Abstract

A series of aromatic Schiff bases, featuring 7-diethylamino-coumarin and with five different substituents at an adjacent phenyl ring, were synthesized and characterized. With the aim of assessing the stability of these dyes in acidic medium, their hydrolysis reactions were kinetically studied in the absence and presence of the macrocycle cucurbit[7]uril (CB[7]). Our results are consistent with a model containing three different forms of substrates (un-, mono-, and diprotonated) and three parallel reaction pathways. The pKa values and the rate constants were estimated and discussed in terms of the presence of a hydroxyl group at the ortho position and electron-releasing groups on the phenyl ring of the dyes. The kinetic study in the presence of CB[7] led to two different behaviors. Promotion of the reaction by CB[7] was observed for the hydrolysis of the Schiff bases containing only one coordination site toward the macrocycle. Conversely, an inhibitor effect was observed for the hydrolysis of a Schiff base with two coordination sites toward CB[7]. The latter effect could be explained with a model as a function of a prototropic tautomeric equilibrium and the formation of a 2:1 host/guest complex, which prevents the attack of water. Therefore, the kinetic results demonstrated a supramolecular control of the macrocycle toward the reactivity and stability of 7-diethylaminocoumarin Schiff bases in acidic medium.
Introduction
After their first synthesis by Hugo Schiff in 1864,1 Schiff bases have continued to be some of the most widely used organic compounds. Their applications involve several areas, such as sensing,2−6 synthesis,7,8 pharmaceutical,9−11 biological,12,13 and materials.14−16
One of the most interesting examples of Schiff bases are those containing a hydroxyl group at the ortho position, since these compounds undergo prototropic tautomerism,17 meaning proton transfer between the phenolic oxygen and the imine nitrogen. This proton-transfer process is related to the phenomena of thermochromism and photochromism.18,19 In fact, Hadjoudis et al. proposed that not only the role of planarity or nonplanarity of the molecules but also the high electron density of the lone electron pair of the imine nitrogen atom, influenced by substituents, are relevant for explaining this phenomenon.19
On the other hand, recent advances in chemical transformations in confined supramolecular architecture spaces provide a versatile platform for the development of novel supramolecular systems.20−22 Depending on the host–guest interactions, these systems can affect the stability, selectivity, and/or reversibility of different chemical molecules or processes. In fact, supramolecular assemblies have played a prominent role in catalytic23−26 and inhibition26,27 phenomena. Among the host molecules that play both roles, it seems likely that cucurbiturils (CB[n]) form assemblies controlling reactivity and selectivity during chemical transformations.28 This macrocycle exhibits a hydrophobic cavity with two identical portals aligned with carbonyl groups, which leads to not only observed catalytic but also inhibitory properties, depending on the associated guest.26
In particular, cucurbit[7]uril (CB[7]) has demonstrated the ability to form inclusion complexes that are stable in aqueous solutions, with an extensive variety of guests, including a wide range of important colorimetric and/or fluorescent dyes.29−31 The latter is of great relevance considering the instability of Schiff bases, caused by their susceptibility to hydrolysis in both acidic and basic media.6,32,33
It is well known that the condensation reaction of aldehydes or ketones with primary amines leads to the reversible formation of a Schiff base (or imines) via iminium ion generation.34 These imines are basic (pKa ≈ 7) and they exist in acidic solutions as iminium ions.34 In water, this equilibrium is shifted toward the starting reactants, while the iminium ion exists only as transient species. Relevant reports in aqueous solutions have demonstrated a novel use of host–guest chemistry to quantitatively generate and stabilize iminium ions from pyrrolidine and various ketones by encapsulation into a hydrophobic tetrahedral assembly.35,36 The authors demonstrated that the formation of inclusion complexes does not only require positively charged iminium ions and hydrophobic coordination cages but also a complementary fit of hosts and guests in terms of size and shape.36
Liu et al.27 demonstrated that CB[7] can play an important role in Schiff base reactions by inhibiting the condensation reactions of aldehydes and primary amines. Although CB[7] could not promote the generation of iminium cations under aqueous conditions, it enhanced their stability through host–guest interactions. This suggests that incorporating CB[7] is an interesting option for reaction mixtures containing a Schiff base in an acidic medium with the aim of assessing their ability to hydrolyze.
In this context, the goal of this work is to kinetically assess the effect of different substituents present in the 7-diethylaminocoumarin Schiff bases (imine coumarins IC, Figure 1) on their acidic hydrolysis reactions. In addition, the effect of CB[7] on the kinetic and the mechanism of the above-mentioned reaction for two representative Schiff bases (IC4 and IC5) is investigated for the first time.
Figure 1.
Series of the studied 7-diethylaminocoumarin Schiff bases (IC).
Results and Discussion
Synthesis and Structural Characterization of 7-Diethylaminocoumarin Schiff Bases
The proposed aromatic Schiff bases (IC1–IC5), featuring a 7-diethylaminocoumarin scaffold and a phenyl ring with different moieties, were synthesized according to the route presented in Scheme 1. For this purpose, 4-(diethylamino)salicylaldehyde (1) was coupled with ethylnitroacetate (2) using catalytic piperidine to obtain 3-nitrocoumarin (3), which was reduced to the corresponding 3-aminocoumarin (4) using SnCl2 in 15% HCl. Finally, 4 was coupled with the corresponding substituted benzaldehydes 5a–e to obtain the desired products IC1–IC5.37
Scheme 1. Synthesis of the Studied 7-Diethylaminocoumarin Schiff Bases (IC1–IC5).
Experimental conditions: (a) n-BuOH, piperidine 0.1 equiv reflux, 12 h; (b) SnCl2, 15% HCl, H2O, RT, 6 h; and (c) dry EtOH, N2, RT, 14 h.
All of the Schiff bases were adequately characterized using 1H and 13C NMR and high-resolution mass spectrometry (HRMS) methods (see Figures S1–S15 in the Supporting Information (SI)).
Effect of the Phenyl Ring Substituents on Schiff Bases’ Reactivities in Water
To assess how various substituents in IC influence the kinetics and the mechanism of the hydrolysis reaction, the first-order rate constants (kobs) were measured for the hydrolysis of the substituted aromatic Schiff bases (IC1–IC5) at different pH values (Table S1 in SI). Figure S16A–C displays plots of the kobs values as a function of [H3O+]. A linear apparent dependence was observed, each one showing an enhancement in the first-order rate constants (kobs) for a decrease in pH value. This supports the hypothesis of an acid-catalyzed hydrolysis reaction of the Schiff bases, also in accordance with a general eq 1, where kc is the acidic-catalytic constant and the k0 term represents the spontaneous hydrolysis.
| 1 |
The kc and k0 values for the hydrolysis of the studied 7-diethylaminocoumarin Schiff bases are obtained from Figure S16. As shown in this figure, the catalytic efficiency represented by kc values strongly depends on the substituents of the phenyl-containing Schiff base.
However, the constant kc is a macroconstant, including at least two factors: the pKa values of the Schiff base (imine coumarin; IC) and the rate constants of the reaction of the protonated imines (ICH+ and IC2H+) with water (kH+ and k2H+, respectively), as shown in Scheme 2.
Scheme 2. Proposed Mechanism for the Hydrolysis Reaction of the Substituted Aromatic Schiff Bases.
The general term IC represents the studied 7-diethylaminocoumarin Schiff bases (IC1–IC5).
In fact, Reeves38 postulated a pre-equilibrium between the Schiff base (IC) and its conjugate acid (ICH+) and then two reversible slow steps as a mechanism for the acidic hydrolysis reaction of Schiff bases. In line with this mechanism, Kim et al.6 have demonstrated that the hydrolysis reaction of the coumarin-based Schiff base (containing an imine bond inverted against IC) involves the addition of water to the imine bond as a first step and the dissociation of the hemiaminal intermediate into an amine and aldehyde as the second step. In acidic conditions, the water addition is the rate-determining step.
It is important to note that the nonprotonated Schiff base IC is also susceptible to undergoing the hydrolysis reaction (k0). However, taking into account the low k0 values obtained for each compound (Table 1), we do not believe that such a reaction is taking place, which only leaves the kH+ and k2H+ routes for the hydrolysis reaction. The IC conjugate acids are probably more susceptible to solvent attack due to the presence of an iminium ion.
Table 1. Kinetic Rate Constants for the Hydrolysis Reaction of the Substituted 7-Diethylaminocoumarin Schiff Bases (IC1–IC5; 8 μM) at T = 25.0 °C.
| Schiff bases | pKa1 | pKa2 | 104kH+ (s–1 M–1)a | k2H+ (s–1 M–1)a | 103k0 (s–1)b |
|---|---|---|---|---|---|
| IC1 | 4.29 ± 0.05 | 1.09 ± 0.01 | 1600 ± 100 | 131 ± 5 | <21 |
| IC2 | 3.66 ± 0.28 | 1.15 ± 0.01 | 21 ± 6 | 0.27 ± 0.01 | <0.1 |
| IC3 | 4.10 ± 0.03 | 1.10 ± 0.01 | 3026 ± 5 | 169 ± 5 | <31 |
| IC4 | 3.00 ± 0.13 | 1.10 ± 0.02 | 36 ± 8 | 0.70 ± 0.02 | |
| IC5 | 3.26 ± 0.07 | 1.17 ± 0.18 | 1.3 ± 0.4 | 0.052 ± 0.001 | <0.03 |
According to the mechanism described in Scheme 2, kobs for the hydrolysis reaction of IC can be expressed as follows (calculations can be found in the SI, eqs S1–S12)
| 2 |
where [H3O+] is determined by eq S10.
Figure 2 shows the experimental results of the hydrolysis rate (kobs) as a function of pH (Table S-1), and the calculated curves, using eq 2. As a consequence, the equilibrium and kinetic constants can be obtained as shown in Table 1.
Figure 2.
pH profile for the hydrolysis reactions at 25.0 °C: graph A (green circles) IC4 and (black circles) IC2; graph B (black circles) IC5; and graph C (blue circles) IC3 and (red circles) IC1. Conditions: [buffer] = 0.01 M (acetic acid/sodium acetate buffers) and a fixed concentration of IC (8 μM). Points show experimental values (Table S1), and curves are calculated with eq 2.
In this context, Misra et al.32 have determined the pKa values for the Schiff bases, N-(2-hydroxybenzylidene)-2-aminobenzothiazole and N-(4-hydroxybenzylidene)-2-aminobenzothiazole, which were calculated to be 3.56 and 4.24, respectively. These values are comparable to those determined for the studied Schiff bases IC1–IC5 (pKa1 values presented in Table 1).
The pKa values of the 7-diethylamino group at the coumarin scaffold determined by fitting kinetic data and presented in Table 1 (≈1.1) are in complete agreement with those reported values of conjugate acids of 3-aryl substituents on the 7-aminocoumarin systems.39
Considering IC1 as a reference compound for the studied hydrolysis reactions, it was observed that the substitution with a hydroxyl group at the para position of the phenyl ring (IC3) leads to a higher reactivity at the reaction site of IC3. This can be hypothesized with the greater electron-withdrawing effect of OH compared to H, which leaves the aldimide carbon of IC3 more positive, and therefore, more prone to a nucleophilic attack by water. In fact, as shown in Table 1, the compound IC3 depicts a higher second-order rate constant (kH+ and k2H+) than IC1.
On the other hand, the presence of a hydroxyl group at the ortho position, such as in IC2, IC4, and IC5, decreases the acidic-catalytic constant (kH+ and k2H+) by one to three orders of magnitude against compounds IC1 and IC3. These results suggest stronger stabilization of the conjugated acid of the Schiff base in the former group, probably due to the existence of a specific hydrogen bond. In this context, two structural forms of o-hydroxy Schiff bases, called phenol-imine and keto-enamine structures, are well known as prototropic tautomers.17,40
Interestingly, Mishra et al. reported on the influence of the neighboring −OH by assessing the hydrolysis of the Schiff bases: N-(2-hydroxybenzylidene)-2-aminobenzothiazole and N-(4-hydroxybenzylidene)-2-aminobenzothiazole.32 However, the authors found a higher reactivity for the first benzothiazole than the second one with regard to their hydrolysis reaction. The most convincing explanation was that the transition state involves the intramolecular participation of the ortho-OH (or O–) and the thiazolyl C=N bond facilitating the attack by water. As a result, we propose that the difference in reactivity compared with the present study arises from the fact that the coumarin scaffold, which is present in the used Schiff bases (Figure 1), is not able to involve a water molecule in the transition state at least not in the same way as a benzothiazole scaffold.
Moreover, for the above-mentioned 2-hydroxy Schiff base, a pKa value of ≈8.3 for −OH at the ortho position was reported.32 Therefore, considering our experimental conditions (pH < 5), the protonation of OH present in IC2 would be completely favored, which indicates that the presence of the ortho(−OH) group would facilitate the direct formation of hydrogen bonds, either with water molecules or with the nitrogen atom of the imine group.18 In fact, other authors have proposed that the ortho(−OH) group, present in similar Schiff bases, exerts a masking effect on the reaction site due to a crowding effect and also because it captures the incoming nucleophilic water molecule through hydrogen bonding.41
Figure S4 shows the 1H NMR spectrum for IC2 with a typical signal at around 13 ppm associated with the proton of −OH at the ortho position, which is an indication of the presence of the phenol-imine tautomer. Thus, the protector role of the OH group is a probable reason for the stabilization of this IC2 tautomer against an attack at the aldimide carbon (of the Schiff base) compared to IC3 and IC1, therefore decreasing the reactivity for IC2, as shown in Table 1.
Furthermore, the lowest reactivity was observed for IC5, which does not only show a protector effect of the OH group at the ortho position of the aromatic ring but also possesses an additional hydroxyl group at the meta position (releasing effect), decreasing the nucleophilic attack at the aldimine carbon.
Effect of the Inclusion of IC4 and IC5 in the Macrocycle CB[7] on Their Reactivity
To study the effect of the macrocycle CB[7] on the reactivity of the 7-diethylaminocoumarin Schiff base derivatives, mass spectra (HRMS) experiments were carried out (Figures S17–S22).
As shown in Figures S18–S22, the analysis in negative ionization mode for each derivative in the presence of CB[7] allowed us to assign the molecular ions with m/z 1605.32 (IC2), 1587.39 (IC3), 1763.51 (IC4), and 1549.45 (IC5). This revealed an association between each derivative and CB[7] in a 1:1 ratio, respectively. No signals were observed with a ratio m/z related to other complex stoichiometries, except for IC4, where the fragment at m/z 1450.3287 [H·IC4·2·CB[7]·2H2O + Cl– + HSO4–]2– (see Figure S21) corresponds to a 2:1 (host/guest) complex stoichiometry.
In the case of IC1, the analysis of Figure S17 demonstrated that the molecular ion associated with the adduct IC1 and CB[7] is not observed, probably due to the fast decomposition of this compound via the hydrolysis reaction. In fact, the major molecular ions observed were 1443.5303 and 1585.4238, which are related to the association between the products of the hydrolysis (benzaldehyde and 3-amino-7-(diethylamino)-2H-chromen-2-one) and CB[7].
Moreover, other reports have determined a binding constant of 1.2 × 105 M–1 for the inclusion complex formed between benzaldehyde and CB[7],42 while binding constants of the same order of magnitude have been reported for 7-(diethylamino)-2H-chromen-2-one derivatives43 and CB[7] complexes. However, as shown below, the incorporation of the substrate into CB[7] is favored in comparison with the products.
Among the tested Schiff base derivatives, IC4 and IC5 were chosen as representative compounds to assess the effect of the inclusion into CB[7] on the reactivity toward their hydrolysis reactions.
Figure 3 shows the 1H NMR spectra for the free IC4 (A) and in the presence of the macrocycle CB[7] at two different ratios, 1:1 (B) and 1:5 (C), respectively. As shown in Figure 3, this inclusion was proven by the upfield-shifted guest 1H NMR resonances of aromatic and aliphatic signals of IC4 in its phenol-imine tautomeric form, in both ratios (Figures 3 and S23). Moreover, the aromatic Hd′, Hf′, and Hα and the Hβ signals all appeared in duplicate form after addition of CB[7] (Figure 3C).
Figure 3.
1H NMR spectra (400 MHz) for (A) dye IC4 (0.5 mM), (B) IC4 in the presence of a 1:2 molar ratio (dye/CB[7]), and (C) IC4 in the presence of a 1:5 molar ratio (dye/CB[7]) in DMSO-d6/D2O 1:1 v/v.
These results suggest the presence of different species after the inclusion while confirming the inclusion of both IC4 extrema (diethylaminocoumarin and phenyl ring) in the macrocycle. The latter is in line with studies demonstrating that CB[7] encapsulates diethylaminocoumarin-derivative dyes44 and flavylium derivatives containing groups45 with high binding constants (≈105 M–1).
Moreover, the signal associated with the imine (N=C–Ha′) proton of IC4 was observed at 8.92 ppm (A) for the free guest in DMSO. In the presence of an equimolar concentration of CB[7], the signal undergoes an upfield shift to 8.69 ppm (B), and in the presence of an excess of CB[7], the signal is observed at 8.62 ppm (C). The shift of this peak to 8.69 ppm (B) and 8.62 ppm (C) can be explained by the interaction of IC4 in its phenol-imine form with the macrocycle, leading to higher shielding due to the inclusion of this group.
Interestingly, Figure 3B,C shows the appearance of a characteristic signal at 6.4 ppm associated with the Ha proton of IC4 in its keto-enamine tautomeric form. Alternatively, the proton Hb, which changes its position from the hydroxyl to the amino group, could be found in low proportion because of the isotopic exchange with the deuterated water.
Therefore, both tautomers would be present in the presence of low CB[7] concentrations. However, when this IC is exposed to a high concentration of CB[7], the keto-enamine tautomer is the predominant form.
It is important to mention that another possible explanation could be the decomposition of the imine group into aldehyde and amine, which could justify the assignment of the signals at 8.69 ppm (B) and 8.62 ppm (C) to the NH2 group. However, considering the previously described kinetic study, this scenario is unlikely, since CB[7] inhibits the acid-catalyzed hydrolysis. Therefore, the occurrence of the keto-enamine form is highly probable in the presence of CB[7].
Once the ability of IC4 to form a stable 1:2 inclusion complex with CB[7] was established, kinetic studies of its hydrolysis reactions in the presence of CB[7] were carried out under pseudo-first-order conditions. To compare different Schiff bases, the hydrolysis reaction of IC5 was also investigated.
In the presence of CB[7], the hydrolysis reactions of these two representative substituted Schiff bases show a great difference between the IC4 derivative, which has a −OH at the ortho position and a diethylamino group at the para position of the aromatic ring, and IC5, which has a −OH at the ortho position and a −OH at the meta position of the aromatic ring.
As shown in Figure 4, the kobs values associated with the hydrolysis of the IC4 decrease with increasing concentration of CB[7]. This inhibition of the CB[7]-induced reaction depicts a fast initial decrease of kobs and then a slight stabilization of the inhibition. This behavior is observed at both pH 2.5 and 3.5 (Figure 4).
Figure 4.
Influence of the CB[7] concentration on the pseudo-first-order rate constants (kobs) for IC4 hydrolysis at pH 2.5 (A) and pH 3.5 (B). Conditions: [buffer] = 0.05 M, Britton–Robinson; T = 25.0 °C.
The inhibition process could be explained by the formation of the keto-enamine tautomer for IC4 after the inclusion into CB[7], which continuously inhibits the reaction with water molecules. The presence of an excess of CB[7] leads to total inhibition due to the inclusion of the coumarin scaffold in CB[7], which would prevent the attack of a water molecule on the aldimide carbon by steric hindrance.
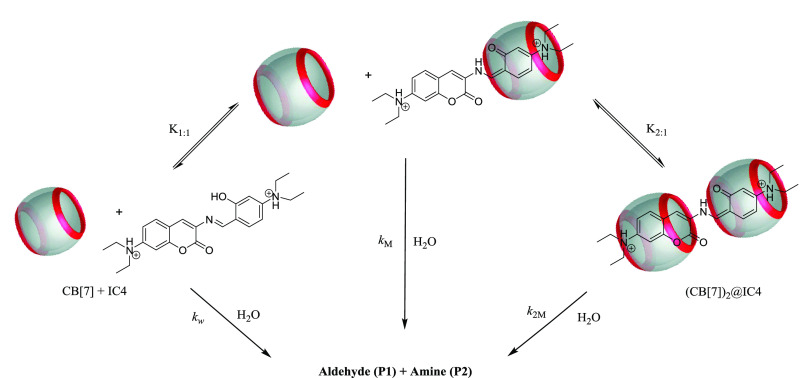
Regarding IC4, NMR results demonstrated the formation of both 1:1 and 2:1 complexes between CB[7] and IC4. As a result, this information was included in our proposed hydrolysis mechanism in the presence of CB[7] (Scheme 3). In this scheme, only the keto-enamine form was included in CB[7] for clarity.
Scheme 3. Proposed Mechanism for the Hydrolysis Reaction of the Substituted Aromatic Schiff Base IC4 in the Presence of CB[7].
Therefore, the correlation between the observed rate constant kobs and the concentration of the macrocycle can be obtained from eq 3
| 3 |
where the rate constants kw, kM, and k2M represent the hydrolysis of the diprotonated Schiff base IC4 free and their CB[7]-complexed forms 1:1 and 2:1, respectively.
Note that CB[7] refers to the concentration of uncomplexed cucurbit[7]uril. Thus, this concentration was obtained by solving a third-order equation according to Thordarson.46
For the fitting procedure, the hydrolysis rate constants in bulk water, kw, were kept constant by using the experimentally obtained values (see above). Figure 4 shows good fits of the kinetic data obtained for IC4 (using eq 3). According to Scheme 3, cationic IC4 is able to accommodate two hosts, implying that two rate constants should be considered for the cucurbituril-inhibited hydrolysis.
Table 2 shows the results obtained from the kinetic data fit for the IC4 hydrolysis reaction in the presence of CB[7] by using eq 3 with kw = 1 × 10–3 s–1 (at pH 3.5).
Table 2. Kinetic Rate Constants and Host:Guest Binding Constants for Hydrolysis of IC4a, IC2b, and IC5b in the Presence of CB[7] at T = 25.0 °C.
| derivative | pH | 10–5K1:1 (M–1) | 10–3K2:1(M–1) | 103kw (s–1) | 104kM(s–1) |
|---|---|---|---|---|---|
| IC4 | 2.5 | 160 ± 50 | 20 ± 14 | 22 ± 0.2 | 8 ± 2 |
| IC4 | 3.5 | 3.5 ± 0.4 | 2.8 ± 0.5 | 1.0 ± 0.1 | 4.4 ± 0.1 |
| IC2 | 2.8 | 110 ± 10 | 10 ± 0.2 | 230 ± 1 | |
| IC5 | 4.0 | 31.0 ± 0.3 | 0.021 ± 0.2 | 0.81 ± 0.1 |
However, when IC5 was studied, promotion of the observed rate constants as a function of the CB[7] concentration was shown under our experimental conditions (Figure 5A). With the aim of obtaining more information about the importance of the presence of the OH– group at the ortho position, another derivative containing only a OH– group at this position (IC2) was kinetically studied as a control experiment (see Figure 5B). Interestingly, the same kinetic behavior as that observed for IC5 was obtained for IC2.
Figure 5.
Influence of CB7 concentration on the pseudo-first-order rate constants for the hydrolysis of IC5 at pH 4.0 (A) and for IC2 hydrolysis at pH 2.8 (B). Conditions: [buffer] = 0.05 M, Britton–Robinson; T = 25.0 °C.
In both cases (IC2 and IC5), kinetic results are consistent with a proposed mechanism similar to Scheme 3 (suggested as an example for IC5), considering only the formation of a 1:1 host:guest complex between CB[7] and the Schiff base (KCB[7]:IC5). The hydrolytic reaction takes place simultaneously in water (kw) and inside the host cavity (kM). Both reaction pathways, kw and kM, involve the Schiff base protonation and carbocation formation as the rate-determining step.
Thus, considering the existence of a 1:1 host/guest complex and that hydrolysis can take place simultaneously in water, kw, and the host cavity, kCB[7], it is possible to derive the rate equation 4
| 4 |
Interestingly, as shown in Table 2, the apparent binding constant (K1:1), obtained for IC4 by fitting kinetic data in our experimental conditions, is lower than other affinities reported for the amino-substituted flavylium guest.47
In the case of IC5, the K1:1 value obtained from the kinetic study is 3.1 × 106 M–1, which is slightly higher than other values reported by the inclusion of 7-(diethylamino)coumarin derivatives (order of ∼105 M–1) in CB[7].43,48 Thus, the K1:1 value for IC5 demonstrates a greater binding of the substrate than with the aldehyde product.
On the other hand, a comparison of the kinetic behaviors observed suggests that the inhibitory effect (IC4) would be explained by the existence of another diethylamino group, which allows for the formation of a 2:1 host/guest complex while preventing the attack of water.
In contrast, there is a rate enhancement for the hydrolysis reaction of Schiff bases (IC2 and IC5) that contain only one inclusion site toward CB[7]. Most likely, the portal CB[7]’s assistance for the IC5 protonation of the imine nitrogen could be the main factor responsible for the observed catalytic effect.
Finally, the kinetic behavior and reactivity toward the hydrolysis reactions of 7-diethylaminocoumarin Schiff base derivatives can be controlled by the presence of the ortho-(OH) group at the phenyl ring or by their encapsulation into CB[7].
Conclusions
The hydrolysis reactivity of a series of 7-diethylaminocoumarin Schiff base derivatives follows the order IC3 > IC1 > IC4 > IC2 > IC5, where the decrease in the reactivity and stabilization of Schiff bases is associated with the presence of an ortho-(OH) group and electron-releasing groups at the aromatic ring.
As representative compounds, IC4, IC2, and IC5 were chosen to assess the effect of the CB[7] on the hydrolysis reactivity. Interestingly, kinetic results demonstrated a dual effect of the macrocycle. IC4 depicts a first inhibition induced by the macrocycle associated with a modification of the prototropic tautomeric equilibrium after the encapsulation into a single CB[7] molecule. Subsequently, a second inhibition process was observed, which was attributed to the steric hindrance that prevents the hydrolysis reaction after a second encapsulation of the IC4. However, a significant rate enhancement was observed in the case of IC2 and IC5, showing the promotion of the acidic hydrolysis reaction by CB[7].
Experimental Section
All solvents and reagents were purchased from Sigma-Aldrich and used as received. Unless indicated, all solutions employed in this study were prepared in aqueous solutions.
Synthesis
The synthesis of the intermediates 3-nitrocoumarin and 3-amino-7-(diethylamino)-2H-chromen-2-one was carried out following a procedure reported by Bharadwaj et al.,37 and their physical and spectroscopic data are in agreement with the literature. To obtain the final aromatic Schiff bases (IC1–IC5), a mixture of 1 equiv of the corresponding 3-aminocoumarin, the corresponding benzaldehyde (1.5 equiv), and ethanol (20 mL) were added under an inert atmosphere and stirred for 12 h. The crude solid product was filtered and immediately crystallized in acetonitrile/diethyl ether 1:1 v/v. The product was stored in the absence of light.
(E)-3-(Benzylideneamino)-7-(diethylamino)-2H-chromen-2-one (IC1)
Yield 50%; yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 9.13 (s, 1H), 7.89 (dd, J = 6.0, 2.6 Hz, 2H), 7.83 (s, 1H), 7.55–7.46 (m, 4H), 6.74 (dt, J = 9.0, 1.6 Hz, 1H), 6.58 (t, J = 1.6 Hz, 1H), 3.45 (q, J = 7.0 Hz, 4H), 1.14 (t, J = 6.9 Hz, 6H); 13C NMR (101 MHz, DMSO) δ 193.67, 160.62, 158.72, 155.06, 150.66, 136.92, 135.05, 131.76, 129.94, 129.30, 128.82, 126.20, 109.89, 108.68, 96.77, 44.57, 12.81. ESI-HRMS, positive mode m/z calculated for C20H21N2O2+, [M + H]+: 321.1598; found: 321.1556.
(E)-7-(Diethylamino)-3-((2-hydroxybenzylidene)amino)-2H-chromen-2-one (IC2)
Yield 58%, yellow solid; 1H NMR (400 MHz, DMSO-d6) δH 13.15 (d, J = 2.7 Hz, 1H), 9.29 (d, J = 2.8 Hz, 1H), 8.02 (d, J = 3.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 8.0 Hz), 7.39 (t, J = 8.0 Hz, 1H), 6.95 (d, J = 8.5 Hz, 2H), 6.80–6.72 (m, 1H), 6.60 (d, J = 2.8 Hz, 1H), 3.45 (q, J = 7.2 Hz, 5H), 1.14 (td, J = 7.1, 2.9 Hz, 6H); 13C NMR (101 MHz, DMSO) δ 162.51, 160.75, 158.82, 155.30, 150.99, 133.69, 133.49, 132.60, 130.25, 126.12, 120.07, 119.65, 117.15, 110.13, 108.37, 96.88, 44.62, 12.83. ESI-HRMS, positive mode m/z calculated for C20H21N2O3+, [M + H]+ = 337.1547; found: 337.1507.
(E)-7-(Diethylamino)-3-((4-hydroxybenzylidene)amino)-2H-chromen-2-one (IC3)
Yield 54%, yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 10.17 (s, 1H), 8.92 (s, 1H), 7.74 (d, J = 8.1 Hz, 2H), 7.68 (s, 1H), 7.45 (d, J = 8.8 Hz, 1H), 6.90 (d, J = 8.1 Hz, 2H), 6.71 (d, J = 8.6 Hz, 1H), 6.55 (s, 1H), 3.42 (dt, J = 14.1, 6.7 Hz, 7H), 1.13 (t, J = 7.0 Hz, 6H); 13C NMR (101 MHz, DMSO) δ 161.15, 160.50, 158.98, 154.77, 150.24, 132.89, 130.91, 130.00, 129.59, 128.21, 116.19, 109.76, 108.80, 96.82, 44.52, 12.81. ESI-HRMS, positive mode m/z calculated for C20H21N2O3+, [M + H]+ = 337.1547; found: 337.1504.
(E)-7-(Diethylamino)-3-((4-(diethylamino)-2-hydroxy-benzylidene)amino)-2H-chromen-2-one (IC4)
Yield 76%, red solid; 1H NMR (400 MHz, CDCl3) δ 13.82 (s, 1H), 9.30 (s, 1H), 7.50 (s, 1H), 7.31 (d, J = 8.8 Hz, 1H), 7.50 (s, 1H), 7.20 (d, J = 8.8 Hz, 1H), 6.62 (dd, J = 8.8, 2.2 Hz, 1H), 6.55 (s, 1H), 6.26 (dd, J = 8.7, 2.2 Hz), 6.19 (s, 1H), 3.31–3.55 (m, 8H), 1.21–1.26 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 162.28, 159.18, 154.40, 154.21, 151.60, 149.94, 134.14, 133.39, 128.54, 109.62, 109.28, 109.10, 103.79, 99.99, 97.74, 97.17, 44.83, 44.57, 12.74, 12.50. ESI-HRMS, positive mode m/z calculated for C24H30N3O3+, [M + H]+ = 408.2282; found: 408.2263.
(E)-7-(Diethylamino)-3-((2,5-dihydroxybenzylidene)amino)-2H-chromen-2-one (IC5)
Yield 50%, yellow solid; 1H NMR (400 MHz, DMSO-d6) δ 13.43 (s, 1H), 9.71 (s, 1H), 8.88 (s, 1H), 7.81 (s, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.04–6.85 (m, 2H), 6.34 (d, J = 9.0 Hz, 1H), 6.07 (s, 1H), 3.41 (q, J = 7.5 Hz, 5H), 1.13 (t, J = 7.0 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 164.61, 163.06, 158.90, 154.44, 152.69, 145.21, 143.41, 134.93, 134.37, 126.70, 120.94, 118.68, 117.16, 112.35, 109.15, 104.89, 97.29, 44.47, 13.03. ESI-HRMS, negative mode m/z calculated for C20H19N2O4–, [M – H]− = 351.1350; found: 351.1329.
Kinetic Studies
Repetitive scans for the hydrolysis reactions of IC1–IC5, in aqueous solutions and for IC4–IC5 at different CB[7] concentrations, were recorded at different times. These were carried out using an HP-8453 diode array spectrophotometer at 25.0 ± 0.1 °C in the pH range of 2–5. The hydrolysis reaction was followed by observing the increase in absorbance at 314 nm (corresponding to aldehyde formation) as a function of time. In all kinetic runs, the initial concentration of the substrate was 2.00 × 10–5 M, whereas the CB[7] concentrations were in the 0–1.60 × 10–4 M range.
The acidity was kept constant by using 0.05 Britton–Robinson buffer, with a difference between the initial and final reaction pH value of less than 0.03 pH units.
In all cases, the observed pseudo-first-order rate constants, kobsd, were obtained by adjusting the experimental absorbance vs time curves to a first-order kinetic equation. The standard error for kobsd was always <5%.
High-Resolution Mass Spectrometry (HRMS-ESI) Studies
High-resolution mass spectra (HRMS-ESI) were obtained from a Thermo Fisher Scientific Exactive Plus mass spectrometer. The analysis for the reaction products was performed with the following relevant parameters: heater temperature, 50 °C; sheath gas flow, 5; sweep gas flow rate, 0; and spray voltage, 3.0 kV, in negative mode. The accurate mass measurements were performed at a resolution of 140.000.
Nuclear Magnetic Resonance (NMR) Studies
1H NMR spectra were obtained at 25 °C on Bruker Avance 400 and 200 MHz spectrometers using TMS as an internal standard. The NMR spectra were processed using MestreNova software v9.0.
Acknowledgments
This work was supported by FONDECYT grant nos. 1170753 and 1160271. J.J.A. is grateful for the CONICYT Doctoral Fellowship 21170793. N.G. thanks the German Academic Exchange Service for a RISE worldwide stipend (project CL-CH-4026). Financial support from the Ministerio de Economia y Competitividad of Spain (project CTQ2017-84354-P), Xunta de Galicia (GR 2007/085; IN607C 2016/03 and Centro Singular de Investigación de Galicia accreditation 2016–2019, ED431G/09), and the European Union (European Regional Development Fund-ERDF) is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00683.
Spectroscopic data for all compounds (IC1–IC5); kinetic data; and equations for the studied hydrolysis reactions (PDF)
Author Present Address
⊥ The Michael Barber Centre for Collaborative Mass Spectrometry, Manchester Institute of Biotechnology, The University of Manchester, Oxford Road, Manchester M13 9PL, UK.
The authors declare no competing financial interest.
Supplementary Material
References
- Schiff H. Mittheilungen Aus Dem Universitätslaboratorium in Pisa: Eine Neue Reihe Organischer Basen. Ann. Chem. Pharm. 1864, 131, 118–119. 10.1002/jlac.18641310113. [DOI] [Google Scholar]
- Reimann M. J.; Salmon D. R.; Horton J. T.; Gier E. C.; Jefferies L. R. Water-Soluble Sulfonate Schiff-Base Ligands as Fluorescent Detectors for Metal Ions in Drinking Water and Biological Systems. ACS Omega 2019, 4, 2874–2882. 10.1021/acsomega.8b02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin Ö.; Özdemir Ü. Ö.; Seferoğlu N.; Genc Z. K.; Kaya K.; Aydıner B.; Tekin S.; Seferoğlu Z. New Platinum (II) and Palladium (II) Complexes of Coumarin-Thiazole Schiff Base with a Fluorescent Chemosensor Properties: Synthesis, Spectroscopic Characterization, X-Ray Structure Determination, in Vitro Anticancer Activity on Various Human Carcinoma Ce. J. Photochem. Photobiol., B 2018, 178, 428–439. 10.1016/j.jphotobiol.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Wang K.; Sun P.; Chao X.; Cao D.; Mao Z.; Liu Z. A Coumarin Schiff’s Base Two-Photon Fluorescent Probe for Hypochlorite in Living Cells and Zebrafish. RSC Adv. 2018, 8, 6904–6909. 10.1039/c8ra00093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinegger A.; Borisov S. M. Zn(II) Schiff Bases: Bright TADF Emitters for Self-Referenced Decay Time-Based Optical Temperature Sensing. ACS Omega 2020, 5, 7729–7737. 10.1021/acsomega.0c01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. Y.; Shi H.; Jung H. S.; Cho D.; Verwilst P.; Lee J. Y.; Kim J. S. Coumarin-Decorated Schiff Base Hydrolysis as an Efficient Driving Force for the Fluorescence Detection of Water in Organic Solvents.. Chem. Commun. 2016, 52, 8675–8678. 10.1039/c6cc04285f. [DOI] [PubMed] [Google Scholar]
- Tyagi P.; Tyagi M.; Agrawal S.; Chandra S.; Ojha H.; Pathak M. Synthesis, Characterization of 1,2,4-Triazole Schiff Base Derived 3d-Metal Complexes: Induces Cytotoxicity in HepG2, MCF-7 Cell Line, BSA Binding Fluorescence and DFT Study. Spectrochim. Acta, Part A 2017, 171, 246–257. 10.1016/j.saa.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Haque J.; Srivastava V.; Chauhan D. S.; Lgaz H.; Quraishi M. A. Microwave-Induced Synthesis of Chitosan Schiff Bases and Their Application as Novel and Green Corrosion Inhibitors: Experimental and Theoretical Approach. ACS Omega 2018, 3, 5654–5668. 10.1021/acsomega.8b00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervez H.; Ahmad M.; Zaib S.; Yaqub M.; Naseer M. M.; Iqbal J. Synthesis, Cytotoxic and Urease Inhibitory Activities of Some Novel Isatin-Derived Bis-Schiff Bases and Their Copper Complexes. MedChemComm 2016, 7, 914–923. 10.1039/C5MD00529A. [DOI] [Google Scholar]
- Divya K.; Pinto G. M.; Pinto A. F. Application of metal complexes of schiff bases as an antimicrobial drug: A review of recent works. Int. J. Curr. Pharm. Res. 2017, 9, 27–30. 10.22159/ijcpr.2017.v9i3.19966. [DOI] [Google Scholar]
- Kajal A.; Bala S.; Kamboj S.; Sharma N.; Saini V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 1–14. 10.1155/2013/893512. [DOI] [Google Scholar]
- Zoubi W. Al. Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int. J. Org. Chem. 2013, 03, 73–95. 10.4236/ijoc.2013.33A008. [DOI] [Google Scholar]
- Przybylski P.; Huczynski A.; Pyta K.; Brzezinski B.; Bartl F. Biological Properties of Schiff Bases and Azo Derivatives of Phenols. Curr. Org. Chem. 2009, 13, 124–148. 10.2174/138527209787193774. [DOI] [Google Scholar]
- Bedair M. A.; Soliman S. A.; Bakr M. F.; Gad E. S.; Lgaz H.; Chung I.-M.; Salama M.; Alqahtany F. Z. Benzidine-Based Schiff Base Compounds for Employing as Corrosion Inhibitors for Carbon Steel in 1.0 M HCl Aqueous Media by Chemical, Electrochemical and Computational Methods. J. Mol. Liq. 2020, 317, 114015 10.1016/j.molliq.2020.114015. [DOI] [Google Scholar]
- More M. S.; Joshi P. G.; Mishra Y. K.; Khanna P. K. Metal Complexes Driven from Schiff Bases and Semicarbazones for Biomedical and Allied Applications: A Review. Mater. Today Chem. 2019, 14, 100195 10.1016/j.mtchem.2019.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiye É. N.; Ribeiro M. F. M.; Katayama J. M. T.; Tadini M. C.; Balbino M. A.; Eleotério I. C.; Magalhães J.; Castro A. S.; Silva R. S. M.; da Cruz Júnior J. W.; Dockal E. R.; de Oliveira M. F. Electrochemical Sensors Containing Schiff Bases and Their Transition Metal Complexes to Detect Analytes of Forensic, Pharmaceutical and Environmental Interest. A Review. Crit. Rev. Anal. Chem. 2019, 49, 1–22. 10.1080/10408347.2018.1561242. [DOI] [PubMed] [Google Scholar]
- Kaştaş G.; Kaştaş Ç. A.; Kırca B. K.; Ersanlı C. C. The Effect of the Change in Substituents’ Positions on the Formation of Supramolecular Networks and the Solvent Type/Substituent Dependence of Prototropic Behavior in Three New o-Hydroxy Schiff Bases. J. Mol. Struct. 2020, 1200, 127109 10.1016/j.molstruc.2019.127109. [DOI] [Google Scholar]
- Fernández-G J. M.; Del Rio-Portilla F.; Quiroz-García B.; Toscano R. A.; Salcedo R. The Structures of Some Ortho-Hydroxy Schiff Base Ligands. J. Mol. Struct. 2001, 561, 197–207. 10.1016/S0022-2860(00)00915-7. [DOI] [Google Scholar]
- Hadjoudis E.; Vittorakis M.; Moustakali-Mavridis I. Photochromism and Thermochromism of Schiff Bases in the Solid State and in Rigid Glasses. Tetrahedron 1987, 43, 1345–1360. 10.1016/S0040-4020(01)90255-8. [DOI] [Google Scholar]
- Sinha I.; Mukherjee P. S. Chemical Transformations in Confined Space of Coordination Architectures. Inorg. Chem. 2018, 57, 4205–4221. 10.1021/acs.inorgchem.7b03067. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang Y.-X.; Yang H.-B. Supramolecular Transformations within Discrete Coordination-Driven Supramolecular Architectures. Chem. Soc. Rev. 2016, 45, 2656–2693. 10.1039/C5CS00301F. [DOI] [PubMed] [Google Scholar]
- Liu J.; Lan Y.; Yu Z.; Tan C. S. Y.; Parker R. M.; Abell C.; Scherman O. A. Cucurbit[n]Uril-Based Microcapsules Self-Assembled within Microfluidic Droplets: A Versatile Approach for Supramolecular Architectures and Materials. Acc. Chem. Res. 2017, 50, 208–217. 10.1021/acs.accounts.6b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. J.; Toste F. D.; Bergman R. G.; Raymond K. N. Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. 10.1021/cr4001226. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Zhang T.; Wang H.; Xu B. Supramolecular Catalysis and Dynamic Assemblies for Medicine. Chem. Soc. Rev. 2017, 46, 6470–6479. 10.1039/c7cs00472a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C. M.; Bergman R. G.; Raymond K. N.; Toste F. D. Self-Assembled Tetrahedral Hosts as Supramolecular Catalysts. Acc. Chem. Res. 2018, 51, 2447–2455. 10.1021/acs.accounts.8b00328. [DOI] [PubMed] [Google Scholar]
- Basilio N.; García-Río L.; Moreira J. A.; Pessêgo M. Supramolecular Catalysis by Cucurbit[7]Uril and Cyclodextrins: Similarity and Differences. J. Org. Chem. 2010, 75, 848–855. 10.1021/jo902398z. [DOI] [PubMed] [Google Scholar]
- Gong W.; Ma J.; Zhao Z.; Gao F.; Liang F.; Zhang H.; Liu S. Inhibition and Stabilization: Cucurbituril Induced Distinct Effects on the Schiff Base Reaction. J. Org. Chem. 2017, 82, 3298–3301. 10.1021/acs.joc.6b02971. [DOI] [PubMed] [Google Scholar]
- Funk S.; Schatz J. Cucurbiturils in Supramolecular Catalysis. J. Inclusion Phenom. Macrocyclic Chem. 2020, 96, 1–27. 10.1007/s10847-019-00956-0. [DOI] [Google Scholar]
- Dsouza R. N.; Pischel U.; Nau W. M. Fluorescent Dyes and Their Supramolecular Host/Guest Complexes with Macrocycles in Aqueous Solution. Chem. Rev. 2011, 111, 7941–7980. 10.1021/cr200213s. [DOI] [PubMed] [Google Scholar]
- Nau W. M.; Mohanty J. Taming Fluorescent Dyes with Cucurbituril. Int. J. Photoenergy 2005, 7, 568352 10.1155/S1110662X05000206. [DOI] [Google Scholar]
- Delgado-Pinar E.; Valente A. J. M.; Sérgio Seixas de Melo J. A Comprehensive Photophysical and NMR Investigation on the Interaction of a 4-Methylumbelliferone Derivative and Cucurbit[7]Uril. J. Mol. Liq. 2019, 1026 10.1016/j.molliq.2018.12.137. [DOI] [Google Scholar]
- Misra P.; Mishra B. K.; Behera G. B. Hydrolysis of Schiff Bases, 1: Kinetics and Mechanism of Spontaneous, Acid, and Base Hydrolysis of N-(2/4-hydroxybenzylidene)-2-aminobenzothiazoles. Int. J. Chem. Kinet. 1991, 23, 639–654. 10.1002/kin.550230709. [DOI] [Google Scholar]
- Bakalorz K.; Przypis Ł.; Tomczyk M. M.; Książek M.; Grzesik R.; Kuźnik N. Unprecedented Water Effect as a Key Element in Salicyl-Glycine Schiff Base Synthesis. Molecules 2020, 25, 1257 10.3390/molecules25051257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkilä A.; Majander I.; Pihko P. M. Iminium Catalysis. Chem. Rev. 2007, 107, 5416–5470. 10.1021/cr068388p. [DOI] [PubMed] [Google Scholar]
- Galan A.; Ballester P. Stabilization of Reactive Species by Supramolecular Encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. 10.1039/C5CS00861A. [DOI] [PubMed] [Google Scholar]
- Dong V. M.; Fiedler D.; Carl B.; Bergman R. G.; Raymond K. N. Molecular Recognition and Stabilization of Iminium Ions in Water. J. Am. Chem. Soc. 2006, 128, 14464–14465. 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D.; Nag A.; Jana A.; Goswami D.; Bharadwaj P. K. Coumarin Derived Chromophores in the Donor–Acceptor–Donor Format That Gives Fluorescence Enhancement and Large Two-Photon Activity in Presence of Specific Metal Ions. Inorganica Chim. Acta 2010, 363, 2824–2832. 10.1016/j.ica.2010.03.058. [DOI] [Google Scholar]
- Reeves R. L. Schiff Bases. Kinetics of Hydrolysis of p-Trimethylammoniumbenzylidene-p’-Hydroxyaniline Chloride in Aqueous Solution from PH 1 to 11.5. J. Am. Chem. Soc. 1962, 84, 3332 10.1021/ja00876a021. [DOI] [Google Scholar]
- Patalakha N. S.; Yufit D. S.; Kirpichenok M. A.; Gordeeva N. A.; Struchkov Y. T.; Grandberg I. I. Luminescence-Spectral and Acid-Base Characteristics of 3-Aryl-7-Diethylaminocoumarins. Chem. Heterocycl. Compd. 1991, 27, 32–37. 10.1007/BF00633212. [DOI] [Google Scholar]
- Albayrak Kaştaş Ç.; Kaştaş G.; Güder A.; Gür M.; Muğlu H.; Büyükgüngör O. Investigation of Two O-Hydroxy Schiff Bases in Terms of Prototropy and Radical Scavenging Activity. J. Mol. Struct. 2017, 1130, 623–632. 10.1016/j.molstruc.2016.11.023. [DOI] [Google Scholar]
- Mishra P.; Mishra B. K.; Behera G. B. Hydrolysis of Schiff Bases 2: Intramolecular Catalysis of an Ortho-hydroxy Group in Nonionic Surfactant Systems. Int. J. Chem. Kinet. 1992, 24, 593–618. 10.1002/kin.550240609. [DOI] [Google Scholar]
- Klöck C.; Dsouza R. N.; Nau W. M. Cucurbituril-Mediated Supramolecular Acid Catalysis. Org. Lett. 2009, 11, 2595–2598. 10.1021/ol900920p. [DOI] [PubMed] [Google Scholar]
- Gupta M.; Maity D. K.; Singh M. K.; Nayak S. K.; Ray A. K. Supramolecular Interaction of Coumarin 1 Dye with Cucurbit[7]Uril as Host: Combined Experimental and Theoretical Study. J. Phys. Chem. B 2012, 116, 5551–5558. 10.1021/jp301266q. [DOI] [PubMed] [Google Scholar]
- Aliaga M. E.; García-Río L.; Pessêgo M.; Montecinos R.; Fuentealba D.; Uribe I.; Martín-Pastor M.; García-Beltrán O. Host-Guest Interaction of Coumarin-Derivative Dyes and Cucurbit[7]Uril: Leading to the Formation of Supramolecular Ternary Complexes with Mercuric Ions. New J. Chem 2015, 3084–3092. 10.1039/c5nj00162e. [DOI] [Google Scholar]
- Basílio N.; Gago S.; Parola A. J.; Pina F. Contrasting pKa Shifts in Cucurbit[7]Uril Host–Guest Complexes Governed by an Interplay of Hydrophobic Effects and Electrostatic Interactions. ACS Omega 2017, 2, 70–75. 10.1021/acsomega.6b00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordarson P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. 10.1039/C0CS00062K. [DOI] [PubMed] [Google Scholar]
- Basílio N.; Petrov V.; Pina F. Host-Guest Complexes of Flavylium Cations and Cucurbit[7]Uril: The Influence of Flavylium Substituents on the Structure and Stability of the Complex. ChemPlusChem 2015, 80, 1779–1785. 10.1002/cplu.201500304. [DOI] [PubMed] [Google Scholar]
- Ahmed S. A.; Seth Duley S.; Gautam R. K.; Seth D. Inclusion of a Coumarin Derivative inside the Macrocyclic Hosts: A Spectroscopic, Thermodynamic and Theoretical Investigation. J. Mol. Liq. 2018, 264, 550–562. 10.1016/j.molliq.2018.05.081. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.