Abstract
Energy supplementation may reduce oxidative stress by correcting a negative energy balance, but in some contexts, it has been shown to increase oxidative stress, especially at peak lactation. The current experiment examined if a pelleted energy supplement with or without the addition of Lactobacillus-fermented seaweed or seaweed plus terrestrial plants extracts affected oxidative stress of ewes from late gestation through to weaning and ewe and lamb production from lambing to weaning. Treatments were either no supplement (CON−), a pelleted supplement only (CON+, 100 g/ewe per d), CON+ with seaweed extract only (SWO, 10 mL/ewe per day), or CON+ with seaweed plus an arrangement of terrestrial plant extract (SWP, 10 mL/ewe per d). Ewes (n = 160; mean initial BW = 72.3 ± 9.5 kg [mean ± SD]) were randomized to pastures (n = 4 pastures per treatment with 10 ewes each). After lambing, ewes with twins were reallocated to pastures (n = 3 pastures per treatment with 10 ewes each) according to lambing date. At 4 wk in milk, supplementation tended to reduce total antioxidant status (TAS; P = 0.10) and increased glutathione peroxidase (GPx) activity compared with nonsupplemented ewes (P = 0.04). The addition of seaweed and terrestrial plants extracts to the concentrate, that is, SWO and SWP, increased TAS and reduced GPx activity compared with CON+ (P < 0.01). Supplementation increased milk yield at weeks 4, 6, and 8 of lactation, and protein, lactose, and total milk solids yield at peak lactation (week 4; P < 0.05). The CON− ewes had greater somatic cell count than the supplemented ewes at weeks 4, 8, and 10 of lactation (P = 0.03). Our results suggest that energy supplementation, alone, increases oxidative stress of lactating ewes, which may relate to increased oxidative phosphorylation. Most importantly, these results indicate that in situations where energy supplementation is needed to increase animal performance, negative effects of energy supplementation around peak lactation can be offset by the addition of Lactobacillus-fermented plant extracts (SWO and SWP) to improve antioxidant status.
Keywords: grazing, sheep milk production, oxidative stress, probiotics
Introduction
Oxidative stress is defined as the imbalance between oxidants (e.g., superoxide anion) and antioxidants (both enzymatic [e.g., superoxide dismutase] and nonenzymatic [e.g., glutathione]; Sordillo and Aitken, 2009). Several stressors have been shown to increase oxidative stress and thereby increase the incidence of morbidity and mortality in livestock (Chirase et al., 2004; Sordillo and Aitken, 2009; Celi, 2010). For example, handling stress (Fidan et al., 2010) and shearing (Fidan et al., 2009) have led to increased blood cortisol (indicating physiological stress), a greater concentration of plasma malondialdehyde (MDA; a marker for oxidative stress), and less glutathione (as a marker of antioxidant status). Transport stress in cattle caused an increase in MDA and reduced total antioxidant capacity in serum (Chirase et al., 2004). Following transportation, calves that required three or more treatments for bovine respiratory disease, throughout a finishing phase, had two times greater MDA concentrations than their healthier cohorts at arrival (Chirase et al., 2004). Specific to mature breeding and lactating livestock, unfulfilled energy demand is a significant source of stress (Sordillo and Aitken, 2009; Celi, 2010; Singh et al., 2011). Such a negative energy balance causes nutritional stress, which in turn produces reactive oxygen metabolites and subsequently oxidative stress, reducing health and welfare of the lactating animals by enabling disorders such as mastitis, metritis, and retained placenta (Sordillo and Aitken, 2009; Celi, 2010).
One possible means to reduce oxidative stress in transition and lactating grazing animals is to reduce the negative energy balance by strategic supplementation of the base forage with energy-rich concentrates (Sordillo et al., 2009). However, energy supplementation through starch may also increase oxidative stress by increasing oxidative phosphorylation, especially around peak lactation (around 80 d in milk in dairy cows, i.e., peak lactation; Gabai et al., 2004). Another alternative to reduce oxidative stress is feeding plant extracts, such as seaweed products (Beck and Gregorini, 2020). For example, Kannan et al. (2007) found that the addition of a brown seaweed (Ascophyllum nodosum) extract (Acadian Seaplants Ltd., Dartmouth, NS, Canada) to the diet of transport-stressed goats improved antioxidant status. Additionally, several experiments determined that probiotic supplements based on Lactobacillus species can reduce oxidative stress in ruminants (Ran et al., 2019; Izuddin et al., 2020). Therefore, it was hypothesized that energy supplementation of the base forage diet of lactating grazing ewes would reduce oxidative stress and that this effect would be enhanced by the supplemental addition of Lactobacillus-fermented extract products based on seaweed (Animal Health Tonic) and seaweed plus terrestrial plants (Fortress+; AgriSea Ltd., Paeroa, New Zealand). The objective of the current experiment was to determine the effect of a pelleted energy supplement with or without the addition of a seaweed or seaweed plus terrestrial plants extracts on oxidative stress and milk production and composition of ewes from late gestation through to weaning.
Materials and Methods
This experiment took place at the Lincoln University sheep farm from 8 July to 14 December 2018. During the experimental period, daily maximum, minimum, and average temperatures were 15.9, 5.4, and 9.5°C, respectively (NIWA, 2021). All procedures outlined here were approved by the Lincoln University Animal Ethics Committee (AEC 2018–25).
Description of products
The current experiment was designed to explore the health, in terms of ameliorating oxidative stress, and rumen fermentation effects of two Lactobacillus-fermented plant products. One of the products is based on the fermentation of a brown seaweed (Ecklonia radiata; SWO; Animal Health Tonic, AgriSea New Zealand Seaweed Ltd.; AgriSea New Zealand Seaweed Ltd., Paeroa, New Zealand). The other product is based on the fermentation of seaweed, plantain (Plantago lanceolota), chicory (Chicorium intybus), broad-leaf dock (Rumex obstusifolius), and lucerne (Medicago sativa; SWP; Fortress+; AgriSea New Zealand Seaweed Ltd., Paeroa, New Zealand). Both extract products followed the extraction process, which was based on a fermentative extraction technique of the seaweed, in the case of SWO, as well as the seaweed and the terrestrial plants for SWP according to proprietary methods (Bradley; AgriSea New Zealand Seaweed Ltd.). These extracts are thought to provide health benefits through providing Lactobacillus fermentation metabolites of compounds extracted from the plant species, prebiotics, and probiotics.
Prior to the start of this experiment, the antioxidant capacities of SWO and SWP were investigated using the stable free-radical compound, 2,2-diphenyl-1-picryl-hyrazyl-hydrate (DPPH), which is purple in color while in the free-radical form. In the presence of antioxidants, that is, free-radical scavengers, the radical is scavenged and DPPH goes from purple to clear in color. Thus, the inhibition of DPPH is measured colorimetrically by measuring changes in absorption at 515 nm (Olejar et al. 2019). Using the methods described by Olejar et al. (2019), we determined that SWO and SWP had a 33.9% ± 1.4 and 48.7% ± 1.2 (mean ± SD) inhibition, respectively. These levels of DPPH inhibition are similar to values reported for methanolic extracts of different brown seaweed species (A. nodosum [25.6%] and Fucus vesiculosus [31.2%]; O’Sullivan et al. 2011).
To determine the specific Lactobacillus species, 1 mL of SWO and SWP products were aseptically transferred into 9 mL of buffered peptone water and proceeded with the serial dilution procedure. The serial dilutions of the samples (10−1, 10−2) were prepared and 1 mL of the sample from 10−2 dilutions were pipetted using a sterile pipette and aseptically transferred to specialized culture media, MRS agar (MRS agar-code 1219 [Fort Richard Laboratories Ltd.; Auckland, New Zealand]; used for isolation of Lactobacillus species), with two plates per extract product. From each plate, five random colonies were characterized using 16S ribosomal RNA sequencing (Lane et al., 1985) using universal primers. The predominant Lactobacillus species were determined as Lactobaccillus paracasei and Lactobaccillus plantarum.
Animals and treatments
Coopworth ewes at the Lincoln University Sheep Farm were ultra-sound scanned to determine whether they were pregnant with a single or multiple lambs. Based on these results, ewes carrying multiple offspring (n = 160; body weight = 72.3 ± 9.5 kg; 3.8 ± 1.2 yr old; 2.8 ± 1.2 parturitions [mean ± SD]) were randomly assigned to one of four dietary treatments: 1) negative control, which received no supplement (CON−); 2) positive control, which was offered the basal supplement only (CON+); 3) the basal supplement with 10 mL per ewe per day of SWO; or 4) the basal supplement plus SWP at 10 mL per ewe per day. The basal supplement was a commercially available pellet (12 mm) labeled to supply 12.2 MJ of metabolizable energy (ME)/kg dry matter (DM), 12.2% crude protein (CP), and 2% fat (Sheep Nuts, Reliance Feeds, Canterbury, New Zealand). All ewes were supplemented once daily at 0800 h by feeding 1 kg of supplement per paddock in a 2-m feed bunk which the ewes had access to both sides of and the ewes typically finished consuming the supplement in <20 min. Addition of the SWO and SWP extracts occurred by adding 2.5 L of the respective extract products to 25 kg of pelleted supplement, mixed in a concrete mixer for 5 min, and then were spread on a concrete pad for 2 d to dry. The proportion of ewes, excluding CON−, that did not approach the feed bunk when supplements were provided were identified by the number on their ear tag and averaged 17.5 ± 10.5% (mean ± standard deviation) and was not affected by treatment. This was done on 29 September and again on 7 October, and it was determined that the same ewes refused to consume the supplements each time. The refusal to consume supplements is common in grazing ruminants and the values seen in the current experiment are similar to those reported by others (Dixon et al., 2003).
After being randomized to treatments, ewes were randomly allocated to spatially different pastures (replicates, four per treatment) of the same vegetative sward (approximately 60% Ryegrass [Lolium perenne L.], 30% white-clover [Trifolium repens L.], and 10% weeds), with each pasture containing 10 ewes per pasture. Ewes remained on their pastures until after lambing, when ewes with twins were reallocated to pastures (n = 3 pastures per treatment with 10 ewes each) according to lambing date. Additionally, pastures were grouped by lambing date so that the twin bearing ewes were placed into pastures with similar aged lambs. Only the pastures with twins were used for data collection to control for any potential effects associated with the number of lambs being raised by the ewes. All pastures were grazed using continuous stocking as the grazing management. Stocking rate was the same at 10 ewes in a 0.75-ha pasture during the whole experimental period. Herbage mass was determined thrice, 29 September, 6 November, and 16 December, using the calibrated rising plate meter at 30 random locations per paddock (Jenquip; Feilding, New Zealand), with one equation being established for all pastures. Calibration equations were developed by taking plate readings at 10 locations with a continuum of compressed herbage heights to encompass the herbage mass present in the pastures and then clipping the herbage at the 10 locations to ground level. The clipped herbage was weighed fresh and a subsample was oven-dried at 60 °C to determine DM percentage. The DM% was used to calculate total DM of herbage in the clipped area, which was then extrapolated to a kg DM per ha basis. These values were fitted with a regression equation along with the plate meter compressed heights readings to develop the calibration equation (R2 = 0.94). Herbage mass throughout the experiment averaged 2,229 ± 456 kg DM/ha and provided 1.72 ± 0.4 kg of herbage DM/kg of body weight. A subsample of herbage clippings was freeze-dried, ground, and analyzed for chemical composition by near-infrared spectroscopy (NIRs), using pre-established equations. Nutritive values used for the calibration of NIRs were obtained for DM and ash (AOAC, 1990; method 930.15 and 942.05, respectively), neutral detergent fiber (NDF; Van Soest et al., 1991), and acid detergent fiber (ADF; AOAC, 1990; method 973.18); CP by combustion (Variomax CN Analyser Elementar), water-soluble carbohydrates (MAFF, 1986); and DM digestibility, organic matter (OM) digestibility, and digestible OM in the DM (DOMD; Iowerth et al., 1975). All calibration equations had R2 ≥ 0.90, and NIRs readings for the forage samples were within the calibration range. The ME content of the herbage provided was then calculated as 0.16 multiplied by DOMD content (Primary Industries Standing Committee, 2007). The herbage was determined to contain (mean ± SD) 30.6 ± 7.5% DM, 90.2 ± 2.8% OM, 46.3 ± 3.1% NDF, 25.9 ± 1.8% ADF, 11.0 ± 1.8% CP, 23.6 ± 0.6% water-soluble carbohydrates, 72.3 ± 3.8% DM digestibility, 77.5 ± 4.2% OM digestibility, and 10.5 ± 0.6 MJ ME/kg DM.
Data and sample collection
All ewes were weighed unfasted prior to lambing at trial initiation (8 July 2018) and weaning (14 December 2018). During lambing (from 26 August 2018 to 18 September 2018), pastures were checked daily (0800 AM), and any new lamb was weighed with birth weight, sex, and date of birth recorded and allocated an electronic identification ear tag. Lambs were also weighed at tailing (9 October 2018) and weaning (14 December 2018) to calculate average daily gain (ADG) during different periods up to weaning.
Blood samples were collected from all ewes prior to lambing. After lambing, samples were collected from focal ewes (n = 5 ewes per pasture, 20 per treatment), which were randomly selected, excluding ewes which did not consume the supplements. The same focal animals were sampled for blood samples 4 wk after lambing and at weaning and for ruminal fluid at weaning. Only samples from the focal animals were analyzed. Sampling occurred in the morning after the supplements were fed and prior to milking, if that occurred. Blood samples were collected (10-mL heparinized blood tube) by jugular venipuncture. Two milliliters of heparinized whole blood was removed from the blood tubes, placed into a 2-mL Eppendorf tube, and stored at −20 °C until analysis. Blood tubes were then centrifuged at 2300 × g for 10 min at 4 °C, and plasma was aspirated and stored at −20 °C until analysis. Two 2-mL subsamples of the ruminal fluid were immediately placed into tubes, one containing sulfuric acid (to inhibit NH3 volatilization) for the determination of NH3 and one without for the measurement of volatile fatty acid (VFA) concentration.
Milk production of ewes from two pastures per treatment containing the earliest lambed ewes (n = 20 ewes per treatment) were measured. The first group of measured pastures lambed on 3 September 2018 ± 3.8 d and the second on 11 September 2018 ± 3.0 d, with one pasture per treatment in each group. Milking of the first group began on 2 October 2018 and the second group on 9 October 2018, so that milking began approximately 4-wk postlambing. Each group of sheep in assigned pastures was milk sampled every-other week, until 20 November 2018. Milking was completed as previously described by Yusuf et al. (2018), implementing the four hour milking interval technique (Hunter et al., 2015). Ewes were brought from pastures to the sheep yards, and lambs were separated from ewes. Ewes were milked using a portable milker (type: DVP170/340; DeLaval; Tumba, Sweden) to remove existing milk from the udder and were then held for 4 h. The ewes were then administered an intramuscular dose of oxytocin (1.0 mL, 0.0167 mg/mL, 10 IU per mL, Kela Health, Hoogstraten, Belgium). After 1-min ewes were milked again using the same portable milking system, but this time fitted with subsampling containers containing bronopol (0.1%). Samples were stored at 4 °C until analysis, which occurred within 4 d of collection.
Sample analysis
Nonacidified ruminal fluid was analyzed for VFA concentration using gas chromatography (GC-2010, Shimadzu, Kyoto, Japan). In brief, the samples were thawed over night at 4 °C. The samples were then centrifuged (Beckman centrifuge JA20 rotor) at 13,000 rpm for 30 min at 4 °C, and 100 μL of the supernatant was aspirated and placed into a 2-mL tube. Twenty microliters of 2-methylvaleric acid was added as an internal standard, and 40 μL of metaphosphoric acid was added to the tube. The tube was then vortexed, diluted with equal parts of acetone and water, and then filtered using a 0.22-μm nylon syringe. One microliter was then injected using an autosampler (AOC-20i) at a split ratio of 1:3 at the injection port (240°C). The specific VFA were separated with a SGE BP21 30 m × 530 μm × 1.0 μm bore capillary column, with a flow of 5.23 mL/min of He. The initial oven temperature was 105°C for 4 min and then increased by 15°C/min to 230°C, which was maintained for 5 min. The flame ionization detector was maintained at 240°C.
The acidified ruminal fluid sample was analyzed for NH3 concentration by an enzymatic UV method (Neeley and Phillipson, 1988) using an automated clinical analyzer (Randox Rx Daytona, Crumlin, County Antrim, UK). Heparinized whole blood samples were analyzed for the antioxidant enzyme, glutathione peroxidase (GPx), according to the Randox kit manual (RANSEL; catalog number RS504), which is an enzymatic based protocol, and was analyzed using an automated clinical analyzer (Randox Rx Daytona, Crumlin, County Antrim, UK). The whole blood was diluted 61 times, as suggested by the manual, so that the values would fall within the technical operating range of 75 to 925 U/L. Plasma samples were analyzed for total antioxidant status (TAS) using a colorimetric method and for plasma urea N using a commercial enzymatic kinetic technique according to their respective manuals (Randox, Crumlin, County Antrim, UK). These assays were analyzed using the Randox Rx Daytona (Crumlin, County, Antrim, UK).
Milk samples were analyzed for fat, protein, lactose, and somatic cell count (SCC) by a MilkoScan (Foss Electric, Hillerød, Denmark), which was calibrated for sheep milk samples. Based on the quantity of milk sampled by the portable milker subsampling unit, the total amount of milk produced during the 4 h between the two milkings were calculated according to the calculations provided by Yusuf et al. (2018). Milk yield data are reported as kilogram produced per 4 h.
Statistical analysis
This experiment was a completely randomized design. Pasture was the experimental unit and individual sheep was used as a random effect. All data were fit by a mixed effects model using the ‘lme4’ package (Bates et al., 2015). The blood results included sampling time, treatment, and the sampling time-by-treatment interaction as fixed effects in the model. Ewe initial and final body weight, ewe weight change, lamb birth, tailing, and weaning weights, and ruminal samples only included treatment as a fixed effect.
Milk composition (SCC and fat, protein, lactose, solids no fat [lactose + protein], and milk solids [MS; fat, protein, and lactose] percent) were fit by a generalized linear model with the ‘glmer’ function from the ‘lme4’ package (Bates et al., 2015). The model included fixed effects for treatment, the week postlambing, and the treatment by week postlambing interaction. Random effects were milking groups and individual animal. For the percentage results (fat, protein, lactose, solids no fat, and milk solids), a gamma distribution was used and a Poisson distribution was used for SCC. Milk yield data (fat, protein, lactose, solids no fat [lactose + protein], and MS [fat, protein, and lactose] yield; kg/4 h) were fit by a linear mixed effects model using ‘lmer’ function of the ‘lme4’ package (Bates et al., 2015). The results from the ‘glmer’ output were then back-transformed to the response level and means were generated using the ‘emmeans’ function and these are the values reported (Lenth, 2018).
Finally, treatment effects were compared using preplanned orthogonal contrasts. The planned contrasts were designed to test 1) differences between extracts (SWP vs. SWO), 2) effect of supplementation (CON− vs. CON+, SWO, and SWP), and 3) effect of extracts (CON+ vs. SWO and SWP). All contrasts were generated using the ‘emmeans’ package (Lenth, 2018). Additionally, Pearson’s correlation coefficients were generated using the ‘cor.test’ function for some of the variables to assist with discussion. Significance was declared at P ≤ 0.05 and a tendency at 0.05 < P ≤ 0.1. All statistical analysis was conducted using R (v.3.4.4; R Core Team, 2020).
Results
Ewe and lamb body weights
There were no effects for initial or final body weight for any of the generated contrasts (P ≥ 0.16; Table 1). Supplementation did not affect ewes body weight change (i.e., CON− vs. supplemented; P ≥ 0.28; Table 1). However, SWP lost less weight, from trial start to weaning, than SWO (i.e., SWO vs. SWP; P = 0.04). Treatment did not affect lamb birth, tailing, or weaning weights (P ≥ 0.14; Table 2). There was no treatment effect on lamb ADG from birth to weaning (P ≥ 0.25; Table 2).
Table 1.
Initial and final body weight and weight change of grazing ewes from late gestation to weaning
| Treatment1 | Orthogonal contrast P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | CON− | CON+ | SWO | SWP | SE | SWO vs. SWP | Eff. of extract | Effect of supp. |
| n 3 | 3 | 3 | 3 | 3 | — | — | — | — |
| Age, yr | 3.9 | 3.7 | 3.9 | 3.6 | 0.2 | 0.46 | 0.80 | 0.25 |
| Initial, kg | 73.9 | 70.6 | 74.1 | 70.8 | 1.6 | 0.16 | 0.36 | 0.27 |
| Weaning, kg | 69.6 | 68.5 | 68.3 | 69.3 | 1.9 | 0.69 | 0.91 | 0.67 |
| BW change, kg/d | −0.03 | −0.01 | −0.04 | −0.01 | 0.01 | 0.04 | 0.35 | 0.50 |
1Treatments were not supplemented (negative control; CON−), a pelleted supplement only (positive control; CON+), CON+ with a seaweed extract (SWO), and CON+ with a seaweed plus terrestrial plant extract (SWP).
2Orthogonal contrasts tested were SWO vs. SWP = differences in the extracts used (SWO compared with SWP), eff. of extract (CON+ compared with SWO and SWP), and eff. of supp. = effect of supplements (CON− compared with CON+, SWO, and SWP).
3Experimental units were the replicated pastures.
Table 2.
Body weight and ADG of lambs from birth to weaning
| Treatment1 | Orthogonal contrast P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | CON− | CON+ | SWO | SWP | SE | SWO vs. SWP | Eff. of extract | Eff. of supp. |
| n 3 | 3 | 3 | 3 | 3 | — | — | — | — |
| Body weight, kg | ||||||||
| Birth | 4.8 | 4.9 | 5.0 | 4.8 | 0.1 | 0.14 | 0.98 | 0.61 |
| Tailing | 12.5 | 12.4 | 12.5 | 11.9 | 0.6 | 0.50 | 0.84 | 0.81 |
| Weaning | 31.3 | 30.3 | 29.9 | 30.5 | 1.0 | 0.63 | 0.91 | 0.38 |
| ADG, kg/d | ||||||||
| Birth-to-tailing | 0.25 | 0.25 | 0.26 | 0.26 | 0.02 | 0.85 | 0.73 | 0.89 |
| Tailing-to-wean | 0.28 | 0.28 | 0.27 | 0.28 | 0.01 | 0.28 | 0.91 | 0.56 |
| Birth-to-wean | 0.27 | 0.27 | 0.26 | 0.28 | 0.01 | 0.25 | 0.94 | 0.68 |
1Treatments were not supplemented (CON−), a pelleted supplement only (CON+), CON+ with a seaweed extract (SWO), and CON+ with a seaweed plus terrestrial plant extract (SWP).
2Orthogonal contrasts tested were SWO vs. SWP = differences in the extracts used (SWO compared with SWP), eff. of extract (CON+ compared with SWO and SWP), and eff. of supp. = effect of supplements (CON− compared with CON+, SWO, and SWP).
3Experimental units were the replicated pastures.
Blood analysis
Plasma urea concentration of ewes was not different at the pre- or postlambing sampling dates (P ≥ 0.17). At weaning, supplementation increased plasma urea compared with the nonsupplemented ewes (P < 0.01). Although at weaning there was no effect of treatment on plasma urea concentrations when comparing both SWO and SWP to the CON+ treatment (P = 0.75), though SWP plasma urea concentrations was lower than SWO (P = 0.05; Table 3). Treatment did not affect total antioxidant status either prior to lambing or at weaning (P ≥ 0.22). Four weeks after lambing, CON− ewes tended to have greater TAS than the supplemented treatments (P = 0.10). At 4 wk in milk, CON+ ewes had lower (11%) TAS than the ewes fed SWO and SWP (P = 0.04; Table 3). Due to a sampling error, the GPx activity at weaning is not available. Before lambing, there were no treatment effects on GPx activity in the whole blood (P ≥ 0.53). However, GPx activity was lower for CON− ewes than ewes fed supplement (P < 0.01) and lower for the ewes provided SWO and SWP compared with CON+ (P = 0.05).
Table 3.
Blood chemistry of ewes
| Treatment1 | Orthogonal contrast P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item3 | CON− | CON+ | SWO | SWP | SE | SWO vs. SWP | Eff. of extract | Eff. of supp. |
| n 4 | 3 | 3 | 3 | 3 | — | — | — | — |
| PU, mmol/L | ||||||||
| Prelambing | 8.07 | 8.14 | 7.11 | 7.89 | 0.50 | 0.25 | 0.26 | 0.50 |
| 4 wk in milk | 7.13 | 6.85 | 7.04 | 8.07 | 0.57 | 0.17 | 0.27 | 0.76 |
| Weaning | 8.49 | 10.25 | 11.20 | 9.71 | 0.56 | 0.05 | 0.75 | <0.01 |
| TAS, mmol/L | ||||||||
| Prelambing | 1.16 | 1.10 | 1.11 | 1.12 | 0.03 | 0.84 | 0.60 | 0.22 |
| 4 wk in milk | 1.16 | 1.02 | 1.12 | 1.13 | 0.04 | 0.91 | 0.04 | 0.10 |
| Weaning | 1.14 | 1.11 | 1.16 | 1.14 | 0.04 | 0.81 | 0.45 | 0.89 |
| GPx, U/mL | ||||||||
| Prelambing | 15.25 | 11.97 | 13.98 | 13.49 | 2.9 | 0.91 | 0.62 | 0.53 |
| 4 wk in milk | 14.85 | 39.35 | 34.09 | 29.22 | 4.0 | 0.36 | 0.05 | <0.01 |
Sampling dates occurred prior to lambing (prelambing), after lambing (postlambing), and at weaning.
1Treatments were not supplemented (CON−), a pelleted supplement only (CON+), CON+ with a seaweed extract (SWO), and CON+ with a seaweed plus terrestrial plant extract (SWP).
2Orthogonal contrasts tested were SWO vs. SWP = differences in the extracts used (SWO compared with SWP), eff. of extract (CON+ compared with SWO and SWP), and eff. of supp. = effect of supplements (CON− compared with CON+, SWO, and SWP).
3PU = plasma urea.
4Experimental unit was the replicated pastures.
Milk composition
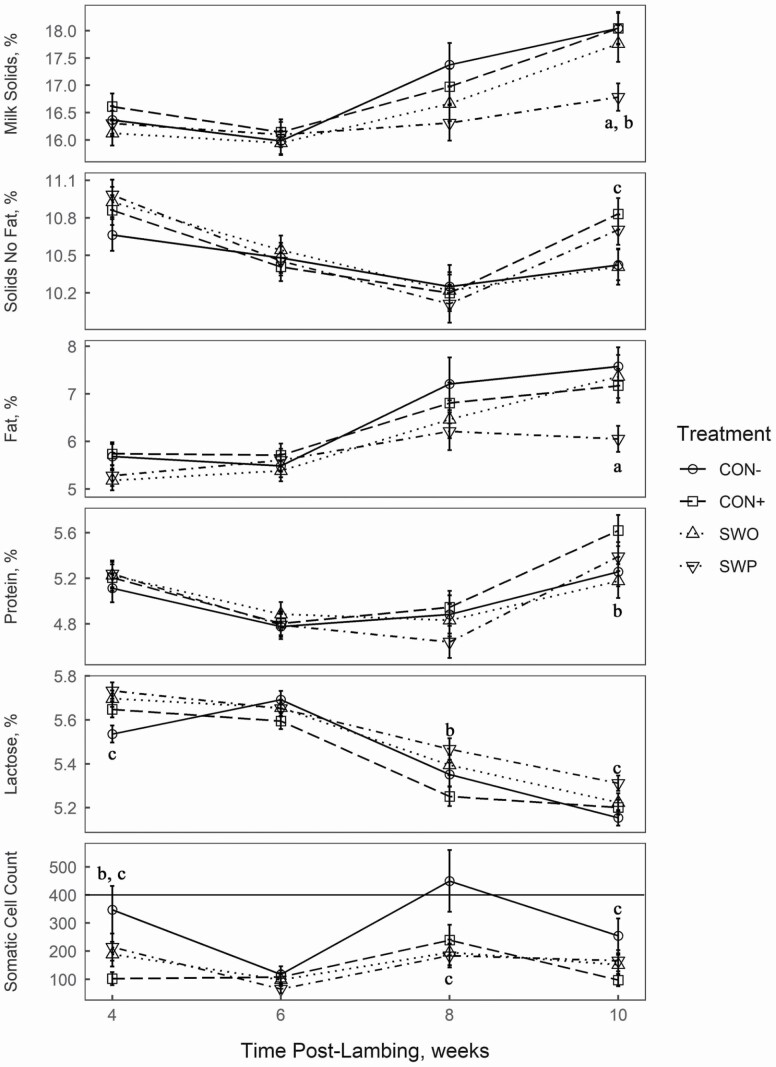
Milk constituents over the milking period (weeks 4 to 10 postlambing) are shown in Figure 1. MS percent (fat, protein, and lactose percent) were not different (P > 0.10) between treatments until week 10 after lambing. Ten weeks after lambing, SWP had less (P = 0.02) MS proportion than SWO, and CON+ greater than SWO and SWP (P = 0.02). The differences seen in MS at week 10 were largely due to difference in fat content, as SWP had lower fat content than SWO (P < 0.01). Additionally, 4 wk after lambing SWO and SWP ewes had lower (P = 0.05) fat concentration than CON+. Protein concentrations were also only different at week 10 after lambing, with CON− ewes producing less than ewes fed supplements (P = 0.05). In addition, 10 wk after lambing, SWO and SWP treatments reduced milk protein concentration compared with CON+ (P = 0.03). Ewes in CON− produced milk with lower lactose concentration (P ≤ 0.03) compared with the ewes fed supplements at weeks 4 and 10 after lambing. Ewes under SWO and SWP treatments had greater milk lactose concentration at week 8 compared with CON+ (P < 0.01). Ewes receiving supplement had lower SCC than CON− at weeks 4, 8, and 10 postlambing (P ≤ 0.03). Additionally, CON+ had lower SCC than ewes under SWO and SWP at week 4 of lactation (P = 0.03).
Figure 1.
Milk composition 4–10 wk of lactation from ewes that either received no supplement (negative control; CON−), a pelleted supplement only (positive control; CON+), CON+ with a seaweed extract (seaweed only; SWO), or CON+ with a seaweed plus terrestrial plant extract (seaweed plus; SWP) from late gestation to weaning. Superscripts within each panel within each week after lambing indicates significance (P ≤ 0.05) for the orthogonal contrasts: aSWO versus SWP, beffect of extract (CON+ compared with SWO and SWP), and ceffect of supplement (CON− compared with CON+, SWO, and SWP). Somatic cell count is in 1 × 103/mL of milk and the flat line in the SCC panel is at the suggested threshold for subclinical mastitis (400 × 103 SCC/mL).
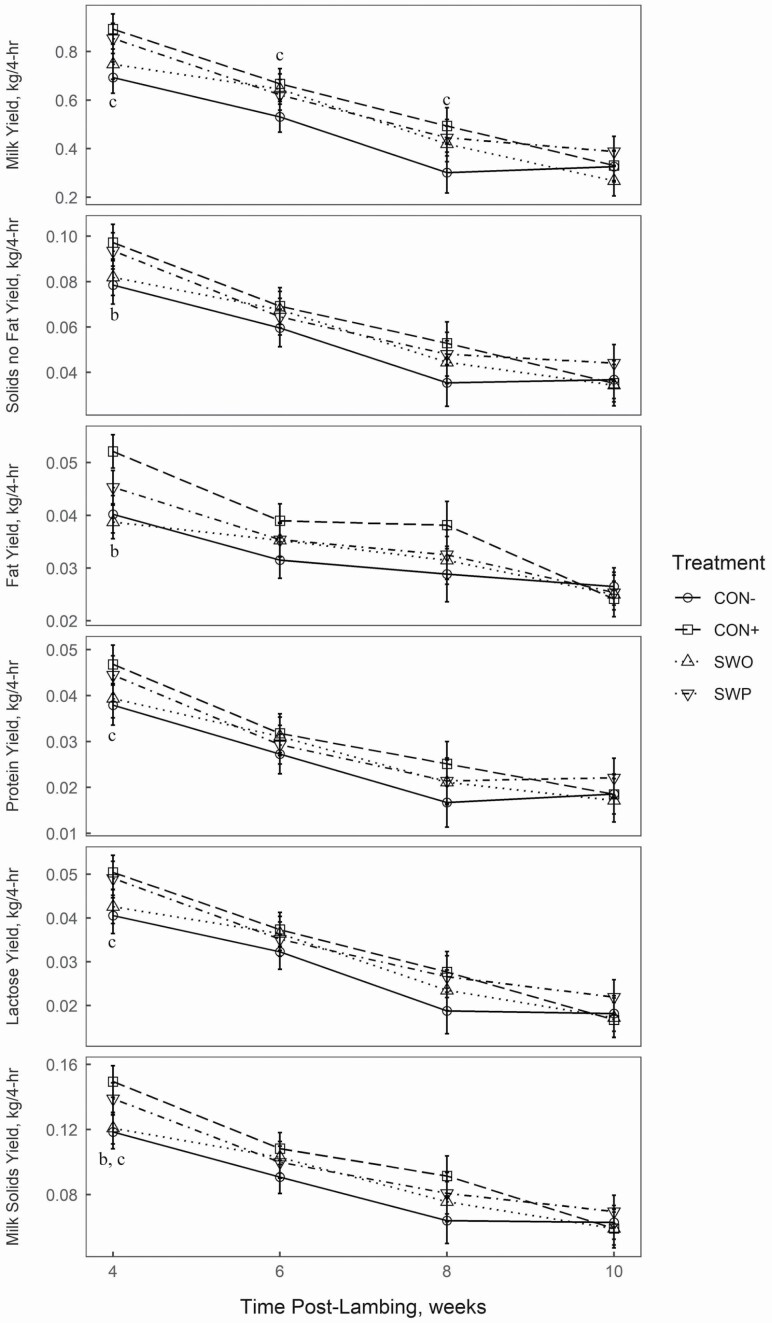
Milk yield results are displayed in Figure 2. Supplementation increased (P < 0.05) milk yield (kg/4 h) during week 4 (+20%), 6 (+22%), and 8 (+50%) of lactation. The only treatment-specific effects for fat yield, protein yield, lactose yield, and total MS yield were observed at 4 wk of lactation. The sheep provided the plant extracts (i.e., SWO and SWP) had less (P < 0.05) MS and fat yield than CON+. Additionally, the supplemented treatments (i.e., SWO, SWP, and CON+) had greater (P < 0.05) protein yield, lactose yield, and milk solids yield than the nonsupplemented ewes. MS (%) and milk yield were negatively correlated (r = −0.25; P < 0.01). Milk yield was not correlated to TAS (r = −0.10; P = 0.56) or GPx (r = −0.03; P = 0.85).
Figure 2.
Milk yields 4–10 wk of lactation from ewes that either received no supplement (negative control; CON−), a pelleted supplement only (positive control; CON+), CON+ with a seaweed extract (seaweed only; SWO), and CON+ with a seaweed plus terrestrial plant extract (seaweed plus; SWP) from late gestation to weaning. Superscripts within each panel within each week after lambing indicates significance (P ≤ 0.05) for the orthogonal contrasts: aSWO versus SWP = differences in the extracts used (i.e., SWO compared with SWP), beffect of extract (i.e., CON+ compared with SWO and SWP), and ceffect of supplement (i.e., CON− compared with CON+, SWO, and SWP).
Ruminal fluid parameters
Supplementation increased (P < 0.01) ruminal NH3 concentrations at weaning (Table 4). The SWO extract increased acetate concentration compared with SWP (P = 0.04), but there were no other treatment differences detected for acetate. There were no treatment-specific effects on propionate concentration (P ≥ 0.13). Ewes receiving supplement had greater butyrate concentration compared with CON− (P = 0.04), and ewes under SWO had greater butyrate concentration than SWP (P = 0.02). Ewes under CON− had lower valerate, iso-butyrate, and iso-valerate concentrations than the ewes receiving supplement (P ≤ 0.02). Supplementation tended to reduce the acetate:propionate ratio compared with CON− (P = 0.07), and SWP had a lower acetate:propionate ratio compared with SWO (P = 0.02). There was no treatment effect on total VFA concentration detected from supplemented ewes compared with CON− (P = 0.44) nor from the addition of the extracts compared with CON+ (P = 0.59); however, SWO tended (P = 0.06) to have greater total VFA concentration than SWP (Table 4). Ruminal NH3 was positively correlated with iso-butyrate (r = 0.82; P < 0.01) and iso-valerate (r = 0.85; P < 0.01). Plasma urea concentration was positively correlated with NH3 (r = 0.64; P < 0.01), iso-butyrate (r = 0.60; P < 0.01), and iso-valerate (r = 0.61; P < 0.01).
Table 4.
Rumen fermentation parameters of ewes sampled at weaning that either received no supplement (CON−), a pelleted supplement only (CON+), CON+ with a seaweed extract (SWO), and CON+ with a seaweed plus terrestrial plant extract (SWP) from late gestation to weaning
| Treatment1 | Orthogonal contrast P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item, mmol/L | CON− | CON+ | SWO | SWP | SE | SWO vs. SWP | Eff. of extract | Eff. of supp. |
| NH3 | 11.22 | 15.42 | 16.78 | 14.78 | 4.8 | 0.27 | 0.81 | <0.01 |
| Acetate | 59.33 | 62.20 | 65.00 | 55.11 | 6.4 | 0.04 | 0.58 | 0.72 |
| Propionate | 14.51 | 16.46 | 15.85 | 14.52 | 1.9 | 0.36 | 0.28 | 0.37 |
| Butyrate | 7.07 | 8.30 | 9.38 | 7.61 | 0.9 | 0.02 | 0.75 | 0.04 |
| Valerate | 0.67 | 0.85 | 0.91 | 0.84 | 0.1 | 0.50 | 0.75 | 0.01 |
| Hexanoate | 0.06 | 0.03 | 0.06 | 0.07 | 0.01 | 0.77 | 0.04 | 0.74 |
| Iso-butyrate | 0.86 | 0.99 | 1.09 | 1.03 | 0.16 | 0.48 | 0.28 | 0.02 |
| Iso-valerate | 1.01 | 1.20 | 1.33 | 1.24 | 0.2 | 0.44 | 0.40 | 0.01 |
| Acetate:propionate | 4.14 | 3.81 | 4.14 | 3.81 | 0.1 | 0.03 | 0.17 | 0.07 |
| Total VFA | 83.47 | 90.02 | 93.58 | 80.40 | 9.6 | 0.06 | 0.59 | 0.44 |
1Treatments were not supplemented (CON−), a pelleted supplement only (CON+), CON+ with a seaweed extract (SWO), and CON+ with a seaweed plus terrestrial plant extract (SWP).
2Orthogonal contrasts tested were SWO vs. SWP = differences in the extracts used (SWO compared with SWP), eff. of extract (CON+ compared with SWO and SWP), and eff. of supp. = effect of supplements (CON− compared with CON+, SWO, and SWP).
Discussion
It was hypothesized that energy supplementation to the dietary base would help account for the negative energy balance experienced during lactation and that such an energy supplementation fed to ewes would cause glucogenic shifts of ruminal fermentation patterns increasing energy supply and thereby ameliorating oxidative stress. Our results indicate that the energy supplementation alters fermentation patterns at weaning by increasing butyrate, valerate, iso-butyrate, and iso-valerate concentrations, increasing ruminal NH3, and tending to reduce the acetate-to-propionate ratio, but increasing oxidative stress at peak lactation. Therefore, the hypothesis was rejected. An additional hypothesis was that oxidative stress is alleviated through the addition of plant extracts (SWO and SWP). The results of the current study support this hypothesis, albeit GPx activities of SWO and SWP ewes were still greater than CON− ewes.
Oxidative stress
Energy supplementation increased oxidative stress 4-wk postlambing, at peak lactation for lactating ewes (Cardellino and Benson, 2002). This is evidenced by the tendency for CON− to have greater TAS and significantly less GPx activity compared with CON+, SWO, and SWP. The reason for supplemental ewes only tending to have lower TAS than CON− ewes was likely due to SWO and SWP being included in the contrast. If we were to have compared CON+ and CON−, they would have likely been significantly different. Total antioxidant status is a biological marker that attempts to measure the overall antioxidant capacity in biological samples and likely provides a better estimate of antioxidant status of animals than simply quantifying individual antioxidants (Ghiselli et al., 2000). Additionally, measurements of antioxidant enzymes, such as GPx, provide estimates of oxidative stress (Celi, 2011). Thus, GPx activity has been used to measure oxidative stress in livestock. Bernabucci et al. (2002) determined increased GPx activity and MDA (product of lipid peroxidation) in dairy cows transitioning from dry to lactating in the summer than in the spring, indicating that heat stress induces oxidative stress. Although the SWO and SWP ewes did not have as low of GPx levels as the CON− ewes, they still had a 19.6% reduction compared with CON+ ewes. Additionally, SWO and SWP ewes had 10.3% lower plasma TAS than CON+ ewes. Therefore, when TAS and GPx results from the current experiment are taken together, energy supplementation increased GPx activity and reduced plasma TAS, but this effect of supplementation was alleviated by the plant extracts (SWO and SWP).
The current results contrast with several reports. Sgorlon et al. (2008) was unable to detect any differences in GPx activity in ewes fed a high starch diet (28% starch in the diet) compared with their control counterparts 35- or 50-d postlambing. Additionally, a lower density energy diet (5.6% corn) fed to goats resulted in a lower antioxidant capacity at parturition compared with a higher energy diet (19.7% corn; Celi et al., 2010). However, the differences between these experiments and the current one could be explained by the physiological stage of the animals or due to differences in milk yield. Several works have linked milk production and oxidative stress (Castillo et al., 2003; Gabai et al., 2004; Lohrke et al., 2004). Castillo et al. (2003) determined that dairy cows at peak lactation (35 L/cow and day) had greater plasma hydroperoxide (measurement of oxidative stress) than cows at sixth month of lactation (20 L/cow and day). This agrees with Lohrke et al. (2004), who found a strong positive correlation (r = 0.86) between daily milk yield and plasma hydroperoxides in dairy cows. However, in the current experiment, we found no correlation between milk yield and TAS or GPx at 4 weeks of lactation, indicating that the increased oxidative stress associated with the starch supplementation may be due to another cause.
Another possibility to explain these results is potential changes in metabolism associated with the different diets given to the sheep. Wullepit et al. (2009) determined that a high energy diet (providing 8 kg of concentrate per d) compared with a lower energy diet (providing 3 kg of concentrate per d) fed to dairy heifers resulted in a lower ferric-reducing ability of plasma, which indicates reduced antioxidant status in the plasma. However, the remaining oxidative stress markers measured were inconclusive (Wullepit et al., 2009). Furthermore, dairy cows provided diets consisting of 20% wheat, had lower TAS, and increased superoxide dismutase and GPx activity than those provided 10 or 0% wheat (Guo et al., 2013). When growing lambs were fed different diets containing 83, 52, or 0% maize in the diet, the lambs provided grain had greater MDA concentrations in erythrocytes (Singh et al., 2011). Additionally, Gabai et al. (2004) found that feeding starch based concentrates to lactating dairy cows increased oxidative stress, as evidenced by the greater (20% increase) MDA levels at day 80 of lactation. Peak lactation for dairy cows generally occurs around day 80 of lactation (Hutjens, 2011) and would thus be a comparable point in the lactation curve to the current experiment, as 4 wk in milk will be near peak lactation for lactating ewes (Cardellino and Benson, 2002). Such a negative effect of a starch-based energy concentrate has been explained by changes related to oxidative phosphorylation (Gabai et al., 2004). Oxidative phosphorylation occurs in the mitochondria and is a major source of oxidants (namely superoxide anion) in mammals (Murphy, 2009). The majority of these oxidants are generated by complex I of the electron transport chain (Murphy, 2009). In ruminants, acetate is the predominant source of energy and after conversion to acetyl-CoA, it can be converted into ketone bodies, which can be used for energy by the peripheral tissues, or enter into the Krebs cycle through condensation with oxaloacetate, which requires propionate, glucogenic amino acids, lactate, or glycerol to generate. In the events where the acetate-to-propionate ratios are high, acetate will be metabolized at greater rates through conversion to ketone bodies and utilization by peripheral tissues. However, when propionate production is at a rate where gluconeogenesis needs are met, a greater proportion of acetate will enter into the Krebs cycle, thus causing increases in normal oxidative metabolism, through oxidative phosphorylation (Van Soest, 1994). Thus, the increased oxidative stress in our ewes consuming supplement could be attributed to changes in metabolic processes, such as increased oxidative phosphorylation. We speculate that when the oxidants produced from increased oxidative phosphorylation were paired with increased energy demand at peak lactation, the production of oxidants outpaced the antioxidant defense, thus resulting in oxidative stress.
Although feeding energy concentrate to the ewes increased oxidative stress, the additional plant extracts alleviated this stress. The SWO and SWP extracts are fermented extract products and therefore contain natural antioxidants from the extracted plants (i.e., seaweed and/or the terrestrial plants), but also lactic acid producing bacteria and Lactobacillus fermentation products (metabolites from microbial fermentation). Plant extracts can provide potent antioxidants due to their plant secondary compounds (PSC; namely mono- and poly-phenols; Rice-Evans et al., 1997). There is evidence to support several modes of actions for PSC as antioxidants. These include by reducing oxidants in the gastrointestinal tract, directly reducing oxidants at the tissue level, and by regulating gene expression (namely nuclear factor erythroid 2-related factor 2; Beck and Gregorini, 2020). Moreover, Lactobacillus fermentation products can exhibit antioxidant activities. When finishing steers in a feedlot system were provided with Lactobacillus fermentation products (at a similar dose to the one used here), there was an increased blood antioxidant status and reduced GPx activity (Ran et al., 2019). Thus, it is proposed that the increased antioxidant status provided by the plant extracts (SWO and SWP) in the current study may provide health benefits through a combination of PSC and Lactobacillus fermentation products.
Ruminal fluid and plasma urea
Supplementation altered ruminal fermentation patterns at weaning. These differences were namely a tendency for reduced acetate-to-propionate ratio, and increased butyrate, valerate, the branch chain VFA, and ruminal NH3 concentrations. A reduced acetate-to-propionate ratio is expected with increased starch intake as propionate production is increased from starch fermentation in the rumen (Orskov, 1986). Increased ruminal NH3 concentrations in the rumen is less expected, on the one hand, as typically starch feeding would namely 1) reduce daily N intake (Castillo et al., 2001) and 2) increase the proportion of N consumed captured as microbial cell protein (i.e., increase the efficiency of N use; Bach et al., 2005). On the other hand, there are measurements at weaning that support this finding, particularly the elevated iso-butyrate and iso-valerate (branch-chained VFA) concentrations in the ruminal fluid. These are a product of ruminal fermentation of branch-chained amino acids, and subsequently, they have been suggested as markers for ruminal fermentation of amino acids, which would explain the increased ruminal NH3 concentrations (Apajalahti et al., 2019). This relationship between ruminal NH3 and branch-chained VFA was observed in this current study, as the correlation between NH3 and iso-butyrate and iso-valerate across animals were strong (r = 0.82 and 0.85, respectively). Additionally, plasma urea N concentration was increased at weaning for the supplemented ewes. Following absorption through the ruminal wall, NH3 is rapidly converted in the liver to urea and either recycled to the rumen, or excreted through the urine. As such, urea in the blood is a strong indicator of rumen fermentation of amino acids (Bach et al., 2005). Consequently, plasma urea concentrations were positively correlated to ruminal NH3 (r = 0.64), iso-butyrate (r = 0.60), and iso-valerate (r = 0.61) concentrations. Therefore, it appears that the supplemented ewes had greater ruminal protein degradation at weaning than the unsupplemented ewes.
While unexpected, further investigation into the literature provides a possible explanation for the starch supplements increasing protein degradation in the rumen at weaning (Bach et al., 2005). This is by potential differences in microbial populations between the supplemented and nonsupplemented treatments. By applying the liquid extracts to the supplement, this may alter the bacterial profile of the feed, which may influence the results determined in this experiment. Additionally, supplemental starch may result in shifts of microbial populations to greater amounts of starch fermenting bacteria which would mean greater amylase activity in the rumen (Nozière and Michalet-Doreau, 1997). Amylase has been shown to increase protein degradation by several experiments (see Bach et al., 2005). This is due to the inability of protein degrading bacteria containing proteolytic enzymes to attach to carbohydrate bound proteins (Bach et al., 2005). However, independently of these possible explanations, plasma urea was not elevated at any of the other time points sampled, which may suggest that these results are an artifact of the sampling time, and not necessarily a result from the starch supplement.
Ewe performance
Ewes under CON− had two incidences of high SCC (>300 × 103 SCC/mL; weeks 4 and 8). Additionally, the SWP and SWO treatments had greater SCC than the CON+ treatment at week 4 of lactation (Figure 1). This greater SCC for CON− was unexpected, since increments in SCC are indicators of mastitis, which is associated with increased inflammation, and thus, oxidative stress (Harmon, 1994). Moreover, the current results indicated that CON− ewes had greater TAS and lower GPx activity, yet shown several incidence of high levels of SCC. Despite such a disagreement, some reports support the current findings. Feeding lactating dairy cow’s pomegranate pulp silage (source of phenolic compounds as natural antioxidants) improved antioxidant status with no subsequent improvement in SCC of milk (Kotsampasi et al., 2017). The authors speculate that the current results are related to the relatively low SCC observed in all of our treatments, even the CON−. A SCC greater than 400 × 103/mL of milk is the threshold for subclinical mastitis in lactating ewes (Kern et al., 2013; Zafalon et al., 2016; Persson et al., 2017). There was only one instance where this was exceeded which was CON− at week 8 of lactation (437.5 ± 105.3 × 103 SCC; mean ± SE). All other SCC were below this threshold (Figure 1). Additionally, at week 4, when the blood samples were collected, the SCC of CON− was less than the suggested threshold (332.3 ± 79.8 × 103 SCC; mean ± SE) and much less for the ewes provided the extract products (≤214.2 ± 47.8 × 103; mean ± SE). Therefore, we speculate that the level of inflammation occurring in the mammary glands of the sheep was not great enough to disrupt the homeostatic balance of oxidants and antioxidants in the blood of the sheep. It is recommended that further research is needed to confirm these results.
There were some observed differences in milk composition, especially at week 10 of lactation. Overall, the trend of the milk composition data followed similar patterns to those in the literature. Generally, as milk yield (kilogram of milk per day) decreases throughout lactation, the corresponding concentration of milk solids will increase (Sakul and Boylan, 1992). What was less expected was the differences in milk solid percent at week 10 of lactation between SWP and the other treatments. However, there were no differences in milk solids yield between the treatments, indicating that the differences in milk solid percent may be more of a function of numerical (albeit not statistically significant) milk yield.
Milk yield was increased by starch supplementation at weeks 4, 6, and 8 of lactation. In production systems based on high-quality forages, a relatively small addition (0.2 to 0.6% of body weight) of energy (both starch based and fiber based) supplement can increase performance. This is because it corrects an imbalance that exists in terms of a large amount of rumen degradable protein compared with digestible OM. Even though the forage, which was harvested to ground level, was measured to contain only 11% CP, this is likely lower than what the ewes actually consumed due to selectivity of the animals. Although the current experiment fed a relatively lower amount of supplement, around 0.15% of BW, we speculate that this mechanism is the reason for the greater milk production, even if the effects on milk production were modest.
Conclusions
Energy supplement feeding increased oxidative stress compared with nonsupplemented lactating ewes 4-wk postpartum, corresponding to peak lactation. Such a stress can be alleviated by feeding fermented seaweed and seaweed plus terrestrial plants. To our knowledge, these are the first results showing how an energy supplement and plant-based fermented products influence the redox balance, milk constituents, and fermentation parameters of ewes from late gestation through to weaning.
Acknowledgments
We acknowledge the beneficial help of Rosy Tung with laboratory work. We also express gratitude toward AgriSea Ltd. and Callaghan Innovation (Wellington, New Zealand) for funding M.B.’s PhD studies. This experiment was funded by AgriSea NZ Seaweed Ltd. (Paeroa, New Zealand).
Glossary
Abbreviations
- ADF
acid detergent fiber
- ADG
average daily gain
- CP
crude protein
- DM
dry matter
- DOMD
digestible OM in the DM
- DPPH
2,2-diphenyl-1-picryl-hyrazyl-hydrate
- GPx
glutathione peroxidase
- MDA
malondialdehyde
- ME
metabolizable energy
- MS
milk solids
- N
nitrogen
- NDF
neutral detergent fiber
- NH3
ammonia
- OM
organic matter
- PSC
plant secondary compounds
- PU
plasma urea
- SCC
somatic cell count
- TAS
total antioxidant status
- VFA
volatile fatty acid
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- AOAC . 1990. Official methods of analysis. 15th ed. Arlington (VA): Association of Official Analytical Chemists. [Google Scholar]
- Apajalahti, J., Vienola K., Raatikainen K., Holder V., and Moran C. A.. . 2019. Conversion of branched-chain amino acids to corresponding isoacids – an in vitro tool for estimating ruminal protein degradability. Front. Vet. Sci. 6:311. doi: 10.3389/fvets.2019.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, A., Calsamiglia S., and Stern M. D.. . 2005. Nitrogen metabolism in the rumen. J. Dairy Sci. 88:E9–E21. doi: 10.3168/JDS.S0022-0302(05)73133-7 [DOI] [PubMed] [Google Scholar]
- Bates, D., Maechler M., Bolker B., and Walker S.. . 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67:1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beck, M. R., and Gregorini P.. . 2020. How dietary diversity enhances hedonic and eudaimonic well-being in grazing ruminants. Front. Vet. Sci. 7:191. doi: 10.3389/fvets.2020.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabucci, U., Ronchi B., Lacetera N., and Nardone A.. . 2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 85:2173–2179. doi: 10.3168/jds.S0022-0302(02)74296-3 [DOI] [PubMed] [Google Scholar]
- Cardellino, R. A., and Benson M. E.. . 2002. Lactation curves of commercial ewes rearing lambs. J. Anim. Sci. 80:23–27. doi: 10.2527/2002.80123x [DOI] [PubMed] [Google Scholar]
- Castillo, A. R., Kebreab E., Beever D. E., Barbi J. H., Sutton J. D., Kirby H. C., and France J.. . 2001. The effect of energy supplementation on nitrogen utilization in lactating dairy cows fed grass silage diets. J. Anim. Sci. 79:240–246. doi: 10.2527/2001.791240x [DOI] [PubMed] [Google Scholar]
- Castillo, C., Hernández J., Lopez-Alonso M., Miranda M., and Benedito J. L.. . 2003. Values of plasma lipid hydroperoxides and total antioxidant status in healthy dairy cows: preliminary observations. Arch. Tierzucht. 46:227–233. doi: 10.5194/aab-46-227-2003 [DOI] [Google Scholar]
- Celi, P. 2010. The role of oxidative stress in small ruminants’ health and production. Rev. Bras. Zootec. 39:348–363. doi: 10.1590/S1516-35982010001300038 [DOI] [Google Scholar]
- Celi, P. 2011. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 33:233–240. doi: 10.3109/08923973.2010.514917 [DOI] [PubMed] [Google Scholar]
- Celi, P., Di Trana A., and Claps S.. . 2010. Effects of plane of nutrition on oxidative stress in goats during the peripartum period. Vet. J. 184:95–99. doi: 10.1016/j.tvjl.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Chirase, N. K., Greene L. W., Purdy C. W., Loan R. W., Auvermann B. W., Parker D. B., E. F.Walborg, Jr., Stevenson D. E., Xu Y., and Klaunig J. E.. . 2004. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am. J. Vet. Res. 65:860–864. doi: 10.2460/ajvr.2004.65.860 [DOI] [PubMed] [Google Scholar]
- Dixon, R. M., White A., Fry P., and Petherick J. C.. . 2003. Effects of supplement type and previous experience on variability in intake of supplements by heifers. Aust. J. Agric. Res. 54:529–540. doi: 10.1071/AR02091 [DOI] [Google Scholar]
- Fidan, A. F., Kucukkurt I., Eryavuz A., Cigerci I. H., Yardimci M., and Ozdemir A.. . 2009. Effects of shearing procedures on oxidant–antioxidant status in Chios sheep. Rev. Med. Vet. 7:349–355. [Google Scholar]
- Fidan, A. F., Pamuk K., Ozdemir A., SaritaŞ Z. K., and Tarakci U.. . 2010. Effects of dehorning by amputation on oxidant–antioxidant status in mature cattle. Rev. Med. Vet. 161:502–508. [Google Scholar]
- Gabai, G., Testoni S., Piccinini R., Marinelli L., and Stradaioli G.. . 2004. Oxidative stress in primiparous cows in relation to dietary starch and the progress of lactation. Anim. Sci. 79:99–108. doi: 10.1017/S1357729800054576 [DOI] [Google Scholar]
- Ghiselli, A., Serafini M., Natella F., and Scaccini C.. . 2000. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic. Biol. Med. 29:1106–1114. doi: 10.1016/s0891-5849(00)00394-4 [DOI] [PubMed] [Google Scholar]
- Guo, Y., Xu X., Zou Y., Yang Z., Li S., and Cao Z.. . 2013. Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with subacute ruminal acidosis and its regulation with pelleted beet pulp. J. Anim. Sci. Biotechnol. 4:31. doi: 10.1186/2049-1891-4-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon, R. J. 1994. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 77:2103–2112. doi: 10.3168/jds.S0022-0302(94)77153-8 [DOI] [PubMed] [Google Scholar]
- Hunter, T. E., Suster D., DiGiacomo K., Dunshea F. R., Cummins L. J., Egan A. R., and Leury B. J.. . 2015. Milk production and body composition of single-bearing East Friesian×Romney and Border Leicester×Merino ewes. Small Rumin. Res. 131:123–129. doi: 10.1016/j.smallrumres.2015.08.006 [DOI] [Google Scholar]
- Hutjens, M. F. 2011. Dairy farm management|Dry lot dairy cow breeds. In: J. W. Fuguay, editor. Encyclopedia of dairy sciences. p. 52–58. [Google Scholar]
- Iowerth, D., Jones H., and Hayward M. V.. . 1975. The effect of pepsin pretreatment of herbage on the prediction of dry matter digestibility from solubility in fungal cellulase solutions. J. Sci. Food Agric. 26:711–718. doi: 10.1002/jsfa.2740260518 [DOI] [Google Scholar]
- Izuddin, W. I., Humam A. M., Loh T. C., Foo H. L., and Samsudin A. A.. . 2020. Dietary postbiotic Lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants 9:1–13. doi: 10.3390/antiox9030250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan, G., Saker K. E., Terrill T. H., Kouakou B., Galipalli S., and Gelaye S.. . 2007. Effect of seaweed extract supplementation in goats exposed to simulated preslaughter stress. Small Rumin. Res. 73:221–227. doi: 10.1016/j.smallrumres.2007.02.006 [DOI] [Google Scholar]
- Kern, G., Traulsen I., Kemper N., and Krieter J.. . 2013. Analysis of somatic cell counts and risk factors associated with occurrence of bacteria in ewes of different primary purposes. Livest. Sci. 157:597–604. doi: 10.1016/j.livsci.2013.09.008 [DOI] [Google Scholar]
- Kotsampasi, B., Christodoulou C., Tsiplakou E., Mavrommatis A., Mitsiopoulou C., Karaiskou C., Dotas V., Robinson P. H., Bampidis V. A., Christodoulou V., . et al. 2017. Effects of dietary pomegranate pulp silage supplementation on milk yield and composition, milk fatty acid profile and blood plasma antioxidant status of lactating dairy cows. Anim. Feed Sci. Technol. 234:228–236. doi: 10.1016/j.anifeedsci.2017.08.017 [DOI] [Google Scholar]
- Lane, D. J., Pace B., Olsen G. J., Stahlt D. A., Sogint M. L., and Pace N. R.. . 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses (reverse transcriptase/dideoxynudeotide). Proc. Natl. Acad. Sci. USA 82:6955–6959. doi: 10.1073/pnas.82.20.6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, R. 2018. emmeans: Estimated marginal means, aka Least-Squares Means. R package version 1.2. Available from https://cran.r-project.org/package=emmeans
- Lohrke, B., Vierguts T. T., Kanitz W., Gollnitz K., Becker F., Hurtienne A., and Schweigert F. J.. . 2004. High milk yield in dairy cows associated with oxidant stress. J. Vet. Res. 8:70–78. [Google Scholar]
- MAFF . 1986. Carbohydrates, soluble, in herbage. 3rd ed. London (UK): HMSO. [Google Scholar]
- Murphy, M. P. 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417:1–13. doi: 10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeley, W. E., and Phillipson J.. . 1988. Automated enzymatic method for determining ammonia in plasma, with 14-day reagent stability. Clin. Chem. 34:1868–1869. doi: 10.1093/clinchem/34.9.1866 [DOI] [PubMed] [Google Scholar]
- NIWA . 2021. The national climate database. Available from https://cliflo.niwa.co.nz/. Accessed 4 January 2021.
- Nozière, P., and Michalet-Doreau B.. . 1997. Effects of amount and availability of starch on amylolytic activity of ruminal solid-associated microorganisms. J. Sci. Food Agric. 73:471–476. doi: [DOI] [Google Scholar]
- O’Sullivan, A. M., O’Callaghan Y. C., O’Grady M. N., Queguineur B., Hanniffy D., Troy D. J., Kerry J. P., and O’Brien N. M.. . 2011. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 126:1064–1070. doi: 10.1016/j.foodchem.2010.11.127 [DOI] [Google Scholar]
- Olejar, K. J., Ricci A., Swift S., Zujovic Z., Gordon K. C., Fedrizzi B., Versari A., and Kilmartin P. A.. . 2019. Characterization of an antioxidant and antimicrobial extract from Cool Climate, White Grape Marc. Antioxidants 8:1–13. doi: 10.3390/antiox8070232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov, E. R. 1986. Starch digestion and utilization in ruminants. J. Anim. Sci. 63:1624–1633. doi: 10.2527/jas1986.6351624x [DOI] [PubMed] [Google Scholar]
- Persson, Y., Nyman A. K., Söderquist L., Tomic N., and Waller K. P.. . 2017. Intramammary infections and somatic cell counts in meat and pelt producing ewes with clinically healthy udders. Small Rumin. Res. 156:66–72. doi: 10.1016/j.smallrumres.2017.09.012 [DOI] [Google Scholar]
- Primary Industries Standing Committee. 2007. Nutrient requirements of domesticated ruminants. CSIRO Publishing. [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Ran, T., Gomaa W. M. S., Shen Y. Z., Saleem A. M., Yang W. Z., and McAllister T. A.. . 2019. Use of naturally sourced feed additives (Lactobacillus fermentation products and enzymes) in growing and finishing steers: effects on performance, carcass characteristics and blood metabolites. Anim. Feed Sci. Technol. 254:114190. doi: 10.1016/j.anifeedsci.2019.05.013 [DOI] [Google Scholar]
- Rice-Evans, C., Miller N., and Paganga G.. . 1997. Antioxidant properties of phenolic compounds. Trends Plant Sci. 2:152–159. doi: 10.1016/S1360-1385(97)01018-2 [DOI] [Google Scholar]
- Sakul, H., and Boylan W. J.. . 1992. Lactation curves for several US sheep breeds. Anim. Prod. 54:229–233. doi: 10.1017/S0003356100036849 [DOI] [Google Scholar]
- Sgorlon, S., Stradaioli G., Gabai G., and Stefanon B.. . 2008. Variation of starch and fat in the diet affects metabolic status and oxidative stress in ewes. Small Rumin. Res. 74:123–129. doi: 10.1016/j.smallrumres.2007.04.004 [DOI] [Google Scholar]
- Singh, V. K., Pattanaik A. K., Sharma K., and Saini M.. . 2011. Effect of dietary energy intake on erythrocytic antioxidant defence in growing lambs fed a wheat straw-based diet. Anim. Prod. Sci. 51:642. doi: 10.1071/an10098 [DOI] [Google Scholar]
- Sordillo, L. M., and Aitken S. L.. . 2009. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 128:104–109. doi: 10.1016/j.vetimm.2008.10.305 [DOI] [PubMed] [Google Scholar]
- Sordillo, L. M., Contreras G. A., and Aitken S. L.. . 2009. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev. 10:53–63. doi: 10.1017/S1466252309990016 [DOI] [PubMed] [Google Scholar]
- Van Soest, P. J. 1994. Nutritional ecology of the ruminant. 2nd ed. Ithaca (NY): Cornell University Press. [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Wullepit, N., Raes K., Beerda B., Veerkamp R. F., Fremaut D., and De Smet S.. . 2009. Influence of management and genetic merit for milk yield on the oxidative status of plasma in heifers. Livest. Sci. 123:276–282. doi: 10.1016/j.livsci.2008.11.013 [DOI] [Google Scholar]
- Yusuf, O. M., Logan C. M., Ridler A., and Greer A. W.. . 2018. Investigation into udder characteristics, mastitis and milk production in crossbred sheep. New Zeal. J. Anim. Sci. Prod. 78:82–87. [Google Scholar]
- Zafalon, L. F., Santana R. C., Pilon L. E., and Júnior G. A.. . 2016. Diagnosis of subclinical mastitis in Santa Inês and Morada Nova sheep in southeastern Brazil. Trop. Anim. Health Prod. 48:967–972. doi: 10.1007/s11250-016-1046-1 [DOI] [PubMed] [Google Scholar]