Abstract
This report describes the direct synthesis of dihydro-pyrrolo-pyrazole heterocycles from allylic azides and methyl vinyl sulfone. The product results from a complex cascade reaction that is operationally straightforward, with aromatization being the result of a concomitant elimination step. A variety of azides could participate in this reaction (12 examples) and the isolated yields of the desired product ranged from 51%–72%. Lastly the ethylene sulfone group could be removed by heating the product in pyrrolidine.
Keywords: Azide, Vinyl sulfone, Pyrrolidine, Pyrazole, Cascade reaction
Graphical Abstract

Many pharmaceuticals, agrochemicals, and natural products contain one or more nitrogen heterocycles.[1,2] Both aromatic and saturated nitrogen heterocycles are prevalent in these societally important molecules. Thus, developing and manufacturing novel agents often requires efficient and robust synthetic access to differentially substituted N-heterocycles. While a plethora of synthetic routes are available for some of the most common N-heterocycles, more complex fused systems often lack expedient and direct access.
The dihydro-pyrrolo-pyrazole ring system is a fused N-heterocyclic framework featuring an aromatic pyrazole ring fused to a pyrrolidine (Fig. 1). This fused ring system is a key substructure in omarigliptin, which is an approved treatment for type 2 diabetes,[3] and danusertib, which entered clinical trials to treat chronic myelogenous leukemia (Fig. 1).[4,5] The dihydro-pyrrolo-pyrazole substructure also appears in a glycine transporter-1 inhibitor, a P21-activated kinase inhibitor, and elsewhere.[6,7] Versatile synthetic routes to dihydro-pyrrolo-pyrazole heterocycles are lacking, which is surprising in light of the stated utility. Current methods to synthesize dihydro-pyrrolo-pyrazoles are primarily based on carbonyl condensation with hydrazine (Scheme 1). Exposing 1,3-diketones or β-keto-nitriles to hydrazine can generate the pyrazole (Scheme 1a).[8] Other prefunctionalized ketones can condense with hydrazine (Scheme 1b).[3,8,9] Alternatively, an intramolecular dipolar cycloaddition is possible (Scheme 1c). [10,11] While effective, these approaches require preformation of the substrate. Ideally, there would be a divergent method to generate a wide variety of these heterocycles directly from simple starting materials.
Fig. 1.
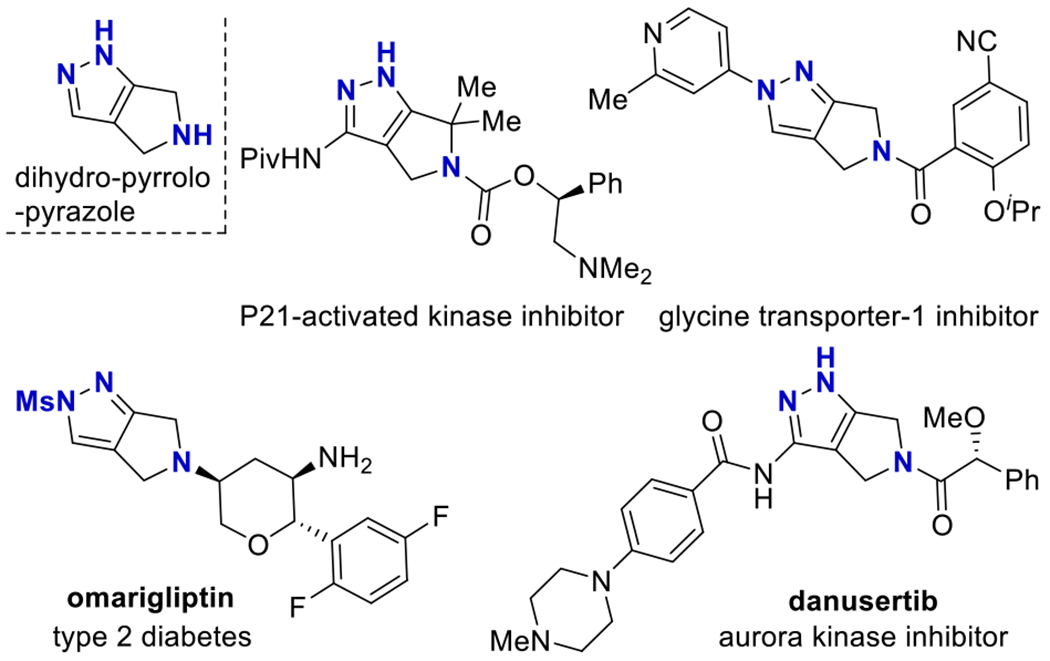
Select Biologically Active Molecules Containing a Dihydro-pyrrolo-pyrazole.
Scheme 1.

Prior Syntheses of Dihydro-pyrrolo-pyrazoles.
We envisioned accessing dihydro-pyrrolo-pyrazole heterocycles from allylic azides. Previously, we reported a cascade reaction between cinnamyl azides and commodity acrylates to form tetrahydro-pyrrolo-pyrazoles (Scheme 2).[12] The proposed mechanism for this cascade reaction begins with a (3 + 2)-cycloaddition between the azide and electron deficient alkene to generate triazoline 3.[13–19] This triazoline can equilibrate to the corresponding diazo species 4 and amine bases can facilitate this equilibrium. [18,20] Diazo 4 can undergo an intramolecular (3 + 2)-cycloaddition to generate tetrahydro-pyrrolo-pyrazole 5.[21,22] Tautomerization to the conjugated isomer (6) generated the product previously reported.[21,22] This reaction was remarkably effective at generating a diverse family of complex heterocycles from a variety of Michael acceptors.
Scheme 2.

Cascade Reaction with Allylic Azides.
Conceivably, by using a Michael acceptor that could subsequently eliminate, the cascade reaction could be extended a step further to aromatization (Scheme 2, step v). This would result in a dihydro-pyrrolo-pyrazole ring system (7). To explore this possibility, our previous reaction conditions were screened against a variety of Michael acceptors. Gratifyingly, when vinyl methyl sulfone was utilized, the reaction afforded the desired dihydro-pyrrolo-pyrazole. However, in the presence of excess methyl vinyl sulfone, the amine underwent a conjugate addition to generate compound 2. A detailed analysis of this reaction mixture also revealed that some of the triazoline had undergone premature elimination to generate the N-substituted 1,2,3-triazole (8).
The cascade reaction was subjected to a systematic optimization (Table 1). The reaction tolerated a range of solvents (entries 1–6), as might be expected for a cycloaddition sequence. Dioxane proved optimal for the dihydro-pyrrolo-pyrazole product. As previously mentioned, premature elimination to the triazole is one of the primary side products. Because of this, alternate amine bases were screened with the hope of slowing the rate of triazole formation. The use of TEA afforded the highest yield (Table 1).
Table 1.
Reaction Optimization with Methyl Vinyl Sulfone.
 | |||||
|---|---|---|---|---|---|
| entry | solvent | base | equiv MVS | 1aa(%) | 2aa(%) |
| 1 | C6H6 | TEA | 3 | n.d. | 65 |
| 2 | MeCN | TEA | 3 | n.d. | 58 |
| 3 | IPA | TEA | 3 | n.d. | 68 |
| 4 | MeOH | TEA | 3 | n.d. | 57 |
| 5 | DCE | TEA | 3 | n.d. | 54 |
| 6 | Dioxane | TEA | 3 | n.d. | 72 |
| 7 | Dioxane | DIPEA | 3 | n.d. | 28 |
| 8 | Dioxane | DIPA | 3 | n.d. | 64 |
| 9 | Dioxane | TEA | 1.2 | 52 | 32 |
| 10 | Dioxane | TEA | 2 | 30 | 57 |
| 11 | Dioxane | TEA | 4 | n.d. | 70 |
Reactions were conducted with azide 1b (70 μmol), methyl vinyl sulfone (84, 140, 210, or 280 μmol), and base (35 μmol) in solvent (0.2 M) at 70 °C for 48 h. Conversion and yield were determined by 1H NMR analysis with dibenzylether as the internal standard. Not determined = n.d. MVS = methyl vinyl sulfone.
The equivalents of methyl vinyl sulfone were reduced in an attempt to prevent the conjugate addition and isolate the free amine (Table 1, entries 9–10). Unfortunately, reducing the equivalents simply led to low conversion. The eliminated sulfinic acid could consume an equivalent of methyl vinyl sulfone, with the dimer being isolated as a side product (shown in header). This prevented reducing the equivalents of methyl vinyl sulfone below three equivalents. Conveniently, this side product is fairly insoluble and can be removed by filtration. Three equivalents of methyl vinyl sulfone in dioxane with TEA (entry 6) were taken as optimal.
Using the optimized conditions, the model substrate (1a) was isolated in 72% yield (Table 2). Ethyl vinyl sulfone and phenyl vinyl sulfone were also competent in the reaction (2b and 2c).
Table 2.
Scope of vinyl sulfones.
 |
Yields are reported for isolated and purified products. Yield values reflect the average of duplicate trials. See Supporting Information for details.
The cascade reaction was performed with a variety of cinnamyl azides (Table 3). The reaction can tolerate electron donating and electron withdrawing substituents on the arene (2d–2g). Also, the benzene ring could be replaced by a thiophene (2h), benzothiophene (2i), or furan (2j). Substrates with electron neutral arenes generally performed worse and were difficult to isolate (not shown). However, when substituents were added onto the azide carbon, the reaction proceeded well (2k–2l). Only substrates with an aryl group were examined in this reaction because alkyl allylic azides undergo the Winstein rearrangement.[23–25] This rearrangement generally causes the starting allylic azide to exist as a mixture of equilibrating isomers and complicates the reaction mixture. Installing an aryl group biases the equilibrium so that only one azide isomer is observed and provides some polarization to the alkene.
Table 3.
Scope of Cascade Reaction with Cinnamyl Azides.
 |
Yields are reported for isolated and purified products. See Supporting Information for details.
During the course of these studies, dynamic NMR behavior was frequently observed. This was attributed to the two different NH tautomers that are possible on the pyrazole ring. The tautomerization often resulted in significant broadening of the resonances in both the 1H and 13C NMR. This was true in many common NMR solvents including CDCl3, CD2Cl2, MeOD, DMSO, and MeCN. It was found that in AcOD, both tautomers were independently visible for some compounds. The optimal NMR solvent was substrate dependent, but a mixture of MeCN and AcOD frequently limited the broadening and easily dissolved the compounds. Compounds 2c and 2g were crystallized and analyzed by X-ray diffraction (Fig. 2). Interestingly, the diffraction pattern was most consistent with each being a different tautomer, which supports a dynamic equilibrium. The location of the NH has been arbitrarily drawn through this report.
Fig. 2.

Crystal structure of compound 2c and 2g.
The ethyl sulfone group attached to the pyrrolidine nitrogen could be useful in its own right or potentially viewed as a protecting group. This group was easily removed by simply heating the compound in pyrrolidine (Scheme 3). Presumably, this is a retroaza-Michael addition followed by an aza-Michael addition with excess pyrrolidine.
Scheme 3.

Removing the protecting group.
In conclusion a new cascade reaction has been developed to generate dihydro-pyrrolo-pyrazoles directly from cinnamyl azides and vinyl sulfones. The yields for this cascade reaction are modest, but acceptable given the large increase in molecular complexity and number of competing reaction pathways. Removal of the ethylene sulfone group can be easily accomplished.
Supplementary Material
Acknowledgements
En-chih Liu, Margaret Clapham, and Dr. Victor Young Jr. are thanked for their assistance with X-ray crystallography. This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM124718. We also acknowledge NIH Shared Instrumentation Grant #S10OD011952. A.S.C. gratefully acknowledges support from the Wayland E. Noland Fellowship and UMN Doctoral Dissertation Fellowship.
Footnotes
Appendix A.: Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tetlet.2021.152860.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Vitaku E, Smith DT, Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals, J. Med. Chem 57 (2014) 10257–10274. [DOI] [PubMed] [Google Scholar]
- [2].Joule JA, Natural Products Containing Nitrogen Heterocycles-Some Highlights 1990-2015. In Advances in Heterocyclic Chemistry; Elsevier Ltd, 2016; Vol. 119, pp 81–106. [Google Scholar]
- [3].Chung JYL, Scott JP, Anderson C, Bishop B, Bremeyer N, Cao Y, Chen Q, Dunn R, Kassim A, Lieberman D, Moment AJ, Sheen F, Zacuto M, Evolution of a Manufacturing Route to Omarigliptin, A Long-Acting DPP-4 Inhibitor for the Treatment of Type 2 Diabetes, Org. Process Res. Dev. 19 (2015) 1760–1768. [Google Scholar]
- [4].Fancelli D, Moll J, Varasi M, Bravo R, Artico R, Berta D, Bindi S, Cameron A, Candiani I, Cappella P, Carpinelli P, Croci W, Forte B, Giorgini ML, Klapwijk J, Marsiglio A, Pesenti E, Rocchetti M, Roletto F, Severino D, Soncini C, Storici P, Tonani R, Zugnoni P, Vianello P, 1,4,5,6-Tetrahydropyrrolo[3,4-c] Pyrazoles: Identification of a Potent Aurora Kinase Inhibitor with a Favorable Antitumor Kinase Inhibition Profile, J. Med. Chem 49 (2006) 7247–7251. [DOI] [PubMed] [Google Scholar]
- [5].Borthakur G, Dombret H, Schafhausen P, Brummendorf TH, Boisse N, Jabbour E, Mariani M, Capolongo L, Carpinelli P, Davite C, Kantarjian H, Cortes JE, A Phase I Study of Danusertib (PHA-739358) in Adult Patients with Accelerated or Blastic Phase Chronic Myeloid Leukemia and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Resistant or Intolerant to Imatinib and/or Other Second Generation C-, Haematologica 100 (2015) 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guo C, McAlpine I, Zhang J, Knighton DD, Kephart S, Johnson MC, Li H, Bouzida D, Yang A, Dong L, Marakovits J, Tikhe J, Richardson P, Guo LC, Kania R, Edwards MP, Kraynov E, Christensen J, Piraino J, Lee J, Dagostino E, Del-Carmen C, Deng YL, Smeal T, Murray BW, Discovery of Pyrroloaminopyrazoles as Novel PAK Inhibitors, J. Med. Chem 55 (2012) 4728–4739. [DOI] [PubMed] [Google Scholar]
- [7].Santora VJ, Almos TA, Barido R, Basinger J, Bellows CL, Bookser BC, Breitenbucher JG, Broadbent NJ, Cabebe C, Chai CK, Chen M, Chow S, Chung DM, Crickard L, Danks AM, Freestone GC, Gitnick D, Gupta V, Hoffmaster C, Hudson AR, Kaplan AP, Kennedy MR, Lee D, Limberis J, Ly K, Mak CC, Masatsugu B, Morse AC, Na J, Neul D, Nikpur J, Peters M, Petroski RE, Renick J, Sebring K, Sevidal S, Tabatabaei A, Wen J, Yan Y, Yoder ZW, Zook D, Design and Synthesis of Novel and Selective Glycine Transporter-1 (GlyT1) Inhibitors with Memory Enhancing Properties, J. Med. Chem 61 (2018) 6018–6033. [DOI] [PubMed] [Google Scholar]
- [8].Deaton DN, Haffner CD, Henke BR, Jeune MR, Shearer BG, Stewart EL, Stuart JD, Ulrich JC, 2,4-Diamino-8-Quinazoline Carboxamides as Novel, Potent Inhibitors of the NAD Hydrolyzing Enzyme CD38: Exploration of the 2-Position Structure-Activity Relationships, Bioorganic Med. Chem 26 (2018) 2107–2150. [DOI] [PubMed] [Google Scholar]
- [9].Fukui H, Inoguchi K, Nakano J, Synthesis of the Bicyclic Secondary Amines via Dimethylaminomethylene Ketones from 3-Pyrrolidone and 4-Piperidone, Heterocycles 56 (2002) 257–264. [Google Scholar]
- [10].Quiclet-Sire B, Zard SZ, Observations on the Reaction of Hydrazones with Iodine: Interception of the Diazo Intermediates, Chem. Commun (17) (2006) 1831, 10.1039/b602580c. [DOI] [PubMed] [Google Scholar]
- [11].Winters MP, Teleha CA, Sui Z, Synthesis of Substituted 2,4,5,6-Tetrahydrocyclopenta[c]Pyrazoles and 2,4,5,6-Tetrahydropyrrolo[3,4-c] Pyrazoles by Intramolecular Nitrilimine Cycloaddition, Tetrahedron Lett. 55 (2014)2150–2153. [Google Scholar]
- [12].Carlson AS, Liu E-C, Topczewski JJ, A Cascade Reaction of Cinnamyl Azides with Acrylates Directly Generates Tetrahydro-Pyrrolo-Pyrazole Heterocycles, J. Org. Chem 85 (2020) 6044–6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huisgen R Proceedings of the Chemical Society. October 1961. Proc. Chem. Soc 1961, 357–396. [Google Scholar]
- [14].Huisgen R, Szeimies G, Mӧbius L, 1.3-Dipolar Cycloadditions. XXXII. Kinetics of the Addition of Organic Azides to Carbon-Carbon Multiple Bonds, Chem. Ber 100 (1967) 2494–2507. [Google Scholar]
- [15].Liddon JTR, Lindsay-Scott PJ, Robertson J, Secondary Products from Intramolecular Cycloadditions of Azidoalkyl Enol Ethers and Azidoalkyl Vinyl Bromides: 1-Azadienes, Their Reactions with Diphenylketene, and Radical Cyclizations To Form Bi- and Tricyclic Lactams, J. Org. Chem 84 (2019) 13780–13793. [DOI] [PubMed] [Google Scholar]
- [16].Xie S, Lopez SA, Ramstrӧm O, Yan M, Houk KN, 1,3-Dipolar Cycloaddition Reactivities of Perfluorinated Aryl Azides with Enamines and Strained Dipolarophiles, J. Am. Chem. Soc 137 (2015) 2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krivopalov VP, Shkurko OP, 1,2,3-Triazole and Its Derivatives. Development of Methods for the Formation of the Triazole Ring, Russ. Chem. Rev 74 (2005) 339–379. [Google Scholar]
- [18].Broeckx W, Overbergh N, Samyn C, Smets G, L’abbé G, Cycloaddition Reactions of Azides with Electron-Poor Olefins, Tetrahedron 27 (1971)3527–3534. [Google Scholar]
- [19].L’abbé G, Decomposition and Addition Reactions of Organic Azides, Chem. Rev 69 (1969) 345–363. [Google Scholar]
- [20].Herdeis Claus, Schiffer Thomas, Synthesis of Nonracemic 2,3,6-Trisubstituted Piperidine Derivatives from Sugar Lactones via Tandem Wittig [2+3] Cycloaddition Reaction. A Novel Entry to Prosopis and Cassia Alkaloids, Tetrahedron 55 (4) (1999) 1043–1056. [Google Scholar]
- [21].Yang C, Shen H, One Pot Multiple-Steps Reactions of Allyl Azide and Alkenes Carrying Electron-Withdrawing Groups, Tetrahedron Lett. 34 (1993) 4051–4054. [Google Scholar]
- [22].Yang C-H, Shen H-J, Wang R-H, Wang J-C, 2,3,7-Triazabicyclo[3.3.0] Octenes Prepared by Tandem Cascade Reaction of Allyl Azides and Olefinic Dipolarophiles, J. Chinese Chem. Soc 49 (2002) 95–102. [Google Scholar]
- [23].Gagneux A, Winstein S, Young WG, Rearrangement of Allyl Azides, J. Am. Chem. Soc 82 (1960) 5956–5957. [Google Scholar]
- [24].Ott AA, Topczewski JJ, On the Winstein Rearrangement: Equilibrium and Mechanism, ARKIVOC vi (2019) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carlson AS, Topczewski JJ, Allylic Azides: Synthesis, Reactivity, and the Winstein Rearrangement, Org. Biomol. Chem 17 (2019) 4406–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


