Abstract
Oxidative stress seriously affects poultry production. Nutritional manipulations have been effectively used to alleviate the negative effects caused by oxidative stress. This study investigated the attenuating effects and potential mechanisms of dietary taurine on the growth performance and meat quality of broiler chickens challenged with hydrogen peroxide (H2O2). Briefly, a total of 192 male Arbor Acres broilers (28 d old) were randomly categorized into three groups: non-injection of birds on basal diets (control), 10.0% H2O2 injection of birds on basal diets (H2O2), and 10.0% H2O2 injection of birds on basal diets supplemented with 5 g/kg taurine (H2O2 + taurine). Each group consisted of eight cages of eight birds per cage. Results indicated that H2O2 administration significantly reduced growth performance and impaired breast meat quality by decreasing ultimate pH and increasing shear force value (P < 0.05). Dietary taurine improved the body weight gain and feed intake and decreased feed/gain ratio of H2O2-challenged broilers. Meanwhile, oxidative stress induced by intraperitoneal injection of H2O2 suppressed the nuclear factor-κB (NF-κB) signaling and initiated autophagy and apoptosis. Compared with the H2O2 group, taurine supplementation restored the redox status in the breast muscle by decreasing levels of reactive oxygen species and contents of oxidative products and increasing antioxidant capacity (P < 0.05). Moreover, upregulated mRNA expression of NF-κB signaling-related genes, including NF-κB subunit 1 (p50) and B-cell CLL/lymphoma 2 (Bcl-2), and enhanced protein expression of NF-κB were observed in the H2O2 + taurine group (P < 0.05). Additionally, dietary taurine decreased the expression of caspase family, beclin1, and microtubule-associated protein 1light chain 3 beta (LC3-II; P < 0.05), thereby rescuing autophagy and apoptosis in breast muscle induced by H2O2. Collectively, dietary supplementation with taurine effectively improves growth performance and breast meat quality of broilers challenged with H2O2, possibly by protecting against oxidative injury and modulating cell death signaling.
Keywords: apoptosis, autophagy, broilers, meat quality, oxidative stress, taurine
Introduction
Oxidative stress constitutes a continuous challenge for the modern poultry industry globally. Oxidative stress occurs when the balance of prooxidants and endogenous antioxidants in living system is disturbed, which can lead to the overproduction of free radicals (Sies et al., 2017). Excessive reactive oxygen species (ROS) attack macromolecules, including nucleic acids, proteins, and lipids, resulting in cellular oxidative damage and the subsequent dysfunction (Lykkesfeldt and Svendsen, 2007). Accumulating evidence suggested that oxidative stress could compromise health condition and impair the growth performance and meat quality of broiler chickens, which negatively impacts poultry production (Estévez, 2015).
Environmental heat, oxidized diets, and exposure to toxins and excessive heavy metals, as well as other predisposing factors, have been well documented to induce oxidative stress in poultry (Xing et al., 2019). As a central redox metabolite, hydrogen peroxide (H2O2) can be generated by almost all the aforementioned oxidative stressors, and it diffuses through cells and tissues, resulting in the disequilibrium of redox status (Sies et al., 2017). Yin et al. (2015) indicated that intragastric administration of H2O2 caused oxidative stress and disrupted intestinal permeability and barrier function in piglets. Recently, we also demonstrated that intraperitoneal injection of H2O2 induced oxidative stress in broiler chickens and led to a decline in growth performance and deterioration in meat quality (Chen et al., 2017, 2018).
Oxidative stress triggered in response to various insults or pathological states is closely associated with the induction of programmed cell death, including apoptosis and autophagy (Ryter et al., 2007). As important signaling molecules, ROS can promote the opening of the mitochondria permeability transition pore and destroy membrane potential, stimulating intrinsic apoptosis (Simon et al., 2000). ROS also regulate autophagy via various signals such as ROS-TP53-induced glycolysis and apoptosis regulator (TIGAR)-autophagy and ROS-hypoxia inducible factor (HIF1)-BNIP3/NIX-autophagy (Li et al., 2015). Accumulating evidence suggests that the nuclear factor-κB (NF-κB) signaling is involved in ROS-mediated autophagy and apoptosis (Sies et al., 2017). Chen et al. (2016) indicated that daily exposure to 0.2, 0.4, or 0.8 mg/kg aflatoxin B2 induced the excessive production of ROS and triggered mitochondria-mediated apoptosis and PI3K/Akt/mammalian target of rapamycin (mTOR)-mediated autophagy in hepatocytes of 21-d-old broilers. Administration of H2O2 caused oxidative stress and initiated the autophagy response mediated by NF-κB signaling in the jejunum of piglets (Yin et al., 2015). Correspondingly, our recent studies indicated that broilers exposed to H2O2 exhibited elevated apoptosis and autophagy responses as well as suppressed NF-κB in both the breast muscle and liver tissues (Chen et al., 2017, 2018). This implied that the oxidative damage caused by H2O2 could be attributed to the induction of apoptosis or autophagy mediated by NF-κB signaling.
Taurine is a ubiquitous sulfur-containing amino acid existing in most of the types of animal tissues, and it plays essential physiological functions, including osmoregulation, calcium homeostasis, and antioxidant function (Huxtable, 1992). Dietary taurine supplementation (5 g/kg) enhanced free radical scavenging activities and improved the antioxidant capacities in the breast muscle of broilers (Xu et al., 2020). Moreover, the ameliorative effects of taurine on the redox status were achieved by reducing ROS generation and protecting mitochondria from oxidative attack in the breast muscle of heat-stress broilers (Lu et al., 2019b). Therefore, taurine appears to be an effective antioxidant in protecting muscle cells from damage by free radicals in broiler chickens. Furthermore, taurine has been reported to counteract arsenic-induced autophagy and apoptotic cell death by modulating ROS generation in mammals (Das et al., 2009; Bai et al., 2016). Additionally, taurine might exert these beneficial roles via regulating the NF-κB pathway (Das et al., 2009; Roy et al., 2009). However, there is limited information on the efficacy and mechanism of taurine on oxidative injury in poultry.
In this study, we hypothesized that dietary taurine can improve the redox status and modulate cell death signaling, thereby alleviating the negative impacts of oxidative stress on growth performance and meat quality in broilers. Therefore, we evaluated the influence of taurine supplementation on the growth performance, meat quality, and oxidative damage of broilers administrated with H2O2. Furthermore, we also examined the expression patterns of factors involved in apoptosis and autophagy as well as the regulation of NF-κB signaling to elucidate the possible alleviating mechanism.
Materials and Methods
Preparation of H2O2 solution
The 10.0% H2O2 solution was prepared by serially diluting a 30% H2O2 solution (Sigma-Aldrich Co. Ltd., Shanghai, China) with 0.75% sodium chloride buffer (saline). The H2O2 solution was protected from light in a dry place. The concentration was determined by using a commercially available assay kit (A064, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Bird management and treatments
The experimental procedures and bird management were approved by the Animal Care and Use Committee of Nanjing Agricultural University under protocol number SYXK 2017-0007. A total of 250 newly hatched male Arbor Acres broiler chicks were obtained from a commercial hatchery (Hewei Agricultural Development Co. Ltd, Xuancheng, China). All chicks were provided with commercial standard diets and management from 1 to 27 d of age. At 28 d of age, a total of 192 broilers with similar body weight (BW; 1,089.24 ± 28.53 g) were selected and randomly allocated into three groups, with eight cages per group and eight birds per cage. In the control group, birds were fed a basal diet and received no injections; in the H2O2 group, birds were fed the basal diet and given an intraperitoneal injection of 10% H2O2, with a dosage of 1.0 mL/kg BW, calculated as 2.96 mmol H2O2/kg BW; in the H2O2 + taurine group, birds were fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group. Taurine (>99.9% purity) was purchased from NOW FOODS (Bloomingdale, IL, USA). The experimental period was 14 d, and all birds in the H2O2 and H2O2 + taurine groups were administered the injections on day 9 of the experiment (at 37 d of age). Birds were reared in steel three-layer cages (length × width × height: 110 × 60 × 50 cm) with plastic floors. All birds were allowed ad libitum access to feed and water from 28 to 42 d old. Room temperature was kept between 32 °C and 34 °C from days 1 to 3 and reduced to a final temperature of 20 °C at the rate of 2 °C to 3 °C per week. All birds were exposed to continuous fluorescent illumination at a rate of 23:1 (L:D) h during the 14-d experiment period. The composition and nutrition levels of the basal diet are given in Supplementary Table 1. After feed deprivation for 12 h, BW and feed consumption were recorded on a replicate basis at 42 d of age to calculate the average daily gain (ADG), average daily feed intake (ADFI), and gain:feed ratio (G:F).
Sample collection and preparation
At the end of the experiment, one bird per cage (close to the replicate average BW) was selected. The birds were euthanized by cervical dislocation and necropsied immediately for pectoralis major (PM) muscle collection. Each sample from the left side of the PM muscle was removed and placed in an RNAase-free tube, frozen in liquid nitrogen, and stored at −80 °C until required for analysis. The other PM muscles were removed from the carcass and stored at 4 °C for meat quality measurement.
Meat quality measurement
Muscle pH at 24 h postmortem (pH24 h) was measured in triplicate by using a glass electronic pH meter (Hanna Instrument Company, Cluj-Napoca, Romania).
Meat color (L* = lightness, a* = redness, and b* = yellowness) at 24 h postmortem was measured with a colorimeter (Minolta CR400, Konica Minolta Co. Ltd., Osaka, Japan). The average of the measurements obtained at three different locations of the thickest part of the breast muscle was recorded.
Drip loss was determined as previously described (Xing et al., 2017). Briefly, an approximately 15 g PM muscle was cut into a rectangle (1 × 1 × 3 cm) and suspended on a hook from the lid of a vacuum bag at 4 °C for 24 h. Any surface moisture on meat sample was absorbed with filter paper. The drip loss was calculated as a percentage: .
Duplicate PM muscles (approximately 40 g of the same size and thickness) were taken from each broiler at 24 h postmortem and packed in polyethylene bags. The samples were cooked in a water bath (TW20, Julabo Labortechnik GmbH, Seelbach, Germany) at 80 °C until the central temperature reached 72 °C. The cooked samples were cooled to room temperature and reweighed. The drip loss was calculated as a percentage: .
After the cooking loss measurement, the cooked meat samples were cut into two strips (1 × 1 × 3 cm) with fibers parallel to the long axis. Then, each strip was sheared perpendicular to the axis of muscle fibers twice using a Digital Meat Tenderness Meter (Model C1LM3, Northeast Agricultural University, Harbin, China) according to Huang et al. (2007). The peak force (N) was recorded as the shear force value.
Detection of ROS
A sensitive fluorescent dichlorofluorescein-diacetate probe was used to detect the intracellular ROS level according to the instructions of the assay kit (E004, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The formation of the fluorescent-oxidized derivative of dichlorofluorescein was monitored using the FACS Calibur flow cytometer (FC500 MCL/MPL, Beckman Coulter Inc., California, USA) with excitation wavelength at 488 nm and emission wavelength at 525 nm. The ROS level was reported as the fold difference of fluorescence intensity between treated and control groups.
Measurement of oxidative parameters
Frozen PM muscle tissue (0.5 g) was homogenized in 4.5 mL of 0.75% ice-cold phosphate saline buffer and centrifuged at 3,000 × g for 10 min at 4 °C. The supernatant was collected for the measurements of total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), protein carbonyl, malondialdehyde (MDA), and total sulfhydryl using the corresponding commercial kits following the manufacturer’s instructions (A015, A001, A005, A087, A003, and A063, respectively; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The contents of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and advanced oxidation protein products (AOPPs) were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (ANG-E33070R and ANG-E12140R; Nanjing Angle Gene Biotechnology Institute, Nanjing, China) per the manufacturer’s instructions. The protein concentration was determined using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, RD, USA) for standardization.
RNA extraction, cDNA synthesis, and real-time quantitative PCR
Total RNA was isolated using Trizol reagent (TaKaRa Biotechnology Co. Ltd., Dalian, China). The purity and quantity of total RNA were assessed using a Nano-Drop ND-100 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The total RNA was treated with DNase I (TaKaRa Biotechnology Co. Ltd., Dalian, China) to remove DNA and reverse-transcribed to cDNA with a Veriti 96-Well Thermal Cycler (Applied Biosystems, Singapore City, Singapore) using a Prime Script RT Master Mix kit (TaKaRa Biotechnology Co. Ltd., Dalian, China).
Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using SYBR Premix Ex Taq Kits (TaKaRa Biotechnology Co. Ltd., Dalian, China) on QuanStudio5 RT-qPCR detection system (Applied Biosystems Co. Ltd., Foster City, CA, USA). RT-PCRs were performed at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s, then 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s as previously described (Chen et al., 2017). The primers were synthesized by Sangon Biotechnology (Shanghai, China) and the sequences are listed in Supplementary Table 2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard gene to normalize the expression of targets genes. Each sample was performed in triplicate and the relative mRNA expression was calculated using the 2−∆∆Ct method.
Total protein extraction and Western blot analysis
Frozen PM muscles were powdered in liquid nitrogen, homogenized in chilled radioimmunoprecipitation assay (RIPA) buffer (Beyotime Biotechnology, Jiangsu, China) containing protease inhibitor (Roche Applied Science, Indianapolis, IN, USA) and then centrifuged at 12,000 × g for 20 min at 4 °C to get the supernatants. The BCA assay was used to determine protein concentration (Thermo Fisher Scientific, RD, USA). Equal amounts of total protein (60 μg) were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore, Merck, Germany). The membranes were blocked with 5.0% fat-free milk for 1 h at room temperature and then incubated in primary antibodies against caspase-3 (1:500; product number: 9662S), microtubule-associated protein 1 light chain 3 beta (LC3)-II (1:1 000; product number: 2775S), NF-κB (1:1 000; product number: 12540S), and β-actin (1:1 500; product number: 3700S, Cell Signaling Technology Inc., Beverly, MA, USA) overnight at 4 °C with gentle shaking. After washing, the membranes were incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (Bioworld, Nanjing, China) at room temperature for 1 h. Finally, the membranes were developed using Super Signal West Pico chemiluminescent substrate (Thermo-Scientific Co. Ltd., Waltham, MA, USA), and semiquantitative analysis of protein was determined by band intensity using Kodak 1D Scientific Imaging Systems (v.3.6.2; Kodak, New Haven, CT, USA). The densities of the bands were quantified using Scion Image software (Scion Corporation, Frederick, MD, USA). The intensity of the specific band was normalized to β-actin.
Statistical analysis
The growth performance data were analyzed using the cage (each cage included eight broilers) as the experimental unit (n = 8), and the other parameters were analyzed using the individual broiler in each cage as the experimental unit (n = 8). The data were checked for normal distribution and homogeneity of variance using the Shapiro–Wilk and Levene’s tests. Overall data from the experiment were analyzed using the one-way analysis of variance (ANOVA) option of the GLM procedure of SAS software (SAS 9.2). Differences between the mean values of each treatment were evaluated using a Tukey’s multiple comparison test of the GLM procedure and considered significant at P < 0.05. Data were reported as means ± SE.
Results
Growth performance
As exhibited in Table 1, broilers exposed to H2O2 showed lower (P < 0.05) ADG (by 10.5%), G:F (by 10.3%), and ADFI (by 1.2%, only 1.7 g reduction) compared with the control group. Dietary taurine increased (P < 0.05) ADG (by 7.7%), G:F (by 7.7%), and ADFI (by 0.8%, only 1.1 g elevation) in broilers administered with H2O2. There was no significant difference (P > 0.05) in the ADFI between the control and H2O2 + taurine groups. However, the ADG and G:F were decreased (P < 0.05) by 3.5% and 3.4%, respectively, in the H2O2 + taurine group compared with the control group. Furthermore, although significant differences (P < 0.05) in ADFI were observed in the H2O2 group as compared with the control and the H2O2 + taurine groups, it should be noted that these differences were negligible. Therefore, the improvement of G:F in H2O2-treated birds was largely being driven by a taurine-induced rescue of ADG rather than through the alteration of ADFI.
Table 1.
Effects of dietary supplementation of taurine on the growth performance of broiler chickens subjected to H2O2 administration1
| Treatments | ||||
|---|---|---|---|---|
| Items2 | Control | H2O2 | H2O2 + taurine | P-value |
| ADG, g/d | 80.3 ± 1.0a | 71.9 ± 0.6c | 77.5 ± 0.6b | <0.01 |
| ADFI, g/d | 138.8 ± 0.2a | 137.0 ± 0.3b | 138.2 ± 0.1a | <0.01 |
| G:F, g/g | 0.58 ± 0.01a | 0.52 ± 0.01c | 0.56 ± 0.01b | <0.01 |
1Values are means ± SE, n = 8.
2Control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group.
a–cMean values within a row followed by different lowercase superscript letters were significantly different (P < 0.05) between groups.
Meat quality traits
As presented in Table 2, broilers in the H2O2 group exhibited significantly decreased (P < 0.05) pH24 h (6.15 vs. 6.45) and increased (P < 0.05) shear force (by 16.7%) than those in the control group. Shear force in the H2O2 + taurine group was lower (P < 0.05, by 8.9%) than that in the H2O2 treatment. No significant difference (P > 0.05) in pH24 h was observed between the H2O2 + taurine and the control group. Moreover, there was no significant difference (P > 0.05) in the L*, a*, b*, drip loss, and cooking loss among the three groups.
Table 2.
Effects of dietary supplementation of taurine on meat quality of broiler chickens subjected to H2O2 administration1
| Treatments | ||||
|---|---|---|---|---|
| Items2 | Control | H2O2 | H2O2 + taurine | P-value |
| pH24 h | 6.45 ± 0.05a | 6.15 ± 0.05b | 6.27 ± 0.03ab | <0.01 |
| a* | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 0.95 |
| b* | 17.0 ± 0.4 | 17.2 ± 0.4 | 17.2 ± 0.4 | 0.89 |
| L* | 50.4 ± 0.4 | 51.5 ± 0.4 | 50.7 ± 0.6 | 0.26 |
| Drip loss, % | 1.50 ± 0.05 | 1.53 ± 0.05 | 1.51 ± 0.03 | 0.70 |
| Cooking loss, % | 15.1 ± 0.2 | 15.3 ± 0.3 | 14.9 ± 0.4 | 0.59 |
| Shear force value, N | 17.8 ± 0.5c | 20.8 ± 0.4a | 19.0 ± 0.3b | <0.01 |
1Values are means ± SE, n = 8.
2a*, redness; b*, yellowness; L*, lightness; control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group.
a–cMean values within a row followed by different lowercase superscript letters were significantly different (P < 0.05) between groups.
Generation of intracellular ROS
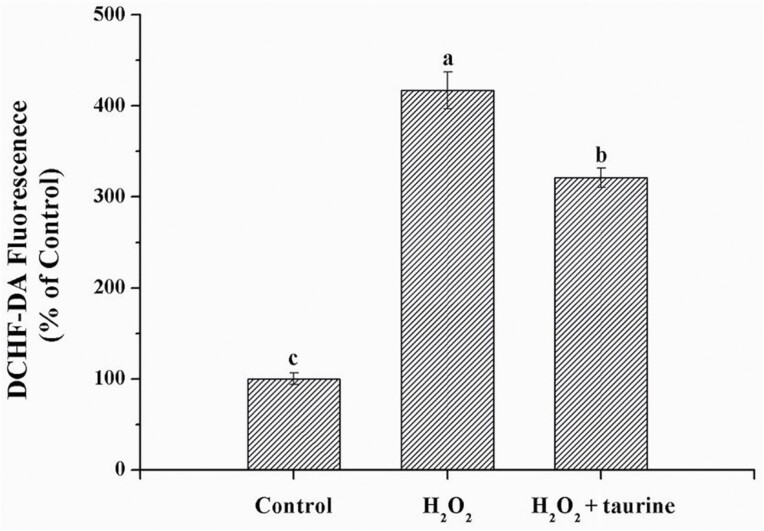
Broilers in the H2O2 and H2O2 + taurine groups exhibited increased (P < 0.05, by 315.9% and 202.2%, respectively) ROS formation in the PM muscle as compared with those of the control group (Figure 1). Compared with the H2O2 group, broilers in the H2O2 + taurine group exhibited lower (P < 0.05, by 27.3%) ROS formation in the PM muscle.
Figure 1.
Effects of dietary supplementation of taurine on the level of ROS in the PM muscle of broiler chickens subjected to H2O2 administration. Control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group. All measurements are expressed as mean ± SE (n = 8) with different superscript letters (a–c) indicating significance (P < 0.05). DCHF-DA, 2,7-Dichlorodi -hydrofluorescein diacetate.
Antioxidants activity and content of oxidative products
H2O2-injected broilers exhibited significantly decreased (P < 0.05) activities of T-AOC, T-SOD, GSH-Px, and content of total sulfhydryl (by 19.1%, 17.3%, 15.6%, and 17.9%, respectively), whereas higher (P < 0.05) contents of protein carbonyl, 8-OHdG, AOPPs, and MDA (by 12.3%, 23.9%, 12.6%, and 17.5%, respectively) as compared with the control (Table 3). Broilers in the H2O2 + taurine group exhibited lower (P < 0.05) T-AOC and T-SOD (by 9.5% and 8.9%) activities as well as higher (P < 0.05) contents of AOPPs and MDA (by 8.1% and 15%) than those in the control group. Compared with the H2O2 group, broilers in the H2O2 + taurine group exhibited higher (P < 0.05) T-AOC, T-SOD, and GSH-Px (by 11.8%, 10.1%, and 16.6%, respectively) activities as well as lower contents of protein carbonyl and AOPPs (by 8.4% and 4.0%).
Table 3.
Effects of dietary supplementation of taurine on antioxidant capacities of breast meat in broiler chickens subjected to H2O2 administration1
| Treatments | ||||
|---|---|---|---|---|
| Items2 | Control | H2O2 | H2O2 + taurine | P-value |
| T-AOC, U/mg of protein | 0.21 ± 0.01a | 0.17 ± 0.01c | 0.19 ± 0.01b | <0.01 |
| T-SOD, U/mg of protein | 83.1 ± 1.5a | 68.7 ± 2.1c | 75.6 ± 2.2b | <0.01 |
| GSH-Px, U/mg of protein | 25.3 ± 0.9a | 21.3 ± 0.7b | 24.9 ± 1.5a | <0.01 |
| Canbonyl, nmol/mg of protein | 2.85 ± 0.13b | 3.20 ± 0.20a | 2.93 ± 0.15b | <0.01 |
| Total sulfhydryl, μmol/g of protein | 58.4 ± 2.2a | 48.0 ± 1.4b | 50.2 ± 1.9ab | 0.02 |
| 8-OHdG, pg/g of protein | 28.4 ± 2.5b | 35.2 ± 1.5a | 30.8 ± 1.3ab | <0.01 |
| AOPPs, nmol/g of protein | 70.2 ± 2.1c | 79.0 ± 3.6a | 75.9 ± 3.6b | <0.01 |
| MDA, nmol/mg of protein | 0.40 ± 0.02b | 0.47 ± 0.01a | 0.46 ± 0.01a | <0.01 |
1Values are means ± SE, n = 8.
2Control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group.
a–cMean values within a row followed by different lowercase superscript letters were significantly different (P < 0.05) between groups.
Apoptosis and autophagy response
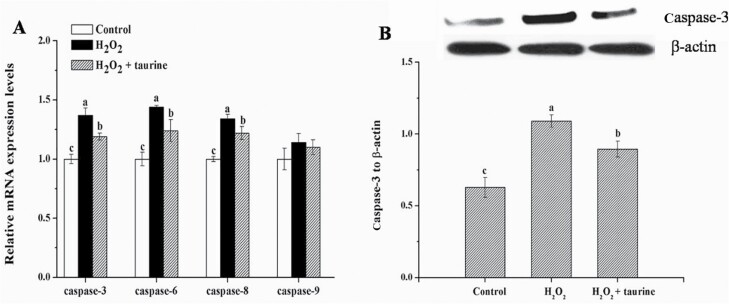
As shown in Figure 2, broilers in the H2O2 and H2O2 + taurine groups exhibited increased (P < 0.05) mRNA expression of caspase-3 (by 34.6% and 19.2%), 6 (by 38.8% and 20.4%), and 8 (by 31.4% and 20.6%) as well as higher (P < 0.05) protein expression of caspase-3 (by 83.5% and 33.2%) in comparison with those of broilers in the control group. Compared with the H2O2 treatment, broilers in the H2O2 + taurine group exhibited decreased (P < 0.05) mRNA expression of caspase-3 (by 11.4%), 6 (by 13.3%), and 8 (by 8.2%) as well as lower (P < 0.05) protein expression of caspase-3 (by 27.3%).
Figure 2.
Effects of dietary supplementation of taurine on the mRNA expression (A) and protein content (B) of the caspases in the PM muscle of broiler chickens subjected to H2O2 administration. Control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group. All measurements are expressed as mean ± SE (n = 8) with different superscript letters (a–c) indicating significance (P < 0.05).
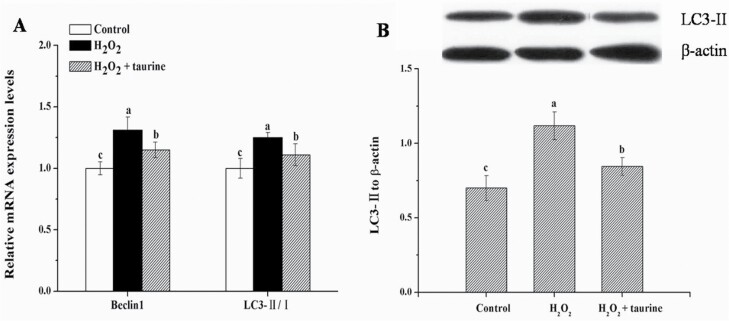
As shown in Figure 3, the administration of H2O2 significantly upregulated (P < 0.05) the mRNA expression of beclin1 (by 26.1%) and LC3-II/LC3-I ratio (by 24.0%) and increased (P < 0.05) the protein expression of LC3-II (by 61.4%) in the PM muscle of broilers as compared with those in the control group. Compared with the H2O2 group, broilers in the H2O2 + taurine group exhibited lower (P < 0.05) mRNA expression of beclin1 (by 10.5%) and LC3-II/LC3-I ratio (by 11.3%) as well as decreased (P < 0.05) protein expression of LC3-II (by 31.8%).
Figure 3.
Effects of dietary supplementation of taurine on the mRNA expression (A) and protein content (B) of autophagy response in the PM muscle of broiler chickens subjected to H2O2 administration. Control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group. All measurements are expressed as mean ± SE (n = 8) with different superscript letters (a–c) indicating significance (P < 0.05).
NF-κB signaling
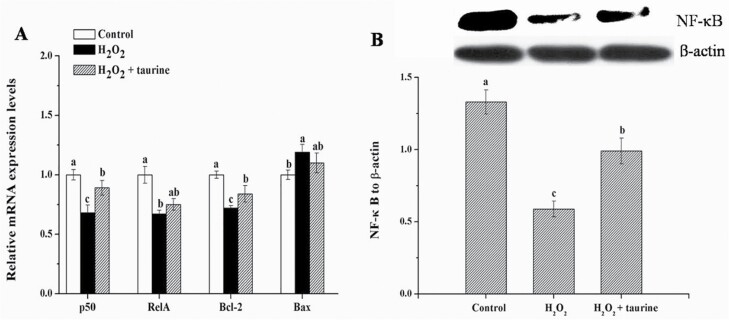
Compared with the control group, H2O2-treated broilers exhibited significantly lower (P < 0.05) mRNA expression of NF-κB subunit 1 (p50), B-cell CLL/lymphoma 2 (Bcl-2), v-rel reticuloendotheliosis viral oncogene homolog A (RelA), and protein expression of NF-κB (by 30.4%, 30.1%, 29.1%, and 57.0%, respectively, Figure 4); broilers in the H2O2 + taurine group exhibited lower (P < 0.05) mRNA expression of p50, Bcl-2, and protein expression of NF-κB (by 15.7%, 25.2%, and 25.8%, respectively) in the PM muscle. Moreover, broilers in the H2O2 + taurine group exhibited higher (P < 0.05) mRNA expression of p50, Bcl-2, and protein expression of NF-κB (by 21.1%, 6.9%, and 72.7%, respectively) in comparison with those in the H2O2 group.
Figure 4.
Effects of dietary supplementation of taurine on the mRNA expression (A) and protein content (B) of NF-κB signaling in the PM muscle of broiler chickens subjected to H2O2 administration. Control, broilers fed a basal diet and received no injections; H2O2, birds fed a basal diet and received an intraperitoneal injection of 10% H2O2 on day 9 of the experiment (at 37 d of age), with a dosage of 1.0 mL/kg BW; H2O2 + taurine, birds fed the basal diet supplemented with 5 g/kg taurine and received the same injection as the H2O2 group. All measurements are expressed as mean ± SE (n = 8) with different superscript letters (a–c) indicating significance (P < 0.05).
Discussion
The negative impacts of oxidative stress on health conditions, production performance, and product quality have been well described in poultry (Fellenberg and Speisky, 2006). The current study indicated a significant decrease in the growth performance of broilers exposed to intraperitoneal injection of 10% H2O2, which was in consistent with a previous study (Chen et al., 2018). Taurine receives much attention in animal production due to its multiple physiological functions. Campbell and Classen (1989) indicated that adding taurine (0, 0.5, 1.0, or 2.0 g/kg) to the diet of broilers reduced feed:gain linearly from 3 to 6 wk of age. However, there were also studies demonstrating that dietary taurine inclusion did not affect ADFI, ADG, and feed:gain of broilers (Huang et al., 2014; Xu et al., 2020). Herein, we found that dietary supplementation of 5 g/kg taurine greatly improved ADG and increased G:F whereas had a negligible effect on the feed intake of H2O2-administered broilers. Similarly, taurine supplementation was reported to enhance BW gain of broiler chickens under chronic heat stress and did not affect the feed intake (Shim et al., 2006). Our recent study also indicated that 5 g/kg taurine addition tended to decrease the feed conversion ratio of heat-stressed birds (Lu et al., 2019a). This implied that taurine might promote the nutrient metabolism of oxidative-stressed broilers to improve the feed efficiency rather than affecting feed intake. Furthermore, it should be noted that dietary supplementation of 5 g/kg taurine could not restore the ADG and G:F of broilers received 10% H2O2 injection to control levels. Overall, the results suggested that the impairment of production performance caused by high dose of H2O2 administration is irreversible, and dietary taurine could only ameliorate these negative effects to some extent.
In addition to the impaired growth performance, H2O2 administration negatively affected meat quality by decreasing pH24 h and increasing shear force. Exsanguination eliminates the oxygen and nutrient supplies of animals, forcing the metabolically active muscle to resort to anaerobic glycolysis for energy supply. Oxidative stress can promote postmortem glycolysis and the resultant overproduction of lactate, thereby accelerating the decline of muscle pH (Xing et al., 2019). Also, oxidative stress can influence the turnover of the intramuscular collagen, resulting in a decrease in collagen solubility and hence increasing meat toughness (Falowo et al., 2014). Taurine supplementation alleviated the decrease in pH24 h and decreased shear force of breast meat in H2O2-treated broilers. These improvements might be attributed to the enhancement of antioxidative capacity and the restoring redox status of PM muscle by taurine supplementation (Xu et al., 2020). In accordance with these results, the beneficial effects of taurine supplementation on the meat quality of broilers have been reported under both normal and stressful conditions (Lu et al., 2019b; Xu et al., 2020).
Genetic selection for fast growing and breast muscle yield as well as intensive breeding have made modern broilers particularly sensitive to oxidative stress (Petracci et al., 2015). Moreover, poultry muscle has been regarded as highly susceptible to oxidative reactions among animal-source foods owing to the high unsaturation degree of the muscle lipids (Min et al., 2008). The overproduction of free radicals constitutes a main cause of lipid peroxidation and protein oxidation, which produces large amounts of oxidative products and further induces biological damage (Estévez, 2015). Taurine exhibits enhanced radical scavenging capacities both in vivo and in vitro as an efficient antioxidant (Patel et al., 2016; Xu et al., 2020), which enables the protection of unsaturated lipids and valuable protein residues against oxidation. Previous studies showed that chronic heat exposure increased ROS levels in the PM muscle of broilers, whereas taurine could scavenge ROS and reduce the contents of oxidative products (Oliveira et al., 2010). Accordingly, in the present study, dietary taurine effectively reduced the elevated ROS levels and relieved the aggravated oxidative damage of biomolecules induced by H2O2 injection in the PM muscle of broilers. Moreover, the ameliorative effect of taurine on the reduction of the antioxidant capacity in H2O2-treated broilers was observed; a similar finding was also reported in heat-stress broilers (Lu et al., 2019b). Overall, we speculated that taurine could protect chicken muscle from oxidative damage induced by intraperitoneal injection of H2O2 by scavenging ROS and restoring redox status.
The overproduction of ROS, especially H2O2, can trigger apoptosis and autophagy in various types of tissues and cells (Simon et al., 2000). Apoptosis is essential for the control of tissue growth during development (Salvesen and Dixit, 1999). Caspases are crucial executors involved in apoptosis; among which, caspase-3 plays a central role in intrinsic and extrinsic apoptotic signaling pathways (Xing et al., 2019). The present results showed that the mRNA expression of caspase-3, 6, and 8 as well as the protein content of caspase-3 were upregulated in the H2O2 group, indicating a significant number of apoptotic cells in the PM muscle of broilers exposed to H2O2. Similar results were reported by Chen et al. (2016) and Xu et al. (2017) for cardiocytes and splenic lymphocytes of broilers in response to oxidative stress. Das et al. (2009) reported that taurine could restore intracellular ROS levels and suppress apoptosis by reducing the activity of caspase-3 in the testicle of mice exposed to arsenic. Similarly, taurine suppressed the apoptosis by downregulating the caspases levels in PM muscle of H2O2-administered broilers in the current study. Autophagy is a highly conserved mechanism for degrading cytoplasmic substrates to protect cells against stresses, which also plays important roles in the homeostasis of organelle integrity and tissue remodeling during development (Scherz-Shouval and Elazar, 2011). Consistent with previous studies (Yin et al., 2015; Chen et al., 2017), we observed that administration of H2O2 enhanced beclin1 mRNA expression and LC3-II/LC3-I ratio and increased protein content of LC3-II, indicating the formation of autophagosome in the PM muscle. A certain degree of autophagy can remove the oxidative-damaged molecules or organelles and, therefore, protect tissues against cell death, whereas excessive and dysregulated autophagy induced by the heightened oxidative injury may promote cell death and aggravate tissue injury (Song et al., 2017). Taurine has been demonstrated to protect against As2O3-induced autophagic cell death by alleviating oxidative stress in the pancreas of rats (Bai et al., 2016). Similarly, H2O2 administration-stimulated autophagic influx in the PM muscle was restored by taurine supplementation in the present study. Furthermore, an in vitro study reported that taurine could attenuate apoptosis and autophagy processes triggered by methylamphetamine in PC12 cells (Li et al., 2012). Together, our results demonstrated that taurine could protect H2O2-induced PM muscle damage by reducing ROS generation and regulating intracellular apoptotic and autophagic influxes.
NF-κB constitutes a major oxidative stress-sensitive signal transduction pathway in skeletal muscle, and the key factors of the NF-κB family are known as molecular redox switches for system-wide oxidative stress response (Sies et al., 2017). NF-κB activation initiates a variety of adaptive responses to protect cells from the apoptotic cascade and control cell survival (Kondylis et al., 2017). Crosstalk between ROS and NF-κB signaling has been extensively studied, whereas the role of ROS in NF-κB activation/inhibition still remains controversial. Yin et al. (2015) demonstrated that the administration of H2O2 caused oxidative stress in the jejunum of piglet and triggered the autophagic process, which was possibly related to the activation of NF-κB signaling. However, dihydroartemisinin induced the accumulation of ROS-stimulated autophagy by repressing NF-κB activation in several cancer lines (Hu et al., 2014). In the current study, H2O2 administration decreased the expression of NF-κB and Bcl-2/bcl2-associated X (Bax) ratio, suggesting that excessive ROS induced by H2O2 in the PM muscle of broilers might have an inhibitory effect on NF-κB activation and thereby promote autophagy and apoptosis. Furthermore, evidence suggests that the impairment of NF-κB activation can lead to the further accumulation of ROS (Gloire et al., 2006). Taurine addition promoted the expression of NF-κB and increased Bcl-2/Bax ratio in the H2O2 + taurine group. Similarly, oral administration of 100 mg/kg taurine restored the redox status of NaAsO2-administered rat and ameliorated apoptosis by controlling the activation of NF-κB and the ratio of Bcl-2/Bax (Das et al., 2009; Roy et al., 2009). Therefore, we speculated that taurine might activate the NF-κB signaling to suppress apoptosis and excessive autophagy in the PM muscle of broilers exposed to H2O2.
Conclusions
In summary, our findings demonstrated that the administration of H2O2 had negative impacts on the growth performance and meat quality of broilers. Dietary taurine could attenuate the decline in growth performance and improve the meat quality of H2O2-challenged birds to some extent possibly by providing protection against oxidative damage through restoring redox status. Furthermore, taurine might also counteract H2O2-induced apoptosis and autophagy by regulating the NF-κB signaling.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31872374), the National Key Research and Development Program of China (2016YFD0500501, 2018YFD0500405), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS [2020]407).
Glossary
Abbreviations
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- ADFI
average daily feed intake
- ADG
average daily gain
- AOPPs
advanced oxidation protein products
- Bcl-2
B-cell CLL/lymphoma 2
- BW
body weight
- FCR
feed conversion ratio
- GSH-Px
glutathione peroxidase
- MDA
malondialdehyde
- NF-κB
nuclear factor-κB
- PM
pectoralis major
- ROS
reactive oxygen species
- T-AOC
total antioxidant capacity
- T-SOD
total superoxide dismutase
Conflicts of interest statement
All authors declare no conflicts of interest.
Literature Cited
- Bai, J., Yao X. F., Jiang L. P., Qiu T. M., Liu S., Qi B. X., Zheng Y., Kong Y., Yang G., Chen M., . et al. 2016. Taurine protects against As2O3-induced autophagy in pancreas of rat offsprings through Nrf2/Trx pathway. Biochimie. 123:1–6. doi: 10.1016/j.biochi.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Campbell, G. L., and Classen H. L.. . 1989. Effect of dietary taurine supplementation on sudden death syndrome in broiler chickens. Can. J. Anim. Sci. 69:509–512. doi: 10.4141/cjas89-060 [DOI] [Google Scholar]
- Chen, X. X., Gu R. Q., Zhang L., Li J. L., and Gao F.. . 2018. Induction of nuclear factor-κB signal-mediated apoptosis and autophagy by reactive oxygen species is associated with H2O2-impaired growth performance of broilers. Animal. 12:2561–2570. doi: 10.1017/S1751731118000903 [DOI] [PubMed] [Google Scholar]
- Chen, B. L., Li D. Y., Li M., Li S. C., Peng K. N., Shi X., Zhou L. Y., Zhang P., Xu Z. X., Yin H. D., . et al. 2016. Induction of mitochondria-mediated apoptosis and PI3K/Akt/mTOR-mediated autophagy by aflatoxin B2 in hepatocytes of broilers. Oncotarget. 7:84989–84998. doi: 10.18632/oncotarget.13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. X., Zhang L., Li J. L., Gao F., and Zhou G. H.. . 2017. Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ROS generation, apoptosis, and autophagy in the NF-κB signal pathway. J. Agr. Food Chem. 65:3986–3994. doi: 10.1021/acs.jafc.7b01267 [DOI] [PubMed] [Google Scholar]
- Das, J., Ghosh J., Manna P., Sinha M., and Sil P. C.. . 2009. Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol. Lett. 187:201–210. doi: 10.1016/j.toxlet.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Estévez, M. 2015. Oxidative damage to poultry: from farm to fork. Poult. Sci. 94:1368–1378. doi: 10.3382/ps/pev094 [DOI] [PubMed] [Google Scholar]
- Falowo, A. B., Fayemi P. O., and Muchenje V.. . 2014. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: a review. Food Res. Int. 64:171–181. doi: 10.1016/j.foodres.2014.06.022 [DOI] [PubMed] [Google Scholar]
- Fellenberg, M. A., and Speisky H.. . 2006. Antioxidants: their effects on broiler oxidative stress and its meat oxidative stability. World. Poult. Sci. J. 62:53–70. doi: 10.1079/WPS200584 [DOI] [Google Scholar]
- Gloire, G., Legrand-Poels S., and Piette J.. . 2006. NF-κB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72:1493–1505. doi: 10.1016/j.bcp.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Hu, W., Chen S. S., Zhang J. L., Lou X. E., and Zhou H. J.. . 2014. Dihydroartemisinin induces autophagy by suppressing NF-κB activation. Cancer Lett. 343:239–248. doi: 10.1016/j.canlet.2013.09.035 [DOI] [PubMed] [Google Scholar]
- Huang, Y., Haley C. S., Wu F., Hu S., Hao J., Wu C., and Li N.. . 2007. Genetic mapping of quantitative trait loci affecting carcass and meat quality traits in Beijing ducks (Anas platyrhynchos). Anim. Genet. 38:114–119. doi: 10.1111/j.1365-2052.2007.01571.x [DOI] [PubMed] [Google Scholar]
- Huang, C. X., Wang B., Min Z., and Yuan J.. . 2014. Dietary inclusion level and time effects of taurine on broiler performance, meat quality, oxidative status and muscle taurine content. Br. Poult. Sci. 55:598–604. doi: 10.1080/00071668.2014.943692 [DOI] [PubMed] [Google Scholar]
- Huxtable, R. J. 1992. Physiological actions of taurine. Physiol. Rev. 72:101–163. doi: 10.1152/physrev.1992.72.1.101 [DOI] [PubMed] [Google Scholar]
- Kondylis, V., Kumari S., Vlantis K., and Pasparakis M.. . 2017. The interplay of IKK, NF-κB and RIPK 1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol. Rev. 277:113–127. doi: 10.1111/imr.12550 [DOI] [PubMed] [Google Scholar]
- Li, Y., Hu Z. T., Chen B., Bu Q., Lu W. J., Deng Y., Zhu R. M., Shao X., Hou J., Zhao J. X., . et al. 2012. Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol. Lett. 215:1–7. doi: 10.1016/j.toxlet.2012.09.019 [DOI] [PubMed] [Google Scholar]
- Li, L., Tan J., Miao Y., Lei P., and Zhang Q.. . 2015. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell. Mol. Neurobiol. 35:615–621. doi: 10.1007/s10571-015-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., He X. F., Ma B. B., Zhang L., Li J. L., Jiang Y., Zhou G. H., and Gao F.. . 2019a. The alleviative effects and related mechanisms of taurine supplementation on growth performance and carcass characteristics in broilers exposed to chronic heat stress. Poult. Sci. 98:878–886. doi: 10.3382/ps/pey433 [DOI] [PubMed] [Google Scholar]
- Lu, Z., He X. F., Ma B. B., Zhang L., Li J. L., Jiang Y., Zhou G. H., and Gao F.. . 2019b. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J. Sci. Food Agr. 99:1066–1072. doi: 10.1002/jsfa.9273 [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt, J., and Svendsen O.. . 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet. J. 173:502–511. doi: 10.1016/j.tvjl.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Min, B., Nam K. C., Cordray J., and Ahn D. U.. . 2008. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J. Food Sci. 73:C439–C446. doi: 10.1111/j.1750-3841.2008.00805.x [DOI] [PubMed] [Google Scholar]
- Oliveira, M. W., Minotto J. B., de Oliveira M. R., Zanotto-Filho A., Behr G. A., Rocha R. F., Moreira J. C., and Klamt F.. . 2010. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep. 62:185–193. doi: 10.1016/s1734-1140(10)70256-5 [DOI] [PubMed] [Google Scholar]
- Patel, S. N., Pandya K., Clark G. J., Parikh M. C., and Lau-Cam C. A.. . 2016. Comparison of taurine and pantoyltaurine as antioxidants in vitro and in the central nervous system of diabetic rats. Exp. Toxicol. Pathol. 68:103–112. doi: 10.1016/j.etp.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Petracci, M., Mudalal S., Soglia F., and Cavani C.. . 2015. Meat quality in fast-growing broiler chickens. World. Poult. Sci. J. 71:363–374. doi: 10.1017/S0043933915000367 [DOI] [Google Scholar]
- Roy, A., Manna P., and Sil P. C.. . 2009. Prophylactic role of taurine on arsenic mediated oxidative renal dysfunction via MAPKs/NF-κB and mitochondria dependent pathways. Free Radic. Res. 43:995–1007. doi: 10.1080/10715760903164998 [DOI] [PubMed] [Google Scholar]
- Ryter, S. W., Kim H. P., Hoetzel A., Park J. W., Nakahira K., Wang X., and Choi A. M.. . 2007. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 9:49–89. doi: 10.1089/ars.2007.9.49 [DOI] [PubMed] [Google Scholar]
- Salvesen, G. S., and Dixit V. M.. . 1999. Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. U. S. A. 96:10964–10967. doi: 10.1073/pnas.96.20.10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval, R., and Elazar Z.. . 2011. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36:30–38. doi: 10.1016/j.tibs.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Shim, K. S., Hwang K. T., Son M. W., and Park G. H.. . 2006. Lipid metabolism and peroxidation in broiler chicks under chronic heat stress. Asian Australas. J. Anim. 19:1206–1211. doi: 10.5713/ajas.2006.1206 [DOI] [Google Scholar]
- Sies, H., Berndt C., and Jones D. P.. . 2017. Oxidative stress. Annu. Rev. Biochem. 86:1–34. doi: 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]
- Simon, H. U., Haj-Yehia A., and Levi-Schaffer F.. . 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418. doi: 10.1023/a:1009616228304 [DOI] [PubMed] [Google Scholar]
- Song, S., Tan J., Miao Y., Li M., and Zhang Q.. . 2017. Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J. Cell. Physiol. 232:2977–2984. doi: 10.1002/jcp.25785 [DOI] [PubMed] [Google Scholar]
- Xing, T., Gao F., Tume R. K., Zhou G. H., and Xu X. L.. . 2019. Stress effects on meat quality: a mechanistic perspective. Compr. Rev. Food Sci. F. 18:380–401. doi: 10.1111/1541-4337.12417 [DOI] [PubMed] [Google Scholar]
- Xing, T., Zhao X., Wang P., Chen H., Xu X., and Zhou G.. . 2017. Different oxidative status and expression of calcium channel components in stress-induced dysfunctional chicken muscle. J. Anim. Sci. 95:1565–1573. doi: 10.2527/jas.2016.0868 [DOI] [PubMed] [Google Scholar]
- Xu, S. W., Lu Z., Ma B. B., Xing T., Li J. L., Zhang L., Jiang Y., and Gao F.. . 2020. Dietary taurine supplementation enhances antioxidative capacity and improves breast meat quality of broiler chickens. Br. Poult. Sci. 61:140–145. doi: 10.1080/00071668.2019.1691147 [DOI] [PubMed] [Google Scholar]
- Xu, J., Tang S., Song E., Yin B., Wu D., and Bao E.. . 2017. Hsp70 expression induced by Co-Enzyme Q10 protected chicken myocardial cells from damage and apoptosis under in vitro heat stress. Poult. Sci. 96:1426–1437. doi: 10.3382/ps/pew402 [DOI] [PubMed] [Google Scholar]
- Yin, J., Duan J., Cui Z., Ren W., Li T., and Yin Y. L.. . 2015. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 5:15479–15486. doi: 10.1039/C4RA13557A [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.