermal treatment at 120 °C, both the shuriken and comb test structures were perfectly replicated (Figures 2a, left and 2c, left). In an attempt to quantify PDMS curing inhibition, the “apparent step width” was measured, as detailed in the Experimental Section.
The apparent step width typically increased with the degree of inhibition. In a perfectly replicated structure, the wall is vertical, so that only its shadow could affect the measurement (Figure 2a, left), leading to an apparent step width <50 μm. The same illumination parameters were kept for all experiments, not to introduce any bias. Partial PDMS curing typically resulted in a damaged PDMS surface after demolding and an increased apparent width (Figure 2a, middle). In the case of severe inhibition, no step could be defined (Figure 2a, right).
Determining an Optimal Post-Treatment Recipe for 16 SLA Resins
Next, we systematically applied this methodology to 16 commercial resins from four manufacturers, exhibiting a wide range of properties (e.g., transparent, flexible, or high resolution) and therefore diverse formulations (Table 1). However, resins from DWS and Formlabs could not be processed with our printer because they strongly adhere to its Teflon-coated tank. Moreover, all resins have differences in penetration depth for the UV light, minimum dose to initiate the polymerization, or horizontal migration of activated species, which all affect the produced features and their sizes. Therefore, the resins were cast against a PDMS countermold to yield identical structures for all resins (Figure 1). All molds were subjected to the same combinations of UV exposure and baking at 60 or 120 °C. The apparent step width and thinnest properly replicated tooth were systematically determined to identify treatment recipes (Figure 2d and Tables S2 and S3). For every resin, a first recipe was defined for an “apparent step width” smaller than 50 μm and successful replication of the finest tooth (aspect ratio 2.1). This recipe typically includes baking at 120 °C, sometimes after a UV step (for 9/16 resins) with a total treatment duration shorter than 135 min for 10 resins (recipe 1, Table 1). For most of the resins (11/16), a second recipe was defined with only UV or baking at 60 °C, or a combination of both (recipe 2, Table 1). Formlabs resins and those sold as biocompatible (E-shell 300 and DS3000) were the easiest to treat. A short baking step at 120 °C (<2 h) or longer at 60 °C was sufficient, which, importantly, does not require any specialized equipment.
It is worth noticing that treatments recommended by manufacturers to complete the polymerization of the resins after 3D printing are shorter that the treatments established in this study to avoid PDMS curing inhibition (see Table S4 for an overview); yet, those “commercial” treatments are not sufficient to prevent PDMS curing inhibition. Flexible resins (flex and GL4000) were successfully treated using only UV exposure. All these recipes, established using cast molds, were next verified on 3D-printed molds for the industrial and deep black resins: for triplicate structures, an apparent step width <45 μm was found and the finest tooth was successfully replicated, confirming the results obtained using cast structures.
All treatment processes were found to work for our two test structures. However, other elements should be considered for other types of structures. In our hands, higher aspect-ratio structures typically required longer UV exposures due to shadowing effects.18,19 Some resins, when exposed to long UV exposure or high-temperature treatments (120 °C), tend to bend and change colors, and molds produced in flexible resins became harder. In the latter case, recipes using a longer thermal treatment at a lower temperature should be preferred.
Decrease in the Mold Mass after Post-Treatment
In a first hypothesis, inhibitory compounds could be eliminated through heating, which was tested by weighing tiles before and after treatment (i.e., 2 h UV exposure, followed by 4 h at 120 °C). All tiles lost mass after treatment (Figure 3), with variations ranging from −0.41 ± 0.02% (DS3000) to −3.13 ± 0.23% (flex). These mass changes could be explained by the release of volatile compounds from the 3D-printed materials, which is confirmed by the condensation of liquids. This loss of mass was associated with negligible shrinkage of less than 2% (see Figure S1).
Figure 3.
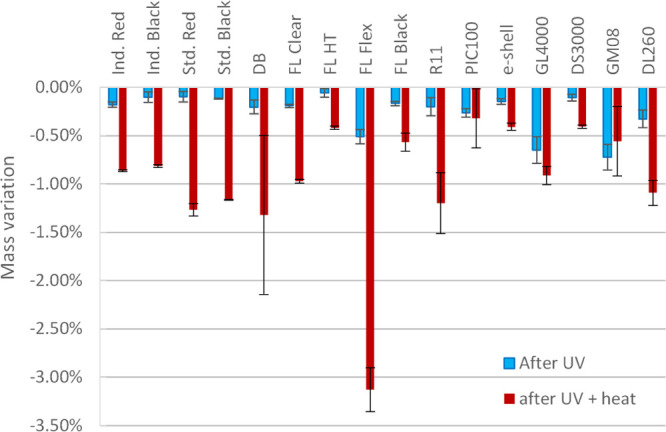
Variation in mass of tiles cast from 16 commercially available SLA resins, after 2 h of UV post-curing at 405 nm (blue), or a combined UV post-curing and 4 h post-baking at 120 °C (red), expressed as % of the initial mass (n = 3).
Raman Spectroscopy of Resins and Condensed Liquids
To identify potential chemical changes induced by the mold post-treatments, Raman spectroscopy analysis was performed on PIC100, clear, industrial red and GL4000 samples (liquid resins, and cast and treated molds). The inherent complexity of the resins precludes a thorough molecular analysis, and only a few major differences were found between samples (indicated by arrows on Figure 4; see Table S5 for a detailed overview). Characteristic bands of methyl methacrylate (3100, 1630, and 1400 rel·cm–1, blue arrows) and hexanediol dimethacrylate monomers (Figure S2) were not found any more after casting and treatment. No significant chemical changes, unless linked to the polymerization, were identified after the post-treatment. The condensed liquids collected for PIC100, clear and industrial red were similarly analyzed. The resulting spectra for PIC100 and clear significantly differed from those of the liquid resins and acrylate monomers, suggesting that the released compounds were not highly abundant in the resins. Similar bands were detected for the industrial red condensed liquid and the methacrylate monomers (black stars in Figure 4; see Table S5 for an overview).
Figure 4.
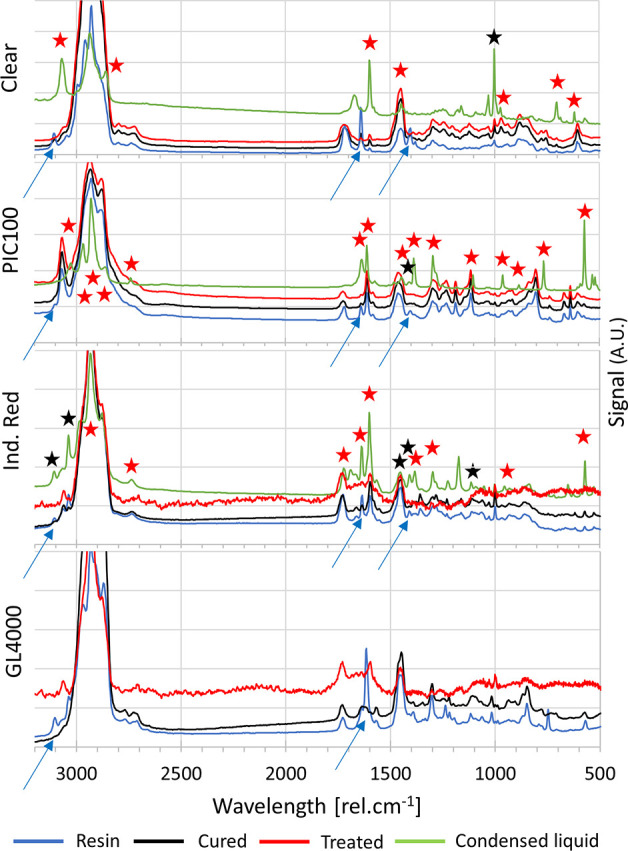
Raman spectra acquired for four resins (clear, PIC100, Industrial Red and GL4000) before casting (blue), after casting (black), after post-treatment (red), and for the corresponding condensed liquids (green). Arrows indicate bands being attributed to the methacrylate monomers that are not present after polymerization. Stars indicate bands found, respectively, in the BAPO and TPO-L spectra (red), and the methyl methacrylate spectrum (black).
Raman Spectroscopy Analysis of Photo-Initiators
We next examined if the condensed liquids obtained from the resins would contain photo-initiators and/or fragments thereof. Two phosphine oxide photo-initiators found in the SLA resins (BAPO and TPO-L) were subjected to several post-treatments: 1 h UV exposure, 2 h at 120 °C or combination thereof, that all yielded a similar brownish paste. BAPO (powder) lost 48.0 ± 0.2% of its mass after treatment, against 16.1 ± 5% for TPO-L (liquid).
Raman analysis of these two photo-initiators after full treatment yielded very similar spectra (Figure S2), which suggests that the post-treatment produces similar fragments for BAPO and TPO-L. Furthermore, a large number of bands detected on the Raman spectra of the resin condensed liquids were also found on the spectra of the photo-initiator condensed liquids (7/11 bands for the clear, 14/15 bands for the PIC100, and 8/15 bands for the industrial red resin, red stars in Figure 4; see Table S5 for an overview). Altogether, these data collectively suggest that the mixtures released from the 3D-printed molds through heating are very likely to contain phosphine oxide-based photo-initiators and/or fragments thereof with yet a small amount of uncured methacrylate monomers.
PDMS Curing Inhibition by Photo-Initiators and Fragments Thereof
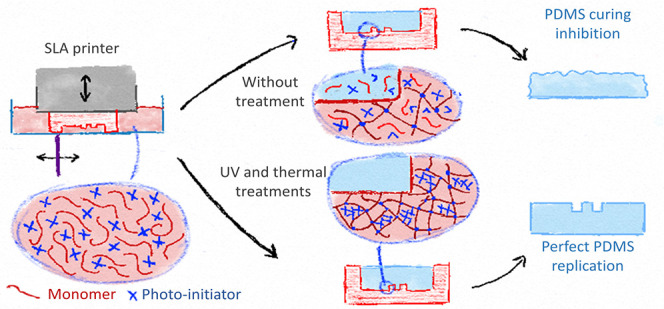
To test if PDMS curing was inhibited by the photo-initiators, fragments thereof, and/or methacrylate monomers, PDMS (10:1 w/w pre-polymer: curing agent) was supplemented with these compounds or one resin (deep black), its condensed liquid, and Irgacure 2959 and 369, two α-ketone photo-initiators (all at 1% w/v). These different mixtures were cured (3 h at 60 °C) on a treated test mold. FTD deep black resin and its condensed liquid strongly inhibited PDMS curing, leading to a viscous silicone paste (Figure S3), indicating that both samples contained a catalyst poison. In contrast, PDMS cured correctly in the presence of methyl methacrylate, while strongly attaching to the mold, most probably through cross-linking with the 3D-printed mold. This result is actually reminiscent of the previous work in which polyacrylate is employed to enhance adhesion between silicone-based and acrylic resin structures, while no mechanisms explaining the underlying chemistry have been proposed so far.28 Both TPO-L and BAPO strongly inhibited PDMS curing, even after either UV or thermal treatment at 120 °C. However, after exposure to a combined treatment, these two photo-initiators formed a paste that did not solubilize anymore in PDMS, so that only a thin layer of PDMS around the paste remained uncured, while the bulk successfully polymerized. PDMS did not cure at all after supplementation with BAPO or TPO-L condensed liquids. Irgacure 2959 and Irgacure 369 which do not contain phosphorus did not inhibit PDMS curing, even at a much higher concentration (10% w/v) (data not shown). These qualitative results (Figure S3) collectively reveal that phosphine oxide-based photo-initiators are likely poisoning the PDMS catalyst, while unreacted methacrylate monomers leaching from untreated molds promote adhesion of the PDMS replica, as previously reported.12,16,17 After a photo-thermal treatment, neither BAPO nor TPO-L did inhibit PDMS curing, in sharp contrast to their condensed liquids.
MS and NMR Analyses of Photo-Initiators
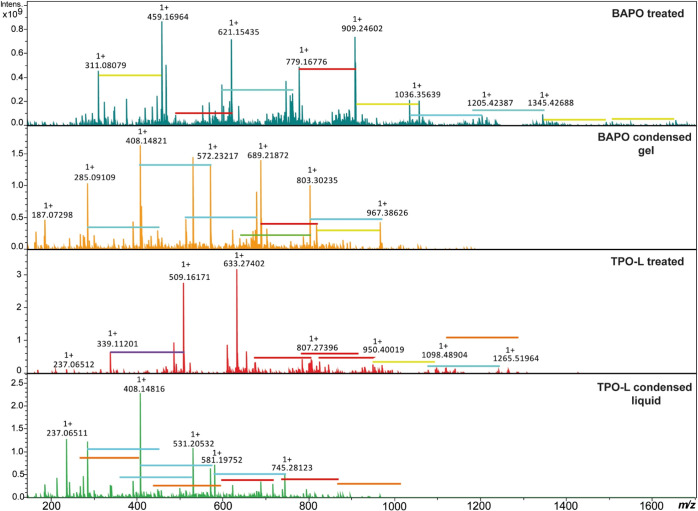
NMR and MS analyses were performed for the BAPO and TPO-L photo-initiators after a combined UV and thermal treatment, as well as on their condensed liquids to elucidate the effect of the post-treatment. 31P NMR spectra (Figure S4) confirmed the presence of phosphorus on all these samples, with compounds belonging to at least two PV families as phosphine oxide and phosphonate, and the absence of PIII compounds such as phosphines.29 MS analysis demonstrated that for both photo-initiators, the treated brown pastes and the condensed liquids were composed of a large variety of molecules containing mesitoyl, phenyl, mesitylethanone, and similar groups attached to one or several phosphorus atoms, with molecular weights ranging from 311 to 1650 g/mol for the treated BAPO and 339 to 1250 g/mol for the treated TPO-L (Figure 5). Such recombination has recently been reported after photolysis of BAPO;30 in this study, at least 16 molecules were identified and formation mechanisms proposed from the molecular formula determined using HR-MS (see Figure S5). The detected molecular weights were lower for both condensed liquids compared to their treated counterparts (from m/z 187–967 for BAPO and m/z 237–795 for TPO-L). Next to this, no striking differences in chemical structures were observed.
Figure 5.
HR-mass spectra of BAPO and TPO-L after 1 h UV exposure and 2 h at 120 °C and of their condensed liquid collected after heat treatment. Colored horizontal lines indicate the addition of: C10H10 (m/z 130.0777, red), C10H10O (m/z 146.0726, orange), C10H12O (m/z 148.0882, yellow), C10H10O2 (m/z 162.0675, green), C10H12O2 (m/z 164.0831, blue), and C8H11O2P (m/z 170.0491, purple).
MS analysis therefore suggests that the applied treatment induces the formation of inert high molecular weight molecules from the photo-initiators and the elimination by vaporization of low molecular weight inhibiting compounds (as the condensed liquids still inhibit PDMS curing). While this result was only obtained for two typical photo-initiators and not for the complex matrix as found in commercial resins, we can nevertheless elaborate on possible PDMS curing inhibition mechanisms. Photo-initiators in excess and their fragments produced during 3D-printing can leach from the mold into the PDMS, inhibiting its curing. During treatment, these fragments partly recombine into larger species, which are trapped in the surrounding poly-acrylic network, while smaller and volatile molecules are released from the mold, both processes reducing drastically the quantity of inhibitors able to diffuse out of the molds during PDMS casting.
Conclusions
We first established a series of post-treatment, combining UV exposure and heat, using 3D-printed test structures produced from 16 SLA resins for their faithful replication in PDMS with no curing inhibition. For at least 11 of those resins, this treatment was shorter than 135 min. We believe that both the simple equipment required for these treatments and the large spectrum of resins tested in this study (resins with different properties and formulations from four manufacturers) will widely support the use of SLA for producing molds for soft lithography. We next explored the mechanisms responsible for PDMS curing inhibition to notably understand how the post-treatment could prevent this inhibition. Leaching phosphine oxide-based photo-initiator fragments were identified as PDMS catalyst poisons, while unreacted monomers would promote PDMS adhesion onto the 3D-printed molds through cross-linking reactions during PDMS curing. UV post-curing alone further polymerizes the resin, thereby limiting the risk of leaching monomers without yet avoiding the curing inhibition. Combining it with thermal treatment, on the one hand, allows for vaporizing the remaining photo-initiators out of the 3D-printed structures and, on the other hand, promotes recombination reactions between the photo-initiator fragments to yield high MW species that remain trapped in the 3D-printed resin. This mechanism is likely to be valid for resins using phosphine-oxide based photo-initiators and silicone polymers such as PDMS using a Pt-based catalyst, as is the case for Sylgard 184 and RTV 615.12 Reducing leaching from 3D-printed objects has already been reported to also improve their biocompatibility and the survival of cells cultured in their direct vicinity.31 While this would require additional studies, our generic treatment is also likely to significantly improve the biocompatibility of resins and support their use to produce organ-on-a-chip models, directly using 3D printing.
Acknowledgments
The authors thank Prof. Nathalie Azaroual and Alexandre Rech from the GRITA laboratory for recording NMR spectra. The NMR and Mass Spectrometry facilities are funded by the European Regional Development Fund, Région Haut-de-France (France), the CNRS, and the University of Lille. Access to the FT-ICR MS in the frame of the EU_FT-ICR_MS network installation is funded by the EU Horizon 2020 grant 731077; support for conducting research is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.0c04944.
Overview of previously reported post-treatments to suppress PDMS curing inhibition on 3D-printed molds; post-treatment screenings for protocols including UV exposure and heating at 120 °C and 60 °C, respectively; comparison between post-treatments recommended by Formlabs and those proposed in this study; influence of the post-treatment on the dimensions of 3D printed structures; overview of the bands in the Raman spectra—comparison between spectra; Raman spectra of monomers and BAPO and TPO-L; pictures of PDMS supplemented with various resins, condensed liquids thereof, photo-initiators and condensed liquids thereof, or monomers after casting; 31P NMR spectra for BAPO and TPO-L; and identification of compounds from the MS analysis of the treated BAPO and TPO-L and possible formation mechanisms (PDF)
Author Contributions
B.V. designed the study, conducted part of the experiments analyzed data, and wrote the manuscript; S.D. conducted experiments on the post-treatment identification for resins and to evaluate mass loss after post-treatment; Z.M. and F.B. conducted MS analysis, analyzed spectra and, together with A.C., identified chemical species and proposed recombination mechanisms; C.R. supervised MS and NMR analysis; A.L. conducted Raman spectroscopy analysis with B.V.; C.O. supervised Raman spectroscopy analysis; S.L.G. designed the study and wrote the manuscript; all authors commented on the manuscript and approved of its content.
The authors declare no competing financial interest.
Supplementary Material
References
- Duffy D. C.; Mcdonald J. C.; Schueller O. J. A.; Whitesides G. M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- Delamarche E.; et al. Patterned Delivery of Immunoglobulins to Surfaces Using Microfluidic Networks. Science 1997, 276, 779–781. 10.1126/science.276.5313.779. [DOI] [PubMed] [Google Scholar]
- M K. R.; Chakraborty S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. 10.1002/app.48958. [DOI] [Google Scholar]
- Yousuff C.; Danish M.; Ho E.; Kamal Basha I.; Hamid N. Study on the Optimum Cutting Parameters of an Aluminum Mold for Effective Bonding Strength of a PDMS Microfluidic Device. Micromachines 2017, 8, 258. 10.3390/mi8080258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigala G. V.; Ho S.; Penterman R.; Backhouse C. J. Rapid Prototyping of Microfluidic Devices with a Wax Printer. Lab Chip 2007, 7, 384. 10.1039/b617764f. [DOI] [PubMed] [Google Scholar]
- Branham M. L.; Tran-Son-Tay R.; Schoonover C.; Davis P. S.; Allen S. D.; Shyy W. Rapid Prototyping of Micropatterned Substrates Using Conventional Laser Printers. J. Mater. Res. 2002, 17, 1559–1562. 10.1557/jmr.2002.0231. [DOI] [Google Scholar]
- Mcdonald J. C.; Chabinyc M. L.; Metallo S. J.; Anderson J. R.; Stroock A. D.; Whitesides G. M. Prototyping of Microfluidic Devices in Poly(Dimethylsiloxane) Using Solid-Object Printing. Anal. Chem. 2002, 74, 1537–1545. 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- Glick C.; et al. Rapid Assembly of Multilayer Microfluidic Structures via 3D-Printed Transfer Molding and Bonding. Microsyst. Nanoeng. 2016, 2, 16063. 10.1038/micronano.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan H. K.; Badar F.; Doeven E. H.; Novak J. I.; Merenda A.; Dumée L. F.; Loy J.; Guijt R. M. 3D Printing: An Alternative Microfabrication Approach with Unprecedented Opportunities in Design. Anal. Chem. 2021, 93, 350–366. 10.1021/acs.analchem.0c04672. [DOI] [PubMed] [Google Scholar]
- Accardo A.; Courson R.; Riesco R.; Raimbault V.; Malaquin L. Direct Laser Fabrication of Meso-Scale 2D and 3D Architectures with Micrometric Feature Resolution. Addit. Manuf. 2018, 22, 440–446. 10.1016/j.addma.2018.04.027. [DOI] [Google Scholar]
- Comina G.; Suska A.; Filippini D.; German Comina A. S.; Filippini D. PDMS Lab-on-a-Chip Fabrication Using 3D Printed Templates. Lab Chip 2014, 14, 424–430. 10.1039/c3lc50956g. [DOI] [PubMed] [Google Scholar]
- Chan H. N.; Chen Y.; Shu Y.; Chen Y.; Tian Q.; Wu H. Direct, One-Step Molding of 3D-Printed Structures for Convenient Fabrication of Truly 3D PDMS Microfluidic Chips. Microfluid. Nanofluid. 2015, 19, 9–18. 10.1007/s10404-014-1542-4. [DOI] [Google Scholar]
- Costa P. F.; et al. Mimicking Arterial Thrombosis in a 3D-Printed Microfluidic in Vitro Vascular Model Based on Computed Tomography Angiography Data. Lab Chip 2017, 17, 2785–2792. 10.1039/c7lc00202e. [DOI] [PubMed] [Google Scholar]
- Dinh T.; Phan H.-P.; Kashaninejad N.; Nguyen T.-K.; Dao D. V.; Nguyen N.-T. An On-Chip SiC MEMS Device with Integrated Heating, Sensing, and Microfluidic Cooling Systems. Adv. Mater. Interfaces 2018, 5, 1800764. 10.1002/admi.201800764. [DOI] [Google Scholar]
- Razavi Bazaz S.; Kashaninejad N.; Azadi S.; Patel K.; Asadnia M.; Jin D.; Ebrahimi Warkiani M. Rapid Softlithography Using 3D-Printed Molds. Adv. Mater. Technol. 2019, 4, 1900425. 10.1002/admt.201900425. [DOI] [Google Scholar]
- Waheed S.; et al. Enhanced Physicochemical Properties of Polydimethylsiloxane Based Microfluidic Devices and Thin Films by Incorporating Synthetic Micro-Diamond. Sci. Rep. 2017, 7, 15109. 10.1038/s41598-017-15408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. H.; Jones G.; Morgan H.; de Planque M. R. R.; Zauner K.-P. Interdroplet Bilayer Arrays in Millifluidic Droplet Traps from 3D-Printed Moulds. Lab Chip 2014, 14, 722–729. 10.1039/c3lc51072g. [DOI] [PubMed] [Google Scholar]
- Ferraz M. d. A. M. M.; Nagashima J. B.; Venzac B.; Le Gac S.; Songsasen N. 3D Printed Mold Leachates in PDMS Microfluidic Devices. Sci. Rep. 2020, 10, 994. 10.1038/s41598-020-57816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz M. d. A. M. M.; Nagashima J. B.; Venzac B.; Le Gac S.; Songsasen N. A Dog Oviduct-on-a-Chip Model of Serous Tubal Intraepithelial Carcinoma. Sci. Rep. 2020, 10, 1575. 10.1038/s41598-020-58507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju A. O.; Robillard A.; Dagher M.; Juncker D. Autonomous Microfluidic Capillaric Circuits Replicated from 3D-Printed Molds. Lab Chip 2016, 16, 3804–3814. 10.1039/c6lc00764c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers G. L.; Switzer S. T.. Background Material Properties of Selected Silicone Potting Compounds and Raw Materials for Their Substitutes; MHSMP-78-18; Mason and Hanger-Silas Mason Co., Inc.: Amarillo, Tex. (USA), 1978. [Google Scholar]
- Faglioni F.; Blanco M.; Goddard W. A.; Saunders D. Heterogeneous Inhibition of Homogeneous Reactions: Karstedt Catalyzed Hydrosilylation. J. Phys. Chem. B 2002, 106, 1714–1721. 10.1021/jp0128933. [DOI] [Google Scholar]
- Kownacki I.; et al. Effect of Triorganophosphites on Platinum Catalyzed Curing of Silicon Rubber. Appl. Catal., A 2009, 362, 106–114. 10.1016/j.apcata.2009.04.027. [DOI] [Google Scholar]
- Lewis L. N.; Stein J.; Colborn R. E.; Gao Y.; Dong J. The Chemistry of Fumarate and Maleate Inhibitors with Platinum Hydrosilylation Catalysts. J. Organomet. Chem. 1996, 521, 221–227. 10.1016/0022-328x(96)06247-x. [DOI] [Google Scholar]
- de Almeida Monteiro Melo Ferraz M.; et al. Potential Health and Environmental Risks of Three-Dimensional Engineered Polymers. Environ. Sci. Technol. Lett. 2018, 5, 80–85. 10.1021/acs.estlett.7b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskui S. M.; Diamante G.; Liao C.; Shi W.; Gan J.; Schlenk D.; Grover W. H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2016, 3, 1–6. 10.1021/acs.estlett.5b00249. [DOI] [Google Scholar]
- Carve M.; Wlodkowic D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines 2018, 9, 91. 10.3390/mi9020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. P.; Hansen N. A.; Phoenix R. D.; Schneid T. R. The Effects of Primers and Surface Bonding Characteristics on the Adhesion of Polyurethane to Two Commonly Used Silicone Elastomers. J. Prosthodont. 2009, 18, 23–31. 10.1111/j.1532-849x.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Kolczak U.; Rist G.; Dietliker K.; Wirz J. Reaction Mechanism of Monoacyl- and Bisacylphosphine Oxide Photoinitiators Studied by 31 P-, 13 C-, and 1 H-CIDNP and ESR. J. Am. Chem. Soc. 1996, 118, 6477–6489. 10.1021/ja9534213. [DOI] [Google Scholar]
- Schmallegger M.; et al. Unprecedented Bifunctional Chemistry of Bis(Acyl)Phosphane Oxides in Aqueous and Alcoholic Media. Chem.—Eur J. 2019, 25, 8982–8986. 10.1002/chem.201900935. [DOI] [PubMed] [Google Scholar]
- Piironen K.; Haapala M.; Talman V.; Järvinen P.; Sikanen T. Cell Adhesion and Proliferation on Common 3D Printing Materials Used in Stereolithography of Microfluidic Devices. Lab Chip 2020, 20, 2372–2382. 10.1039/d0lc00114g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.