Abstract
During extensive studies on π-allylpalladium chemistry, we have developed classical β-keto ester and malonate chemistry to a new generation by discovering a variety of palladium-catalyzed reactions of their allylic esters. Palladium enolates are generated from allyl β-keto esters after decarboxylation and undergo the following transformations; a) reductive elimination to provide α-allyl ketones, b) elimination of β-hydrogen to give α, β-unsaturated ketones, c) formation of α-methylene ketones, d) hydrogenolysis to give ketones, e) aldol condensation, and f) Michael addition. Allyl malonates and cyanoacetes undergo similar reactions. Results of these studies, including several applications carried out by other researchers are summarized.
Keywords: Carroll rearrangement, palladium enolates, palladium-catalyzed aldol condensation, palladium- catalyzed Michael addition
Introduction
Palladium is now regarded as the most versatile metal among a number of transition metals used for organic synthesis.1) We started research on organopalladium chemistry in early 1960s, and discovered carbon-carbon bond formation by using palladium complexes for the first time. Since then we have carried out extensive studies on organopalladium. One topic of these studies is palladium-catalyzed reaction of allylic compounds via π-allylpalladium complexes. Among a number of new catalytic reactions discovered in this area, synthetically useful catalytic reactions of allyl β- keto carboxylates and malonates via palladium enolates are summarized in this review.
The first palladium-mediated carbon-carbon bond formation
At first we investigated the possibility of carbon-carbon bond formation by using palladium complexes. When we treated the stable PdCl2 complex of cyclooctadiene (COD) 1 with diethyl malonate in ether in the presence of sodium carbonate at room temperature, a facile carbopalladation occurred to give the new stable complex 2. This reaction is the first example of carbopalladation of an alkene in palladium chemistry, resulting in the carbon-carbon bond formation. The palladium-carbon σ-bond in the complex 2 is stabilized by coordination of π-olefin bond. By the treatment of the complex 2 with a base, malonate anion was generated and attacked the palladium-carbon bond, affording the bicyclo[6.1.0]nonane 3. The reaction of the complex 2 with diethyl malonate gave rise to the bicyclo[3.3.0]octane 4 by transannulation.2)
Then we discovered a nucleophilic attack of a carbanion to π-allylpalladium chloride (5).3) The reaction of 5 with the carbanion generated by the treatment of diethyl malonate with NaH proceeded in DMSO, and diethyl allylmalonate (6) was obtained as expected with precipitation of palladium metal. Furthermore the reaction of π-allylpalladium chloride (5) with the enamine 7 of cyclohexanone, which is regarded as a pseudo-carbanion, afforded 2-allylcyclohexanone after hydrolysis of the reaction product. These reactions constitute the basis of π-allylpalladium chemistry. Then catalytic allylation of nucleophiles including carbon nucleophiles with allyl acetate and allyl phenyl ether to afford 8 was reported by two groups in 1970.4) Discovery of these reactions marked the birth of π-allyl-palladium chemistry which has made steady and remarkable progress in the last thirty years.
The facile reactions of PdCl2 complex of COD (1) and π-allylpalladium chloride (5) with the carbon nucleophiles are significant in history of organometallic chemistry by the following reason. It is well-established that organometallic compounds known at that time, typically allylmagnesium halide, are nucleophilic, and react with carbonyl groups. At the same time Mg(II) is generated, showing that Grignard reaction involves the oxidation of Mg(0) to Mg(II). Thus the Grignard reaction is intrinsically stoichiometric, because in situ reduction of Mg(II) to Mg(0) is practically impossible. On the other hand, we have shown that π-allylpalladium chloride (5) is electrophilic, offering a new concept in organometallic chemistry. The reaction of the palladium complexes with nucleophiles accompanies the reduction of Pd(II) to Pd(0). Formation of Pd(0) suggests the possibility of a catalytic reaction. The generation of Pd(0) after the reactions is the most characteristic feature of palladium complexes.
Reactions of allyl β-keto carboxylates and related compounds.5)
Acetoacetates and malonates are important compounds in organic chemistry. They are extensively used in organic synthesis for the preparation of a variety of β-alkylated ketones 9, esters 10, and carboxylic acids 11 via alkylation, hydrolysis, and decarboxylation. We expected that acetoacetate (β-keto esters in general) and malonate chemistry can be expanded further by introduction of palladium-catalyzed reactions of their allylic esters. We found that π-allylpalladium enolate 13 and α-(π-allylpallada)ketones 14 are generated after facile decarboxylation by the treatment of allyl β-keto carboxylates 12 with palladium catalyst, and undergo several transformations. Thus we expanded the usefulness of β-keto esters based on the palladium-catalyzed reactions of their allylic ester, offering new synthetic methodologies which are not attainable by conventional methods. In addition to β-keto esters, it was confirmed that allyl acetates 15 which have electron-withdrawing groups (EWG) such as alkoxycarbonyl, cyano, nitro, and sulfonyl groups at α-carbon undergo similar transformations via 16. In this review, palladium-catalyzed reactions of allyl β-keto carboxylates 12 and allyl acetates 15 bearing EWG via the intermediates 13 and 16 are summarized.
In order to give a general view, six types of palladium-catalyzed reactions of allyl β-keto carboxylates are summarized in the following scheme, citing allyl cyclohexanonecarboxylate 17 as a model compound. Allyl β-keto carboxylate 17 undergoes palladium-catalyzed oxidative addition, followed by facile decarboxylation to form π-allylpalladium enolate 18 and α-(π-allylpallada) ketone 19. Also 18 and 19 are generated from enol carbonates 20 of the corresponding ketones. When these reactions were discovered, almost nothing was known about palladium enolates, and we started to explore the chemistry of palladium enolates. The palladium enolates, generated in this way, undergo reductive elimination, β-hydrogen elimination, and other transformations as expected. As summarized in the scheme, six transformations to afford the respective products 21–27 occur under different conditions and depending on the substituents R’s.5) In addition to allyl β-keto carboxylates, other allyl acetates bearing electron-withdrawing groups at α-carbon such as allyl malonates, cyanoacetates, and nitroacetates undergo similar transformations. Each transformation is explained in the followings.
Allylation by reductive elimination
The first reaction is decarboxylation-allylation of allyl β-keto carboxylates 17 or acetoacetate 28 to afford α-allyl ketones 21 by reductive elimination of 18, or γ, δ-unsaturated methyl ketones 29. Formation of allyl ketones 21 and 29 from allyl β-keto esters 17 and 28 is known as the Carroll rearrangement, which is carried out at temperatures as high as 200 ˚C. The rearrangement is useful for the synthesis of terpenoids. We found that the palladium-catalyzed Carroll rearrangement proceeds smoothly under mild conditions.6)–8) Mechanism of the thermal rearrangement is explained by [3.3]sigmatropic rearrangement and palladium-catalyzed reaction proceeds via π-allylpalladium enolate formation. Difference in mechanisms can be shown by the reaction of allyl α, α-dimethyl acetoacetate (30). No thermal [3.3]sigmatropic rearrangement occurs because there is no possibility of enolization. On the other hand, the palladium-catalyzed rearrangement proceeds smoothly under mild conditions in THF to afford the α-allyl ketone 31 regioselectively. Palladium-catalyzed reaction of geranyl acetoacetate (32) in THF afforded geranylacetone (34) selectively. On the other hand, a mixture of 34 and nerylacetone (35) was obtained from linalyl acetoacetate (33). Shimizu and Ishii reported a useful synthetic method of trifluoromethyl ketone 37 by the palladium-catalyzed reaction of allyl γ, γ, γ-trifluoroacetoacetate 36.9)
Allyl β-keto carboxylate 38 having allyl carbonate moiety was converted to 2-allyl-3-vinylcyclohexanone (40). In this case, 39 was formed preferencially by intramolecular allylation of the β-keto ester, and the subsequent Carroll rearrangement of 39 afforded 40.10)
Several compounds related to allyl β-keto esters undergo similar allylation reactions. Allyl enol carbonates 20 are typical examples. The enol carbonate 41 of 2-methyl-4-(tert-butyl)cyclohexanone underwent facile rearrangement at 0 ˚C in DME to afford a mixture of the stereoisomers 42 and 43 in 80% yield.8) As a recent application of regio- and stereoselective α-allylation of cyclohexanone derivative, Nicolaou and coworkers carried out the palladium-catalyzed decarboxylation-allylation of the allyl enol carbonate 44 at room temperature to give the α-allyl ketone 45 in 58% yield and a regioisomer (24%) using triphenylphosphine as a ligand in their total synthesis of colomviasin A.11) Paquette and coworkers applied a similar reaction of allyl enol carbonate to synthetic approach toward a phorbol framework.12)
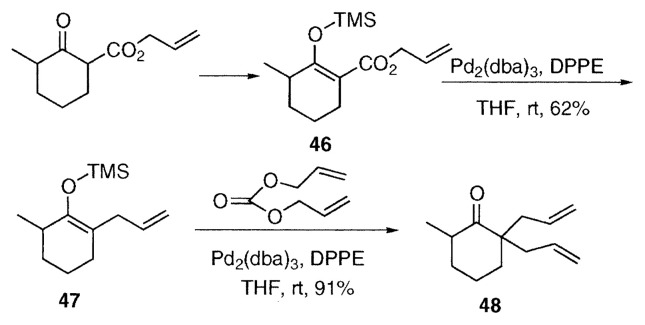
As a related reaction, the silyl enol ether 47 was prepared regioselectively from the silyl enol ethers 46 of allyl β-keto carboxylate at room temperature using diphenylphosphinoethane (DPPE) as a ligand. The silyl enol ether 47, prepared in this way, was allylated further to give diallyl ketone 48 by the palladium-catalyzed reaction with diallyl carbonate.13)
In addition to allyl β-keto esters, derivatives of diallyl malonate 49 and allyl cyanoacetate 51 undergo similar decarboxylation-allylation to give allyl α-allyl carboxylate 50 and α-allylnitrile 52 accompanied by 53 respectively. The nitro ester 54 is very reactive and the allylation proceeds even at −50 ˚C to give the α-allyl nitro alkane 55.7)
Preparation of α, β-unsaturated carbonyl compounds by β-hydrogen elimination
As the second reaction, we confirmed that elimination of β-hydrogen from the palladium-enolates 18 or 19 affords α, β-unsaturated ketones 22 as expected in boiling acetonitrile. 14),15) α, β-Unsaturated ketone 57 was obtained from allyl α, α-dimethylacetoacetate derivative 56 in refluxing acetonitrile. The allyl group trapps the hydrogen and generates propene. As a supporting evidence, reaction of the cinnamyl ester 58 afforded the enone 59 and 1-phenylpropene (60) in equal amounts.
2-Substituted cyclopentenones are conveniently prepared by Dieckmann condensation of diallyl adipate (61), and alkylation, followed by palladium-catalyzed decarboxylation-dehydrogenation. Facile preparation of 2-methylcyclopentenone (63) from 62 is an example. 16) A commercial process for methyl cis-jasmonate (67) was developed applying the enone formation as a key reaction. Preparation of α-(2-pentynyl)cyclopentenone (65) in 85% yield from allyl α-(2-pentynyl)cyclopentanonecarboxylate (64) was carried out by the palladium-catalyzed β-hydrogen elimination in boiling acetonitrile. Methyl cis-jasmonate (67) was obtained by hydrogenation of the triple bond in 65 to cis double bond, and Michael addition of dimethyl malonate to form 66, followed by decarboxylation.17)
The enones are also prepared from enol carbonates 20. 2-Methyl-2-cyclohexenone (69) was prepared regioselectively from the enol carbonate 68 of 2-methylcyclohexanone.18) The α, β-unsaturated aldehyde 72 was obtained from the enol carbonate 71 of aldehyde 70.15) Similarly α, β-unsaturated nitrile 74 was prepared from the disubstituted allyl cyanoacetate 73.15),19)
Synthesis of α-methylene carbonyl compounds by deacetoxylation
Acetoxymethyl group is introduced at α-carbon of allyl cyclopentanonecarboxylate by the reaction of formaldehyde, followed by acetylation to give 75. Unexpectedly the ester 75 underwent facile palladium-catalyzed decarboxylation and deacetoxylation to give α-methylenecyclopentanone 77 and 23.19),20) Interestingly, the acetoxy group in 76 was eliminated selectively as allyl acetate (24) more easily than β-hydrogen, and the cyclopentenone 78 was not formed. Diallyl malonates undergo similar transformation. As a synthetic example, itaconate 82 was prepared from diallyl malonate. The appropriately substituted diallyl malonate 80 was prepared from diallyl malonate 79, and subjected to the palladium-catalyzed reaction at 40 ˚C to provide allyl ethyl itaconate (82) as shown by 81 in 83% yield.
The reaction can be extended to other allyl acetates bearing EWG. The α-methylenelactone 84 was prepared from allyl lactonecarboxylate 83 in acetonitrile at 50 ˚C. Nitro and sulfonyl groups are more reactive, and the reactions of 85 and 87 proceeded smoothly at 25 ˚C to afford 1-alkyl-1-nitroethylene 86 and 1-alkyl-1-sulfonylethylene 88 in good yields. Lactams are less reactive, and α-methylene-γ-lactam 90 was prepared at somewhat higher temperature (80 ˚C) from the allyl γ-lactamcarboxylate 89.21) α-Methylenelactones and cyclic α-methyleneketones are present in natural products and show interesting biological properties, but the preparation of these labile functional groups in high yields under mild conditions is not easy. The palladium-catalyzed reaction which can be carried out under mild neutral conditions offers a useful synthetic method of these compounds.
Removal of the ester groups from substituted β-keto esters and malonates by hydrogenolysis
In the classical uses of β-keto esters and malonates, alkylated β-keto esters and malonates are hydrolyzed and decarboxylated to afford alkylated ketones and esters, or acids. But the hydrolysis of congested disubstituted β-keto esters and malonates is not easy, and usually harsh conditions are required. Sometimes rather strong bases or acids are used for hydrolysis. In addition, decarboxylation occurs at high temperature. On the other hand, palladium-catalyzed decarboxylation-hydrogenolysis of substituted allyl β-keto esters and malonates proceeds at room temperature under neutral conditions using a mixture of formic acid and triethylamine, which is soluble in organic solvents, to give 25. The reaction offers a useful method particularly for labile compounds.22) The reaction can be understood by the following mechanism. The palladium enolate 92, formed from 91 is attacked by a proton from formic acid to afford the ketone 93 and π-allylpalladium formate 94. Decarboxylation of 94 generates π-allylpalladium hydride 95 which collapses to propene and Pd(0).
Acid-sensitive esters such as THP-protected allyl β-keto ester 96 was converted to 97 at room temperature without deprotection of THP group, and the dike-tone 99 was obtained from a base-sensitive ester 98 without undergoing retro-Michael addition.22) The method was utilized by Murai and coworkers in the synthesis of the glycinoeclepin intermediate 101. Only the allyl ester in 100 was cleaved selectively to give the ketone, which was converted to trflate 101 without attacking the methyl ester and acetate in 100.23)
Shimizu and coworkers reported an interesting synthetic method of α-keto carboxylic acids such as 105. The diallyl 2-oxosuccinate 104 was prepared from diallyl oxalate (102) and allyl 3-phenylpropionate (103), and chemoselective decarboxylation of only the allyl β-keto carboxylate in 104 took place to give the α-keto carboxylic acid 105.24) Also Shimizu and Ishii obtained α-fluorocyclododecanone (106) by hydrogenolysis of allyl α-fluoro-β-cyclododecanonecarboxylate (107). In addition, they prepared α-fluorocyclododecenone (108) from 107.25)
Disubstituted diallyl malonates react smoothly with a mixture of formic acid and triethylamine. The free monocarboxylic acid 110 was obtained smoothly in a good yield in boiling dioxane from the disubstituted diallyl malonate 109.26) As an application, reaction of the diallyl malonate attached to β-lactam 111 proceeded stereoselectively to give the monocarboxylic acid with the β-oriented methyl group 112.27)
γ-Methylene-γ-butyrolactone 116 was prepared in one-pot in 81% yield from diallyl alkyl(2-propynyl)malonate 113 by palladium-catalyzed cyclization and hydrogenolysis with formic acid.28) In this case, after oxidative addition, intramolecular oxypalladation of the triple bond in the intermediate 114 takes place to afford the (π-allylpalladium)alkenyllactone 115. Finally hydrogenolysis of 115 with formic acid provides γ-methylene-γ-butyrolactone 116.
Palladium-catalyzed aldol condensation
Typical reactions of metal enolates are aldol condensation and Michael addition. As expected, we found that palladium enolates undergo these reactions smoothly. So far intramolecular reactions proceed efficiently, and intermolecular reactions are competitive with other reactions and hence selectivity is low. Palladium-catalyzed aldol condensation of allyl acetoacetate derivative 117 at room temperature gave the aldol 118 in 82% yield. When an aldehyde group is present in allyl β-keto ester 119, intramolecular aldol condensation took place yielding the cyclic aldol 122 in 96% yield and the diketone 122 as a minor product. In this reaction, the palladium alkoxide 121 is generated at room temperature via palladium enolate 120 and provides the aldol product 122. No allylation occurs.29) The reaction of the allyl cyanoacetate 124 provided the lactone 126 in 56% yield as a main product and the alcohol 127 in 16% by intramolecular reaction of the intermediate 125, followed by lactonization. The aldol condensation proceeds under neutral conditions.
Palladium-catalyzed Michael addition
Intra-molecular Michael addition via palladium enolates occurs under mild conditions.30) Michael addition of the allyl β-keto ester 128 proceeded as shown by 129 to provide the cyclized palladium enolate 130 as an intermediate, and the enolate 130 was converted to the diketone 131 in 77% yield by protonation together with the cyclopentanones 132 and 133 as minor products formed by reductive elimination and β-hydrogen elimination. Intramolecular Michael addition of the substituted diallyl malonate 134 occurred as shown by 135 to afford the cyclized product 136 as a main product and 137 as a minor one. Yamamoto and coworkers obtained the ketone 140 by the reaction of allyl acetoacetate (138) with an activated olefin 139. The reaction is explained by Michael addition of the enolate 141 to 139 to give 142, followed by allylation.31)
An interesting strategy for convergent steroid synthesis has been reported by Deslongchamps based on palladium-catalyzed domino decarboxylation-Michael addition of allyl β-keto ester (bicyclic Nazarov reagent) 143 to the cyclohexenone 145. The intermolecular Michael addition of the first palladium enolate 144, generated from 143, to 145 affords the second palladium enolate 146. Intramolecular Michael addition of 146 provides the third enolate 147, constructing finally the tetracyclic steroid skeleton 148 by β-hydrogen elimination. 32)
Conclusions
Palladium enolates are generated from allyl β-keto carboxylates after facile decarboxylation by the treatment of with a zero-valent palladium complex as a catalyst, and undergo several transformations. Reductive elimination provides α-allyl ketones, and α, β-unsaturated ketones are obtained by elimination of β-hydrogen. A new synthetic method for α-methylene ketones was discovered. The allyl ester group can be removed easily by the treatment with formic acid and triethylamine to give ketones. Intramolecular aldol condensation and Michael addition proceed under neutral conditions. In addition to allyl β-keto carboxylates, allyl acetates bearing electron-withdrawing groups such as malonates, cyanoacetate, and nitroacetates undergo similar transformations. Developments of these reactions expanded the classical chemistry of β-keto carboxylates and malonates in a large extent.
Acknowledgement
Research reported in this review has been carried out in Basic Research Laboratories, Toray Industries Ltd., Tokyo Institute of Technology, and Okayama University of Science. I want to thank sincerely for important contributions made by a number of coworkers whose names appear in references.
References
- 1.Books, Tsuji J. (1995) Palladium Reagents and Catalysts, Innovations in Organic Synthesis, John Wiley & Sons.Tsuji J. (2004) Palladium Reagents and Catalysts, New Perspective for the XXI Century, John Wiley & Sons.
- 2.(a) Tsuji J., Takahashi H. (1965) J. Am. Chem. Soc. 87, 3275–3276 14324316 [Google Scholar]; (b) Takahashi H., Tsuji J. (1968) J. Am. Chem. Soc. 90, 2387–2392. [Google Scholar]
- 3.Tsuji J., Takahashi H., Morikawa M. (1965) Tetrahedron Lett. 6, 4387–4388. [Google Scholar]
- 4.(a) Hata G., Takahashi K., Miyake A. (1970) J. Chem. Soc. D: Chem. Commun. 1392–1393. [Google Scholar]; (b) Atkins K. E., Walker W. E., Manyik R. M. (1970) Tetrahedron Lett. 11, 3821–3824. [Google Scholar]
- 5.Reviews, Tsuji J. (1986) Tetrahedron 42, 4361–4401.Tsuji J., Minami I. (1987) Acc. Chem. Res. 20, 140–145.
- 6.Shimizu I., Yamada T., Tsuji J. (1980) Tetrahedron Lett. 21, 3199–3202. [Google Scholar]
- 7.Tsuda T., Chujo Y., Nishi S., Tawara K., Saegusa T. (1980) J. Am. Chem. Soc. 102, 6381–6384. [Google Scholar]
- 8.Tsuji J., Yamada T., Minami I., Yuhara M., Nisar M., Shimizu I. (1987) J. Org. Chem. 52, 2988–2995. [Google Scholar]
- 9.(a) Shimizu I., Ishii H., Tasaka A. (1989) Chem. Lett. 1127–1128. [Google Scholar]; (b) Shimizu I., Ishii H. (1989) Chem. Lett. 587–588. [Google Scholar]
- 10.Shimizu I., Ohashi Y., Tsuji J. (1983) Tetrahedron Lett. 24, 3865–3868. [Google Scholar]
- 11.Nicolaou K. C., Vassilikogiannakis G., Mägerlein W., Kranich R. (2001) Angew. Chem. Int. Ed. 40, 2482–2486. [DOI] [PubMed] [Google Scholar]
- 12.Paquette L. A., Gallou F., Zhao Z., Young D. G., Liu J., Yang J., Friedrich D. (2000) J. Am. Chem. Soc. 122, 9610–9620. [Google Scholar]
- 13.Tsuji J., Ohashi Y., Minami I. (1987) Tetrahedron Lett. 28, 2397–2398. [Google Scholar]
- 14.Shimizu I., Tsuji J. (1982) J. Am. Chem. Soc. 104, 5844–5846. [Google Scholar]
- 15.Minami I., Nisar M., Yuhara M., Shimizu I., Tsuji J. (1987) Synthesis 992–998. [Google Scholar]
- 16.Tsuji J., Nisar M., Shimizu I., Minami I. (1984) Synthesis 1009–1010. [Google Scholar]
- 17.Kataoka H., Yamada T., Goto K., Tsuji J. (1987) Tetrahedron 43, 4107–4112. [Google Scholar]
- 18.Tsuji J., Minami I., Shimizu I., Kataoka H. (1984) Chem. Lett. 1133–1136. [Google Scholar]
- 19.Minami I., Yuhara M., Shimizu I., Tsuji J. (1986) J. Chem. Soc., Chem. Commun. 118–119. [Google Scholar]
- 20.Tsuji J., Nisar M., Minami I. (1986) Tetrahedron Lett. 27, 2483–2486. [Google Scholar]
- 21.Tsuji J., Nisar M., Minami I. (1987) Chem. Lett. 23–24. [Google Scholar]
- 22.Tsuji J., Nisar M., Shimizu I. (1985) J. Org. Chem. 50, 3416–3417. [Google Scholar]
- 23.Murai A., Tanimoto N., Sakamoto N., Masamune T. (1988) J. Am. Chem. Soc. 110, 1985–1986. [Google Scholar]
- 24.Shimizu I., Makuta T., Ohshima M. (1989) Chem. Lett. 1457–1460. [Google Scholar]
- 25.Shimizu I., Ishii H. (1994) Tetrahedron 50, 487–495. [Google Scholar]
- 26.Mandai T., Imaji M., Takada H., Kawata M., Nokami J., Tsuji J. (1989) J. Org. Chem. 54, 5395–5397. [Google Scholar]
- 27.Murayama T., Yoshida A., Kobayashi T., Miura T. (1994) Tetrahedron Lett. 35, 2271–2274. [Google Scholar]
- 28.Mandai T., Ohta K., Baba N., Kawada M., Tsuji J. (1992) Synlett 671–672. [Google Scholar]
- 29.Nokami J., Mandai T., Watanabe H., Ohyama H., Tsuji J. (1989) J. Am. Chem. Soc. 111, 4126–4127. [Google Scholar]
- 30.Nokami J., Watanabe H., Mandai T., Kawada M., Tsuji J. (1989) Tetrahedron Lett. 30, 4829–4832. [Google Scholar]
- 31.Shim J. G., Nakamura H., Yamamoto Y. (1998) J. Org. Chem. 63, 8470–8474. [DOI] [PubMed] [Google Scholar]
- 32.Lepage O., Deslongchamps P. (2003) J. Org. Chem. 68, 2183–2186. [DOI] [PubMed] [Google Scholar]