Abstract
The quality of Dendrobium nobile Lindl. is related to its endophytic fungi. It has been reported that the mycorrhizal fungus MF23 helps to increase the content of dendrobine in Dendrobium, but few studies have explained the mechanism underlying this phenomenon. In a previous study, we verified the mechanism of symbiosis between MF23 and D. nobile on agar medium. The research carried out in this study on bark medium, similar to the natural environment, is of great importance because of its benefits for wide application. We found a significant effect, especially in the later period of cultivation, in which the highest dendrobine content in the experimental group was 0.147%, which is equivalent to 2.88 times that of the control group, and suggesting that MF23 promoted D. nobile in the natural environment, which verifies the application of the technique in field conditions. This result also implied that post-modification enzyme genes might play an important role in stimulating the biosynthesis of dendrobine.
1. Introduction
Dendrobium nobile Lindl., Orchidaceae, is a herbal plant commonly used in Chinese traditional medicine, and it is one of the several Dendrobium species that were specified in the Chinese Pharmacopeia.1,2 Previous research has shown that Dendrobium has pharmacological activities such as regulating immunity, regulating blood sugar and blood lipids, anticoagulation, and anti-tumor.3 Many compounds have been extracted and isolated from the Dendrobium species, including alkaloids,4,5 polysaccharides,6,7 phenanthrenes,8 phenolics,9,10 and bibenzyls.11,12 Dendrobine, an index component of D. nobile, has anti-tumor,13 hypoglycemic,14 anti-flu,15 and other pharmacological activities.16
The growth of Orchidaceae is highly affected by mycorrhizal fungi, and it has been reported that fungi can promote the germination of orchid species.17 Orchidaceae seeds that contain only undifferentiated embryos are as small as dust. In this case, these species must rely on fungal symbiosis to provide nutrients for germination under natural conditions. The DMEs (Dendrobium myco-endophytes, DMEs) are cross-transmitted inside the host plant cells, playing an important role in plant host development, resistance, and alkaloid stimulation.18 Guo et al.19,20 obtained a number of fungi from Dendrobium that could effectively promote the germination of Dendrobium seeds. Fungi also affect the growth of the plant21 by invading the root cells and forming mycorrhizal symbioses with the roots. These fungi supply water and nutrients such as nitrogen and phosphorus to the host plant.22 One study has shown that stress resistance can be promoted by mycorrhizal fungi. Fungal elicitors may induce the activity of lipoxygenase, peroxidase, and phenylalanine ammonia lyase, which are involved in the synthesis of protective substances, in the protocorms of D. candidum.(23) The effect of endophytic fungi on host plants has been examined in great detail. It has been shown that an effective increase in active compounds, such as steroids and alkaloids, occurs after infection.24,25
The Orchidaceae-fungal endophytes’ biodynamics for sustainable development of bioproducts and its applications are supported in the large-scale biosynthesis of industrially and pharmaceutical important biomolecules.26 MF23 is a mycorrhizal Mycena species that has been obtained from D. officinale Kimura et Migo. It has been reported that MF23 promotes the growth of Dendrobium plants.27,28 In recent studies, MF23 was shown to promote the growth of D. officinale and increase the dendrobine content of D. nobile.29,30 Our previous research31 was carried out with agar medium, which offered good repeatability and controllability for the analysis of the results. However, such conditions do not well represent the natural environment of Dendrobium, which includes stones or bark lacking many necessary nutritional compounds. For this reason, to provide research results with practical applications in real-life production, this study was performed on chestnut bark. This material offered a natural environment to study the effect of MF23 on D. nobile, providing basic materials and evidence for the MF23-induced symbiotic growth-promoting mechanism in the natural environment. The purpose of the study is to explore the effects of mycorrhizal fungi on D. nobile cultivated on bark medium as well as the underlying mechanisms of these effects and provide a foundation and guidance for the possibility of using mycorrhizal fungi to promote the production of D. nobile in the field.
2. Results
2.1. The Effect of MF23 on the Growth of D. nobile
The survival rate of D. nobile showed a decreasing trend overall both in the experimental and control groups, but with the extension of the time after cultivation, the experimental groups had a higher survival rate than the control groups and maintained a relatively stable trend during the period of inoculation. At the 12th week, the survival rate was significantly different between the control and the experimental groups, and at the 24th week, the survival rate of the control group was only 72.9%, whereas the experimental group had a higher rate at more than 85.4%, forming a striking contrast with the control group (Figure 1a). In addition, the stem diameter of D. nobile presented a significant difference between the experimental and the control groups from the 9th week onward (Figure 1b). In terms of fresh stem weight, the experimental groups showed a significant increase compared to the control groups from the 3rd week onward (Figure 1c). In general, MF23 promoted the growth of D. nobile.
Figure 1.
Changes in morphology of D. nobile grown on bark medium between the control (gray line) and the experimental groups (color line). Values are presented as the means ± SDs (n = 12); values with * are of statistical significance at P < 0.05. (a) Comparison of survival rates. (b) Comparison of stem diameter. (c) Comparison of fresh stem weight.
2.2. The Effect of MF23 on the Content of Dendrobine
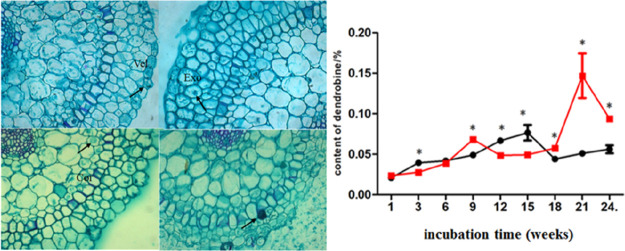
In general, the dendrobine content was significantly different between the experimental and control groups. The dendrobine content was not significantly different during the first 6 weeks (except the 3rd week), but as the experiment continued, dendrobine was accumulated obviously both in the control and the experimental group (Figure 2); in particular, at the 21st week, a clear difference in the dendrobine content occurred in the experimental group (P < 0.01), reaching 0.146%, which was almost three times more than that in the control groups (0.051%).
Figure 2.

Difference in the dendrobine content between the control and the experimental groups. Values are presented as the means ± SD (n = 3); values with * are of statistical significance at P < 0.05.
2.3. The Infection Process of MF23 in Roots of D. nobile
Hyphae were present in the velamen at the 6th week after inoculation (Figure 3A) and were found in the exodermis by 9 weeks (Figure 3B), while they appeared in the cortex by 15 weeks (Figure 3C) and in passage cells by 21 weeks (Figure 3D). Additionally, dense mycelium was present in the cortex of D. nobile at the 18th week but was rarely observed by the 21st and 24th weeks.
Figure 3.

Light micrographs of interactions between the roots of D. nobile and MF23 (A–D). (A) Mycelium entered the velamen of the root after 6 weeks (×20). (B) Mycelium entered the exodermis of the root after 9 weeks (×20). (C) Mycelium entered the cortex of the root after 15 weeks (×20). (D) The hyphal coils appeared after 21 weeks (×20). vel represents velamen; exo represents exodermis; cor represents cortex.
2.4. Validation and Expression Analysis of Genes Involved in the Formation of the Backbone of the Sesquiterpene Alkaloid, Dendrobine
The key enzyme genes involved in the MVA pathway in D. nobile were selected for qRT-PCR analysis. As shown in Figure S1, the genes MK, MVD, GPPS, FPPS, and TPS21 were upregulated during the early weeks (6 weeks), MVD, FPPS, and TPS21 were upregulated during the middle weeks (15 weeks), and all of the genes were upregulated at the 18th week but showed a lower expression level and had no significant difference compared with the control group at the 21st week, the time when the experimental group exhibited the maximum dendrobine content. At the 24th week, the expression levels of all the key enzyme genes in the mevalonate (MVA) pathway were downregulated except those of HMGR.
The qRT-PCR analysis of key enzyme genes in the methyl-d-erythritol 4-phosphate (MEP) pathway (Figure S2) was almost the same as that in the control group in the pre-inoculation stage, and only the gene DXR was upregulated during the early period (6 weeks). At the middle period (18 weeks), all the genes (DXS, DXR, MCT, CMK, MDS, HDS, and HDR) were upregulated, and their activation was much greater than that at any other time. However, during the late stage of inoculation (24th week), the expression pattern of key enzyme genes was similar to that of the MVA pathway and was downregulated to a low level.
2.5. Validation and Expression Analysis of Genes Involved in Post-modification
The qRT-PCR analysis of genes involved in the post-modification process was more consistent with the change in dendrobine content (Figure S3). At the early weeks (6 weeks), genes CYP71D10, At4g10440, and ATX4 were upregulated, while the genes had no significant change during the middle period. All the genes exhibited high expression levels at 18 weeks after inoculation. The levels of expression were higher in the experimental groups than those of the control groups, reaching a significant difference at 21 weeks, and almost all the genes except CYP94C1, At4g10440, and ATX4 exhibited higher expression levels after inoculation than at any other stages, which was consistent with the maximization of the dendrobine content. In conclusion, the enzyme genes involved in post-modification had a close relationship with the content of dendrobine.
3. Discussion
3.1. MF23 Had a Significant Effect on the Growth of D. nobile on Bark Medium
MF23 had a significant effect on the growth of the D. nobile on bark medium. The bark matrix is closer to the natural cultivation condition, which makes our results more suitable for practical production. The time point at which the growth index of D. nobile in the experimental group began to increase significantly (9th week) was highly consistent with the time when the MF23 hyphae began to enter the interior of the root. It can be inferred that the increase in D. nobile growth was closely related to infection by the hyphae. As early as 1983, Ames et al.(32) used radioisotope labeling technology to find that AM fungal hyphae can absorb nitrogen from soil outside the root and transport it to the root of the host. In addition, Li et al.(33) proved that, compared with the root system of plants, the absorption area of extraroot hyphal networks is wider, which is conducive to the absorption of water and a large number of mineral elements. Moreover, endophytic fungi can increase the pH of the mycelium by secreting protons and organic acids to activate substances such as slightly soluble phosphates in the matrix so that nutrients such as phosphate can be increased in the rhizosphere.34 This is probably one of the important reasons why D. nobile can still maintain good growth on nutrient-deficient bark. This shows that the bark substrate is more suitable for the establishment of a symbiosis system between Dendrobium nobile and MF23, and it also shows to some extent that MF23 has a good potential in the wild or imitated state of Dendrobium nobile.30
3.2. Infection of D. nobile by MF23 from the Velamen to the Exodermis Then to the Cortex
The hyphae of MF23 appeared in the velamen, the exodermis, and the cortex and gradually advanced, and it is worth noting that a small number of hyphae were distributed in nonchannel cells. Two reasons might explain this phenomenon: one is that the process of MF23 influx into the cortex from passage cells occurred precisely at the time that we did not investigate. Otherwise, passage cells are probably not the only channel by which MF23 enters cortical cells. MF23 could pass into the cortex through nonchannel cells directly. We found that the mycelium densely covered the cortex of D. nobile at the 18th week but was rarely observed at the 21st and 24th weeks, and it has been reported that Orchidaceae might digest endophytic fungi to obtain nutrients for growth.35 Therefore, we may regard this as the period of large-scale digestion of fungal hyphae by the plant to obtain energy and nutrients. In addition, it was also reported that there were differences in the distribution of hyphae in different parts of the root. Pan et al.36 found that most Orchidaceae have no mycorrhizal infection at their root tips; in general, our findings may also be related to the sampling site.
3.3. Significant Change in the Dendrobine Content on Bark Medium
In this study, MF23 showed a significant effect on the content of dendrobine, and the maximum increase was 187.7% that of the control group. (Cultured on bark substrate for 21 weeks, the culture bottles were kept in a conventional greenhouse with 12 h of light and an illumination intensity of 2000 LX. The temperature was controlled at 24–26 °C.) It has been reported that there may be a negative feedback regulatory mechanism in the salicylic acid synthesis pathway of Scutellaria baicalensis Georgi that is involved in the accumulation of secondary metabolites such as baicalin and baicalein.37 It was speculated that salicylic acid may participate in or trigger the regulatory process to prevent excessive accumulation of this compound in plants. Other studies have38,39 also shown that feedback inhibition mainly exists in biosynthesis pathways of plants. Therefore, it could be hypothesized that after 21 weeks of inoculation, the biosynthetic pathway of the experimental group was negatively regulated and inhibited, leading to the decrease in the content of dendrobine after 24 weeks, which suggests that the secondary metabolites of D. nobile may maintain a balance to protect this plant from the risks of excessive alkaloid levels. In addition, during the entire experimental period, the dendrobine content of the experimental group changed to increase first, then decrease, then increase, and then decrease. In the second increase interval (15–21 weeks) of the experimental group, the content of dendrobine increased from 0.047% (15 weeks) to 0.147% (21 weeks), an increase of about 0.1%, which may be related to the bacteria in the cortex of D. nobile silk infection. As discussed in Section 3.2, D. nobile consumes a large amount of fungal hyphae to obtain energy in 21 weeks, thereby synthesizing dendrobine, and the content of dendrobine reaches its peak.
3.4. MF23 Is Effective against the Enzyme Genes of the MVA Pathway—The Upstream Dendrobine Synthesis Pathway in the Natural Environment
The molecular results indicate that the key enzyme gene in the MVA pathway of D. nobile grown on bark medium is involved in the induction of MF23, but it appeared that an unequal relationship existed in the MVA pathway between the biosynthesis of dendrobine and the expression of key enzyme genes. Studies have shown that genes often have an unequal relationship at the transcriptomic and proteomic levels, leading to unequal symmetry with catalytic products, which may result from enzymatic processing and changes in regulation before and after gene translation.40 For instance, when Fusarium was induced for 2 months, the sesqui-TPS gene was active, but an increased sesquiterpenoid content was observed at the next adjacent sampling point as well as the time when the sesqui-TPS gene was significantly downregulated.41 Likewise, a similar phenomenon appeared in our experiment because, in addition to dendrobine having a basic sesquiterpene skeleton, its biosynthesis also requires a series of post-modification processes. Specifically, it could be speculated that the sharp increase in the content of dendrobine after 21 weeks may be the result of gene activation in the early stage, while the key enzyme genes in the later stage were at relatively low expression levels, suggesting that the key enzyme genes of the MVA pathway might be affected by dendrobine negative feedback regulation to ensure that the content of dendrobine in plants is balanced. However, according to the latest report, the T. longibrachiatum MD33 endophytic fungal strain isolated from the stem of D. nobile has the potential to produce dendrobine compounds.42 Whether this mechanism also exists in MF23 remains to be further explored.
Through the analysis of the MEP pathway genes, we found that this pathway may not be directly involved in the regulation of dendrobine biosynthesis by MF23, but it has been reported that these two pathways are not completely separate.43 For example, the addition of the DXR (a key enzyme gene in the MEP pathway) inhibitor not only inhibited the subsequent reaction of the MEP pathway but also promoted the flow of IPP and DMAPP, the basic components of terpenoid synthesis, to the MVA pathway, which promoted an increase in the catalytic product of the MVA pathway without activating this pathway.44−46 Therefore, it may be speculated that, under the strong effect of MF23, the MEP pathway may become involved in the biosynthesis of dendrobine and cooperate with the MVA pathway to promote the biosynthesis of dendrobine.
3.5. The Relationship between the Key Genes in Downstream Synthetic Pathways and the Content of Dendrobine Was Clear with the Use of Bark Medium
Studies have reported that the synthesis pathways of some secondary metabolites, such as terpenoids, are inseparable from the effects of the modified enzymes.46 For example, the synthesis pathway of paclitaxel includes seven kinds of hydroxylation reactions and an epoxidation reaction.47 To date, the key enzyme genes of the six hydroxylation reactions have been successfully cloned48 and all of them showed good catalytic activity. In this study, through the exploration of the expression levels of the post-modification enzyme genes P450, methyltransferase, and aminotransferase, we found that these genes were significantly upregulated when the dendrobine content increased. Nine weeks after inoculation, the corresponding relationship between the expression level of the post-modification enzyme gene and the content of dendrobine was significant. In particular, the aminotransferase DAT was significantly upregulated in the experimental group at the 9th, 15th, and 21st weeks compared to the control group and reached maximum expression at the 21st week when the highest content of dendrobine was observed. In general, post-modification enzyme genes may play an important role in the process by which MF23 promotes increases in the D. nobile dendrobine content in the natural environment.
4. Materials and Methods
4.1. Source of Materials
Twenty-four weeks of healthy tissue-cultured seedlings of D. nobile were obtained from Chishui Xintian humantang Pharmaceutical Company Limited (Guizhou, China).
4.2. Biological Materials and Culture Conditions31
The chestnut bark was washed, soaked for 24 h, and cut into pieces, and a 70 g portion was placed into each medium bottle. Then, 80 mL of deionized water was added, and the bottles were autoclaved at 121 °C for 180 min. Tissue-cultured seedlings of D. nobile, 3–5 cm in height, were cultured on bark medium, an artificial primary environment, which was similar to the natural surroundings of this species. Mycelial plugs from 20 day-old MF23 grown on potato dextrose agar medium were placed on the roots of D. nobile and covered with bark. Then, the culture bottles were kept in a conventional greenhouse with 12 h of light and an illumination intensity of 2000 LX. The temperature was controlled at 24–26 °C, and the plants without mycelial plugs were designated as the control.
Plant samples were collected at different periods: 1, 3, 6, 9, 12, 15, 18, 21, and 24 weeks, (Figure S4) from the control group and the experimental group. Twelve repetitions were carried out at each point in time, and the samples were selected randomly. All samples were divided into two portions: samples were used for morphological and chemical research and samples frozen in liquid nitrogen and stored for RNA extraction.
4.3. Cultivation Conditions of D. nobile(31)
One culture bottle with 12 plants was used as a repetition. We recorded the growth changes of D. nobile, such as the number of roots and stem diameter, at each point of time. The fresh stems were weighed and dried at 55 °C.
4.4. Determination of the Dendrobine Content of D. nobile(31)
Dendrobine standard and the internal standard naphthalene were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) and Beijing Bei Na Chuang Lian Biotechnology Institute (Beijing, China), respectively. The sample preparation for the GC analysis was performed according to the Pharmacopoeia of China (2015). Chromatography was performed on an Agilent 6890 GC-FID (California, USA) with a DB-1 capillary column (0.25 μm × 0.25 mm × 30 m, California, USA) and nitrogen as the carrier gas.
Three replications were carried out to ensure the reliability of the experimental results. One microliter of the derivatized sample was injected, and components of the total ion chromatogram were extracted by flame ionization detection. The linear regression equation y = 0.1861x – 0.1474 (r = 0.9995) implied that the dendrobine concentration was linear, with a peak area in the range of 1.1–11 mg/L.
4.5. Light Microscopy31
Fresh roots of D. nobile were fixed in formalin-acetic acid-alcohol (FAA) following the approach described by Feder and O’Brien.49 The samples were dehydrated in a graded ethanol series, embedded in paraffin, stained with safranine and fast green, sealed with Gel Damar, and then observed and photographed with a light microscope equipped with a camera (Zeiss Axio Imager A1).
4.6. RNA Extraction31
An RNeasy Plant Mini Kit (cat. nos. 74903 and 74904) (Qiagen, Hilden, Germany) was used to extract the total RNA from the D. nobile samples following the manufacturer’s instructions. Total RNA degradation and contamination were verified by electrophoresis on a 1.0% agarose gel in 0.5 × TBE (44.5 mM Tris-HCl, 44.5 mM boric acid, and 1.25 mM Na2EDTA). The Qubit RNA Assay Kit was used with a Qubit 2.0 A fluorometer (Life Technologies, Carlsbad, CA, USA) to determine the concentration of total RNA. In addition, an RNA Nano 6000 Assay Kit, equipped with a Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA), was used to assess the integrity of the RNA.
4.7. Construction of the cDNA Library of D. nobile(31)
Stem samples of the two D. nobile groups were collected for RNA extraction at the 9th and 21st weeks. An average of 1.5 μg of RNA was extracted from each sample, verified as high quality, and used for RNA sample preparations. Then, eight samples with RNA integrity number (RIN) values above 8 were used for library construction, and two repetitions were performed per treatment. The specific process of RNA extraction and other procedures were as described in our previously published study.31
4.8. Confirmation of the Infection-Response Expression Profiles by qRT-PCR
The key enzyme genes involved in the formation of dendrobine, including the mevalonate (MVA) pathway (Table S1), the methyl-d-erythritol 4-phosphate (MEP) pathway (Table S2), and the post-modification pathway (Table S3), were selected from the transcriptome library created by our group.
The transcriptome library of D. nobile has already been established.31 Selected differentially expressed genes (DEGs) were validated with qRT-PCR. qRT-PCR was performed with SYBR Premix ExTaqTM (TaKaRa, Dalian, China) on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The actin gene of D. nobile was used as a reference control.50 The reaction was performed using the following conditions: denaturation at 95 °C for 30 s followed by 40 cycles of amplification (95 °C for 5 s, 60 °C for 34 s). For each sample, three technical replicates of the qRT-PCR assay were used with a minimum of three biological replicates. The 2–ΔΔCt method51 was used to evaluate the gene expression results.
4.9. Statistical Analysis31
Statistical software including GraphPad Prism 5.0 and SPSS 19.0 was used to evaluate the resulting data. An unpaired t-test was performed, with P <0.05 indicating significance.
5. Conclusions
Some findings revealed that Orchidaceae growth and developments were affected indirectly or directly as a result of complex microbial ecological interactions. Our results of the dendrobine content determination and microscopic observations showed that the fungus MF23 had a significant effect on D. nobile grown on bark medium. The D. nobile survival rate was significantly increased in the later period following inoculation. In addition, microscopic observation revealed that the MF23 infection gradually progressed inward, from the root to the exodermis and then to the cortex. The expression levels of post-modification enzyme genes, such as cytochrome P450, aminotransferase, and N-methyltransferase, were studied. The results showed that these enzyme genes had a significant contribution to the content of dendrobine, especially DAT, an aminotransferase enzyme gene, which was positively correlated with dendrobine biosynthesis. This implies that post-modification enzyme genes might play an important role in stimulating the biosynthesis of dendrobine as a result of the effects of MF23. This research lays an important foundation for understanding the MF23-induced increase in the dendrobine content of D. nobile and provides a scientific basis for regulating the biosynthesis of dendrobine with molecular methods. In conclusion, this study reveals the mechanism of mycorrhizal symbiosis in the wild and lays a foundation for the possibility of using mycorrhizal fungi to promote the production of D. nobile in the field.
Most importantly, the findings of this study strongly suggest that the endophytic fungus MF23 is beneficial to the growth of D. nobile on bark medium, which closely mimics natural conditions, indicating that MF23 is suitable for field production applications.
Additionally, further exploration of the role and molecular mechanism of MF23 in improving the quality of D. nobile with experimental field trials should be carried out in the future. Focusing on the use of transgenic, RNAi, and other technologies to verify the functions of candidate genes, future proteomic studies will be performed to intuitively and accurately elucidate the molecular mechanism by which MF23 increases the effective component content of D. nobile. In short, this study, carried out on bark medium, not only has importance for its applicability in the field but also lays the foundation for further research.
Acknowledgments
We gratefully thank the reviewers for their suggestions and comments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06325.
The qRT-PCR analysis of key enzyme-coding genes involved in the MVA pathway, MEP pathway, and post-modification in D. nobile; the effect of MF23 on the growth morphology of D. nobile cultivated on bark medium; and DEGs involved in the MVA pathway, MEP pathway, and post-modification in D. nobile (PDF)
Author Contributions
B.L. and S.X.G. conceived and designed the experiments. X.Y.C. and Q.L. performed the experiments and analyzed the data. X.L.X. wrote the paper. G.D. provided technological guidance for the chemical analyses and edited the paper. All authors read and approved the final manuscript.
This research was financially supported by the National Key R&D Program of China (2018YFC1706200 for L.B.), the CAMS Initiative for Innovative Medicine (CAMS-2016-I2M-2-003 for L.B.), the Special Project for Academic Construction of Peking Union Medical College (Tsinghua 211-201920100902), and the National Science & Technology Fundamental Resources Investigation Program of China (2018FY100700). We gratefully acknowledge financial support from these funding sources, which assisted us in completing the study, including the collection of the materials and the establishment of the gene library of D. nobile.
The authors declare no competing financial interest.
Notes
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary Material
References
- Zhang Y.; Wang H.; Mei N.; Ma C.; Lou Z.; Lv W.; He G. H. Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol. 2018, 107, 230–235. 10.1016/j.ijbiomac.2017.08.175. [DOI] [PubMed] [Google Scholar]
- Xu J.; Han Q.-B.; Li S.-L.; Chen X.-J.; Wang X.-N.; Zhao Z.-Z.; Chen H.-B. Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. 10.1007/s11101-013-9310-8. [DOI] [Google Scholar]
- Xu Y.; Liu H. C.; Li X. Research progress in chemical composition, fingerprint and pharmacological activity of dendrobii caulis. Chin. J. Inf. Tradit. Chin. Med. 2019, 26, 129–132. 10.3969/j.issn.1005-5304.2019.02.030. [DOI] [Google Scholar]
- Nie J.; Jiang L.-S.; Zhang Y.; Tian Y.; Li L.-S.; Lu Y.-L.; Yang W.-J.; Shi J.-S. Dendrobium nobile Lindl. alkaloids decreases the level of intracellular β-amyloid by improving impaired autolysosomal proteolysis in APP/PS1 mice. Front. Pharmacol. 2018, 9, 1479. 10.3389/fphar.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Wu Q.; Liu H.; Ling H.; He Y.; Wang C.; Wang Z.; Lu Y.; Lu Y. Alkaloids of dendrobium nobile lindl. altered hepatic lipid homeostasis via regulation of bile acids. J. Ethnopharmacol. 2019, 241, 111976. 10.1016/j.jep.2019.111976. [DOI] [PubMed] [Google Scholar]
- Li X.-L.; Xiao J.-J.; Zha X.-Q.; Pan L.-H.; Asghar M.-N.; Luo J.-P. Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohydr. Polym. 2014, 106, 247–254. 10.1016/j.carbpol.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Huang K.; Li Y.; Tao S.; Wei G.; Huang Y.; Chen D.; Wu C. Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. 10.3390/molecules21060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. S.; Lee S. A.; Hong S. S.; Han X. H.; Lee C.; Kang S. J.; Lee D.; Kim Y.; Hong J. T.; Lee M. K.; Hwang B. Y. Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2010, 20, 3785–3787. 10.1016/j.bmcl.2010.04.054. [DOI] [PubMed] [Google Scholar]
- Klongkumnuankarn P.; Busaranon K.; Chanvorachote P.; Sritularak B.; Jongbunprasert V.; Likhitwitayawuid K. Cytotoxic and antimigratory activities of phenolic compounds from Dendrobium brymerianum. Evidence-Based Complementary Altern. Med. 2015, 2015, 350410. 10.1155/2015/350410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Zhang S.; Gao B.; Qian Z.; Liu J.; Wu S.; Si J. Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of Dendrobium catenatum flowers and selection of quality control herb-markers. Food Res. Int. 2019, 123, 732–745. 10.1016/j.foodres.2019.05.040. [DOI] [PubMed] [Google Scholar]
- Chaotham C.; Pongrakhananon V.; Sritularak B.; Chanvorachote P. A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 2014, 34, 1931–1938. [PubMed] [Google Scholar]
- Guo S. X.; Gao W. Q.; Gao W. W. Isolation and biological activity of mycorrhizal fungi from Dendrobium candidum and D. nobile. China J. Chin. Mater. Med. 2000, 25, 338–341. [PubMed] [Google Scholar]
- Song T.-H.; Chen X.-X.; Lee C. K.-F.; Sze S. C.-W.; Feng Y.-B.; Yang Z.-J.; Chen H.-Y.; Li S.-T.; Zhang L.-Y.; Wei G.; Shi J.; Xu K.; Ng T.-B.; Zhu L.-L.; Zhang K. Y. Dendrobine targeting JNK stress signaling to sensitize chemotoxicity of cisplatin against non -small cell lung cancer cells in vitro and in vivo. Phytomedicine 2019, 53, 18–27. 10.1016/j.phymed.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Jia B.; Feng Q.; Zhang Y.; Chen Y.; Meng J. Dendrobine attenuates gestational diabetes mellitus in mice by inhibiting Th17 cells. Basic Clin. Pharmacol. Toxicol. 2021, 379–385. 10.1111/bcpt.13524. [DOI] [PubMed] [Google Scholar]
- Li R.; Liu T.; Liu M.; Chen F.; Liu S.; Yang J. Anti-influenza a virus activity of dendrobine and its mechanism of action. J. Agric. Food Chem. 2017, 65, 3665–3674. 10.1021/acs.jafc.7b00276. [DOI] [PubMed] [Google Scholar]
- Chen X. Y.; Gong D. Y.; Guo S. X.; Li B.. Research Progress of Dendrobine-type Alakaloids and Synthetic Biology of Dendrobine Journal of Chongqing Technology and Business University(Natural Science Edition). 2021, 38 ( (3), ), 8–18.
- Selosse M.-A.; Boullard B.; Richardson D. Noël Bernard (1874–1911): orchids to symbiosis in a dozen years, one century ago. Symbiosis 2011, 54, 61–68. 10.1007/s13199-011-0131-5. [DOI] [Google Scholar]
- Sarsaiya S.; Jain A.; Jia Q.; Fan X.; Shu F.; Chen Z.; Zhou Q.; Shi J.; Chen J. Molecular identification of endophytic fungi and their pathogenicity evaluation against Dendrobium nobile and Dendrobium officinale. Int. J. Mol. Sci. 2020, 21, 316. 10.3390/ijms21010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. Studies on the effects of fungi on the course of seed germination of Dendrobium lohohens and Dendrobium candidum. Acta Acad. Med. Sin. 1991, 13, 46–49. [PubMed] [Google Scholar]
- Shunxing G.; Li F.; Wenquin C.; Xiaomei C. Mycena dendrobii, a new mycorrhizal fungus. Mycosystema 1999, 18, 141–144. [Google Scholar]
- Szűcs Z.; Plaszkó T.; Cziáky Z.; Kiss-Szikszai A.; Emri T.; Bertóti R.; Sinka L. T.; Vasas G.; Gonda S. Endophytic fungi from the roots of horseradish (Armoracia rusticana) and their interactions with the defensive metabolites of the glucosinolate - myrosinase - isothiocyanate system. BMC Plant Biol. 2018, 18, 85. 10.1186/s12870-018-1295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrle H.; Penningsfeld F.; Hock B. The role of nitrogen concentration in determining the outcome of the interaction between Dactylorhiza incarnata (L.) Soo and Rhizoctonia sp. New Phytol. 1991, 117, 665–672. 10.1111/j.1469-8137.1991.tb00971.x. [DOI] [Google Scholar]
- Hou P.; Guo S. Influences of fungus mycenae sp. on activities of some enzymes and extracellular ph of protocorms of dendrobium candidum. Microbiology 2004, 31, 26–29. [Google Scholar]
- Kapoor R.; Chaudhary V.; Bhatnagar A. K. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 2007, 17, 581–587. 10.1007/s00572-007-0135-4. [DOI] [PubMed] [Google Scholar]
- Wang Q.-X.; Yan N.; Ji D.-G.; Li S.-Y.; Hu J.-M.; Hu H. Mycorrhizal fungi promote growth and nitrogen utilization by Dendrobium nobile (Orchidaceae). Plant Diversity Resour. 2014, 36, 321–330. 10.7677/ynzwyj201413117. [DOI] [Google Scholar]
- Sarsalya S.; Shi J.; Chen J. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: current research, challenges, and future possibilities. Bioengineered 2019, 10, 316–334. 10.1080/21655979.2019.1644854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. T.; Zhang Y. Q.; Dong W. H.; Bai Y. B.; Li Z. S.; Sun W. L. Growth promoting of Dendrobium endophytic fungus on Dendrobium officinale tissue culture seedlings. Southwest China J. Agric. Sci. 2014, 27, 317–324. [Google Scholar]
- Zhang L.; Chen J.; Lv Y.; Gao C.; Guo S. Mycena sp., a mycorrhizal fungus of the orchid Dendrobium officinale. Mycol. Prog. 2012, 11, 395–401. 10.1007/s11557-011-0754-1. [DOI] [Google Scholar]
- Xiaomei C.; Shunxing G.; Zhixia M. Effects of fungal elicitor on the protocorms of Dendrobium candidum. Zhongguo Yaoxue Zazhi 2008, 39, 423–426. [Google Scholar]
- Yan H. L.; Mei C. X.; Liao F. H.; Deng X. F.; Yang J. Y. Effects of mycorrhizal fungi on the growth and dendrobine and polysaccharide accumulation of Dendrobium nobile Lindl seedlings. Chinese Pharmaceutical Journal. 2016, 51, 1450–1454. [Google Scholar]
- Li Q.; Ding G.; Li B.; Guo S.-X. Transcriptome Analysis of genes involved in Dendrobine biosynthesis in Dendrobium nobile Lindl. infected with Mycorrhizal Fungus MF23 (Mycena sp.). Sci. Rep. 2017, 7, 316. 10.1038/s41598-017-00445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames R. N.; Reid C. P. P.; Porter L. K.; Cambardella C. Hyphal uptake and transport of nitrogen from two 15N-labelled sources by glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1983, 95, 381–396. 10.1111/j.1469-8137.1983.tb03506.x. [DOI] [Google Scholar]
- Li X.-L.; George E.; Marschner H. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 1991, 136, 41–48. 10.1007/BF02465218. [DOI] [Google Scholar]
- Guo Y.-E.; Li F.; Li Y.-D.; Duan T.-Y. Progress in the elucidation of the mechanisms of arbuscular mycorrhizal fungi in promotion of phosphorus uptake and utilization by plants. Pratacult. Sci. 2016, 10, 2379–2390. 10.11829/j.issn.1001-0629.2015-0747. [DOI] [Google Scholar]
- Hadley G.; Williamson B. Features of mycorrhizal infection in some malayan orchids. New Phytol. 1972, 71, 1111–1118. 10.1111/j.1469-8137.1972.tb01989.x. [DOI] [Google Scholar]
- Pan C. M.; Chen R. M.; Ye Q. S. Infection characteristics and physiological features of mycorrhizal fungi in roots of Cymbidium sinense and C.ensifolium. Soil Environ. Sci. 1999, 8, 287–289. 10.3969/j.issn.1674-5906.1999.04.012. [DOI] [Google Scholar]
- Su H.; Song S.; Yan X.; Fang L.; Zeng B.; Zhu Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS One 2018, 13, e0192114 10.1371/journal.pone.0192114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling R. J.; Kamiya Y.; Seto H.; Harberd N. P. Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol. 1998, 117, 1195–1203. 10.1104/pp.117.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. L.; Ward D. A.; Uknes S.; Appleford N. E. J.; Lange T.; Huttly A. K.; Gaskin P.; Graebe J. E.; Hedden P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995, 108, 1049–1057. 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q.; Stepaniants S. B.; Mao M.; Weng L.; Feetham M. C.; Doyle M. J.; Yi E. C.; Dai H.; Thorsson V.; Eng J.; Goodlett D.; Berger J. P.; Gunter B.; Linseley P. S.; Stoughton R. B.; Aebersold R.; Collins S. J.; Hanlon W. A.; Hood L. E. Integrated Genomic and Proteomic Analyses of Gene Expression in Mammalian Cells. Mol. Cell. Proteomics 2004, 3, 960–969. 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- Chen X. D.; Xie M. R.; Liu S. F.; Zhou W. P.; Zhang W. M.; Gao X. X. Relationship between Expression of Sesquiterpene Synthase Gene and Sesquiterpene Content in Artificial Agarwood Induced by Fusarium sp. A2. J. Chin. Pharm. 2014, 39, 197–203. 10.11669/cpj.2015.21.006. [DOI] [Google Scholar]
- Sarsaiya S.; Jain A.; Fan X.; Jia Q.; Xu Q.; Shu F.; Zhou Q.; Shi J.; Chen J. New insights into detection of a dendrobine compound from a novel endophytic Trichoderma longibrachiatum strain and its toxicity against phytopathogenic bacteria. Front. Microbiol 2020, 10.3389/fmicb.2020.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawari A. H.; Mohamed-Hussein Z.-A. Simulation of a petri net-based model of the terpenoid biosynthesis pathway. BMC Bioinf. 2010, 11, 83. 10.1186/1471-2105-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J. S.; Wang H.; Dai H. M.; Qing Z. H.; Yang J. F. Effects of lovastatin and phosphoamimycin on the activity of tobacco HMGR and DXR. Agriculture and Technology. 2015, 35, 4–7. [Google Scholar]
- Laule O.; Furholz A.; Chang H.-S.; Zhu T.; Wang X.; Heifetz P. B.; Gruissem W.; Lange M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. PNAS 2003, 100, 6866–6871. 10.1073/pnas.1031755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.; Liu D.; Ge F.; Chen C. Advances in studies on key post-modification enzymes in triterpenoid saponins biosynthesis. Acta Bot. Boreali-Occident. Sin. 2014, 34, 2137–2144. 10.7606/j.issn.1000-4025.2014.10.2137. [DOI] [Google Scholar]
- Li J.; Luo X.; Zhao P.; Zeng Y. Post-modification enzymes involved in the biosynthesis of plant terpenoids. Acta Bot. Yunnanica 2009, 31, 461–468. 10.3724/SP.J.1143.2009.09108. [DOI] [Google Scholar]
- Croteau R.; Ketchum R. E. B.; Long R. M.; Kaspera R.; Wildung M. R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. 10.1007/s11101-005-3748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder N.; O’Brien T. P. Plant microtechnique: some principles and new methods. Am. J. Bot. 1968, 55, 123–142. 10.1002/j.1537-2197.1968.tb06952.x. [DOI] [Google Scholar]
- Chen W.; Cheng X.; Zhou Z.; Liu J.; Wang H. Molecular cloning and characterization of a tropinone reductase from Dendrobium nobile Lindl. Mol. Biol. Rep. 2013, 40, 1145–1154. 10.1007/s11033-012-2156-0. [DOI] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.