Abstract
Background
Reference-based pricing limits reimbursement for a group of drugs that are deemed therapeutically equivalent to the cost of the lowest-priced product within that group. We estimated the effect of reference-based pricing of nitrate drugs used for long-term prophylaxis on prescribing of and expenditures on nitrates and other anti-anginal drugs dispensed to senior citizens in British Columbia.
Methods
We assessed trends in the monthly volume of prescriptions of anti- anginal drugs and the associated drug ingredient cost paid by the province's publicly funded drug subsidy program, Pharmacare, and by the patients themselves for the period April 1994 to May 1999. Trends in monthly rates of nitrate expenditures per 100 000 senior citizens before the introduction of reference-based pricing were extrapolated to infer what expenditures would have been without the policy.
Results
During the 31/2 years after reference-based pricing was introduced, Pharmacare expenditures on nitrates prescribed to senior citizens declined by $14.9 million (95% confidence interval $10.7 to $19.1 million). Most of these savings were due to the lower prices that Pharmacare paid for sustained-release nitroglycerin tablets and the nitroglycerin patch, which were the 2 most frequently prescribed nitrates before the introduction of reference-based pricing; $1.2 million (8%) of the savings represented expenditures by senior citizens who purchased drugs that were only partially reimbursed. There were no compensatory increases in expenditures for other anti-anginal drugs. Use of sublingual nitroglycerin — a marker for deteriorating health in patients with angina — did not increase after the introduction of reference-based pricing. The nitroglycerin patch is now the most frequently prescribed nitrate, owing to the fact that Pharmacare resumed the provision of full subsidies for the drug after its manufacturers voluntarily reduced retail prices.
Interpretation
Evidence to date suggests that reference-based pricing of nitrates has achieved its primary goal of reducing drug expenditures. The effects of this policy on patient health, associated health care costs and administrative costs remain to be investigated.
Reference-based pricing has been adopted both within Canada (in British Columbia and Nova Scotia) and in other countries (including the United States, Australia, New Zealand and Germany1) as a means of limiting expenditures for drug subsidy and insurance programs. Reference-based pricing limits reimbursement for a group of drugs with similar therapeutic application but different active ingredients to the price of the lowest-cost drug within the group (the reference standard). Patients have the option of purchasing drugs that are partially subsidized, in which case they pay the difference between the retail price and the reference price. Reference-based pricing policies differ in terms of the groups of drugs that are subject to reimbursement restrictions, the mechanisms by which patients can be exempted from the restrictions and the coexisting regulations that might affect drug prices (and hence savings), such as mandatory substitution of generic drugs, direct price regulation and patent protection.
This paper evaluates the effect of reference-based pricing, as applied by Pharmacare, the British Columbia Ministry of Health's drug subsidy program, on expenditures for nitrate drugs used for long-term prophylaxis, 1 of the 5 drug groups that have been subject to the policy. Given that drug prices within a group of drugs that are deemed interchangeable can vary substantially, limiting reimbursement to the cost of the lowest-priced drug could be expected to reduce costs. But there may be offsetting factors. First, physicians can apply for a “special authority” exemption from reference-based pricing for Pharmacare beneficiaries for whom they believe a switch to another drug would be inadvisable. Second, physicians may substitute relatively expensive drugs that are used for the same indication but are not directly targeted by the reference-based pricing policy.2,3 For example, during the 2-month period after reference-based pricing of nitrates was introduced, the average cost to Pharmacare per defined daily dose of the lowest-cost calcium-channel blocker (CCB) was greater than that of the highest-priced nitrate. Third, economic theory suggests that setting reimbursement rates according to the prices of a set of reference standard drugs might encourage the manufacturers of those drugs to raise prices.4,5,6 Fourth, patients whose angina worsened as a result of the policy might use more acute “rescue” therapy (sublingual nitroglycerin being the first choice for such therapy), which would result in additional drug expenditures. Finally, to the extent that reference-based pricing is effective in reducing Pharmacare expenditures, it might do so by shifting the costs to beneficiaries, who pay the difference between the retail price and the reference price.
We used aggregate Pharmacare claims data to examine the mean price that Pharmacare paid for nitrates, as well as prescribing patterns and Pharmacare-reimbursed expenditures for nitrates and other anti-anginal drugs (specifically, β-blockers and CCBs) and patients' out-of-pocket spending on nitrates, in the periods before and after introduction of reference-based pricing.
Methods
The BC Ministry of Health applied reference-based pricing to nitrate drugs in 2 stages: Pharmacare beneficiaries whose first prescription for a nitrate was dispensed on or after Oct. 1, 1995, were immediately affected by the policy, but Pharmacare beneficiaries who had prescriptions for nitrates before this date were not affected until Nov. 1, 1995. Residents of long-term care facilities were automatically exempted. Under the reference-based pricing policy, reimbursement for a daily dose of isosorbide mononitrate or pentaerythritol, as well as for sustained-release isosorbide dinitrate (ISDN) and nitroglycerin tablets, was restricted to the price of the lowest-cost brand of regular-release ISDN. Reimbursement for the transdermal nitroglycerin patch was limited to the cost of nitroglycerin ointment, although the 0.2- and 0.4-mg patches were exempted from reference-based pricing starting in January 1996 and the 0.6- and 0.8-mg patches were exempted starting in March 1996, after the manufacturers reduced retail prices. The nitrate drugs used for acute treatment (0.3- and 0.6-mg nitroglycerin tablets, 5-mg ISDN tablets and nitroglycerin spray) were never affected by the policy. Hereafter, we refer to the nitrates for which reimbursement was restricted under the reference-based pricing policy as restricted nitrates. Reference standard nitrates are the nitrates whose prices were used to set the level of reimbursement for the restricted nitrates, and exempt nitrates are those exempted from the reference-based pricing policy. We categorized the patch separately because its reimbursement status reverted from restricted to exempt. The remaining anti-anginal drugs — CCBs and β-blockers — were initially exempt from the policy, although some CCBs became subject to reference-based pricing on Jan. 1, 1997. Reimbursement for nifedipine, nicardipine and amlodipine were restricted to the price of the reference standard CCB, felodipine. Regular-release versions of diltiazem and verapamil were exempt from reference-based pricing.
BC Pharmacare provided monthly data for the period April 1994 to May 1999 on the volume of prescriptions and the units of anti-anginal drugs (nitrates, CCBs and β-blockers) dispensed to senior citizens (65 years of age and older) and the associated costs (not including drug dispensing fees) paid by Pharmacare and the component paid by patients. Each unique combination of active ingredient and dosage form was grouped, although tablet and capsule formulations of the same drug and dosage strength were combined. For each anti-anginal drug and for each month we calculated the number of prescriptions dispensed per 100 000 senior citizens,7 the Pharmacare reimbursement per defined daily dose, the Pharmacare expenditure per 100 000 senior citizens, and the patients' expenditures per 100 000 senior citizens.
To determine the Pharmacare reimbursement per defined daily dose, we calculated weighted mean prices for different brands of the same active ingredient, dosage form and strength, with the weights being equal to each brand's share of the total monthly volume of units dispensed. These brand-averaged prices were then averaged across different dosage strengths of the drugs with identical active ingredients and dosage form. (We first found the price per milligram of each dosage strength of each drug–dosage combination and then computed the weighted averages of these per- milligram prices, with the weights being equal to each drug's share of the total monthly volume of milligrams dispensed.) Finally, prices per milligram were converted to prices per defined daily dose according to the World Health Organization definitions.8
We considered the effects of reference-based pricing on nitrates in conjunction with 2 other Pharmacare policies that could have affected the use and costs of nitrates: exemption of the transdermal nitroglycerin patch from reference-based pricing beginning in January 1996 and implementation of reference-based pricing for CCBs in January 1997. It is possible, for example, that physicians faced with the reimbursement restrictions on CCBs started substituting nitrates. To describe the effects of these policy changes, we calculated the means of our outcome variables during periods around the 3 policy changes: April 1994 to October 1995, November and December 1995, January to December 1996, and January 1997 to May 1999. To describe changes in rates of prescribing and expenditures over time, we constructed an index of each period's mean rate relative to the period before reference-based pricing was implemented (April 1994 to October 1995). Monthly values of the outcome variables were graphed to better illustrate dynamics.
In addition to describing trends in the data, we estimated the effect of reference-based pricing on Pharmacare's nitrate expenditures. This effect was defined as the difference between what Pharmacare expenditures would have been had reference-based pricing not been introduced and actual Pharmacare expenditures with reference-based pricing in place. To estimate the former, we extrapolated trends in nitrate expenditures from before the introduction of reference-based pricing (April 1994 to October 1995) to the period when the policy was in place (November 1995 to May 1999). The slope and position of the baseline trends were estimated by linear regression. We constructed 95% confidence intervals (CIs) around the predicted values to reflect sampling error in our estimates.
Results
Although the introduction of reference-based pricing did not affect the overall volume of nitrates dispensed (Table 1; an expanded version of this table is available on eCMAJ at www.cma.ca/cmaj/vol-165/issue-8/grootendoorsttable1.pdf), it did affect the mix. The mean monthly number of prescriptions for restricted nitrates fell by 64% during the 2 months after introduction of the policy (from 750 to 267 prescriptions dispensed per 100 000 senior citizens). This decline was due almost entirely to a reduction in prescribing of sustained-release nitroglycerin. There was a 47% drop in the mean monthly number of prescriptions for the nitroglycerin patch during the 2-month period after introduction of reference-based pricing (from 793 to 424 prescriptions dispensed per 100 000 senior citizens). Some of this change can be attributed to patients stockpiling the patch before implementation of the policy, which would exaggerate the observed decline in drug use immediately after reference-based pricing was introduced (Fig. 1). However, rates of prescribing for the patch returned to baseline levels within 12 months after the patch was exempted from the policy, and, in contrast to the other nitrates, prescribing of the patch gradually increased over time.
Table 1
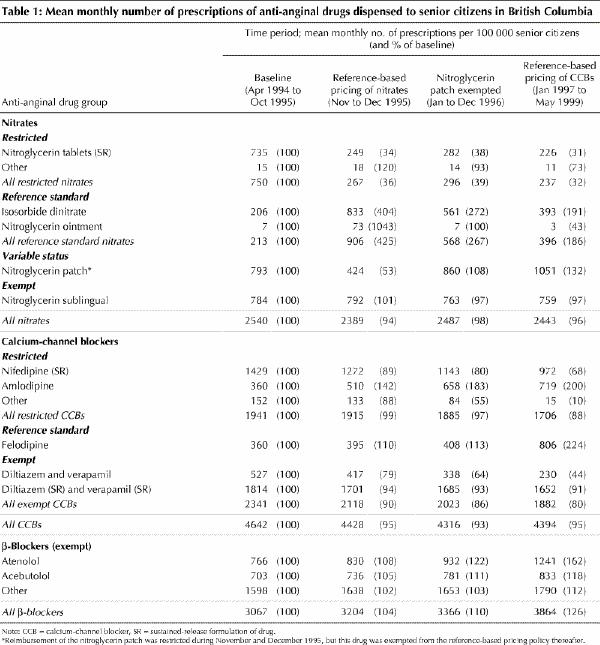
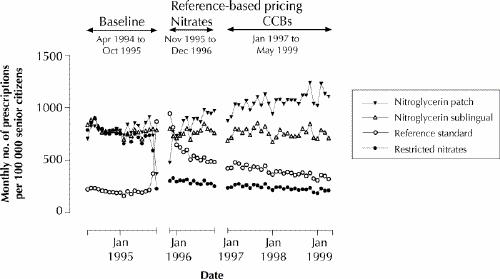
Fig. 1: Monthly numbers of nitrate prescriptions dispensed to senior citizens in British Columbia. To improve clarity, the 2-month period during which remuneration of the nitroglycerin patch was restricted (November and December 1995) has been combined with the following period, January to December 1996. Beginning in January 1996, the nitroglycerin patch was exempt from the reference-based pricing policy. CCB = calcium-channel blocker.
Prescribing of the reference standard nitrates increased sharply immediately after reference-based pricing was introduced. Prescribing of ISDN increased by 304% (from 206 to 833 prescriptions per 100 000 senior citizens), while prescriptions of the ointment increased by 943% (from 7 to 73 prescriptions per 100 000 senior citizens) (Table 1). This increase was short-lived, however: after the patch was exempted from reference-based pricing, rates of prescribing of the reference standard nitrates dropped to less than half their peak levels, and, although they remained above their baseline levels, prescribing rates declined over time. Rates of prescribing of the acute-use nitrates remained stable over the entire study period.
After the introduction of reference-based pricing, rates of prescribing of CCBs decreased, while rates of prescribing of β-blockers (primarily atenolol and metoprolol) increased to 110% and then to 126% of baseline levels (from 3067 to 3366 and 3864 prescriptions per 100 000 senior citizens) during the periods January to December 1996 and January 1997 to May 1999 respectively.
Once the reference-based pricing was in place, the mean price that Pharmacare paid per defined daily dose of the nitroglycerin patch and the restricted nitrates eventually declined to 43% and 66%, respectively, of their baseline levels (Table 2). There were no increases in the prices paid for the reference standard nitrates. As Table 3 (an expanded version of this table is available on eCMAJ at www.cma.ca/cmaj/vol-165/issue-8/grootendoorsttable3.pdf) indicates, the net effect of the price decreases was to lower monthly Pharmacare expenditures on nitrates to 50% of the baseline level (from about $139 000 to about $70 000 per 100 000 senior citizens). By December 1996, monthly spending on the reference standard nitrates had increased by 83% (from $1454 to $2659 per 100 000 senior citizens), but this increase was more than offset by spending reductions of 45% for the patch (from $67 198 to $37 179 per 100 000 senior citizens) and 67% for the restricted nitrates (from $60 403 to $20 171 per 100 000 senior citizens) (Table 3, Fig. 2). There is no evidence that spending on other anti-anginal drugs increased after introduction of the policy.
Table 2
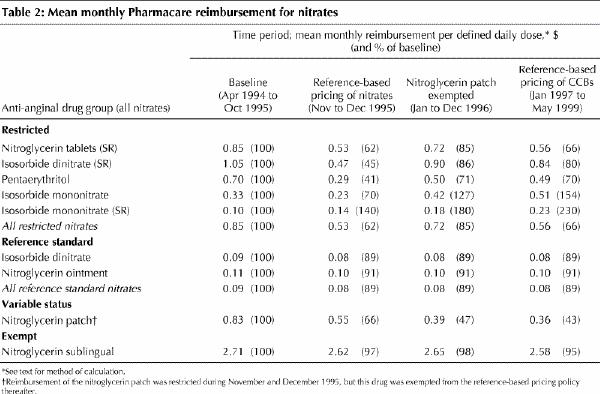
Table 3
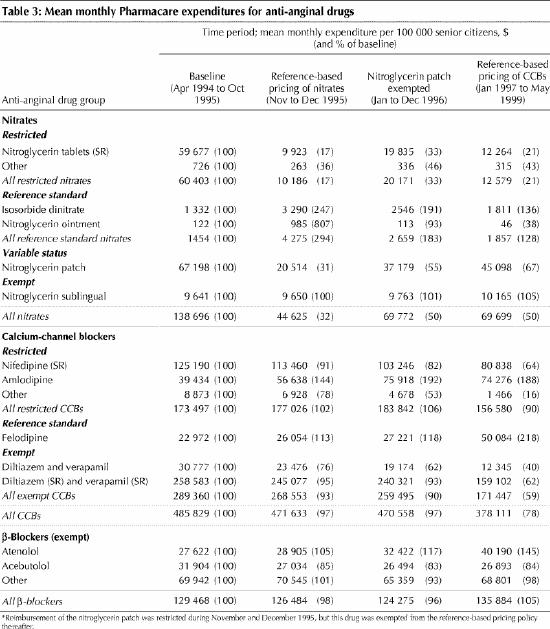
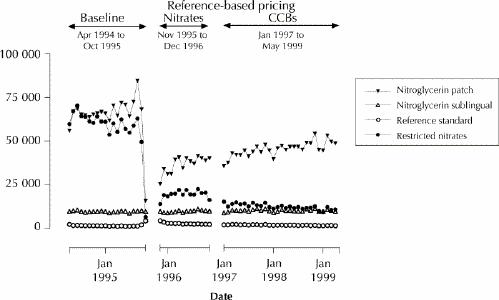
Fig. 2: Monthly Pharmacare expenditures for nitrates dispensed to senior citizens in British Columbia. To improve clarity, the 2-month period during which remuneration of the nitroglycerin patch was restricted (November and December 1995) has been combined with the following period, January to December 1996. Beginning in January 1996, the nitroglycerin patch was exempt from the reference-based pricing policy.
Fig. 3 displays actual Pharmacare spending on nitrates per 100 000 senior citizens and predictions of what Pharmacare would have spent had reference-based pricing not been introduced. Total (undiscounted) savings over the 43-month period November 1995 to May 1999 were $2.9 million (95% CI $2.1 to $3.7 million) per 100 000 senior citizens. This corresponds to total savings of $14.9 million (95% CI $10.7 to $19.1 million) or approximately $4.2 million annually.
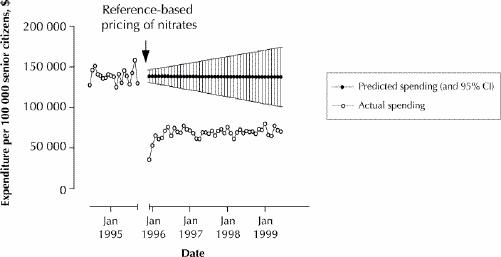
Fig. 3: Actual monthly Pharmacare expenditures on nitrates before and after introduction of reference-based pricing, and predicted expenditures if reference-based pricing had not been introduced. CI = confidence interval.
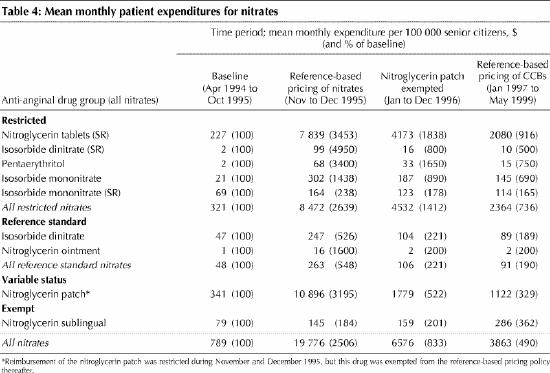
Some of the savings to Pharmacare represented additional out-of-pocket costs to beneficiaries. For example, private spending on the nitroglycerin patch increased by more than 3000% (from $341 to $10 896 per 100 000 senior citizens) immediately after reference-based pricing was introduced, but rates of spending dropped to $1779 per 100 000 in the year after the patch was exempted (Table 4). (The fact that senior citizens paid for nitrates before the introduction of reference-based pricing is due to the introduction in April 1994 of a policy requiring senior citizens to pay a surcharge for brand-name drugs if generic equivalents existed.) Private spending on the restricted nitrates, in particular sustained-release nitroglycerin tablets, increased sharply during the first 2 months of the reference-based pricing policy (from $321 to $8472 per 100 000 senior citizens) but dropped by about 75% (to $2364 per 100 000 senior citizens) in the final 17 months of the study period. The trend in the patient share of total (private plus Pharmacare) spending on nitrates (not shown) tells a similar story: initially beneficiaries paid as much as 37% of costs, but this share dropped to about 5%, as patients reduced the use of nitrates that incurred copayments. Over the period November 1995 to May 1999, inclusive, elderly beneficiaries directly contributed $1.2 million for restricted nitrates and the patch. This represents 8% of estimated Pharmacare savings over the same period.
Table 4
Interpretation
In this study we focused on elderly beneficiaries of BC Pharmacare. These people constitute the single largest beneficiary group (approximately 487 000 in 1996, accounting for 60% of Pharmacare spending), and they have the highest per capita rates of consumption of anti-anginal drugs. Although reference-based pricing has to date been applied to several drug groups other than nitrates (specifically histamine-2 receptor antagonists, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors and dihydropyridine CCBs), we focused on nitrates because there were no apparent concomitant changes in either the pharmacological management of angina or the Pharmacare reimbursement policy for these drugs over our study period. This stability facilitated estimation of the effects of reference-based pricing of nitrates, although the presence of time-varying confounders could have affected our results.
We estimate that reference-based pricing of nitrate drugs has reduced Pharmacare expenditures on nitrates taken by senior citizens by approximately $15 million in the first 31/2 years after introduction of the policy. This is equivalent to $4.2 million annually or approximately 2% of the $202 million that Pharmacare spent on drugs (excluding dispensing fees) for senior citizens in 1996. Reference-based pricing was also applied to social assistance recipients and members of households with high drug costs who qualify for coverage by exceeding an income-contingent deductible; their combined 1996 drug costs were $119 million. However, because most members of these groups are less than 65 years of age and probably have lower rates of nitrate use than senior citizens, reference-based pricing of nitrates probably saved a lower proportion of drug costs for these groups.
Most of Pharmacare's savings are attributable to lower reimbursement prices for sustained-release nitroglycerin tablets and the nitroglycerin patch, which is now the nitrate most widely prescribed in British Columbia. There is no evidence that the reductions in Pharmacare expenditures on nitrates were offset by higher expenditures on other anti-anginal drugs, nor did we find that the reimbursement prices of the reference standard drugs (ISDN and nitroglycerin ointment) increased after the introduction of reference-based pricing.
Rates of prescribing of the sublingual nitrates — the use of which might indicate compromised health status in people with angina — remained virtually unchanged after introduction of reference-based pricing. This finding, coupled with the fact that nitrates offer symptomatic relief only, is consistent with, although certainly not conclusive evidence of, the view that the health of elderly beneficiaries was unaffected by the policy. Indeed, our aggregated data might mask increases in sublingual nitrate use among particularly vulnerable populations. There did not appear to be widespread substitutions between nitrates and other anti-anginal drugs after introduction of reference-based pricing. Rates of prescribing of all nitrates dropped only 2% in the 14 months after introduction of the policy (to December 1996), and rates of CCB use declined by 7% in the same period. Although there was a 10% increase in rates of prescribing of β-blockers over the same period, there is some evidence that rates of prescribing of these drugs were increasing contemporaneously in jurisdictions where reference-based pricing was not in effect.9
About 8% of cumulative Pharmacare savings represent the additional costs to beneficiaries who elected to pay out of pocket to acquire the higher-priced nitrates not fully reimbursed by Pharmacare. Rates of out-of-pocket spending by senior citizens were highest immediately after the policy was implemented. It is plausible that these patients, initially unaware of the policy when refilling prescriptions for restricted nitrates and unable to contact their physicians to have their prescriptions changed, elected to pay the out-of-pocket expense but avoided paying for subsequent prescriptions by receiving a special authority exemption or by switching to a fully reimbursed nitrate.
Our finding that reference-based pricing reduced drug expenditures is consistent with a report by Narine and associates,10 who found that reference-based pricing of histamine-2 receptor antagonists in 1995 reduced Pharmacare expenditures in the following year. Evidence from studies of reference-based pricing in Europe2,3,11 indicates that such policies cannot control drug costs over the long term, but the strength of some of this evidence has been questioned.12
There is some evidence that reference-based pricing in British Columbia has reduced public drug expenditures, but the impact of the policy on other health care costs remains ambiguous for several reasons. Patients taking a drug that is no longer fully reimbursed might consult their physician about treatment options (e.g., switching to a fully reimbursed drug, applying for an exemption or paying out-of-pocket costs), which would increase the number of physician visits, as would the monitoring of patients whose medication has been switched. If a patient cannot tolerate a switch in drugs or if drugs are not interchangeable, the patient's health might be compromised, and use of both pharmaceuticals and other types of health care might increase.13,14,15,16,17,18,19,20 Finally, physicians, pharmacists and patients might spend time and incur other costs in complying with the policy, in addition to the direct costs of program administration.21,22 Additional research into the “downstream” consequences of reference-based pricing is therefore necessary to determine the overall effects of the policy.
Footnotes
This article has been peer reviewed.
Acknowledgements: We thank Colin Dormuth, Malcolm Maclure and seminar participants at the Centre for Evaluation of Medicines for helpful comments. We thank Sean Burnett and Robert Hart at BC Pharmacare for assistance with data retrieval. Dr. Grootendorst acknowledges the career support of the Rx&D Health Research Foundation and the Canadian Institutes for Health Research (CIHR). Dr. Holbrook is the recipient of a CIHR Career Investigator Award.
We appreciate support for this project from the Health Transition Fund, Health Canada; the Canadian Health Services Research Foundation; Brogan Inc.; the BC Ministry of Health; and the Drug Information Association.
The views expressed here do not necessarily represent the official policy of federal, provincial or territorial governments, nor of the project funders.
Competing interests: None declared.
Correspondence to: Dr. Paul Grootendorst, Centre for Evaluation of Medicines, St. Joseph's Hospital, 105 Main St. E, P1, Hamilton ON L8N 1G6; fax 905 528-7386; grootend@mcmaster.ca
References
- 1.Dickson M, Redwood H. Pharmaceutical reference prices. How do they work in practice? Pharmacoeconomics 1998;14(5):471-9. [DOI] [PubMed]
- 2.Selke G. Reference price systems in the European Community. In: Mossialos E, Ranos C, Abel-Smith B, editors. Cost containment, pricing and financing of pharmaceuticals in the European Community: the policy makers' view. Athens: LSE Health and Pharmetrica SA; 1994. p. 147-60.
- 3.Zammit-Lucia J, Dasgupta R. Reference pricing: the European experience. No. 10 in Health Policy Review Paper series, St. Mary's Hospital Medical School. London: University of London; 1995.
- 4.Morton FS. The strategic response by pharmaceutical firms to the Medicaid most-favored-customer rules. Rand J Econ 1997;28(2):269-90. [PubMed]
- 5.Zweifel P, Crivelli L. Price regulation of drugs: lessons from Germany. J Regul Econ 1996;10:257-73.
- 6.Anis A, Wen Q. Price regulation of pharmaceuticals in Canada. J Health Econ 1998;17:21-38. [DOI] [PubMed]
- 7.Canadian socio-economic information management system (data matrix 6377). Ottawa: Statistics Canada; 2000.
- 8.The integrated version of the ATC Index with DDDs and the guidelines for ATC classification and DDD assignment. Oslo: WHO Collaborating Centre of Drug Statistics Methodology; 2000.
- 9.Wang TJ, Stafford RS. National patterns and predictors of beta-blocker use in patients with coronary artery disease. Arch Intern Med 1998;158:1901-6. [DOI] [PubMed]
- 10.Narine L, Senathirajah M, Smith T. Evaluating reference-based pricing: initial findings and prospects. CMAJ 1999;161:286-8. Available: www.cma.ca/cmaj/vol-161/issue-3/0286.htm [PMC free article] [PubMed]
- 11.Busse R, Howorth C. Fixed budgets in the pharmaceutical sector in Germany: effects on costs and quality. In: Schwartz FW, Glennerster H, Saltman RB, editors. Fixing health budgets: experience from Europe and North America. New York: John Wiley & Sons; 1996. p. 109-27.
- 12.Schneeweiss S, Schoffski O, Selke GW. What is Germany's experience on reference based drug pricing and the etiology of adverse health outcomes or substitution? Health Pol 1998;44(3):253-60. [DOI] [PubMed]
- 13.Olley PM, McLaughlin PR. The Canadian Cardiovascular Society and reference-based drug pricing. Can J Cardiol 1998;14(5):669-70. [PubMed]
- 14.Boulet AP, Tessier G. Reference-based pricing in British Columbia: implications for cardiologists — an analysis. Can J Cardiol 1997;13(1):46-51. [PubMed]
- 15.Bourgault C, Elstein E, Le Lorier J, Suissa S. Reference-based pricing of prescription drugs: exploring the equivalence of angiotensin-converting-enzyme inhibitors. CMAJ 1999;161(3):255-60. Available: www.cma.ca/cmaj/vol-161/issue-3/0255.htm [PMC free article] [PubMed]
- 16.Ikeda U, Shimpo M, Shimada K. Thrombotic vascular events after change of statin [letter]. Lancet 1999;353:844-5. [DOI] [PubMed]
- 17.McNee W. Re: M Thomas. The change of cost: reference-based pricing and the statins [letter]. Can J Cardiol 1999;15(11):1287. [PubMed]
- 18.Thomas M. The change of cost: reference-based pricing and the statins. Can J Cardiol 1999;15(5):535-8. [PubMed]
- 19.Thomas MC, Mann J, Williams S. The impact of reference pricing on clinical lipid control. N Z Med J 1998;111(1071):292-4. [PubMed]
- 20.Weiss NS, Heckbert SR. Thrombotic vascular events after change of statin [letter]. Lancet 1999;353:844. [DOI] [PubMed]
- 21.Kent H. BC's reference-based pricing stirs controversy. CMAJ 2000;162:1190. Available: www.cma.ca/cmaj/vol-162/issue-8/1190a.htm [PMC free article] [PubMed]
- 22.Woollard RF. Opportunity lost: a frontline view of reference-based pricing [editorial]. CMAJ 1996;154:1185-8. Available: www.cma.ca/cmaj/vol-154/1185.htm [PMC free article] [PubMed]


