Abstract
Background
Monitoring the success of soil-transmitted helminth (STH) control programs relies on accurate diagnosis and quantitative assessment of infection prevalence and intensity. As preventative chemotherapeutic program coverage for STH expands, the necessity of gaining insights into the relative or comparative sensitivities, in terms of limits of detection (LOD) and egg-recovery-rates (ERR) for microscopy and quantitative polymerase chain reaction qPCR-based diagnostic techniques becomes imperative to inform suitability for their intended use for large scale STH monitoring and treatment efficacy studies.
Methodology/Principal findings
The diagnostic performance in terms of ERR and LOD of the Kato-Katz (KK) thick smear technique, sodium nitrate (NaNO3) faecal floatation (FF) and qPCR for the accurate detection and enumeration of STH eggs were calculated and expressed in eggs per gram (EPG), by experimentally seeding parasite-free human faeces with Ascaris spp., Trichuris spp. and Necator americanus eggs representing low, medium and high intensity infections. The efficiency of NaNO3 flotation was also calculated over a range of specific gravities (SpGr) for the optimum recovery of STH eggs. FF of SpGr 1.30 recovered 62.7%, 11% and 8.7% more Trichuris spp., Necator americanus and Ascaris spp. eggs respectively, than the recommended SpGr of 1.20. All diagnostic methods demonstrated strong direct correlation to the intensity of seeded EPG. KK and FF (SpGr 1.30) resulted in significant lower ERRs compared to qPCR (p <0.05). qPCR demonstrated significantly (p <0.05) greater sensitivity with an ability to detect as little as 5 EPG for all three STH, compared to 50 EPG by KK and FF (SpGr 1.30).
Conclusions/Significance
This study compares the diagnostic parameters in terms of LOD and ERRs of STHs for the KK, FF and qPCR. These results indicate that the diagnostic performance of qPCR assays should be considered by control programs in the phase that aims to seek confirmation of transmission break and cessation of preventive chemotherapy in low-transmission settings, in line with the control targets of the WHO neglected tropical diseases 2030 Roadmap.
Author summary
STH infections predominately affect resource-poor communities and negatively impact on child and maternal health. Diagnostics play a critical role in guiding and informing existing STH control programs and the implementation and evaluation of intervention strategies. The KK technique is most commonly used as per WHO guidelines and is the basis for determining quantitative thresholds for low, moderate and heavy infections. More recently, FF and laboratory-based quantitative PCR techniques have provided alternative options of diagnosing STH infections. A number of studies correlating the relative sensitivity, diagnostic agreement, and egg enumeration of these techniques by comparing field-generated diagnostic data have attempted to correlate quantitative outputs to those defined by the WHO, with little resolution. Moreover, as large-scale deworming programs levels scale up, the necessity to apply techniques that can precisely detect light infections is of outmost importance to establish preventive chemotherapy end-points using more accurate means. In this study we compared the LOD and ERR of seeded non-infected human faecal samples with Ascaris, Trichuris and Necator spp. eggs using KK, FF and qPCR. When compared to copro-microscopy methods, qPCR showed the highest sensitivity for the detection of light-intensity infections and was more accurate in enumerating the original number of eggs per gram of seeded faeces. In addition to this, the results indicated that the diagnostic performance of both KK and FF (using solution at a SpGr of 1.30) were equally efficient and should be considered with their diagnostic limitations in mind, when aiming to monitor STH infections in low-transmission settings.
Introduction
Over 1.5 billion people are infected with at least one soil-transmitted helminth (STHs; Ascaris lumbricoides, Necator americanus, Ancylostoma spp. and Trichuris trichiuria) worldwide. Children, pregnant women and women of child-bearing age from the poorest and most marginalized communities are severely impacted in terms of morbidity [1]. The World Health Organization (WHO) endorsed a resolution to eliminate morbidity caused by the STHs by 2020 through periodical deworming of all at-risk populations in endemic areas [2] and has now set new global targets to build on this target by 2030 [3]. The current global control strategy aims to control morbidity of STHs through large-scale deworming programs using preventive chemotherapy (PC; albendazole or mebendazole) for all at-risk populations (pre-school and school children, women of reproductive age). To date, 50.25% of the world population still requires PC. As STH morbidity and transmission are directly related to the infection prevalence and intensity [4], accurate diagnosis and quantitation of infections is paramount for assessing impact and informing control programs. More precisely, the sensitivity of diagnostic techniques becomes critical in later stages of PC programs, when infection prevalence and intensity decrease, to identify areas of low-transmission and make informed decision on PC interruption [5].
The diagnosis and measure of morbidity attributed to STHs currently relies on the detection and quantitation of eggs in faeces through the assessment of faecal egg counts (FEC; expressed in eggs per gram of stool or EPG). The Kato-Katz (KK) thick smear is the most widely utilized and accepted technique recommended by the WHO and the thresholds for low, moderate and heavy infections have traditionally been based on this technique [6,7]. The technique is simple, inexpensive, reproducible and commonly used in field-based epidemiological surveys. However, a major critique of the KK is its reduced sensitivity in detecting low intensity infections, its predisposition to false negative results and the need for several slides per sample to obtain accurate FEC [5,8–10]. Alternatively, coproscopy-based flotation methods such as the simple or centrifugal faecal float (FF), FLOTAC, mini-FLOTAC, FECPAK, McMaster and most recently DNA based diagnostic methods such as quantitative PCR (qPCR) have demonstrated advantages over KK[10–12]. FF has been demonstrated superior to KK performed in duplicate and quadruplicate for detecting light intensity infections [10,11]. On the other hand, the egg intensity levels as determined by FF using a floatation solution with a specific gravity (SpGr) of 1.20, have tended to produce on average, lower values compared to those of KK [10,13]. FF like KK is inexpensive and provides clean preparations that allow clear observation of ova.
A number of studies have reported the superiority of qPCR compared to microscopy-based methods in terms of sensitivity, its ability to detect higher levels of mixed STH species infections and ability to identify hookworm eggs at species level [12,14,15]. This is important given the emerging distribution of the zoonotic hookworm Ancylostoma ceylanicum [14,16].
Despite the availability of literature assessing the field-based comparative sensitivities of the aforementioned STH diagnostic techniques [11,12,17–20] to our knowledge, no data exists on the assessment of the true ERR and/or limit of detection (LOD) of three STHs by KK, FF and qPCR in human stool. In this study we report a comparison of the ERR and LOD of seeded Ascaris, Trichuris and Necator eggs in parasite-free human faeces using KK, sodium nitrate (NaNO3) FF (SpGr 1.20, 1.25, 1.30 and 1.35), and multiplex qPCR that quantifies genera- and species-specific infection intensities using a pre-determined cycle-threshold to EPG formula.
Methods
Egg purification
Given difficulties in obtaining fresh eggs from infected humans and the similarities in egg morphology between human (T. trichiuria and A. lumbricoides) and swine STH, we used gravid A. suum female worms and T. suis positive faeces sourced from a local pig abattoir in Victoria, Australia. Unmatured eggs of Ascaris were recovered by mechanical dissection of the uterus of an adult female worm followed by repeatedly filtering eggs through a double layer of 10 cm sq surgical gauze with 1x PBS. 10 μL of solution with eggs was quantified in triplicate using light microscopy. Ascaris eggs (n = 1000) were checked in triplicate prior seeding to ensure eggs were fertilized and not unfertilized to avoid egg ploidy. N. americanus eggs were sourced from fresh human stool provided by Prof. Alex Loukas (James Cook University, Qld, AUS). Unmatured eggs of N. americanus and Trichuris were purified from human and pig faeces using gradient centrifugal flotation using Sheather’s solution (1.20 SpGr; 355ml distilled water (dH2O) and 454 g sucrose). Briefly, 3–5 g of faeces were strained through surgical gauze using 20 ml of dH20 and transferred into a 50 ml centrifuge tube. The filtrate was centrifuged for two minutes at 2000 x rpm. The supernatant was discarded leaving behind the faecal pellet. Faeces were homogenized with Sheather’s solution using a wooden stick, filled to the rim of the centrifuge tube and allowed to sit for 15 min. The top layer was carefully aspirated and placed into 15 ml centrifuge tubes followed by a washing step with 1x PBS and centrifugation at 2000 rpm for 5 min. Following removal of the supernatant,10 μl of sediment consisting of purified eggs was quantified (eggs/μl) in triplicate using light microscopy. All purified egg species were identified using previous molecular methods [14,21,22] and stored at 4°C. Eggs were microscopically checked to avoid inclusion of samples in which egg embryonation occurred.
Stool collection and parasitological procedures
Stool samples were sourced from an anonymous donor living in Victoria, Australia, with no history of travel to a STH-endemic area. Vials (50 ml) with popsicle sticks were provided to the participant and once collected, examined by FF and qPCR to ensure the sample was parasite-free prior to the egg seeding experiment. STH-free stool samples were placed into 15 ml centrifuge tubes for KK and FF (1gr each) and into 1.5 ml Eppendorf tubes (200 mg each) for qPCR. A range of infection intensities, measured by EPG was selected to be seeded into faecal samples in triplicate based on current WHO classifications of light, moderate and heavy STH infection intensities [23]. Between 1–8,000 eggs of Necator spp., 1–15,000 eggs of Trichuris spp. and 1–50,000 eggs of Ascaris spp. were seeded in triplicate into each STH-free stool sample within individual tubes, as detailed in Table 1.
Table 1. Serial replicates (n = 3) of soil-transmitted helminth eggs spiked in one gram of parasite-free faeces according to infection intensities selected from WHO guidelines.
| Trichuris spp. | Ascaris spp. | Necator americanus |
|---|---|---|
| Low intensity infections | ||
| 1–999* | 1–4,999* | 1–1999* |
| 5 | 5 | 5 |
| 10 | 10 | 10 |
| 50 | 50 | 50 |
| 100 | 100 | 100 |
| 500 | 150 | 500 |
| 500 | 1,000 | |
| 2,000 | 1,500 | |
| Moderate-Intensity infections | ||
| 1,000–9,999* | 5,000–49,999* | 2,000–3,999* |
| 1,000 | 5,000 | 2,000 |
| 2,000 | 10,000 | 3,000 |
| 5,000 | 25,000 | |
| 7,500 | ||
| High-intensity infections | ||
| ≥10,000* | ≥50,000* | ≥4,000* |
| 10,000 | 50,000 | 4,000 |
| 15,000 | 6,000 | |
| 8,000 | ||
* Classes of infection intensities for soil-transmitted helminths as per WHO guidelines.
Kato-Katz procedure
For each stool sample (n = 3), KK smears (Sterlitech, USA) were performed in duplicate as per WHO’s recommendations [7]. The slide was examined by light microscopy within 60 min following preparation and the number of Ascaris, Trichuris and Necator eggs enumerated. The absolute egg count for each triplicate were summed and multiplied by 12 to obtain EPG value for each seeded stool sample.
Specific gravity for optimal egg recovery using sodium nitrate solution
To assess the optimal SpGr for achieving the highest egg recovery yield by standing FF, the ERR for each parasite species was determined by subjecting one gram of STH-egg seeded stool (100 EPG for each Necator, Ascaris and Trichuris) to flotation using NaNO3 solution with a range of SpGr (i.e. 1.20, 1.25, 1.30 and 1.35, in triplicate).
Once optimised, FF was carried out previously described [24], with parasite-free samples inoculated with a range of egg concentrations for each parasite species in triplicate as shown in Table 1, using a double cover slip method. Briefly, one gram of faeces was homogenized with NaNO3 and strained through a 10 cm sq surgical gauze into a sterile specimen container. The suspension was transferred to a 15 ml centrifuge tube and centrifuged for 5 min at 2500 × rpm. The remaining faecal material remaining was recorded for further EPG calculation. Further NaNO3 was added to the rim of the tube, forming a positive meniscus and allowed to stand for 10 min with a coverslip (22 mm × 22 mm). Once removed, a second coverslip was placed and allowing to stand for a further 5 min after which both coverslips were placed on a slide and examined at 100 magnification by light microscopy. The entire slide was examined in a zig-zag fashion and the total number of eggs was recorded.
ERR were calculated on the basis of total numbers of eggs recovered per gram and number of eggs seeded (for detailed protocol see S1 File).
Generation of formula to convert Ct values to EPG for STH
Genomic DNA was extracted from each triplicate individually (200mg of stool) containing serial dilution of known intensity of seeded eggs of Ascaris, Necator and Trichuris using a Maxwell RSC PureFood GMO and Authentication Kit, Promega (Promega Corporation, US) as per manufacturer’s instructions, with the following modifications; an additional bead-beating step with 400μL CTAB buffer using a FastPrep-24 5G Instrument, (MP Biomedicals) and 0.5 mm Zirconia/Silica beads (Daintree Scientific, AUS). Following bead-beating, cell lysis was proceeded in a Maxwell RSC 48 Instrument, Promega. The final eluted sample (100 μL) was stored at -20°C for further downstream analyses. DNA samples were subjected to two multiplex qPCR (M-qPCR): 1. Hookworm-human four-plex qPCR; 2. Ascaris-Trichuris four-plex qPCR. Details of the probes and primers are listed in Table 2. Both qPCR assays were performed in triplicate for each individual sample using Taq Man hydrolysis probes (Integrated DNA Technologies, USA) in a Mic qPCR Cycler system (Bio molecular systems, AUS). Assays were performed in 20 μl reactions containing 10 μl of GoTaq Probe qPCR Master mix (Promega Corporation, USA), 1 μL of known quantity of EHV4 DNA as an internal qPCR control, and 2 μL of template DNA. Nuclease free-water was added to reach the final reaction volume. Non-template controls (assay master-mix with no template) were included with each run. The cycling conditions for both assays consisted of the following parameters: denaturation at 95°C for 2 min, followed by 40 cycles of 15 sec at 95°C and annealing at 60°C for 1 min, with no extension phase. Egg counts expressed in EPG were calculated by multiplying the absolute egg count by a factor of 5, as 200 mg of faecal sample was subjected to DNA extraction for the current study. Log10 transformations of original egg count (EPG) for each STH species were plotted against Ct values to predict the ability of the assay to estimate original egg intensities in EPG for each STH species, assuming a 100% run efficiency [14].
Table 2. Quantitative multiplex PCR oligonucleotide primers and probes for the detection of soil-transmitted helminths.
| Multiplex qPCR | Target species | Oliglonucleotide sequence 5’-‘3 | Product size | Gene target | Final conc. in nM | Source |
|---|---|---|---|---|---|---|
| Ascaris-Trichuris-EHV4 qPCR | Ascaris spp. | 88 bp | ITS1 | |||
| Asc Fwd | GTAATAGCAGTCGGCGGTTTCTT | 50 | [25] | |||
| Asc Rev | GCCCAACATGCCACCTATTC | 50 | ||||
| Asc Probe | /5HEX/TT GGC GGA C/ZEN/A ATT GCA TGC GAT /3IABkFQ/ | 100 | ||||
| Trichuris spp. | 76bp | 18S | [26] | |||
| Tri 18 S Fwd | TTGAAACGACTTGCTCATCAACTT | 250 | ||||
| Tri 18 S Rev | CTGATTCTCCGTTAACCGTTGTC | 250 | ||||
| Tri 18S Probe | /CY5 -CGATGGTAC/TAO/GCTACGTGCTTACCATGG- 3IAbRQSp/ | 100 | ||||
| Human DNA | ||||||
| Mammal F | CGACCTCGATGTTGGATCAG | 92bp | 16S | 50 | This study | |
| Mammal R | GAACTCAGATCACGTAGGACTTT | 50 | ||||
| Human Probe | /FAM/CCCGATGGT/ZEN/GCAGCCGCTATTAAA /3IABkFQ/ |
100 | ||||
| Equine Herpes Virus | 81 bp | gB | [12] | |||
| EHV Fwd | GATGACACTAGCGACTTCGA | 40 | ||||
| EHV Rev | CAGGGCAGAAACCATAGACA | 40 | ||||
| EHV probe | /ROX/TTTCGCGTGCCTCCTCCAG/3IAbRQSp/ | 100 | ||||
| Hookworm qPCR | Necator americanus | 101 bp | ITS2 | [27] | ||
| Nec Fwd | CTGTTTGTCGAACGGTACTTGC | |||||
| Nec Rev | ATAACAGCGTGCACATGTTGC | |||||
| Nec Probe | /5Cy5/CTG+TA+CTA+CG+CAT+TGTATAC/3IAbRQSp/* | |||||
| Ancylostoma spp. | [14] | |||||
| Anc F | CGGGAAGGTTGGGAGTATC | 104 bp | ITS1 | 300 | ||
| Anc R | CGAACTTCGCACAGCAATC | 300 | ||||
| A.cey Probe | /56-FAM/CCGTTC+CTGGGTGGC/3IABkFQ/ | 100 | ||||
| A.duo Probe | /5HEX/TCGTTAC+T+GGGTGACGG/3IABkFQ/ | 100 | ||||
| Equine Herpes Virus | 81 bp | gB | [12] | |||
| EHV Fwd | GATGACACTAGCGACTTCGA | 40 | ||||
| EHV Rev | CAGGGCAGAAACCATAGACA | 40 | ||||
| EHV probe | /ROX/TTTCGCGTGCCTCCTCCAG/3IAbRQSp/ | 100 |
Multiplex STH qPCRs
Genomic DNA was extracted as per previously described and the seeded stool samples containing Ascaris, Necator and Trichuris eggs representing light, moderate and heavy infection intensities (Table 1) subjected to the two four-plex qPCR assays in duplicate 1. Hookworm-human four-plex qPCR; 2. Ascaris-Trichuris- four-plex qPCR assays as previously described. EPG for each sample were estimated using the Ct value to EPG formula generated.
Data analysis
Analyses were performed using GraphPad Prism version 8.4.2 for Windows (GraphPad Software, La Jolla California USA, http://www.graphpad.com) and Excel 2017 (Microsoft).
Specific gravity for optimal egg recovery using sodium nitrate solution
The ability of NaNO3 to recover Ascaris, Trichuris and hookworm eggs from human faeces was compared using a range of SpGrs from 1.20–1.35. Absolute FECs were recorded and transformed to EPG using the equation to convert FECs to EPG from FF. Descriptive statistics was conducted to observe the distribution of the data and obtain the geometric mean of each SpGr and their respective 95% confidence intervals. Ordinary One-way ANOVA test was used to compare the overall ERR means of the SpGrs tested. A p-value of less than 0.05 was required for significance. Because the overall test was significant, a post-hoc test using Tukey’s multiple comparisons, with a single pooled variance between SpGr means was conducted to assess the point or level of difference for which a significant difference laid.
Diagnostic performance of qPCR and microscopy techniques
Normality and lognormality tests and frequency distribution of the data was evaluated following Shapiro-Wilk normality test for each parasite, with a significance level of α = 0.05. Agreement in EPG between the seeded experiment and the ERR for each technique was evaluated by the non-parametric Spearman’s rank correlation coefficient (ρ). In addition, the agreement between each technique excluding seeded EPG was further evaluated for pair-wise comparisons. Descriptive statistics were conducted to generate data on the ERR frequency of each of the techniques followed by one-way ANOVA to test for the significance of the ERR variability among means. Tukey’s multiple comparison test was used to further analyse the ERR performance of each technique. A p value of 0.05 was required for significance.
Results
Specific gravity for optimal egg recovery using sodium nitrate solution
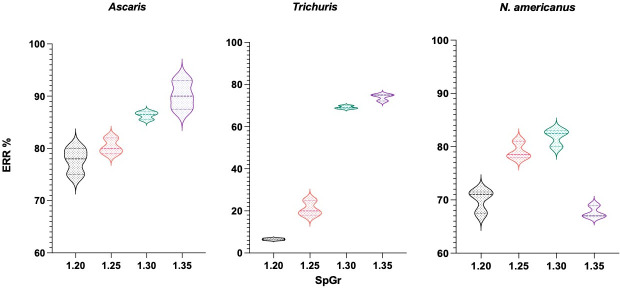
A range of SpGrs was tested to compare the absolute number of eggs recovered using NaNO3 from 100 seeded Ascaris, Trichuris and Necator eggs in 1 gr of human parasite-free faeces. One-way ANOVA analyses revealed a significant difference among the ERR means for the different SpGrs tested for Ascaris (p = 0.003, R2 0.90), Trichuris (p <0.001, R2 0.99) and N. americanus (p <0.001, R2 0.95) (Table 3). High F-values were observed for the three parasites (Ascaris F = 22.92; Trichuris F = 843; N. americanus F = 50.63) indicating evidence for differences among means. Mean ERRs for each species across different SpGrs and their respective Tukey’s test values are reported in Table 4. The empirical distribution of the data for each parasite is shown in Fig 1. In general, ERR increased as the SpGr increased for the three STHs (Table 4). Ascaris ERR’s increased from 77.6% using 1.20 SpGr to 90.3% when using a SpGr of 1.35. However, only a SpGr increase from 1.25 to 1.30 provided a significant higher Ascaris ERR (p = 0.025) from 80.3% to 86.3%. SpGr of 1.20 and 1.30 resulted in comparable ERRs for N. americanus of 70.1% and 81.8% (p 0.007), respectively. However, ERR decreased significantly (p = 0.003) for Necator when the SpGr was increased from 1.30 (81.8%) to 1.35 (67.7%). Trichuris egg recovery rates increased from 6.5% using a 1.20 SpGr to 69.2% with a 1.30 SpGr solution (p 0.006). Thus, a SpGr of 1.30 was deemed optimum for maximizing the ERR for all STHs.
Table 3. ANOVA for egg recovery rates from Ascaris spp., Trichuris spp. and N. americanus 100 seeded eggs using 1.20, 1.25, 1.30 and 1.35-SpGr NaNO3.
| Parasite | Source of variation | Sum of squares | Degrees of freedom | Mean sum of squares | F (DFn, DFd) | P value | R2 |
|---|---|---|---|---|---|---|---|
| Ascaris | |||||||
| Between SpGrs | 289.4 | 3 | 96.47 | F (3, 8) = 22.92 | 0.003* | 0.90 | |
| Within SpGrs | 33.67 | 8 | 4.208 | ||||
| Total | 323.1 | 11 | |||||
| Trichuris | |||||||
| Between SpGrs | 10397 | 3 | 3466 | F (3, 8) = 843 | <0.001*** | 0.99 | |
| Within SpGrs | 33 | 8 | 4.1 | ||||
| Total | 10430 | 11 | |||||
| N. americanus | |||||||
| Between SpGrs | 427.2 | 3 | 142.4 | F (3, 8) = 50.63 | <0.001*** | 0.95 | |
| Within SpGrs | 22.50 | 8 | 2.813 | ||||
| Total | 449.7 | 11 |
*P<0.05
**P<0.002
***P<0.001
Table 4. Egg recovery rate (ERR) comparison from triplicate absolute faecal egg counts of 100 seeded Ascaris spp., Trichuris spp. and N. americanus eggs.
| Parasite | 1.20 SpGr | 1.25 SpGr | 1.30 SpGr | 1. 35 SpGr | |||
|---|---|---|---|---|---|---|---|
| ERR mean % [95% CI] | p-value 1.20–1.25 | ERR mean % [95% CI] | p-value 1.25–1.30 | ERR mean % [95% CI] | p-value 1.30–1.35 | ERR mean % [95% CI] | |
| Ascaris | 77.6 [71.4; 83.9] | 0.409 | 80.3 [76.5; 84.1] | 0.025* | 86.3 [84.4; 88.2] | 0.145 | 90.3 [83.9; 96.7] |
| Trichuris | 6.50 [5.26; 7.74] | 0.047* | 21 [12.0; 29.9] | 0.006* | 69.2 [67.3; 71.1] | 0.088 | 74.1 [70.0; 78.1] |
| N. americanus | 70.1 [65.1; 75.1] | 0.007* | 79.2 [75.4; 83.1] | 0.285 | 81.8 [77.8; 85.8] | 0.003* | 67.7 [64.8; 70.5] |
*P<0.05
**P<0.002, ***P<0.001
α = 0.5 for every 0.5 SpGr increase from Tukey’s multiple comparison test from two-way ANOVA
Fig 1. Violin plots for egg recovery rates with sodium nitrate faecal floatation at different specific gravity concentrations.
Empirical distributions of data sets obtained from triplicate faecal float counts of 100 seeded Ascaris, Trichuris and N. americanus eggs in parasite-free faeces using four specific gravity concentrations of sodium nitrate solution.
Relationship between known numbers of STH seeded eggs and Ct values from multiplex STH qPCRs
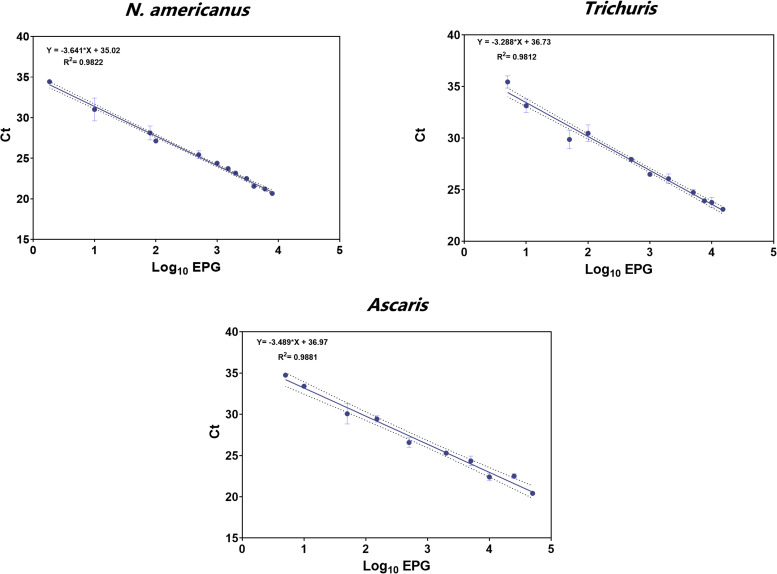
Quantitative results derived from well-defined series of purified known seeded STH eggs (Table 1) plotted against Ct values for Ascaris, Trichuris and N. americanus are shown in Fig 2. The interpolation of qPCR Ct-value to log10 EPG for the three-helminth species showed a strong linear relationship between the two variables (Ascaris R2 = 0.988, Trichuris R2 = 0.981 and N. americanus R2 = 0.982). The conversion formulas for Ct to EPG were determined as the following:
Fig 2. Agreement in eggs per gram (EPG) and cycle threshold (Ct) values by qPCR.
The scatterplots illustrate a strong agreement (Ascaris: R2 = 0.9881; Trichuris: R2 = 0.9812; N. americanus: R2 = 0.982) between faecal egg counts (FECs) expressed as EPG of stool from seeded known numbers of Ascaris, Trichuris and N. americanus eggs and the mean Ct values obtained from qPCR reads from two multiplex qPCR assays. Dotted lines adjacent to the best line of fit represent 95% confidence intervals; graph error bands indicate the standard error of the plotted means (SEM) which measures the variability/dispersion of the triplicate Ct values.
Diagnostic performance of qPCR and microscopy techniques
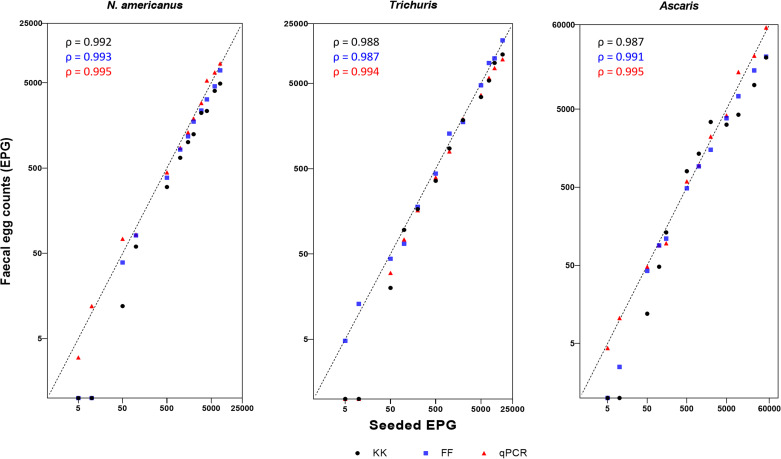
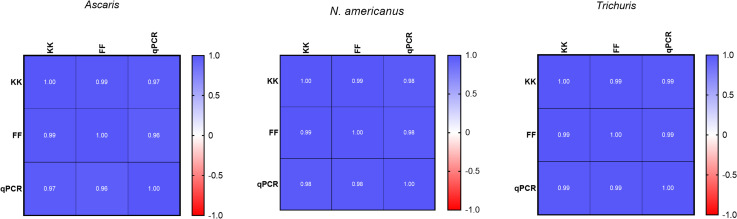
The data distribution of each parasite failed to follow a Gaussian distribution based on the Shapiro-Wilk test for normality (p <0.05). Agreement in recovered EPG from the two microscopy techniques, and qPCR compared to the seeded EPG for each STH using the Spearman’s rank correlation are shown in Fig 3. Overall, a strong positive linear correlation was observed between ERR and seeded EPG for the three diagnostic techniques among all STHs. The highest correlation coefficient was between qPCR and seeded EPG (ρ = 0.994–0.995, p <0.001), followed by FF (ρ = 0.987–0.993, p < 0.001) and KK (ρ = 0.987–0.992 p < 0.001). The highest correlation coefficient for each pair-wise comparison between diagnostic techniques was observed between FF vs KK (Rs >0.99) followed by KK vs qPCR (Rs = 0.97–0.99) and qPCR vs FF (Rs = 0.96–0.99) (Fig 4). Therefore, the observed FECs across the techniques resulted in concordance in classifying the intensity of infection from light to heavy according to the current WHO guidelines.
Fig 3. Agreement of faecal egg counts by the two microscopy techniques, qPCR and seeded EPG.
The scatter plots illustrate the agreement between faecal egg counts, expressed in eggs per gram (EPG) based on each technique versus known numbers of seeded eggs per gram of stool. Black dots (Kato Katz); blue squares (Faecal floats); red triangles (qPCR). In each panel, the Spearman’s rank correlation coefficient (ρ) is given and a diagonal stripped line represents the line of equivalence.
Fig 4. Pairwise correlation matrix heatmaps of each diagnostic technique for each of the STHs.
Each square illustrates the correlation between the performance of each technique. Correlation ranges from -1 to +1 (values closer to zero means there is no linear correlation between the variables examined). The plot is symmetrical on the diagonal since the same two techniques are being paired together in that same square.
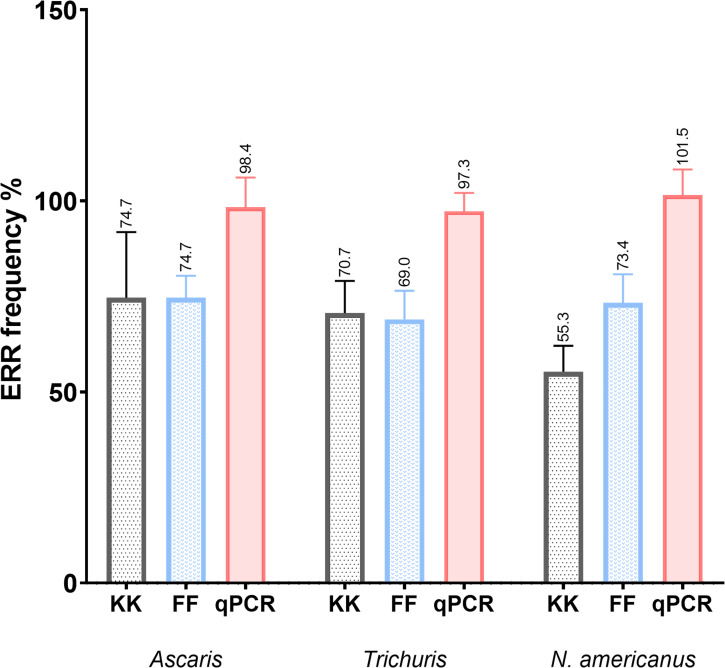
Both KK and FF resulted in lower ERR as compared to qPCR (Fig 5). The overall mean ERR increased from 66.87% [95% CI: 41.48;92.27] with KK, to 72.36% [95% CI: 65.04;79.68] with FF, to 99% [95% CI: 93.61;104.5] with qPCR. ANOVA analyses revealed significant differences between the mean ERR of each technique for each parasite (F = 22.60 p 0.001)(Table 5). This result allowed to reject the null hypothesis that all means are equal. Because the overall test was significant, a post hoc test using Tukey’s multiple comparison test was conducted to compare the mean variation of each technique. The results indicated that KK and FF had no significant differences in ERR among parasites (p 0.564). However, KK vs qPCR and FF vs qPCR showed significant mean differences with p values of 0.001 and 0.004 respectively, contributing to the overall ANOVA significance difference (Table 5). Hence, qPCR outperformed microscopy by recovering around 1/3 (27% and 32%) more eggs than FF and KK respectively. FF and KK recovered eggs at a similar average frequency with FF recovering only 5.5% more eggs than KK.
Fig 5. Egg recovery rate (ERR) frequencies of three diagnostic techniques.
The bar plots represent the recovery percentage of each technique KK (Kato-Katz thick smear), FF (Sodium nitrate faecal floatation 1.30) and qPCR (quantitative qPCR) across the three most common STHs; Ascaris, Trichuris and N. americanus. Error bars represent standard error mean obtained from the overall ERR obtained from a series of known numbers of seeded egg experiments (Table 2).
Table 5. ANOVA- comparison of the egg recovery means among three diagnostic techniques.
| Source of variation | Sum of squares | Degrees of freedom | Mean sum of squares | F (DFn, DFd) | P value | |||
| Variation between techniques | 1777 | 2 | 888.6 | F (2,6) = 22.60 | 0.001* | |||
| Variation within techniques | 235.9 | 6 | 39.32 | |||||
| Total | 2013 | 8 | ||||||
| Tukey’s multiple comparison test between techniques | Mean difference | 95% Confidence interval | Adjusted P value | |||||
| KK vs FF | -5.483 | -21.19 to 10.23 | 0.564 | |||||
| KK vs qPCR | -32.17 | -47.88 to -16.46 | 0.001* | |||||
| FF vs qPCR | -26.69 | -42.40 to -10.98 | 0.004* | |||||
α = 0.05*
Limit of detection of microscopy and qPCR of low intensity infections
When low infection intensities were measured by means of qPCR, the lowest detection limit was 5 EPG corresponding to mean Ct values of 34.75 (S.D ± 0.22) for Ascaris spp., 35.19 (S.D ± 0.47) for Trichuris spp. and 35.42 (S.D ± 0.26) for N. americanus (Fig 2). In comparison, the limit of detection of FF from absolute egg counts was as low as 50 seeded eggs/gr for Trichuris (30 EPG), Ascaris (24 EPG) and N. americanus (39 EPG; Table 6). The limit of detection of the KK technique was the same as for FF (50 seeded eggs/gr), but EPGs resulted in comparable FECs for Ascaris (24 KK vs 42 FF EPG), Trichuris (12 KK vs 36 FF EPG), and N. americanus (12 KK and 39 FF EPG). According to WHO guidelines, all three techniques for all STHs can detect infections classified as light intensity infections. Nonetheless, qPCR outperformed by establishing light-moderate intensity cut-offs more accurately than FF and KK and by LOD for the three-main STHs.
Table 6. Seeded vs observed EPG counts for light-moderate cut-off infection intensities.
| Seeded eggs per gram of faeces | Kato Katz observed EPG | NaNO3 Faecal float 1.3 SpGr observed EPG | M-qPCR observed EPG |
|---|---|---|---|
| Ascaris spp. | |||
| 5 | 0 | 0 | 4 |
| 10 | 0 | 0 | 10 |
| 50 | 24 | 42 | 48 |
| 100 | 48 | 90 | 90 |
| 150 | 132 | 110 | 130 |
| 500 | 804 | 485 | 595 |
| 1000 | 1344 | 925 | 953 |
| 2000 | 3432 | 1513 | 2233 |
| Trichuris spp. | |||
| 5 | 0 | 0 | 5 |
| 10 | 0 | 0 | 13 |
| 50 | 12 | 36 | 44 |
| 100 | 96 | 74 | 66 |
| 200 | 168 | 163 | 179 |
| 500 | 360 | 395 | 438 |
| N. americanus | |||
| 5 | 0 | 0 | 3 |
| 10 | 0 | 0 | 12 |
| 50 | 12 | 39 | 74 |
| 100 | 60 | 81 | 83 |
| 500 | 300 | 385 | 445 |
| 1000 | 660 | 825 | 865 |
| 1500 | 1008 | 1178 | 1311 |
Discussion
To our knowledge this is the first study to accurately test the ERR and LOD of the KK, simple flotation and multiplex qPCR assays for STH eggs in human faeces.
Based on the ERR obtained from the graded SpGrs tested, a NaNO3 solution of SpGr 1.30 resulted in optimum recovery for Ascaris, Trichuris and Necator eggs in human stool. The SpGr of an egg is dependent on its volume and mass (water and solid content) [28]. Heavier eggs have a higher mass per unit volume and less per vitellus space in the egg. For parasite eggs to buoy up in a solution, the SpGr of the solute must be higher [29]. Common floatation solutions used in diagnostic practice include sodium/calcium chloride, sugar (Sheather’s solution), zinc sulphate (ZnSO4), magnesium sulphate and NaNO3. Nonetheless, compared to other solutions, NaNO3 has shown to be superior for the recovery of helminth eggs in canine faeces, including A. caninum and Toxocara canis. [17]. The data generated in this study supported those of previous work demonstrating that SpGrs of more than 1.20 deemed higher egg recovery rates in canine species [17,30,31]. Of importance, was the significantly superior ERR observed for Trichuris when using a SpGr of 1.30 (69.2%; 95% CI: 67.3;71.1) instead of the recommended SpGr of 1.20 routinely used for floatation techniques such as FLOTAC/mini-FLOTAC to detect parasites from human and animal faeces (6.5%, 95% CI: 5.26;7.74) [10,32].This observation may explain the lower prevalence observed of Trichuris spp. compared with qPCR [11,19] and FF [13] reported in previous epidemiological surveys and comparative studies in which a solution of SpGr of 1.20 was employed for flotation-based diagnosis of STH eggs. Failure to detect infections or underestimate the intensities of STH eggs has significant implications for accurately informing STH control programmes [33]. Previous studies have also supported our findings that allowing the cover slip to stand on the solution meniscus for less than 15 min prior to examination results in missed Trichuris infections in canine stool [17]. In addition to this, other variables such as the use of faecal preservatives, preservation times, egg developmental stage and ratio of different parasite egg species present in the faecal specimen, may influence egg recoveries even when using an optimal SpGr [29]. For example, Sawitz [28] tested the buoyancy of fertilized vs unfertilized Ascaris eggs showing that unfertilized eggs floated more readily in solutions of higher SpGr (1.25) when using ZnSO4 as compared to fertilized eggs which floated better in solutions of SpGrs of 1.20. Even though, ERR increased for Ascaris and Trichuris spp. using a SpGr of 1.35, this was not the case for N. americanus, that achieved optimum ERR at SpGr 1.30. Utilisation of a SpGr solution of 1.30 was deemed optimal as increasing this to 1.35 resulted in greater amounts of faecal debris and rapid crystallisation of NaNO3, which made eggs difficult to visualise. It must be noted that egg recovery of 100% is not achievable with any solution or SpGr as inevitably some eggs will adhere to the surface of the filter or may be attached within the faecal sediment [30]. To ensure floatation-based STH detection techniques perform optimally, investigators must ensure solutions attain correct SpGr and standing or centrifugation times are strictly adhered to when applying this technique.
A good correlation between multiplex qPCR assay Ct values and seeded EPG was obtained in agreement with previous experimental egg seeding studies demonstrating quantitation potential of the qPCR assays for enumeration of hookworm eggs in human faeces [14]. In addition, a strong direct correlation between the ERRs of qPCR, FF and KK for the experimentally seeded faecal samples were obtained across a range of egg intensities for all three STHs. Nevertheless, KK underestimated the seeded egg counts by 25–45% as compared to FF (~30%) and qPCR (2–3%). Both microscopy techniques performed equally for Ascaris and Trichuris, but not for Necator for which FF significantly outperformed KK in terms of ERR. This may be owing to the ability of hookworms to degrade and disappear in slides that are left to clear on KK smears for longer than 30–60 min, resulting in egg miscounts and/or false negative results, even though slide examination times were strictly adhered to in this study [13,23]. In accordance with previous studies [11] we observed a bell shape-like trend for the ERR for both coproscopy techniques, whereby light and very heavy intensity infections were underestimated, and moderate infections were more accurately quantified. In the case of light intensity infections, this may be due simply to the low sensitivity of detection microscopy techniques hold, which is well documented [10,12,34,35]. However, for heavy intensity infections, this may be explained by the superimposition of eggs by other eggs or faecal debris that may result in the underestimation of egg counts, in particular for the KK.
Our experimental data supports field epidemiological data demonstrating the superior sensitivity of molecular assays to that of coproscopic techniques for the detection of STH eggs in faeces [12,20,36–38]. For the detection and enumeration of STH eggs in faeces, the present qPCR assay performed best, exhibiting least ERR variation compared with double KK and FF. While a previous study [19] indicated that qPCR could not detect STHs in faecal samples with < 150 EPG, here we established that LOD was as low as 5 EPG for all three STHs studied. Other studies [11,19] have inferred EPG based on a calculation of genome equivalents per ml; such an inference is only valid if the genomic copy number(s) of each genetic marker used in qPCR is/are known, or can be estimated for developmental stages/sexes of STHs present in faecal samples [39]. For instance, it is well-known for nuclear ribosomal markers, such as internal transcribed spacers, that copy number can vary substantially among egg, embryonated egg, larvae and adult stages [40]; such variation can have a direct and significant impact on EPGs inferred from genome equivalents per ml, and on qPCR results. Similarly, copy numbers of ribosomal non-coding DNA markers may potentially vary between different strains of STH species.
In the present study, we employed EPG as an estimate of infection intensity, so that the qPCR results could be compared directly with conventional, quantitative coprodiagnostic methods, which follow current guidelines for the evaluation of faecal egg reduction after chemotherapeutic treatment. Nonetheless, there are still challenges that require further investigation to effectively incorporate qPCR as a diagnostic tool for STH diagnosis from field samples. For instance, repeatable standard operating procedures for DNA extraction and qPCR, effect of different stool preservatives and thermocyclers are still barriers that require attention [39].
Primary advantages of qPCR over coproscopic techniques include i) the ability to preserve faecal samples immediately in 100% ethanol for transport at ambient temperature to centralised laboratories for analysis; thereby negating the requirement for mobilising large teams of technicians and equipment to field sites that also require stable power supply and running water; ii) higher sensitivity and accuracy for detection and enumeration of STH egg intensities iii) ability to differentiate between species of hookworms that inform both morbidity and whether One Heath control measures are required.
To achieve and maintain elimination of STH morbidity in pre- and school aged children in context for WHO 2030 targets, prevalence with STH infections of moderate and heavy intensity must be lowered to <2%. Our study shows that microscopy techniques cannot accurately detect the cut-offs between light and moderate infection intensities by underestimating ERRs by approximately 20%, in particular when utilising KK for hookworm enumeration. This poses a challenge when programs aim to establish breakpoint of transmission, and more importantly, monitor recrudescence. If moderate infections are misclassified as light-infections by WHO, then MDA programs would be terminated too early.
Conclusions
Compared to FF and to the WHO-recommended KK technique, only qPCR was able to accurately classify light-moderate infection cut-offs and to detect very low infection intensities, highlighting the suitability of qPCR to detect STH DNA in stool even in very low-infection settings. Further testing using clinical samples will be necessary to cross validate our results. Even though, KK is the only method that meets all the criteria for the planning, implementation and monitoring phase of MDA, the sensitivity of qPCR must be considered as a diagnostic tool by current programs aiming at maintaining infection levels at <2% as a threshold for MDA cessation and disease re-emergence monitoring.
Supporting information
(PDF)
Acknowledgments
We would like to extend our gratitude to Dr. Dinh Nguyen, Prof Alex Loukas and Luke Becker for the donation of STH eggs for this study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: Adding up the numbers—A review. International Journal for Parasitology. 2010. 10.1016/j.ijpara.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. World Health Organization. 2017. Available from: https://www.who.int/nutrition/publications/guidelines/deworming/en/ [PubMed] [Google Scholar]
- 3.Lankester F, Davis A, Kinung’Hi S, Yoder J, Bunga C, Alkara S, et al. An integrated health delivery platform, targeting soil-transmitted helminths (STH) and canine mediated human rabies, results in cost savings and increased breadth of treatment for STH in remote communities in Tanzania. BMC Public Health. 2019;19: 1–12. 10.1186/s12889-018-6343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bundy DAP, Medley GF. Immuno-epidemiology of human geohelminthiasis: Ecological and immunological determinants of worm burden. Parasitology. 1992;104: S105–S119. 10.1017/s0031182000075284 [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Lu L, Zhang L, Bai Y, Medina A, Rozelle S, et al. More poop, more precision: Improving epidemiologic surveillance of soil-transmitted helminths with multiple fecal sampling using the Kato-Katz technique. Am J Trop Med Hyg. 2017;97: 870–875. 10.4269/ajtmh.16-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosoma mansoni. Rev Inst Med Trop Sao Paulo. 1972;14: 397–400. [PubMed] [Google Scholar]
- 7.World Health Organization. Bench aids. 2019. Available from: https://www.who.int/intestinal_worms/resources/9789241515344/en/ [Google Scholar]
- 8.Coulibaly JT, Ouattara M, Becker SL, Lo NC, Keiser J, N’Goran EK, et al. Comparison of sensitivity and faecal egg counts of Mini-FLOTAC using fixed stool samples and Kato-Katz technique for the diagnosis of Schistosoma mansoni and soil-transmitted helminths. Acta Trop. 2016;164: 107–116. 10.1016/j.actatropica.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 9.Bärenbold O, Raso G, Coulibaly JT, N’Goran EK, Utzinger J, Vounatsou P. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl Trop Dis. 2017;11: 1–14. 10.1371/journal.pntd.0005953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inpankaew T, Schär F, Khieu V, Muth S, Dalsgaard A, Marti H, et al. Simple Fecal Flotation Is a Superior Alternative to Guadruple Kato Katz Smear Examination for the Detection of Hookworm Eggs in Human Stool. PLoS Negl Trop Dis. 2014;8: 8–13. 10.1371/journal.pntd.0003313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools P, Vlaminck J, Albonico M, Ame S, Ayana M, Antonio BPJ, et al. Diagnostic performance of a single and duplicate Kato-Katz, Mini-FLOTAC, FECPAKG2 and qPCR for the detection and quantification of soil-transmitted helminths in three endemic countries. PLoS Negl Trop Dis. 2019;13: 1–22. 10.1371/journal.pntd.0007446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements ACA, et al. Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity Of Intestinal Parasite Infections in a Controlled Clinical Trial. PLoS Negl Trop Dis. 2016;10: 1–19. 10.1371/journal.pntd.0004380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson A, Dennis J. Comparison of Kato–Katz Direct Smear and Sodium Nitrate Flotation for Detection of Geohelminth Infections. 2008;75: 339–341. 10.1654/4340.1 [DOI] [Google Scholar]
- 14.Hii SF, Senevirathna D, Llewellyn S, Inpankaew T, Odermatt P, Khieu V, et al. Development and evaluation of a multiplex quantitative real-time polymerase chain reaction for hookworm species in human stool. Am J Trop Med Hyg. 2018;99: 1186–1193. 10.4269/ajtmh.18-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin-Chung J, Pilotte N, Ercumen A, Grant JR, Maasch JRMA, Gonzalez AM, et al. Comparison of multi-parallel qPCR and double-slide Kato-Katz for detection of soil-transmitted helminth infection among children in rural Bangladesh. PLoS Negl Trop Dis. 2020;14: e0008087. 10.1371/journal.pntd.0008087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massetti L, Cantacessi C, Colella V, Zendejas PA, Ng-Nguyen D, Harriott L, et al. High-throughput multiplex qPCRs for the surveillance of zoonotic species of canine hookworms. PLoS Negl Trop Dis. 2020. 10.1371/journal.pntd.0008392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dryden MW, Payne PA, Ridley R, Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther. 2005;6: 15–28. [PubMed] [Google Scholar]
- 18.Khieu V, Muth S, Dalsgaard A, Inpankaew T, Scha F. Simple Fecal Flotation Is a Superior Alternative to Guadruple Kato Katz Smear Examination for the Detection of Hookworm Eggs in Human Stool. 2014;8: 1–7. 10.1371/journal.pntd.0003313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlaminck J, Cools P, Albonico M, Ame S, Ayana M, Cringoli G, et al. Therapeutic efficacy of albendazole against soil-transmitted helminthiasis in children measured by five diagnostic methods. PLoS Negl Trop Dis. 2019;13: 1–23. 10.1371/journal.pntd.0007471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaz Nery S, Qi J, Llewellyn S, Clarke NE, Traub R, Gray DJ, et al. Use of quantitative PCR to assess the efficacy of albendazole against Necator americanus and Ascaris spp. in Manufahi District, Timor-Leste. Parasites and Vectors. 2018;11: 1–7. 10.1186/s13071-017-2573-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pecson BM, Barrios JA, Johnson DR, Nelson KL. A real-time PCR method for quantifying viable Ascaris eggs using the first internally transcribed spacer region of ribosomal DNA. Appl Environ Microbiol. 2006;72: 7864–7872. 10.1128/AEM.01983-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phosuk I, Sanpool O, Thanchomnang T, Sadaow L, Rodpai R, Anamnart W, et al. Molecular identification of Trichuris suis and Trichuris trichiura eggs in human populations from Thailand, Lao PDR, and Myanmar. Am J Trop Med Hyg. 2018;98: 39–44. 10.4269/ajtmh.17-0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Helminth control in school age children: a guide for managers of control programmes - 2nd ed. WHO Libr Cat Data. 2011. Available from: https://apps.who.int/iris/bitstream/handle/10665/44671/9789241548267_eng.pdf?sequence=1 [Google Scholar]
- 24.Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RCA. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008;155: 67–73. 10.1016/j.vetpar.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 25.Wiria AE, Prasetyani MA, Hamid F, Wammes LJ, Lell B, Ariawan I, et al. Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study). BMC Infect Dis. 2010;10. 10.1186/1471-2334-10-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, et al. A laboratory-developed taqman array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51: 472–480. 10.1128/JCM.02658-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verweij JJ, Brienen EAT, Ziem J, Yelifari L, Polderman AM, Van Lieshout L. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum biforcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg. 2007;77: 685–690. 10.4269/ajtmh.2007.77.685 [DOI] [PubMed] [Google Scholar]
- 28.Sawitz W. The buoyancy of certain nematode eggs. 1976;62: 657–663. 10.2307/3272719 [DOI] [Google Scholar]
- 29.David ED, Lindquist WD. Determination of the Specific Gravity of Certain Helminth Eggs Using Sucrose Density Gradient Centrifugation Author (s): Erwin D. David and William D. Lindquist Published by: Allen Press on behalf of The American Society of Parasitologists Stable URL. J Parasitol. 1982;68: 916–919. 10.2307/3281005 [DOI] [PubMed] [Google Scholar]
- 30.O’Grady MR, Slocombe JOD. An investigation of variables in a fecal flotation technique. Can J Comp Med. 1980;44: 148–154. [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbaum L, Kwong LH, Ercumen A, Negash MS, Lovely AJ, Njenga SM, et al. Detecting and enumerating soil-transmitted helminth eggs in soil: New method development and results from field testing in Kenya and Bangladesh. PLoS Negl Trop Dis. 2017;11: 1–15. 10.1371/journal.pntd.0005522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cringoli G, Rinaldi L, Maurelli MP, Utzinger J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc. 2010;5: 503–515. 10.1038/nprot.2009.235 [DOI] [PubMed] [Google Scholar]
- 33.Lim MD, Brooker SJ, Belizario VY, Gay-Andrieu F, Gilleard J, Levecke B, et al. Diagnostic tools for soil-transmitted helminths control and elimination programs: A pathway for diagnostic product development. PLoS Negl Trop Dis. 2018;12: 1–18. 10.1371/journal.pntd.0006213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarafder MR, Carabin H, Joseph L, Balolong E, Olveda R, McGarvey ST. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a “gold standard.” Int J Parasitol. 2010. 10.1016/j.ijpara.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolay B, Brooker SJ, Pullan RL. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: A meta-analysis in the absence of a true gold standard. Int J Parasitol. 2014;44: 765–774. 10.1016/j.ijpara.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mejia R, Vicuña Y, Broncano N, Sandoval C, Vaca M, Chico M, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg. 2013;88: 1041–1047. 10.4269/ajtmh.12-0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Id KS, Clarke N, Awburn C V, Nery SV, Khieu V, Traub RJ, et al. Development and validation of a multiplexed- tandem qPCR tool for diagnostics of human soil-transmitted helminth infections. 2019; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knopp S, Salim N, Schindler T, Voules DAK, Rothen J, Lweno O, et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg. 2014;90: 535–545. 10.4269/ajtmh.13-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papaiakovou M, Gasser RB, Littlewood DTJ. Quantitative PCR-Based Diagnosis of Soil-Transmitted Helminth Infections: Faecal or Fickle? Trends Parasitol. 2019;35: 491–500. 10.1016/j.pt.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 40.Roeber F, Jex AR, Gasser RB. A real-time PCR assay for the diagnosis of gastrointestinal nematode infections of small ruminants. Methods Mol Biol. 2015;1247:145–52. 10.1007/978-1-4939-2004-4_10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.