Abstract
Background
Positive and negative effects on bone mineral density (BMD) have been described as a result of the premenopausal use of oral contraceptives (OCs); increased fracture rates have also been reported. This study assessed the relation between OC use and BMD in a population-based, 9-centre, national sample of women aged 25–45 years.
Methods
Premenopausal women who had been enrolled in the Canadian Multicentre Osteoporosis Study were classified as having ever been OC users (≥ 3 months) or as having never been OC users (0 to < 3 months). Data were obtained through extensive questionnaires and measuring of participants' weight, height and the BMD of lumbar vertebrae and the proximal femur.
Results
Of the sample of 524 women, whose mean age was 36.3 (standard deviation [SD] 5.9) years, 454 had used OCs; their mean age when they started using OCs was 19.8 (SD 3.5) years and the mean duration of use was 6.8 (SD 4.8) years. Women who had ever and those who had never used OCs showed no differences in age, age at menarche, parity, current calcium intake, exercise, body mass index (BMI), education, past irregular cycles or amenorrhea. OC users reported more alcohol and cigarette use and more use of medications to create regular cycles. Mean BMD values (adjusted for age, BMI and height) were 0.02–0.04 g/cm2 (that is, 2.3%–3.7%) lower in OC users, and were significantly lower in the spine and trochanter. The BMD of the spine in OC users was 1.03 (SD 0.12) g/cm2 >versus 1.07 (SD 0.12) g/cm2 >(95% confidence interval [CI] of difference –0.07 to –0.001) in those who had never used OCs. BMD was neither related to the duration of OC use nor to gynecological age at first use. Current and past users had similar BMD values.
Interpretation
National, population-based data show lower BMD values for the trochanter and spine in premenopausal women who have used OCs compared with those who have never used OCs.
The way in which oral contraceptive (OC) use, premenopausal bone mineral density (BMD) and risk of osteoporotic fracture are related remains controversial. For example, controversy exists about whether or not OC use is positive for bone. Retrospective studies of past use in menopausal women1,2 and prospective studies in perimenopausal women suggest that OC use is beneficial for bone.3,4 Reviews of the effects of OCs on BMD are universally positive,5,6 however, relatively few cross-sectional studies of oral contraceptive use and bone density have focused on premenopausal women. Studies by Fogelman and colleagues of 2 separate, selected populations of Caucasian women (57 retired professional dancers and 102 nulliparous women respectively) detected no effect of OC use or duration of use on dual-energy x-ray absorptiometry (DXA) measurements of the BMD of the lumbar spine or the proximal femur.7,8 Likewise, a study by Lloyd and colleagues of 25 premenopausal women, 14 of whom had used OCs for more than 5 years and 11 of whom had never used OCs, showed no cross-sectional difference in cancellous vertebral BMD by quantitative CT.9 The study by Lindsay and colleagues,2 however, of 57 premenopausal women showed significantly higher BMD by dual photon absorptiometry in past users of OCs. A larger study in a selected population of 186 premenopausal Finnish women showed a small positive correlation between OC use and age-adjusted DXA measurements of the BMD of the femoral neck (r = 0.189, p = 0.05).10
Only a few prospective studies of BMD in premenopausal women have assessed OC use. One study showed no difference in 3-year BMD change between users and nonusers.11 Another study showed a positive effect on BMD change in premenopausal women in their mid-twenties who were described as “normally menstruating” based on experiencing 6 or more menstrual cycles per year.12 However, prospective studies that lasted 5 years and one year respectively in premenopausal women in their late teens or early twenties found that OC use interferes with achievement of peak bone mass.13,14 Similar results were reported from a large, 2-year randomized controlled study in late-adolescent monkeys.15 Furthermore, 2 very large, prospective epidemiological studies of fracture in premenopausal women showed a higher risk ratio for incident fracture in women who had ever used OCs compared with those who had never used OCs.16,17 In the Royal College General Practitioners Oral Contraception Study, OC users experienced a 20% increase in relative risk of fracture (1.20, 95% confidence interval [CI] 1.08–1.34).16 A similar relative risk of fracture (1.3, 95% CI 1.1–1.5) was reported in the Oxford Family Planning Association Contraceptive Study; this fracture risk increased with longer duration of OC use.17
The primary purpose of this study was to determine whether there were differences in BMD between premenopausal women aged 25–45 years who had ever taken OCs and those who had never used OCs.
Methods
The present study used data from all women enrolled in the Canadian Multicentre Osteoporosis Study (CaMOS) aged 25–45 years who had not undergone a bilateral ovariectomy. Briefly, the objectives of CaMOS, which has been described in detail elsewhere,18 were to ascertain the number of prevalent and incident fractures, obtain clinical measures of BMD and describe the distribution of proposed risk factors for osteoporosis in a population-based sample of 9423 noninstitutionalized adult Canadian women and men. CaMOS is both a cross-sectional survey and a longitudinal cohort study. The present study used data from the baseline evaluation collected in 1995–1997.
A stratified random sampling technique was employed to recruit men and women aged 25 years or more in 7 geographic regions across Canada. The sampling frame for recruitment consisted of all residential telephone subscribers in postal code areas within a 50-km radius of 9 urban centres located in 7 Canadian provinces. Based on data from partial participants whose age and sex were known, a participation rate of about 63% was achieved for premenopausal women. All participants gave signed informed consent. This study was approved by the ethics review boards at McGill University and at the universities affiliated with each of the 9 centres.
Data collected via an interviewer-administered questionnaire (CaMOS Questionnaire 1995) included sociodemographic, lifestyle and medical history information. Reproductive history included age at menarche, number of pregnancies, parity, characteristics of initial menstrual flow (regular or irregular), the occurrence and duration of absence of menstrual flow, and whether the woman had been treated to cause her periods to become regular. Information about exercise within the last year (strenuous sports, vigorous work and moderate activity) reported as kilojoules per week was obtained, as well as information about exercise in comparison with peers during childhood, the teen years and at the age of 30 years (scored from 1 to 5 for much less to much more than peers). A food frequency questionnaire concerning calcium-rich food servings consumed, plus current calcium and vitamin supplements, was used to assess calcium intake (in mg per day) and whether or not vitamin D supplements were taken. The questionnaire documented the use of cigarettes in numbers of 20-unit packs per lifetime and the use of alcohol in drinks per week during the past year.
Oral contraceptive use was documented by the age at which a woman initiated use, her gynecological age at that time, her age at discontinuing use and, if currently using OCs, the brand name. Gynecological age at first use was calculated as the age at which OC use was started minus age at menarche. Following criteria used in epidemiological studies of hormonal effects from OC use,19 this study considered women who had used OCs for 3 or more months to be OC ever users and those who had never used OCs (or had used OCs for < 3 months) to be OC never users.
Clinical assessments included measurement of height, weight (summarized as body mass index [BMI], calculated as mass [kg] divided by height [m]2) and BMD by DXA of the lumbar vertebrae (L1–4) and the proximal femur regions of the femoral neck, trochanter and Ward's area. A European spine phantom20 was measured systematically at least once per year in each centre, which allowed researchers to assess the linearity of data from all the centres and its adjustment to a common reference. Seven centres used Hologic instruments and 2 used Lunar instruments; all data were converted to Hologic values.21 Because no methodology has been developed to convert Lunar data for the proximal femur trochanter and Ward's area into Hologic values, comparable values at these sites were available for participants in only 7 of the 9 centres.
Statistical analysis
The primary analysis of this study compared women who had ever used OCs with those who had never used OCs. Descriptive statistics are presented as means and standard deviations (SDs) for continuous variables and as proportions for categorical variables. A Pearson correlation matrix of all independent variables was constructed to assess possible colinearities. Logistic regression models were fitted to assess whether OC use was associated with study centre or with other potentially confounding variables (age, height and BMI) or with alcohol and cigarette use and weight- cycling behaviour. Differences in BMD levels were determined using 95% confidence intervals.
Results
A total of 524 women met the criteria for inclusion; their mean age was 36.3 (SD = 5.9) years. Eighty-seven percent of women (n = 454) reported having ever used OCs, including 17% who were current users (n = 90) and 70% who were past users (n = 364). The percentage of ever users was 89.9 in the 25–29-year age group and 85.5%, 87.2% and 85.2% in the 30–34-year, 35–39-year and 40–45-year age groups respectively. In this population, 13.4% of women (n = 70) reported never having used OCs. The percentages of women who had never used OCs, however, tended to be lower in the 25–29-year age stratum (at 10.1%) than in the 40–45-year age stratum (at 14.8%). There were no clinically important differences in OC use by region or centre.
The mean duration of OC use was 6.8 (SD 4.8) years, with a median duration of 6.0 (interquartile range 2.5–10.0, maximum 28.0) years. Twenty-nine percent of women who used OCs (n = 130) had been doing so for 10 or more years. The mean age at which OC use was started was 19.9 (SD 3.5) years. Gynecological age when starting OCs was 7.2 (SD 3.8) years. Women who had used OCs longer tended to have started at a younger gynecological age.
Women who had ever used OCs did not generally differ from those who had never used OCs (Table 1). The groups were similar in terms of age at menarche, calcium intake, vitamin D supplementation (data not shown), BMI and years of education. However, women who were current and past OC users reported greater consumption of alcohol, and past users and those who had ever used OCs reported more pack-years of smoking than those who had never used OCs. Current OC users, as expected, were younger and had lower parity than past users and those who had never used OCs. Current levels of exercise did not differ between the groups of women who had ever or never used OCs.
Table 1
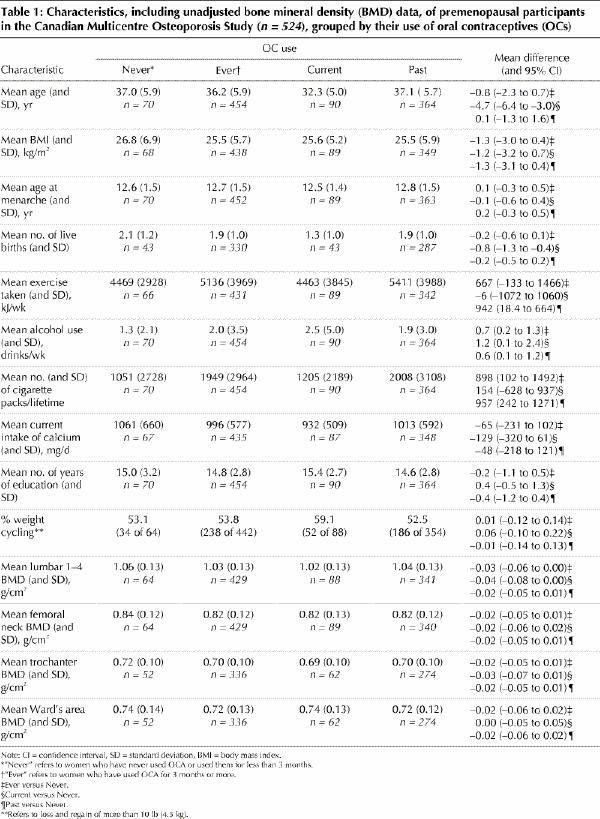
Women who had ever used OCs had numerically lower mean BMD values at every measurement site compared with those who had never used OCs. The unadjusted data are shown in Table 1. After adjustment for the important variables relating to BMD (age, BMI, and height), differences were important at the lumbar spine and trochanter BMD sites (Fig. 1). BMD values in the lumbar spine were 1.03 (SD 0.12) g/cm2 >versus 1.07 (SD 0.12) g/cm2 for OC users and women who had never used OCs respectively and 0.70 (SD 0.09) g/cm2 >versus 0.72 (0.09) g/cm2 respectively at the trochanter. Point estimates of mean BMD values from the femoral neck and Ward's areas showed differences in a similar direction that were not statistically significant.
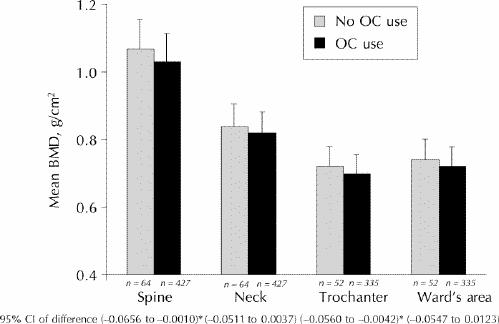
Fig. 1: Bone mineral density (BMD) of premenopausal women adjusted for age, body mass index (BMI) and height at each BMD measurement site. OC use refers to women who have used oral contraceptives (OCs) for 3 months or more, and no OC use refers to women who have never used OCs or have used OCs for less than 3 months. *These confidence intervals are statistically significant.
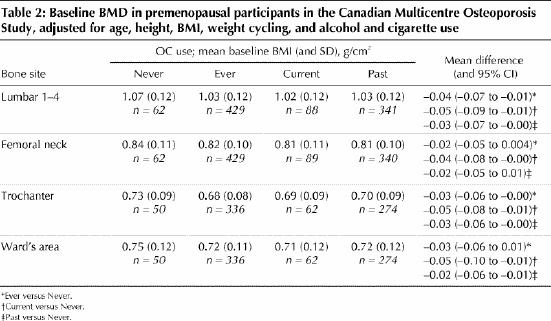
Because lifestyle habits differed between the ever and never users of OCs, the BMD data were further adjusted for alcohol and cigarette use, although neither was significantly related to BMD in this data set. In addition, a history of “weight cycling” (loss and regain of more than 10 lb [4.5 kg]), although it did not differ by OC use, was related to BMD. Data adjusted for age, BMI, height, weight cycling, alcohol and cigarette use are shown in Table 2. BMD remained lower in women who had ever used OCs compared with never users and became significantly lower in current versus never users at the femoral neck and Ward's areas of the proximal femur.
Table 2
Young age or reproductive immaturity when starting OCs13,15 might explain the lower BMD values found in users of OCs. Therefore, the relation between BMD and gynecological age at first OC use was examined and found to have no effect. Furthermore, there was no effect of duration of OC use on BMD. There was also no interaction between exercise and the effects of OC use on BMD, in contrast to previous suggestions.22,23
We postulated that the lower BMD in OC users might be related to different reproductive histories in the 2 groups but found no differences in the regularity of the menstrual cycle after menarche, experience of oligomenorrhea or amenorrhea, and no effect of these variables on BMD. The only potentially important menstruation-related difference between women who had ever used OCs and those who had never used OCs was that more OC users reported taking some medication to make their menstrual cycles regular. Spine BMD (adjusted for age, BMI, height and OC use) was 1.08 (SD 0.12) g/cm2 in those reporting medication use (commonly OCs) to create regular cycles versus 1.04 (SD 0.12) g/cm2 in those who did not take such medication (95% CI of difference –0.01 to 0.09).
Finally, if the menstrual cycle or ovulatory changes on discontinuation of OCs were related to the lower BMD at the spine and trochanter in women who had ever used OCs, past users might be expected to have lower BMD levels than current users. That postulate was not supported: past and current OC users did not differ in BMD at any site.
Interpretation
Oral contraceptive use in this population-based national sample of premenopausal women was associated with lower BMD measurements in the lumbar vertebrae and trochanter, and numerically lower levels were consistent across all measurement sites. The difference is large enough between groups to be clinically important (adjusted differences of 2.4% at the femoral neck to 4.3% at the trochanter). If a 1% increase in BMD is related to a 7% decrease in vertebral fractures (as controlled trial data of antiresorptive therapies in menopausal women suggest24), the lower BMD values in OC users could translate into increased fracture risks of the order of 20%–30%. Furthermore, these observations provide an explanation for the higher fracture rates in 2 large epidemiological studies of premenopausal OC users compared with those who had never used OC.16,17 These negative BMD effects are also congruent with the results of 2 prospective studies in young women.13,14
The observed association between OC use and lower BMD does not appear to arise from features unique to this national premenopausal population. The proportion of premenopausal Canadian women who had used OCs for 3 or more months (87%) is similar to the 86% use reported in a recent study from California and Washington.25 The 17% current use of OCs is also similar to other populations, namely, 18.5% in Seattle's Group Health Cooperative,26 19% in the New England states27 and 23% in France,28 and identical to that in a previous population-based study from Calgary.29 The 7-year mean duration of OC use in Canadian premenopausal women is also similar to that reported elsewhere.28,30
The results of this study confirm those of other studies in showing that premenopausal women who ever or never use OCs differ regarding some lifestyle variables. More cigarette use25 or more cigarette and alcohol use combined have been reported in women who have ever used OCs.31 In another study, women who had ever used OCs not only smoked more cigarettes but also had lower BMI values and higher educational and income levels than nonusers.27
Reviews that cite positive effects of OC use on bone health32,33,34,35,36,37,38 are probably based on evidence that combined estrogen and progestin therapy is positive for BMD in menopausal women. The largest studies of OC use and bone have been retrospective studies of menopausal cohorts. These data on OC use and BMD in menopausal women are confounded, because OC use is a predictor of menopausal ovarian hormone therapy,39 which, in turn, is associated with higher BMD values.
The reasons for lower BMD values in premenopausal women who have ever used OCs are currently unclear. These data did not confirm that women using OCs had more menstrual cycle disturbances than those who had never used OCs. There also was no significant relation between BMD and young gynecological age when starting OCs. Because abnormal menstrual cycles are commonly treated by OC use, it is possible that a higher percentage of women using OCs would report that they had taken some kind of medication to make their cycles regular. Eighty-eight percent of current and 58% of past OC users compared with 13% of women who had never used OCs gave this history (95% CI of difference between ever and never users 0.35–0.72). The robustness of this finding is questionable, however, because the group who had never used OCs was small. Furthermore, this history did not significantly relate to BMD at any site.
Two further hypotheses cannot be tested using these data. One is that in OC users the week-long withdrawal from high-dose steroids every month triggers increased rates of bone resorption similar to those documented within one week following premenopausal ovariectomy.40 The other postulate is that, on discontinuing OC use, the 6–12 months needed for the recovery of fertility41 are a time of ovulatory disturbances that have been associated with accelerated bone loss.42 If the latter were the case, current OC users should have higher BMD values than past users; these data do not show this.
This study suggests an important negative relation between OC use and BMD in cross-sectional data from over 500 premenopausal women who are part of a national, population-based sample. Furthermore, there was little difference between the 2 groups who had ever versus never used OCs, and extensive questionnaire data allowed adjustment for many potential covariates or confounders. This study revealed no effect of the duration of OC use on BMD: that fact makes a direct pharmacological effect of OC use on BMD less likely. Finally, it is possible that unmeasured environmental or other variables accounted for the BMD differences documented between women who had ever or never used OCs.
The Canadian Multicentre Osteoporosis Study, a 5-year prospective study, is currently ascertaining rates of BMD change and incident fractures and is documenting OC use. The 5-year questionnaire will also determine the reason for first OC use; that will allow differentiation of contraceptive users from those using OCs as therapy for gynecological problems. Eventually, the results of this cross-sectional study may be confirmed in prospective studies of the relations among OC use, BMD change and incident fracture.
Acknowledgments
We are grateful to all those who participated in this study and to Health Canada for a contract (no. 213304-02901-4494-257049) that partially funded the BC Centre Coordinator, Yvette Vigna, and allowed continuation of the BC Centre. Lawrence Joseph is supported by a Senior Investigator Award from the Canadian Institutes of Health Research.
Footnotes
Additional members of the CaMOS Research Group are listed in the Acknowledgements section.
This article has been peer reviewed.
The Canadian Multicentre Osteoporosis Study was funded by the Seniors' Independence Research Programme (through the National Health and Development Research Programme of Health Canada [Project no. 6605-4003-OS]), the Medical Research Council of Canada (now the Canadian Institutes of Health Research), MRC-PMAC Health Program, Merck Frosst Canada Inc., Eli Lilly Canada Inc., Procter and Gamble Pharmaceuticals Canada Inc. and the Dairy Farmers of Canada.
We also thank other CaMOS Centre Directors: Dr. T. Anastassiades, Queen's University, Kingston, Ont.; Dr. J. P. Brown, Laval University, Ste-Foy, Que.; Dr. W. Olszynski, Saskatoon Osteoporosis Centre, Saskatoon, Sask.; and Dr. C. Joyce, Memorial University, St. John's, Nfld. We appreciate the work of Suzette Poliquin (National Coordinator), S. Godmaire and Andrew Kmetic of the Montreal coordinating centre. We thank Centre Coordinators J. Allan, B. Stanfield, Laura Pickard, Barbara Matthews, Evelyne Lejeune, Barbara Gardner-Bray, Jola Kedra and Minnie Parsons and other essential people: Loralee Robertson in the Quality Assurance centre in Edmonton, Lori-Ann Larmand who worked with Dr. Kreiger and Sharon V. Cox who worked with Dr. Prior.
Finally, thank you to Dr. Lucy Williams (volunteer research fellow from Australia), and Dr. Moira A. Petit who enthusiastically reviewed early drafts, and to other University of British Columbia colleagues, Dr. Susan I. Barr, Dr. Brian C. Lentle, and Dr. Christine Hitchcock and Dr. Heather McKay for their support.
Competing interests: None declared.
Correspondence to: Dr. Jerilynn C. Prior, Professor of Endocrinology and Metabolism, Department of Medicine, University of British Columbia, Suite 380, 575 West 8th Ave., Vancouver BC V5Z 1C6; fax 604 875-5915; jprior@vanhosp.bc.ca
References
- 1.Kritz-Silverstein D, Barrett-Connor E. Bone mineral density in postmenopausal women as determined by prior oral contraceptive use. Am J Public Health 1993;83:100-02. [DOI] [PMC free article] [PubMed]
- 2.Lindsay R, Tohme JF, Kanders B. The effect of oral contraceptive use on vertebral bone mass in pre- and post-menopausal women. Contraception 1986; 34:333-40. [DOI] [PubMed]
- 3.Tuppurainen M, Kroger H, Saarikoski S, Honkanen R, Alhava E. The effect of previous oral contraceptive use on bone mineral density in perimenopausal women. Osteoporos Int 1994;4:93-8. [DOI] [PubMed]
- 4.Gambacciani M, Ciaponi M, Cappagli B, Benussi C, Genazzani AR. Longitudinal evaluation of perimenopausal femoral bone loss: effects of a low-dose oral contraceptive preparation on bone mineral density and metabolism. Osteoporos Int 2000;11:548 [DOI] [PubMed]
- 5.Corson SL. Oral contraceptives for the prevention of osteoporosis. J Reprod Med 1993;38:1015-21. [PubMed]
- 6.Upton GV. Contraception for the perimenopausal patient. Obstet Gynecol Clin North Am 1987;14(1):207-27. [PubMed]
- 7.Keay N, Fogelman I, Blake G. Bone mineral density in professional female dancers. Br J Sports Med 1997;31:143-7. [DOI] [PMC free article] [PubMed]
- 8.Rodin A, Chapman M, Fogelman I. Bone density in users of combined oral contraception. Br J Fam Plann 1991;16:125-9.
- 9.Lloyd T, Buchanan JR, Ursino GR, Myers C, Woodward G, Halbert DR. Long-term oral contraceptive use does not affect trabecular bone density. Am J Obstet Gynecol 1989;160(2):402-4. [DOI] [PubMed]
- 10.Laitinen K, Valimaki M, Keto P. Bone mineral density measured by dual energy x-ray absorptiometry in healthy Finnish women. Calcif Tissue Int 1991 ; 48:224-31. [DOI] [PubMed]
- 11.Mazess RB, Barden HS. Bone density in premenopausal women: effects of age, dietary intake, physical activity, smoking, and birth-control pills. Am J Clin Nutr 1991;53(1):132-42. [DOI] [PubMed]
- 12.Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992;268:2403-8. [PubMed]
- 13.Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception 1995;51:221-4. [DOI] [PubMed]
- 14.Drake III AJ, Armstrong DW, McDevitt ER, Phull A, Shakir RMM. Bone mineral density and menstrual status in U.S. Naval Academy female midshipmen [abstract]. Program Abstr Endocr Soc Annu Meet 1996;P3-799.
- 15.Register TC, Jayo MJ, Jerome CP. Oral contraceptive treatment inhibits the normal acquisition of bone mineral in skeletally immature young adult female monkeys. Osteoporos Int 1997;7:348-53. [DOI] [PubMed]
- 16.Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women: a prospective study. Bone 1993;14(1):41-5. [DOI] [PubMed]
- 17.Vessey M, Mant J, Painter R. Oral contraception and other factors in relation to hospital referral for fracture — findings in a large cohort study. Contraception 1998;57:231-5. [DOI] [PubMed]
- 18.Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JB, et al. The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging 1999;18:376-87.
- 19.The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987;316(1):650-5. [DOI] [PubMed]
- 20.Pearson J, Dequeker J, Henley M, Bright J, Reeve J, Kalender W, et al. European semi-anthropomorphic spine phantom for the calibration of bone densitometers: assessment of precision, stability and accuracy. The European Quantitation of Osteoporosis Study Group. Osteoporosis Int 1995;5(3):174-84. [DOI] [PubMed]
- 21.Genant HK, Grampp S, Gluer CC, Faulkner KG, Jergas M, Engelke K, et al. Universal standardization for dual-x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 1994;9(10):1503-14. [DOI] [PubMed]
- 22.Hartard M, Bottermann P, Bartenstein P, Jeschke D, Schwaiger M. Effects on bone mineral density of low-dosed oral contraceptives compared to and combined with physical activity. Contraception 1997;55:87-90. [DOI] [PubMed]
- 23.Burr DB, Yoshikawa T, Teegarden D, McCabe G, McCabe L, Weaver C. Exercise and oral contraceptives suppress the normal age-related increase in bone mass and strength in the femoral neck of young women 18–31 years old. Bone 2000;27:855-63. [DOI] [PubMed]
- 24.Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000;85(1):231-6. [DOI] [PubMed]
- 25.Sidney S, Siscovick DS, Petitti DB, Schwartz SM, Quesenberry CP, Psaty BM, et al. Myocardial infarction and use of low-dose oral contraceptives. Circulation 1998;98(11):1058-63. [DOI] [PubMed]
- 26.Porter JB, Jick H, Walker AM. Mortality among oral contraceptive users. Obstet Gynecol 1987;70:29-32. [PubMed]
- 27.Hume AL, Barbour MM, Lapane KL, Flint PM, Carleton RA. Correlates of oral contraceptive use in two New England Communities: 1981–1993. Pharmacotherapy 1996;16:1172-8. [PubMed]
- 28.Garnero P, Sornay-Rendu E, Delmas PD. Decreased bone turnover in oral contraceptive users. Bone 1995;16:499-503. [DOI] [PubMed]
- 29.Ramcharan S, Love EJ, Frick GH, Goldfien A. The epidemiology of premenstrual symptoms in a population-based sample of 2,650 urban women: attributable risk and risk factors. J Clin Epidemiol 992;45:377-92. [DOI] [PubMed]
- 30.Beral V, Hermon C, Kay C, Hannaford P, Darby S, Reeves G. Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners' oral contraceptive study. BMJ 1999;318(7176):96-100. [DOI] [PMC free article] [PubMed]
- 31.Romieu I, Willett WC, Colditz GA, Stampfer MJ, Rosner B, Hennekens CH, et al. Prospective study of oral contraceptive use and risk of breast cancer in women. J Natl Cancer Inst 1989;81(17):1313-21. [DOI] [PubMed]
- 32.Burkman RT. Non-contraceptive effects of hormonal contraceptives: bone mass, sexually transmitted disease and pelvic inflammatory disease, cardiovascular disease, menstrual function and future fertility. Am J Obstet Gynecol 1994; 170: 1569-75. [DOI] [PubMed]
- 33.Mehta S. Bone loss, contraception and lactation. Acta Obstet Gynecol Scand 1993;72:148-56. [DOI] [PubMed]
- 34.Speroff L. Hormonal contraception. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive endocrinology, surgery and technology. Philadelphia: Lippincott-Raven Publishers; 1996. p. 1683-708.
- 35.Shaaban MM. The perimenopause and contraception. Maturitas 1996;23:181-92. [DOI] [PubMed]
- 36.Volpe A, Silferi M, Genazzani AD, Genazzani AR. Contraception in older women. Contraception 1993;47:229-39. [DOI] [PubMed]
- 37.Connell EB. Rational use of oral contraceptives in the perimenopausal woman. J Reprod Med 1993;38:1036-40. [PubMed]
- 38.Van Winter JT, Bernard ME. Oral contraceptive use during the perimenopausal years. Am Fam Physician 1998;58(6):1373-5. [PubMed]
- 39.Johannes CB, Crawford SL, Posner JG, McKinlay SM. Longitudinal patterns and correlates of hormone replacement therapy use in middle-aged women. Am J Epidemiol 1994;140:439-52. [DOI] [PubMed]
- 40.Prior JC, Vigna YM, Wark JD, Eyre DR, Lentle BC, Li DK, et al. Premenopausal ovariectomy-related bone loss: a randomized, double-blind one-year trial of conjugated estrogen or medroxyprogesterone acetate. J Bone Miner Res 1997;12(11):1851-63. [DOI] [PubMed]
- 41.Bracken MB, Hellenbrand KG, Holford TR. Conception delay after oral contraceptive use: the effect of estrogen dose. Fertil Steril 1990;53:21-7. [PubMed]
- 42.Prior JC, Vigna YM, Schechter MT, Burgess AE. Spinal bone loss and ovulatory disturbances. N Engl J Med 1990;323:1221-27. [DOI] [PubMed]


