Abstract
We report a nickel-catalyzed one pot synthesis of 9-arylmethylanthracene motifs, which find applications in medicinal and material chemistry. In this synthesis, we apply three component alkene dicarbofunctionalization of 2-vinylaldimines with aryl iodides and arylzinc reagent to generate a 1,1,2-diarylethyl scaffold, which then undergoes an acidpromoted cyclization followed by aromatization to furnish 9-arylmethylanthracene cores. With the new method, a number of differently-substituted 9-arylmethylanthracene derivatives can be synthesized in good yields.
Keywords: Arylmethylanthracene, cyclization, dicarbofunctionalization, nickel-catalyzed, regioselective, vinylaldimines
Graphical abstract

Anthracene, a polycyclic aromatic hydrocarbon that exhibits blue fluorescence under ultraviolet light, plays an important role as electron-transporting material.[1] Anthracene derivatives have been extensively studied in multiple fields such as material chemistry, thermochromic or photochromic chemistry and as starting materials in organic light-emitting materials.[2] Literatures have shown that anthracene derivatives are also useful for examining DNA cleavage acting as a potential anticancer drug.[3] Due to their chemical stability and excellent photoluminescence property, they have been widely used in the development of fluorescence sensors, which can be applied to function as selective imaging agents.[3–4] These molecular scaffolds are also known to show antitubercular activity[5] (Figure 1).
Figure 1.
9-Substituted anthracene derivatives and their applications.
Although several synthetic approaches have been known for the synthesis of anthracenes,[6] methods to prepare 9-substituted anthracene derivatives are still limited. An acid catalyzed synthesis of 9-arylanthracene have been reported by Meier et. al.[7] in which the methoxy group in the aromatic ring plays an important role to perform the intramolecular electrophilic aromatic substitution (Scheme 1). In addition, Ye et. al. have described the synthesis of alkyl and 9-aryl anthracene derivatives through gold-catalyzed cyclization of o-alkynyldiarylmethane.[8] In a separate report by Majumdar et. al.[9] describes the synthesis of 9-benzyl and related 9-allenyl substituted anthracene derivative employing phase transfer catalyst and an anthrone as a starting material. Mou et. al. report the synthesis of anthracene derivatives using Negishi coupling of aryl halides with organozinc chlorides catalyzed by a palladium bipyridyl complex.[10] Herein, we report an efficient one pot synthesis of 9-arylmethylanthracene from vinylarenes, aryl iodides and arylzinc reagents. In this process, we utilize a Ni-catalyzed alkene dicarbofunctionalization reaction[11] in tandem with an acid-promoted cyclization-aromatization process to construct the 9-arylmethylanthracene scaffold.
Scheme 1.
Some other methods to synthesize anthracene derivatives.
Transition-metal catalyzed three-component difunctionalization of alkenes[12] involving the addition of two carbon-based entities across a double bond, known as alkene dicarbofunctionalization, is an efficient technique to construct complex molecular architectures from the readily available feedstock chemicals.[13] A significant progress has been made in this area recently.[14] However, the application of this new method in the construction of synthetically useful complex molecules remains limited.[14i,15] Recently, we reported a Ni-catalyzed diarylation of 2-vinylaldimines in which two carbon–carbon (C–C) bonds were constructed across the styryl double bond (Scheme 2).[11] This method furnished a variety of differently substituted 1,1,2-triarylethane scaffolds.
Scheme 2.
Coordination assisted 1,2-dicarbofunctionalization of alkene.[11]
In our continued efforts to expand the application of alkene difunctionalization to generate synthetically important compounds, we envisioned that 9-arylmethylanthracene derivatives could be readily accessed from the 1,1,2-triarylethane scaffold (Scheme 3). In this process, we anticipated that the 2-aldimine group in the diarylated product would undergo an acidpromoted cyclization with the ortho-position of the proximal aryl group if this aryl group could be made sufficiently electron-rich followed by the elimination of aniline prompted by the presence of the acid.
Scheme 3.
Retrosynthetic disconnection to access 9-arylmethylanthracene derivatives by alkene dicarbofunctionalization approach.
We initially examined the difunctionalization of 2-vinylaldimine 1 with iodobenzene and 3-methoxylphenylzinc iodide in the presence of 2 mol% Ni(cod)2 under our previously reported reaction conditions (Table 1). The crude reaction mixture, without further purification, was then subjected to cyclization in the presence of HCl. The reaction furnished 9-benzyl-2-methoxyanthracene 2 in 67% yield when the crude product was cyclized with 0.50 mL of 6.0 M HCl (3.0 mmol) (entry 1). Cylization with lower concentrations of HCl or in shorter reaction time generated the anthracene product 2 in lower yields (entries 2–4). Organic acids, such as p-toluene-sulfonic acid, trifluoromethanesulfonic acid and acetic acid, furnished the anthracene product 2 in slightly lower yields (entries 5–7).
Table 1.
Optimization of reaction conditionsa.
 | ||
|---|---|---|
| entry | modified reaction conditions | yields of 2 (%)b |
| 1 | none | 67 (58) |
| 2 | with 1 M (0.5 mL, 0.5 mmol) HCI | 0 |
| 3 | with 3 M (0.5 mL, 1.5 mmol) HCI | 20 |
| 4 | 3 h instead of 6 h | 48 |
| 5 | with 3 mmol p-TsOH | 55 |
| 6 | with 3 mmol TfOH | 62 |
| 7 | with 3 mmol AcOH | 63 |
0.1 mmol scale reactions in 0.5 mL solvent.
1H NMR yields using pyrene as an internal standard. Value in parenthesis is the isolated yield from 0.5 mmol.
After optimizing the reaction conditions for cyclization, we examined the scope of the current reaction with respect to 2-vinylaldimines such as N-phenyl-1-(2-vinylphenyl)methanimine (1), and 1-(4-methoxy-2-vinylphenyl)-N-phenylmethanimine1-(5-fluoro-2-vinylphenyl)-N-phenylmethanimine), along with 3-methoxyphenylzinc and 3,4-dimethoxyphenylzinc iodide, and different aryl iodides (Table 2). The reaction proceeded well with both electron-rich and electron-poor aryl iodides, and tolerated substituents such as alkyl, OMe, Cl, CO2Me, CN, SMe and CF3 on aryl iodides. In addition, the reaction also tolerated ortho-substitution on aryl iodides as demonstrated by 1-naphthyl (7) and ortho-iPr (8). The reaction also tolerated F and OMe groups on vinylaldimine. Examination of reaction with 3,4-dimethoxyphenylzinc iodide (6, 8–11) showed better product yield than the phenylzinc iodide containing one OMe group at meta-position (2–5 and 7) most probably due to the activation of aryl ring for electrophilic cyclization on the imine group.[16] The current reaction produced variously substituted 9-arylmethylanthracene derivatives, the structures of which were further confirmed by an X-ray crystallographic analysis of the anthracene product 6 (Figure 2).
Table 2.
Scope with imine, arylzinc iodide and aryl iodidea.
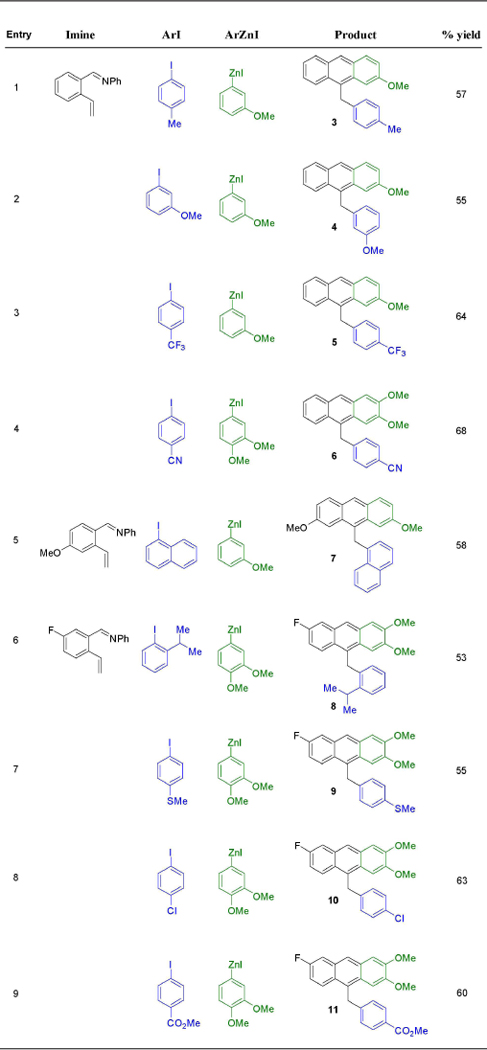 |
Isolated from 0.5 mmol.
Figure 2.
X-ray structure of 6.
We propose the following catalytic cycle (Scheme 4), which involves both the Ni-catalyzed alkene dicarbofunctionalization and the acid-catalyzed cyclization-aromatization reactions, to account for the formation of the 9-arylmethylanthracene derivatives with the current method. 2-Vinylaldimine first binds to a Ni(0) species, which oxidatively adds aryl iodide to generate a Ni(II) intermediate 13. The aryl group in species 13 then undergoes migratory insertion to the bound alkene to generate the nickellacycle 15. The intermediate 15 subsequently undergoes transmetalation followed by reductive elimination to furnish the diarylated product 16 and regenerate the Ni(0)-catalyst. The crude product 16 undergoes further cyclization upon the imine group in the presence of HCl to furnish the final 9-arylmethylanthrancene derivative (Scheme 5). The cyclization occurs with the electron-rich aryl group originally derived from arylzinc iodide, which proceeds sequentially through protonation, cyclization and re-aromatization steps to re-aromatize the OMe containing ring as well as create a new central aryl ring of the anthracene core.
Scheme 4.
Proposed catalytic cycle.
Scheme 5.
Possible mechanistic pathways for the cyclization of dicarbofunctionalized product.
In summary, we have developed a new catalytic protocol for the synthesis of a wide range of 9-arylmethylanthracene derivatives. In this process, we implement a Ni-catalyzed alkene dicarbofunctionalization reaction to assemble all the carbon fragments required to generate the final product in the form of 1,1,2-triarylethyl scaffold. This scaffold is then readily converted to the 9-arylmethylanthracene core upon cyclization of the proximal electron-rich aryl ring onto the imine group. The cyclization process is followed by a re-aromatization event, which constructs the central aryl ring of the anthracene core.
Supplementary Material
Acknowledgements
We thank the Pennsylvania State University and the NIH NIGMS (No. R35GM133438) for financial support.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting information for this article is available on the WWW under https://doi.org/10.1002/ijch.201900158
References
- [1].Becker HD, Chem. Rev 1993, 93, 145–172. [Google Scholar]
- [2].Hayashi H, Kato Y, Matsumoto A, Shikita S, Aizawa N, Suzuki M, Aratani N, Yasuda T, Yamada H, Chem. Eur. J 2019, 25, 1–8. [DOI] [PubMed] [Google Scholar]
- [3].Parkesh R, Clive Lee T, Gunnlaugsson T, Tetrahedron Lett. 2009, 50, 4114–4116. [Google Scholar]
- [4].a) Somashekar PR, Chetana MN, Res. Rev.: J. Chem 2016, 5, 45–52; [Google Scholar]; b) McLaughlin B, Surender EM, Wright GD, Daly B, de Silva AP, Chem. Commun 2018, 54, 1319–1322; [DOI] [PubMed] [Google Scholar]; c) Uchiyama S, Fukatsu E, McClean GD, de Silva AP, Angew. Chem. Int. Ed 2016, 55, 768–771; Angew. Chem. 2016, 128, 778–781. [DOI] [PubMed] [Google Scholar]
- [5].Panda G, Mishra JK, Sinha S, Gaikwad AK, Srivastava AK, Srivastava R, Srivastava BS, Arkivoc 2005, ii, 29–45. [Google Scholar]
- [6].a) de Koning CB, Manzini SS, Michael JP, Mmutlane EM, Tshabidi TR, van Otterlo WAL, Tetrahedron 2005, 61, 555–564; [Google Scholar]; b) Imoto M, Takeda M, Tamaki A, Taniguchi H, Mizuno K, Res. Chem. Intermed 2009, 35, 957; [Google Scholar]; c) Ishibashi JSA, Marshall JL, Mazière A, Lovinger GJ, Li B, Zakharov LN, Dargelos A, Graciaa A, Chrostowska A, Liu S-Y, J. Am. Chem. Soc 2014, 136, 15414–15421; [DOI] [PubMed] [Google Scholar]; d) Fitzgerald JJ, Drysdale NE, Olofson RA, J. Org. Chem 1992, 57, 7122–7126 [Google Scholar]; e) Fukutani T, Hirano K, Satoh T, Miura M, Org. Lett 2009, 11, 5198–5201; [DOI] [PubMed] [Google Scholar]; f) Geiger T, Haupt A, Maichle-Mössmer C, Schrenk C, Schnepf A, Bettinger HF, J. Org. Chem 2019, 84, 10120–10135; [DOI] [PubMed] [Google Scholar]; g) Škalamera Đ, Veljković J, Ptiček L, Sambol M, Mlinarić-Majerski K, Basarić N, Tetrahedron 2017, 73, 5892–5899; [Google Scholar]; h) Li G, Zhou S, Su G, Liu Y, Wang PG, J. Org. Chem 2007, 72, 9830–9833. [DOI] [PubMed] [Google Scholar]
- [7].Gao C, Cao D, Xu S, Meier H, J. Org. Chem 2006, 71, 3071–3076 [DOI] [PubMed] [Google Scholar]
- [8].a) Shu C, Chen C-B, Chen W-X, Ye L-W, Org. Lett 2013, 15, 5542–5545; [DOI] [PubMed] [Google Scholar]; b) Gao C, Cao D, Xu S, Meier H, J. Org. Chem 2006, 71, 3071–3076; [DOI] [PubMed] [Google Scholar]; c) Namashivaya SSR, Oshchepkov AS, Ding H, Förster S, Khrustalev VN, Kataev EA, Org. Lett 2019, 21, 8746–8750. [DOI] [PubMed] [Google Scholar]
- [9].Majumdar KC, Khan AT, Chattopadhyay SK, J. Chem. Soc. Perkin Trans 1 1990, 2219–2223. [Google Scholar]
- [10].Wu W-Y, Lin T-C, Takahashi T, Tsai F-Y, Mou C-Y, ChemCatChem 2013, 5, 1011–1019. [Google Scholar]
- [11].Shrestha B, Basnet P, Dhungana RK, Kc S, Thapa S, Sears JM, Giri R, J. Am. Chem. Soc 2017, 139, 10653–10656. [DOI] [PubMed] [Google Scholar]
- [12].a) Dhungana RK, Basnet SKCP, Giri R, Chem. Rec 2018, 18, 1314–1340; [DOI] [PubMed] [Google Scholar]; b) Giri R, Kc S, J. Org. Chem 2018, 83, 3013–3022; [DOI] [PubMed] [Google Scholar]; c) Thapa S, Dhungana RK, Magar RT, Shrestha B, Kc S, Giri R, Chem. Sci 2018, 9, 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS, Science 2016, 352, 801–805; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guo H-C, Ma J-A, Angew. Chem. Int. Ed 2006, 45, 354–366; Angew. Chem. 2006, 118, 362–375; [DOI] [PubMed] [Google Scholar]; c) Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP, Morken JP, Science 2016, 351, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Nakamura M, Hirai A, Nakamura E, J. Am. Chem. Soc 2000, 122, 978–979; [Google Scholar]; b) García-Domínguez A, Li Z, Nevado C, J. Am. Chem. Soc 2017, 139, 6835–6838; [DOI] [PubMed] [Google Scholar]; c) Terao J, Saito K, Nii S, Kambe N, Sonoda N, J. Am. Chem. Soc 1998, 120, 11822–11823; [Google Scholar]; d) Liao L, Jana R, Urkalan KB, Sigman MS, J. Am. Chem. Soc 2011, 133, 5784–5787; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wu X, Lin H-C, Li M-L, Li L-L, Han Z-Y, Gong L-Z, J. Am. Chem. Soc 2015, 137, 13476–13479; [DOI] [PubMed] [Google Scholar]; f) Mizutani K, Shinokubo H, Oshima K, Org. Lett 2003, 5, 3959–3961; [DOI] [PubMed] [Google Scholar]; g) Kuang Z, Yang K, Song Q, Org. Lett 2017, 19, 2702–2705; [DOI] [PubMed] [Google Scholar]; h) Kc S, Dhungana RK, Shrestha B, Thapa S, Khanal N, Basnet P, Lebrun RW, Giri R, J. Am. Chem. Soc 2018, 140, 9801–9805; [DOI] [PubMed] [Google Scholar]; i) Gao P, Chen L-A, Brown MK, J. Am. Chem. Soc 2018, 140, 10653–10657; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Anthony D, Lin Q, Baudet J, Diao T, Angew. Chem. Int. Ed 2019, 58, 3198–3202; [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Klauck FJR, Yoon H, James MJ, Lautens M, Glorius F, ACS Catal. 2019, 9, 236–241; [Google Scholar]; l) Yong X, Han Y-F, Li Y, Song R-J, Li J-H, Chem. Commun 2018, 54, 12816–12819; [DOI] [PubMed] [Google Scholar]; m) Ouyang X-H, Cheng J, Li J-H, Chem. Commun 2018, 54, 8745–8748; [DOI] [PubMed] [Google Scholar]; n) Ouyang X-H, Li Y, Song R-J, Hu M, Luo S, Li J-H, Sci. Adv 2019, 5, eaav9839; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Basnet P, Dhungana RK, Thapa S, Shrestha B, Kc S, Sears JM, Giri R, J. Am. Chem. Soc 2018, 140, 7782–7786; [DOI] [PubMed] [Google Scholar]; p) Li W, Boon JK, Zhao Y, Chem. Sci 2018, 9, 600–607; [DOI] [PMC free article] [PubMed] [Google Scholar]; q) Dhungana RK, Shrestha B, Thapa-Magar R, Basnet P, Giri R, Org. Lett 2017, 19, 2154–2157; [DOI] [PubMed] [Google Scholar]; r) Thapa S, Basnet P, Giri R, J. Am. Chem. Soc 2017, 139, 5700–5703. [DOI] [PubMed] [Google Scholar]
- [15].Kc S, Dhungana RK, Aryal V, Giri R, Org. Process Res. Dev 2019, 23, 1686–1694. [Google Scholar]
- [16].The diarylated imine products formed after initial dicarbofunctionalization reaction did not undergo the acid-catalyzed cyclization to form anthracene rings when p-OMe, p-Me, m-Me and m-F substituted ArZnI were used as coupling partners. In addition, heteroaryl iodide, such as 4-iodopyridine, was also not tolerated in the reaction.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









