Abstract
Thermoset coatings have been used extensively to protect and enhance the appearance of substrates for industrial maintenance and architectural applications. Here, we demonstrate that anionic polymerization can be used to first graft hydroxyethyl methacrylate methylene malonate (HEMA-MM) onto a latex particle at ambient conditions, while subsequent ultraviolet (UV) exposure enabled their crosslinking into robust coatings. At room temperature, in the presence of air and water, the polymerization of HEMA-MM was initiated by anionic carboxyl groups present on the MAA latex particles and subsequently grafted onto the surface of particles. The pendent hydroxyethyl methacrylate (HEMA) group enabled UV-curing via free radical polymerization and the formation of a crosslinked network. Systematic investigations were conducted to study the formation and performance of the crosslinked coatings as a function of HEMA-MM incorporation. The incorporation of 10 wt% HEMA-MM into MAA latex yielded crosslinked coatings with decreased swelling, a heightened glass transition temperature (by ~20 °C) and a 2.9-fold improvement in the Young’s moduli compared to controls (without HEMA-MM). Here, we demonstrate a facile method that provides a one-step grafting-functionalization approach using functional methylene malonates to produce UV-curable and high-performance coatings at room temperature and under atmospheric environments.
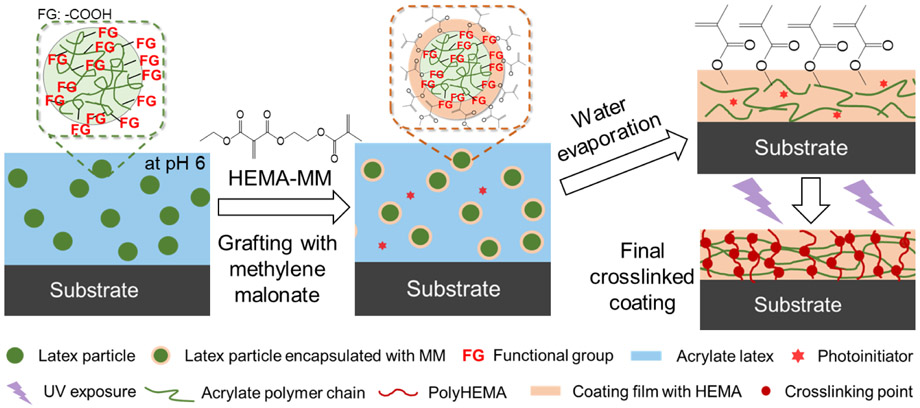
Graphical Abstract

Introduction
Thermoset coatings have been extensively used to protect and enhance the appearance of substrates for industrial maintenance and architectural applications.1 For example, they can prevent steel and concrete structures from corroding, enable high block resistance and hardness to wood coatings,2 and provide high-quality, durable finishes for varnish and automotive coatings.3 Crosslinked thermoset coatings provide enhanced mechanical strength, water/solvent-resistance, and improved abrasion and barrier properties versus non-crosslinked thermoplastic coatings.4 Currently, there are several commercial crosslinking methods that can be used to prepare high-performance thermoset coatings, including the evaporative curing of solvent-borne coatings,5 condensation reaction curing,6 radiation curing (i.e., using ultraviolet (UV),7 infrared,8 or electromagnetic waves9), and room-temperature crosslinking via a variety of different functional groups (i.e., carboxylic acid10, acetoacetyl groups11, acrylamide12 and oxygen13). Unfortunately, these methods are not always practical or economical and they have additional disadvantages. For example, the release of volatile organic compounds (VOCs) from solvent-borne coatings poses concerns, including environmental pollution and risks to human health and safety.14-15 Condensation reactions require curing at elevated temperatures and thus, a high energy consumption is required. Additionally, room temperature crosslinking has potential problems due to its slow reaction rate and limited pot life.6, 16 Thus, UV-curing holds potential to provide outstanding properties, such as high durability, while allowing fast-curing, long pot life, and low VOCs coatings.
UV-curing is a light-induced polymerization of multifunctional monomers or oligomers. Upon absorbing UV-light, photo initiators dissociate into free radicals, cations or anions that initiate monomers to polymerize and generate dense polymer networks.17-19 UV-curing has been widely used in a variety of applications, including protective coatings, printing inks, adhesives and composites, due to its fast polymerization kinetics, ambient temperature curing, potential for solvent-free formulation, and excellent coating performance.20-21 Waterborne systems are a rapidly growing category of UV-curable coatings because they offer several outstanding advantages versus traditional systems, for example low odor/VOCs/irritation, low viscosity formulations that are ideal for spray or roll-on applications, reduced shrinkage, and good hardness.22-24 Most water-based UV-curable polymers consist of dispersions or emulsions of acrylate functionalized oligomers that are photo-crosslinked in their solid state.25 For example, Decker, et al. studied UV-curable coatings with five acrylate oligomers containing carboxylate groups and demonstrated that emulsion-based resins cured faster and more extensively than dispersion-based resins.22 Hwang, et al. synthesized high-quality UV-curable polyurethane coatings using a two-component chemistry with diisocyanate and polycarbonate diol as polyols.26 Additionally, monomers, such as allyl methacrylate (AMA) have been employed to make UV-curable polymers. AMA has an asymmetric structure that provides different reactivity of its two double bonds. By first polymerizing the more reactive bond, a polymer is produced with pendant allyl groups that are available for subsequent on-demand crosslinking (by irradiation or thermal treatment).27 However, gelation might occur during the initial free radical polymerization if large concentrations of AMA are used, and chain transfer agents are needed to prevent pre-crosslinking, which restrains the coatings’ extent of crosslinking.28 Therefore, new strategies are needed to enable a one-component waterborne UV-curable coating that exhibits high crosslinking and enhanced performance.
Recently, our lab has successfully incorporated a novel methylene malonate chemistry into thermoplastic polymers, including acrylic latexes to prepare high-performance coatings.29 Our previous study verified the high reactivity of diethyl methylene malonate (DEMM) initiated by sodium methacrylate; we achieved a ~89% conversion of DEMM to polyDEMM in 5 min under facile conditions (room temperature, in air, in the presence of water).29 The high reactivity and anionically-polymerizable nature of DEMM enabled a fast and easy approach to graft methylene malonate onto polymers and surfaces with incorporated nucleophiles. In this study, we used a novel monomer, hydroxyethyl methacrylate methylene malonate (HEMA-MM, Figure 1) to modify conventional emulsion polymers and convert them to UV-curable polymers without the premature crosslinking observed with other approaches.28, 30 HEMA-MM incorporates an anionically polymerizable methylene malonate and a methacrylate group for free radical polymerization. By employing HEMA-MM as an additive to carboxyl-functional latex, the MM group of HEMA-MM spontaneously oligomerizes from the anionic sites on the latex surface to form grafted coatings while leaving the methacrylate groups unreacted and pendent for on-demand curing. After grafting with HEMA-MM and UV-curing, the performance of the coatings were characterized for their gel content, swelling ratio, glass transition temperature, hardness and mechanical properties. Here, we demonstrate a straightforward approach to introducing unsaturated double bonds under facile conditions and offer a route to controllably tailoring the degree that the coatings are crosslinked.
Figure 1.

Chemical structure of hydroxyethyl methacrylate methylene malonate (HEMA-MM).
MATERIALS AND METHODS
Materials.
Diethyl methylene malonate (Chemilian™ M1000, DEMM) and hydroxyethyl methacrylate methylene malonate (HEMA-MM) were provided by Sirrus Inc. (Loveland, OH). Methyl methacrylate (MMA, 99%, stabilized, Fisher), butyl acrylate (BA, ≥ 99%, Acros Organics), methacrylic acid (MAA, 99%, Sigma-Aldrich) were used as monomers for latex synthesis. Potassium persulfate (KPS, ≥ 99%, Sigma-Aldrich), 2,2-dimethoxy-2-phenylacetophenone (DMPA, 99%, Acros Organics) were used as initiators, ethanol (≥ 98%, Sigma-Aldrich) was used to dissolve DMPA, sodium hydroxide (NaOH, ≥ 98%, pellets, Certified ACS, Sigma-Aldrich) was used to modify the pH value of the latex, sodium dodecyl sulfate (SDS, 10 wt% in water, Sigma-Aldrich) was used as a surfactant. Aluminum oxide (activated, basic, Brockmann I, Sigma-Aldrich) was used to remove the inhibitor from monomers. N, N-dimethylformamide (DMF, 99.9%, Fisher) was used as an extractor. Deionized (DI) water was used throughout the work.
Latex synthesis.
Latex was synthesized through a semi-batch emulsion polymerization method. The solid weight of latex was maintained at 30 wt%. The base monomers were MMA and BA, which simultaneously co-polymerized with MAA to provide functional carboxyl groups. The glass transition temperature of the latex was controlled by holding the monomer ratio constant at MMA:BA:MAA (36:54:10). First, 40 g DI water with 0.2 g SDS was added into the flask with constant stirring at 400 rpm. MMA and BA (3.5 wt%) were added as a monomer mixture. The flask was heated to 80 °C with nitrogen purge and 2 g of 6 wt% KPS solution was fed into the flask at a constant rate of 0.06 g/min to nucleate seed particles. Second, the remaining 93 wt% monomer mixture was mixed with 0.4 g SDS and 15 g DI water, the mixture was then fed into the flask at 0.4 ml/min along with 8 g 6 wt% KPS solution. The feeding step lasted for 2 h. After the addition of all reactants, the temperature was increased to 85 °C for another 30 min. The latex was cooled to room temperature, the pH value was neutralized to 6 using NaOH solution (1M, confirmed using pH-indicator strips (Fisher)) and centrifuged (Thermo Fisher Scientific, Suzhou, China) at 3000 rpm for 10 min to eliminate agglomerations.
Particle size characterization.
Various amount of DEMM (0 wt%, 10 wt%, 20 wt%, 30 wt% and 40 wt% with respect to the solid content of the latex) was added into the latex (pH 6) at room temperature and allowed to react for 2 h. The grafted latex was diluted to 0.3 wt% using DI water for dynamic light scattering measurements (ZEN5600, Worcestershire, UK). The refractive index was set as 1.6, temperature was held constant at 25 °C and the solvent was water. Each sample was measured three times.
Contact angle.
Latex (1 g) grafted with polyDEMM (0 wt%, 10 wt%, 20 wt%, 30 wt% and 40 wt% with respect to the solid content of the latex) was coated onto polypropylene substrates using a drawdown tool (thickness of 100 μm, The Paul N. Gardner Company, Pompano Beach, FL) and dried for 2 h. The contact angle was measured using a tensiometer (Data Physics Corporation, San Jose, CA) by dropping 4 μL DI water and acquiring photographs as soon as water droplet touched the coating’s surface. The images were analyzed using ImcigeJ 1.51j8 software (National Institutes of Health, Bethesda, MD).31 Experiments were conducted 15-20 times for each sample.
Fabrication of UV-crosslinked coatings.
MAA latex (10 g) was added into a 20 ml disposable scintillation vial (Fisher) that was wrapped with aluminum foil. Methylene malonate (0 wt%, 2.5 wt%, 5 wt%, 7.5 wt% and 10 wt% with respect to the solid content of the latex) was added into the latex, while stirring at room temperature for 12 h. The photoinitiator DMPA (3 wt%) was added into the latex by dissolving it in 0.2 g ethanol and mixing the solution for 1 h in the dark. The latex mixture (10 g) was then spread homogeneously on a polytetrafluorethylene surface (PTFE, Dynalon Labware, Rochester, NY) or applied on a stainless steel substrate (Q-lab, Westlake, OH) using a drawdown tool with a controlled thickness of 30 μm. The coating was allowed to dry in air at room temperature (25 °C) for 24 h. Films that were dried film on PTFE surfaces were easily peeled off, placed in a petri-dish, filled with nitrogen, sealed with parafilm, and exposed to a UV light (LL-UV P40 UV Panel HP, ADJ Products, LLC, Los Angeles, CA).32 The intensity of UV radiation reaching the sample was measured to be 4.40 mW/cm2 using a UV powermeter (Optical Associates Inc., Milpitas, CA) and the samples were placed 10 cm below the UV light. The UV exposure time was set as 1 h for adequate polymerization taking place.
Characterization of swelling ratio and gel content.
To determine the swelling ratio of the UV-crosslinked coatings, the initial weight of the free-standing film was taken as m1, whereas after immersing the film in DMF for 24 h, a second weight was taken as m2. The swelling ratio was calculated based on Equation 1:
| (Equation 1) |
Next, the swollen film was taken out of vial and heated at 200 °C to evaporate the remaining DMF. The dried weight was taken as m3. The gel content was calculated based on Equation 2:
| (Equation 2) |
Swelling ratio and gel content were measured in triplicate.
Characterization of glass transition temperature.
The prepared grafted films (before and after 1 h UV-exposure) were cut into 0.5 cm × 2.0 cm rectangles and their thickness was measured using a digital caliper. The glass transition temperature of the films before and after UV-crosslinking were measured using dynamic mechanical analyzer (DMA Q800, TA Instruments).33 The temperature range was set from −40 °C to 100 °C, the preload force was 0.01 N, and the force track was 1.25%. The equipment was connected to liquid nitrogen for cooling. The experiment was conducted in triplicate.
Characterization of film hardness and tensile properties.
The hardness of the films before and after UV-curing was measured using a pendulum hardness tester (QBY film pendulum hardness tester, AliExpress) based on GB 1730-79.34 The films were placed on the panel table with the pendulum (120 g) on top of the film. The pendulum was deflected and released, the number of oscillations were counted using an electronic counter while the angle changed from 5° to 2°. The ultimate tensile strength of the films was measured using a tensile tester (Stable Micro Systems, TA-XT plus).35 The free-standing film was cut into a rectangle (0.5 cm × 2.0 cm), clamped using two grips, and stretched at 5 cm/min until breakage occurred. Experiments conducted using the pendulum hardness tester and the tensile tester were conducted in triplicate.
RESULTS AND DISCUSSION
Methylene malonate chemistry enables the facile grafting of polymers onto nucleophiles (i.e., hydroxyl, carboxylate, borate, phenolate groups) via anionic polymerization under ambient condition.29 Here, latex was synthesized using standard emulsion polymerization, and 10 wt% methacrylic acid (MAA) was incorporated to provide anionic carboxyl groups as initiators to polymerize DEMM. The pH value of the latex was modified to 6, above the pKa of MAA, in order to fully deprotonate carboxyl groups.36 Thus, DEMM was initiated by anionic carboxyl groups, followed by polymerization, and the subsequent grafting onto the latex particles at ambient conditions, Figure 2(a).
Figure 2.
(a) Schematic of the process by which polyDEMM grafts on latex particles using anionic carboxyl groups to form core-shell structures. (b) Average size of the latex particles. (c) Contact angle of the polyDEMM-grafted-latex. The asterisk (*) denotes 99.9% significance between samples, and n.s. indicates no statistical significance. Error bars indicate standard deviation.
The increasing trend in particle size post-grafting of DEMM was confirmed using DLS, Figure 2(b). The size of latex particles increased with a higher concentration of DEMM (10 wt%, 20 wt%, 30 wt% and 40 wt%). The average particle size of the control latex was 85.3 ± 0.09 nm which increased to 91.0 ± 1.2 nm upon 40 wt% DEMM addition; an average increase of ~6 nm was observed, Figure 2(b). Additionally, the particle size distribution was monodispersed, as shown in Figure S1, which demonstrates the homogeneity of the grafting process. Water contact angle measurements were conducted on coatings formed from grafted latex. The contact angle was 33.7 ± 1.8° for the control latex and 51.1 ± 2.5° for polyDEMM-grafted-latex; the statistical increase of ~17° indicates that the hydrophobicity increased after polyDEMM grafting, Figure 2(c). There was no significant difference in the contact angle measurements when the DEMM content increased from 10 wt% to 40 wt%, which qualitatively suggests that a surface modification of 10 wt% was sufficient to change the coating’s hydrophobicity. While peaks characteristic to latex appeared in the FTIR spectra of the control latex (558 cm−1, 591 cm−1, 690 cm−1, 1058 cm−1, 1271 cm−1, 1314 cm−1), upon grafting with polyDEMM, these peaks were no longer apparent (Figure S2). Thus, we suggest from the change in the particle size and surface properties that the grafting of DEMM, a model methylene malonate chemistry onto MAA latex particles was successfully demonstrated.
Based on this discovery, with our collaborators at Sirrus Inc. (Loveland, OH), a novel methylene malonate derivative, HEMA-MM, where DEMM was modified with a hydroxyethyl methacrylate group, was introduced onto the MAA latex particles using the same grafting procedure and mechanism. MM derivatives offer a high reactivity and they are anionically-polymerizable. Notably, HEMA-MM provides a methacrylate group for post-functionalization, which we hypothesized would enable a subsequent UV-crosslinking reaction.
The synthesis of the UV-curable coatings using HEMA-MM chemistry was conducted, as displayed in Figures 3 and S5. First, various amounts of HEMA-MM (0 wt%, 2.5 wt%, 5 wt%, 7.5 wt% and 10 wt%) were added and covalently grafted to the latex particles via anionic initiation with carboxyl group,29 leaving an unreacted methacrylate group pendent on the particle surface. The covalent grafting of poly(HEMA-MM) to the carboxyl groups was verified using 13C NMR via the identification of a covalent bond at 63 ppm, Figure S3. The grafting was again verified using Equation S1 by performing a chloroform extraction study using highly crosslinked latex, and the efficiency (shown on Table S1) further confirms the covalent grafting of HEMA-MM onto the MAA latex particles. The grafting process occurred in an ambient environment (i.e. room temperature, in air, in the presence of water) due to the high reactivity of the methylene malonate chemistry with the carboxylate anion. In the second step, a free radical photoinitiator, DMPA, was added before the poly(HEMA-MM)-grafted-latex was drawdown onto a polytetrafluorethylene substrate. The latex was then dried following film formation, and the unsaturated methacrylate groups were distributed throughout the film. Finally, upon the exposure of UV-light, the methacrylate groups underwent free radical polymerization to form a crosslinked network. Therefore, the grafting of one-component HEMA-MM onto latex particles provides a simple approach to synthesize crosslinked acrylate coatings.
Figure 3.
Schematic of the process used to synthesize UV-curable coatings. First, HEMA-MM was grafted onto MAA latex particles using carboxyls as the functional groups (FG). Next, using a drawdown tool, films of the grafted latex were formed on substrates (stainless steel or PTFE). UV-exposure enabled the formation of a crosslinked coating.
To verify that UV-curing successfully crosslinked the films, swelling experiments using DMF as an extraction solvent were conducted. Films prepared without UV-exposure completely dissolved and had a 0 wt% gel content. In contrast, swollen gels were obtained with poly(HEMA-MM) grafted films after UV-exposure, and a stable gel formed with 10 wt% HEMA-MM addition, as displayed in Figure S4. Additionally, the gel content increased with more HEMA-MM incorporation in the coating formulation, from 0 wt% without HEMA-MM to 67.2 ± 1.1 wt% with 10 wt% HEMA-MM addition, Figure 4(a). This result directly supports the increased extent of crosslinking upon greater HEMA-MM addition. Moreover, the swelling ratio of the films decreased from 41.6 ± 3.8 to 15.4 ± 0.3 upon the addition of 2.5 wt% and 10 wt% HEMA-MM, respectively, Figure 4(b). This data suggests that the crosslinked network increased due to the addition of HEMA-MM; this post-functionalization method introduced more methacrylate groups onto the film, which significantly increased the crosslinking degree and, though beyond the scope of this study, has been demonstrated to improve the solvent-resistance performance of coatings.37 Notably, the UV lamp used in the lab has a very low power (0.0044 W/cm2) so we held the UV time constant and in excess to allow for adequate crosslinking. Interestingly, in order to compare our UV-curing with existing UV-curable coatings, we also conducted experiments in collaboration with Sirrus Inc. using an industrial UV lamp. Here, we determined that a 30 μm thick film (on a stainless steel substrate) was fully cured with UV dosage of 1.5 J/cm2 within several seconds (Figure S5).
Figure 4.
(a) Gel content and (b) swelling ratio of UV-crosslinked films as a function of HEMA-MM incorporation. Error bars indicate standard deviation.
The glass transition temperature of the films was measured using DMA by recording the peak changes of tan δ.38 After crosslinking, the films that had poly(HEMA-MM) incorporated showed an increasing trend in the glass transition temperature (Tg), where the Tg changed from 38.3 ± 1.1 °C without HEMA-MM to 59.3 ± 0.4 °C with 10 wt% HEMA-MM incorporation, Figure 5(a). There was a linear relationship between the Tg and the gel content that had a coefficient of determination (R2) of 0.96, as shown in Figure 5(b).39 The increase in Tg after UV-crosslinking could be attributed to a significant reduction in mobility that results in more densely crosslinked networks.40-41
Figure 5.
(a) The glass transition temperature (Tg) of the films before and after UV-crosslinking. Error bars indicate standard deviation. (b) The glass transition temperature increases as function of gel content.
The hardness of the films before and after crosslinking were measured using a pendulum hardness tester as displayed in Figure 6.34 Without HEMA-MM addition, UV-irradiation did not change the hardness appreciably. In contrast, the pendulum hardness increased from 38 ± 1 cycles to 82 ± 3 cycles with 5 wt% HEMA-MM and 151 ± 2 cycles with 10 wt% HEMA-MM incorporation. These results correspond to a 2.2-fold and a 4.0-fold increase in hardness, respectively. By introducing more methacrylate groups on the latex particles, a higher crosslinking density was obtained in the films. The dense crosslinked network enabled an increased hardness, that significantly improved the mechanical properties of the films. Therefore, the hardness of the coating could be easily modified to satisfy different coating applications by varying the addition of HEMA-MM.
Figure 6.
Evaluation of the films before (nonX) and after UV-crosslinking (X) using the pendulum hardness test. An electronic sensor counted each time the pendulum passed over the film and each pass corresponds to one cycle. Error bars indicate standard deviation.
The mechanical properties of the films after crosslinking were also characterized using a tensile tester. Significant improvements in ultimate tensile strength were observed by incorporating more HEMA-MM as evident from the stress-strain curves provided in Figure 7(a). Young’s modulus was calculated from the slope of the elastic stage of the curve, wherein a 1.5-fold and a 2.9-fold improvement in the Young’s moduli were observed after a 5 wt% and a 10 wt% HEMA-MM incorporation, respectively, see Figure 7(b). Yield strength also showed a remarkable improvement with a 3.0-fold and a 4.2-fold increase upon 5 wt% and 10 wt% HEMA-MM incorporation, respectively. The yield strength was 7 to 11 times higher than previously reported for acrylate copolymer films with a similar Tg.42-43 A higher degree of crosslinking gave rise to an improvement in both Young’s modulus and yield strength.41 Therefore, the mechanical properties of the UV-curable films were controlled by the concentration of HEMA-MM addition and subsequent UV-curing.
Figure 7.
(a) Representative stress-strain curves, (b) Young’s modulus, and yield strength as a function of HEMA-MM incorporation. Error bars indicate standard deviation.
CONCLUSION
In summary, we report that anionic polymerization can be used to first graft HEMA-MM onto latex particles while subsequent ultraviolet (UV) exposure enabled their crosslinking into robust coatings. The grafting process was conducted at ambient conditions (room temperature, in air, in the presentence of water) with easy processing. Next, we demonstrated the feasibility of synthesizing a UV-curable coating using a one-step grafting-functionalization method with hydroxyethyl methacrylate functionalized methylene malonate monomer, where methylene malonate polymerizes and grafts onto the anionic latex particle surface while leaving methacrylate groups unreacted for subsequent free radical polymerization. The introduction of the unsaturated double bonds on the latex particles enabled the formation of a crosslinked network upon UV-exposure. The degree of crosslinking increased upon the addition of more HEMA-MM. The glass transition temperature, film hardness and tensile strength tremendously improved as the degree of crosslinking increased. In conclusion, because of its high reactivity, easy processing and facile polymerization characteristics, HEMA-MM provides a novel route for the synthesis of UV-curable coatings using a post-functionalization approach. This approach provides an on-demand cure of robust coatings prepared from acrylates and other chemistries, such as polyurethanes and polyesters.
Supplementary Material
ACKNOWLEDGEMENTS.
M.H. was supported by National Research Service Award T32 GM008515 from the National Institutes of Health. We thank Sirrus Chemistry for their support on providing DEMM and HEMA-MM.
Footnotes
Supporting information. Latex particle size distribution, FTIR and NMR spectra, extraction study on the grafting of HEMA-MM, digital images acquired during swell testing, and UV-curing using an industrial UV lamp are provided. The Supporting Information is available and free of charge http://pubs.acs.org.
REFERENCES
- (1).Aguirre-Vargas F, Thermoset coatings. In Thermosets, Elsevier: 2018; pp 369–400. [Google Scholar]
- (2).Schuler B; Baumstark R; Kirsch S; Pfau A; Sandor M; Zosel A, Structure and properties of multiphase particles and their impact on the performance of architectural coatings. Prog. Org. Coat 2000, 40 (1-4), 139–150. [Google Scholar]
- (3).Gheno G; Ganzerla R; Bortoluzzi M; Paganica R, Determination of degradation kinetics of two polyester thermosetting powder coatings using TGA and colorimetric analysis. Prog. Org. Coat 2015, 78, 239–243. [Google Scholar]
- (4).Chattopadhyay DK; Raju K, Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci 2007, 32 (3), 352–418. [Google Scholar]
- (5).Drescher J; Muller L; Tokash R; Neubeck H, One-component flexible etch resistant clearcoat. US Patent US20050123781 A1, 2005
- (6).Tillet G; Boutevin B; Ameduri B, Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci 2011, 36 (2), 191–217. [Google Scholar]
- (7).Pappas SP, Radiation curing: science and technology. Springer Science & Business Media: 2013. [Google Scholar]
- (8).Mabbett I; Elvins J; Gowenlock C; Jones P; Worsley D, Effects of highly absorbing pigments on near infrared cured polyester/melamine coil coatings. Prog. Org. Coat 2013, 76 (9), 1184–1190. [Google Scholar]
- (9).Kelly WM; Gilliard A; Burnett G; Dilworth JM, System and method for curing polymeric moldings having a masking collar. US Patent US7387759 B2, 2008
- (10).Liu P; Zhang Q; He L; Xie Q; Ding H, Synthesis and properties of poly (urethaneimide) diacid/epoxy composites cured with an aziridine system. J. Appl. Polym. Sci 2009, 113 (4), 2628–2637. [Google Scholar]
- (11).Mori A; Kitayama T; Takatani M; Okamoto T, A honeymoon-type adhesive for wood products based on acetoacetylated poly (vinyl alcohol) and diamines: Effect of diamines and degree of acetoacetylation. J. Appl. Polym. Sci 2004, 91 (5), 2966–2972. [Google Scholar]
- (12).Volfova P; Chrastova V; Cernakova L; Mrenica J; Kozankova J In Properties of polystyrene/poly (butyl acrylate) core shell polymers modified with N-methylol acrylamide, Macromol Symp, Wiley Online Library: 2001; pp 283–290. [Google Scholar]
- (13).Chen FB; Bufkin BG, Crosslinkable emulsion polymers by autoxidation. II J. Appl. Polym. Sci 1985, 30 (12), 4551–4570. [Google Scholar]
- (14).Yu C; Crump D, A review of the emission of VOCs from polymeric materials used in buildings. Build. Environ 1998, 33 (6), 357–374. [Google Scholar]
- (15).Yang G; Xie W; Huang M; Champagne VK; Lee J-H; Klier J; Schiffman JD, Polymer Particles with a Low Glass Transition Temperature Containing Thermoset Resin Enable Powder Coatings at Room Temperature. Ind. Eng. Chem. Res 2018, 58 (2), 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lin KF; Shieh YD, Core-shell particles designed for toughening the epoxy resins. II. Core-shell-particle-toughened epoxy resins. J. Appl. Polym. Sci 1998, 70 (12), 2313–2322. [Google Scholar]
- (17).Decker C, Kinetic study and new applications of UV radiation curing. Macromol. Rapid Commun 2002, 23 (18), 1067–1093. [Google Scholar]
- (18).Xu H; Qiu F; Wang Y; Wu W; Yang D; Guo Q, UV-curable waterborne polyurethane-acrylate: preparation, characterization and properties. Prog. Org. Coat 2012, 73 (1), 47–53. [Google Scholar]
- (19).Corrales T; Catalina F; Peinado C; Allen N, Free radical macrophotoinitiators: an overview on recent advances. J. Photochem. Photobiol. A: Chem 2003,159 (2), 103–114. [Google Scholar]
- (20).Graziola F; Girardi F; Bauer M; Di Maggio R; Rovezzi M; Bertagnolli H; Sada C; Rossetto G; Gross S, UV-photopolymerisation of poly (methyl methacrylate)-based inorganic-organic hybrid coatings and bulk samples reinforced with methacrylate-modified zirconium oxocluster. Polymer 2008, 49 (20), 4332–4343. [Google Scholar]
- (21).Asif A; Shi W, UV curable waterborne polyurethane acrylate dispersions based on hyperbranched aliphatic polyester: effect of molecular structure on physical and thermal properties. Polym. Advan. Technol 2004,15 (11), 669–675. [Google Scholar]
- (22).Decker C; Lorinczova I, UV-radiation curing of waterborne acrylate coatings. JCT Res. 2004, 1 (4), 247–256. [Google Scholar]
- (23).Schwalm R; Häußling L; Reich W; Beck E; Enenkel P; Menzel K, Tuning the mechanical properties of UV coatings towards hard and flexible systems. Prog. Org. Coat 1997, 52 (1-4), 191–196. [Google Scholar]
- (24).Buschow KJ; Cahn RW; Flemings MC; Ilschner B; Kramer EJ; Mahajan S, Encyclopedia of materials. Sci. Technol 2001, 7, 11. [Google Scholar]
- (25).Masson F; Decker C; Jaworek T; Schwalm R, UV-Radiation curing of waterbased urethane–acrylate coatings. Prog. Org. Coat 2000, 39 (2-4), 115–126. [Google Scholar]
- (26).Hwang H-D; Park C-H; Moon J-I; Kim H-J; Masubuchi T, UV-curing behavior and physical properties of waterborne UV-curable polycarbonate-based polyurethane dispersion. Prog. Org. Coat 2011, 72 (4), 663–675. [Google Scholar]
- (27).Nagelsdiek R; Mennicken M; Maier B; Keul H; Höcker H, Synthesis of polymers containing cross-linkable groups by atom transfer radical polymerization: poly (allyl methacrylate) and copolymers of allyl methacrylate and styrene. Macromolecules 2004, 27 (24), 8923–8932. [Google Scholar]
- (28).Matsumoto A; Kodama K; Aota H; Capek I, Kinetics of emulsion crosslinking polymerization and copolymerization of allyl methacrylate. Eur. Polym. J 1999, 35 (8), 1509–1517. [Google Scholar]
- (29).Huang M; Liu Y Yang G; Klier J; Schiffman JD, Anionic Polymerization of Methylene Malonate for High-Performance Coatings. ACS Appl. Polym. Mater 2019, 1 (4), 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Clemons CM; Sabo RC; Hirth KC, The effects of different silane crosslinking approaches on composites of polyethylene blends and wood flour. J. Appl. Polym. Sci 2011, 120 (4), 2292–2303. [Google Scholar]
- (31).Kurtz IS; Sui S; Hao X; Huang M; Perry SL; Schiffman JD, Bacteria-Resistant, Transparent, Free-Standing Films Prepared from Complex Coacervates. ACS Appl. Bio Mater 2019, 2 (9), 3926–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nikafshar S; Zabihi O; Ahmadi M; Mirmohseni A; Taseidifar M; Naebe M, The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10 (2), 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hu X; Shen X; Huang M; Liu C; Geng Y; Wang R; Xu R; Qiao H; Zhang L, Biodegradable unsaturated polyesters containing2, 3-butanediol for engineering applications: Synthesis, characterization and performances. Polymer 2016, 84, 343–354. [Google Scholar]
- (34).Shang S.-b.; Xie H; Huang H; Wang D.-x., 1 Study on amino polyester baking varnish modified by tung oil. Chem. Ind. For. Prod 2000, 20 (4), 1–5. [Google Scholar]
- (35).Zhang C; Wang H; Zeng W; Zhou Q, High biobased carbon content polyurethane dispersions synthesized from fatty acid-based isocyanate. Ind. Eng. Chem. Res 2019, 58 (13), 5195–5201. [Google Scholar]
- (36).Rasib S; Ahmad Z; Khan A; Akil H; Othman M; Hamid Z; Ullah F, Synthesis and evaluation on pH-and temperature-responsive chitosan-p (MAA-co-NIPAM) hydrogels. Int J Biol Macromol 2018,108, 367–375. [DOI] [PubMed] [Google Scholar]
- (37).Said HM; Nik Salleh NG; Alias MS; El-Naggar AWM, Synthesis and characterization of hard materials based on radiation cured bio-polymer and nanoparticles. J. Radiat. Res. Appl. Sci 2013, 6 (2), 71–78. [Google Scholar]
- (38).Lin HT; Lin CH; Hu YM; Su WC, An approach to develop high-Tg epoxy resins for halogen-free copper clad laminates. Polymer 2009, 50 (24), 5685–5692. [Google Scholar]
- (39).Motulsky H; Christopoulos A, Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. GraphPad Software. Inc., San Diego, CA: 2003. [Google Scholar]
- (40).Rahman M; Zainuddin S; Hosur M; Robertson C; Kumar A; Trovillion J; Jeelani S, Effect of NH2-MWCNTs on crosslink density of epoxy matrix and ILSS properties of e-glass/epoxy composites. Compos. Struct 2013, 95, 213–221. [Google Scholar]
- (41).Xie T; Rousseau IA, Facile tailoring of thermal transition temperatures of epoxy shape memory polymers. Polymer 2009, 50 (8), 1852–1856. [Google Scholar]
- (42).Zamyshlyayeva O; Ionychev B; Baten’kin M; Kopylova N; Markin A; Zaitsev S; Semchikov YD, Properties of methacrylic acid–methyl acrylate copolymers of varied structure. Russ. J. Appl. Chem 2018, 91 (8), 1332–1337. [Google Scholar]
- (43).Mohammed S; Daniels E; Sperling L; Klein A; El-Aasser M, Isocyanate-functionalized latexes: Film formation and tensile properties. J. Appl. Polym. Sci 1997, 66 (10), 1869–1884. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.