Abstract

Two new phosphine ligands, diphenylmethylphosphine (DPMP) and triphenylphosphine (TPP), were introduced onto cesium lead bromoiodide nanocrystals (CsPbBrI2 NCs) to improve air stability in the ambient atmosphere. Incorporating DPMP or TPP ligands can also enhance film-forming and optoelectronic properties of the CsPbBrI2 NCs. The results reveal that DPMP is a better ligand to stabilize the emission of CsPbBrI2 NCs than TPP after storage for 21 days. The increased carrier lifetime and photoluminescence quantum yield (PLQY) of perovskite NCs are due to the surface passivation by DPMP or TPP ligands, which reduces nonradiative recombination at the trap sites. The DPMP and TPP-treated CsPbBrI2 NCs were successfully utilized as red emitters for fabricating perovskite light-emitting diodes with enhanced performance and prolonged device lifetime relative to the pristine one.
1. Introduction
Since the first literature concerning all-inorganic cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I) was published,1 related research studies have been performed in large quantities. Cesium lead halide semiconductors have attracted much attention in the fields of perovskite light-emitting diodes (PeLEDs),2−4 perovskite solar cells,5−8 and photodetectors9,10 due to their superior optoelectronic properties. In particular, perovskite nanocrystals (NCs) are emerging as a new generation in light-emitting area based on several advantages, including low cost, solution process ability, tunable band gap, narrow full width at half-maximum (FWHM), and high photoluminescence quantum yield (PLQY).
There have been several studies on green cesium lead bromide (CsPbBr3) perovskite NCs in the past 5 years; in contrast, relatively fewer reports on red cesium lead iodide (CsPbI3)11,12 or cesium lead bromoiodide (CsPbBrxI3–x, x = 0.6–1.2)13−19 were discussed and published in the literature. It is reported that perovskite CsPbI3 is extremely unstable as its red light-emitting cubic phase (α-phase) easily turns into nonfunctional orthorhombic phase (δ-phase) under ambient conditions.15,20,21 Some studies have demonstrated that partial replacement of I by Br could easily prolong perovskites lifetime.22,23 The obtained CsPbBrxI3–x NCs show better stability than CsPbI3 in air. Therefore, our work is focused on the modification and stability investigation of the red CsPbBrxI3–x NCs.
The α-phase of CsPbBrxI3–x NCs, due to its narrow direct band gap (Eg) of ca. 1.9–2.0 eV, have stimulated great interest in photovoltaics5,23 and perovskite light-emitting diodes (PeLEDs).14,24 To further enhance the stability of α-CsPbBrxI3–x NCs, preventing spontaneous transformation of the metastable α-CsPbBrxI3–x into the nonfunctional δ-phase,12 several groups have launched doping of less toxic, slightly smaller-sized cations Sr2+, Cu2+, or Mn2+ to substitute Pb2+ in the perovskite lattices.12,24,25 The formation energy of α-CsPbBrxI3–x NCs was enhanced, consequently reducing the structural distortion and making the α-phase of CsPbBrxI3–x more stable.12,24
All-inorganic perovskite NCs are found to have a high PLQY because of the long alkyl chains as capping ligands.26 Despite their high luminescent properties, these alkyl ligands have low conductivity and form insulating shells outside the NCs, which hinder carrier injection into the perovskite cores and reduce the performance of the light-emitting devices.3,27 To passivate surface defects and improve the quality of the perovskite NCs, using functional ligands regulation has been proposed by different research groups. Tang and co-workers treated CsPbX3 (X = Br or I) quantum dots (QDs) with phenethylammonium bromide (PEABr), phenethylammonium iodide (PEAI), or corresponding derivatives with different alkyl chain lengths to effectively passivate the trap states in CsPbX3, resulting in high PLQY.28,29 Moreover, the short conjugated benzene ring brought remarkable enhancement of carrier injection and transport. Wu and co-workers proposed a branched capping ligand n-propyltrimethoxysilane-dimethyloctadecyl-ammonium bromide (PDB) to improve the stability of CsPbBr3 QDs.30 Our group also reported a surface ligand diphenylammonium bromide (DPABr) to passivate the surface vacancies of the CsPbBr3 NCs and improve PLQY.31 Furthermore, two π-electron-rich phenyl rings help to increase the conductivity of the resulting CsPbBr3 NCs to achieve better device performance. It is clearly seen that those ligands have similar quaternary ammonium halide (−N+X–) structures. The incorporation of surface ligands containing unwanted halide ions brings a risk of color change via anion exchange.32 Besides, those ammonium halides are derived from the corresponding amines by reacting with hydrobromic acid or hydriodic acid. It requires additional synthesis and purification steps and leads to higher cost and lower yield.
Apart from ammonium halide ligands, alkylated phosphine and phosphine oxide have also been reported as surface ligands to stabilize the perovskite NCs. Li and his co-workers proposed trioctylphosphine (TOP), tributylphosphine (TBP), and diphenylphosphine (DPP) as capping ligands.33 By introducing organic phosphine ligands, the tolerance of CsPbBr3 QDs to ethanol, water, and UV light was dramatically improved. Geyer and his co-workers also introduced TOP as the surface ligand to increase the stability of CsPbI3 QDs.11 They found that the treated CsPbI3 retained its α-phase over a 9 h measurement period, while the transformation from the α-phase to the nonluminescent δ-phase occurred within 2 h for the untreated CsPbI3. Sun and co-workers utilized trioctylphosphine oxide (TOPO) as a ligand to prepare CsPbBr3 NCs.34 The presence of TOPO improved the stability of CsPbX3 NCs against ethanol treatment to maintain long-term luminescent property. Parkin and co-workers prepared CsPbBr3 NCs using trioctylphosphine oxide (TOPO) and diisooctylphosphinic acid instead of long-chain amine and carboxylic acid.35 The Pb–O–P bond was formed on the surface of NCs. From literature survey, the utilization of phosphine-based ligands for the preparation of perovskite NCs is less reported. In addition, the phosphine ligands can be utilized directly in perovskite NCs instead of converting to the ionic state. The development of diverse phosphine-related ligands for synthesizing red CsPbBrxI3–x NCs is investigated in this research.
The structural and optical stability remains the foremost challenge for all-inorganic perovskites NCs. To achieve highly efficient and high luminance red emissive PeLEDs, it is necessary to prepare uniform α-CsPbBrxI3–x NCs within a few nanometers. It has been reported that disintegration of the perovskite NCs is accelerated by moisture,13 especially for red emission perovskite NCs.36 Based on the above consideration, we propose two phosphine ligands, i.e., diphenylmethylphosphine (DPMP) and triphenylphosphine (TPP), to incorporate into perovskite NCs. Both DPMP and TPP have multiple phenyl groups, which can enhance carrier injection into the perovskite core and show resistance to water. The phosphine ligands also provide good resistance to moisture and prohibit a transformation from the α-phase to the δ-phase. The pristine and DPMP (or TPP)-treated CsPbBrxI3–x NCs were synthesized and characterized. The morphological, optical, and electrical characteristics of all samples were investigated with miscellaneous instruments to analyze the ligand effects on the properties of perovskite NCs.
2. Results and Discussion
Figure 1a displays the chemical structures of the two phosphine ligands DPMP and TPP, while Figure 1b shows the schematic illustrations of the DPMP- and TPP-treated CsPbBrI2 NCs. The pristine CsPbBrI2 NCs were also synthesized for comparison in this study. Figure 2 shows all CsPbBrI2 NCs films under UV light (365 nm) irradiation with different kinds and different amounts of phosphine ligands. All fresh samples showed bright red emission and were stored in the ambient atmosphere (ca. 28 °C, relative humidity ∼ 85%) and aged for 24 days. Taking the top left photo as an example, from top to bottom, it shows pristine, TPP-treated, and DPMP-treated CsPbBrI2 NCs; from left to right, it shows CsPbBrI2 NCs treated with different amounts (0.125, 0.25, and 0.5 mg) of phosphine ligands. It is clearly seen that surface modification of the perovskite NCs with phosphine ligands has a dramatic influence on film stability. The TPP and DPMP ligands possess bulky phenyl groups, which bring larger steric hindrance and moisture resistance compared with long-chain ligands, oleic acid (OA), and oleylamine (OAm).37 After 3 days storage, the pristine and TPP (0.5 mg)-treated CsPbBrI2 thin films changed from red to green under UV light irradiation, while the DPMP (0.5 mg)-treated CsPbBrI2 thin films changed to orange. The phenomenon of color change from red to green suggests that those thin films were relatively unstable, possibly due to the composition alteration from CsPbBrI2 to CsPbBr3. Zhang and co-workers observed a green emission at 520 nm from CsPbBrI2 NCs after aging for 2 days, which is originated from the decomposition of CsPbBrI2 to CsPbBr3.17,24 The photoluminescence (PL) emission spectra of our samples after aging were also collected and discussed below, suggesting that CsPbBr3 NCs were formed. After storage for 5 days, the DPMP (0.5 mg)-treated CsPbBrI2 thin films partially changed to green, and completely turned green after storage for 11 days. The result reveals that DPMP is a better ligand to stabilize the emission of CsPbBrI2 NCs than TPP. After storage for 17 days, both TPP (0.25 mg) and DPMP (0.25 mg)-treated CsPbBrI2 NCs became orange, and only the DPMP (0.125 mg)-treated CsPbBrI2 NCs (Figure 2, bottom left) showed red emission after storage for 21 days. The incorporation of excess phosphine ligands led to ease of anionic exchange to form CsPbBr3. To further explore the lower limit of the phosphine ligands, 0.0625 mg of DPMP and TPP ligands were introduced to synthesize CsPbBrI2 NCs. However, the modified perovskite NCs with such low doses of DPP or TPP could not be observed by Fourier transform infrared (FT-IR) characterization, which is discussed in the next paragraph. It is concluded that the addition of 0.125 mg of DPMP has the best passivation effect.
Figure 1.

(a) Chemical structures of DPMP and TPP; (b) schematic illustration of the DPMP- and TPP-treated CsPbBrI2 NCs.
Figure 2.
Photos of fresh and aged (1–25 days) perovskite thin films under UV light (365 nm) irradiation. From top to bottom, the samples were pristine, TPP-, and DPMP-treated CsPbBrI2 NCs. From left to right, the samples were doped with different amounts of phosphine ligands (0.125, 0.25, and 0.5 mg).
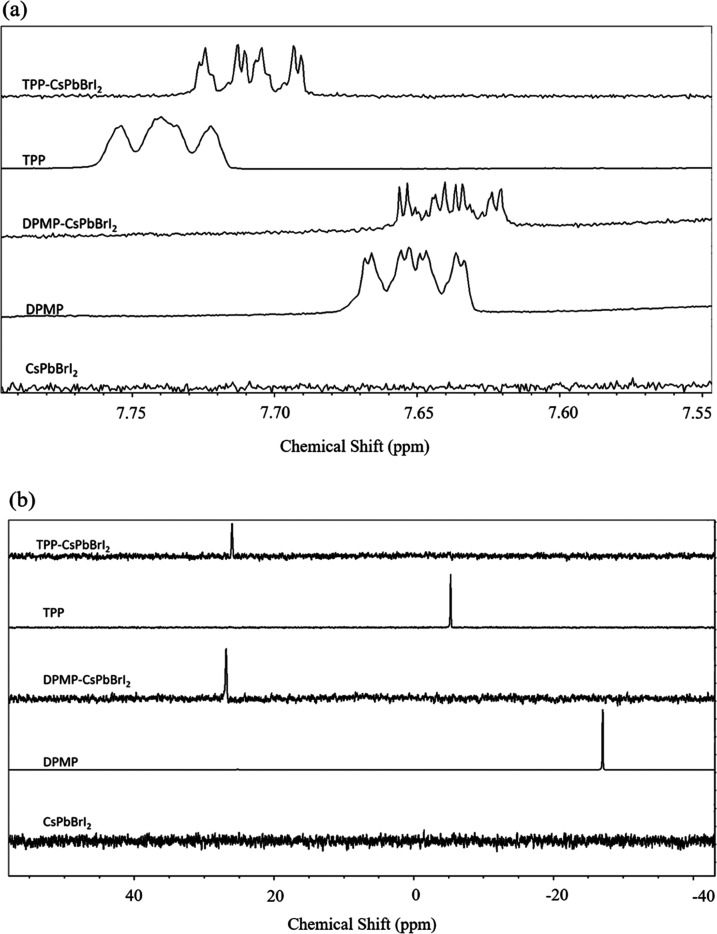
Figure 3a,b shows the FT-IR spectra corresponding to the pristine, TPP-, and DPMP-containing CsPbBrI2 NCs. In this case, 0.125 mg of phosphine ligands (TPP and DPMP) was chosen since the formed CsPbBrI2 NCs showed better stability, as described in the previous paragraph. In Figure 3a, three characteristic peaks at 2957, 2925, and 2852 cm–1 can be found for all samples, which are assigned to the asymmetric C–H stretching from long-chain ligands. Moreover, the occurrence of two absorption peaks at 1465 and 1380 cm–1 represents the −CH2 and terminal −CH3 vibrational modes, respectively. Figure 3b shows the enlarged FT-IR spectra from 2900 to 3200 cm–1. The TPP- and DPMP-treated CsPbBrI2 NCs possess two additional peaks at 3076 and 3003 cm–1 compared to the pristine perovskite, which are ascribed to the =C–H stretching of the benzene ring (referred to the spectral database for organic compounds (SDBS) No. 11322 for DPMP and No. 2967 for TPP). In this study, 0.0625 mg of DPMP and TPP ligands were also introduced individually to synthesize CsPbBrI2 NCs, and the corresponding FT-IR spectra are shown in Figure S1 in the Supporting Information. It is seen that the three samples had similar transmission behaviors around 3076 and 3003 cm–1, implying that DPMP or TPP ligands were not successfully anchored onto the perovskite surface. To further confirm the incorporation of phosphine ligands onto CsPbBrI2 NCs, 1H and 31P nuclear magnetic resonance (NMR) spectra were recorded. Figure 4a reveals the 1H NMR spectra of the pristine, TPP-, and DPMP-treated CsPbBrI2 NCs. The 1H NMR spectra of TPP and DPMP are also included in the same figure for comparison. No proton signal could be found for the pristine CsPbBrI2 NCs in the range of 7.6–7.8 ppm, since no aromatic proton was involved. The multiple signals around 7.74 ppm belong to aromatic protons on TPP, and these signals show an upfield shift to 7.71 ppm after chelating to CsPbBrI2 NCs. Similarly, the multiple signals around 7.65 ppm are assigned to aromatic protons on DPMP, which show a slight upfield shift to 7.64 ppm after tethering to CsPbBrI2 NCs. This phenomenon can be explained by the acid–base interaction between TPP (or DPMP) and octylamine (OA). Almeida et al. reported that the signal shift in the 1H NMR spectra was attributed to the acid–base interaction between TOPO and OA.38 Turning to the 31P NMR spectra in Figure 4b, the 31P signals from TPP and TPP-treated CsPbBrI2 NCs were observed at −5.33 and 25.99 ppm, respectively. Meanwhile, the 31P signals from DPMP and DPMP-treated CsPbBrI2 NCs were located at −27.07 and 26.85 ppm, respectively. The large shift was ascribed to the formation of P–Pb bond since these phosphine ligands were anchored onto the surface of NCs.33
Figure 3.

(a) Entire and (b) enlarged FT-IR spectra of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs using 0.125 mg of phosphine ligands.
Figure 4.
(a) 1H and (b) 31P NMR spectra of the pristine CsPbBrI2, DPMP, DPMP-treated CsPbBrI2, TPP, and TPP-treated CsPbBrI2.
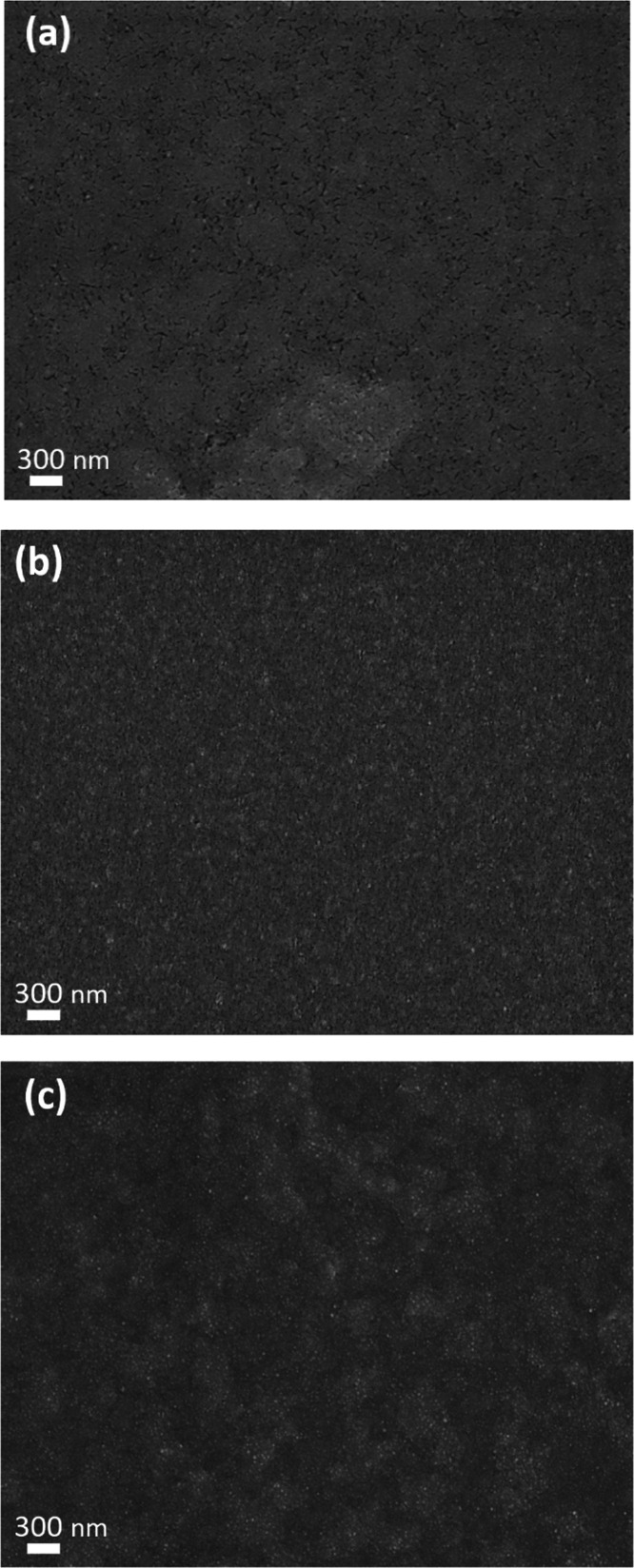
For the devices fabricated by solution processing, the uniformity of the perovskite film is essential for the construction of high-performance PeLEDs. In contrast, the existence of pinholes or rough surface may affect the device performance severely. The top-view scanning electron microscopy (SEM) images of the pristine, DPMP-, and TPP-treated CsPbBrI2 thin films by spin-coating are shown in Figure 5. For the thin film of the pristine CsPbBrI2 NCs in Figure 5a, many pinholes and cracks were observed on the surface of the film. By treating with DPMP or TPP, the resulting CsPbBrI2 dense films show nearly no cracks or holes in Figure 5b,c. In addition to SEM observation, atomic force microscopy (AFM) was also utilized to examine the surface morphology and average roughness (Ra) of the obtained perovskite thin films. The corresponding topographic images of the pristine, TPP-, and DPMP-treated CsPbBrI2 thin films are presented in Figure S2 in the Supporting Information. The topology of the pristine CsPbBrI2 film was rougher, and its Ra value was estimated to be 5.19 nm. By treating with DPMP or TPP, the grains became smaller and homogenously dispersed, revealing reduced Ra values of 3.91 and 3.48 nm, respectively. The results from AFM experiments are consistent with the SEM observation. We conclude that the film-forming property of the perovskite NCs can be ameliorated by introducing appropriate surface ligands, which is seldom reported in the literature.
Figure 5.
Top-view SEM images of (a) pristine, (b) DPMP-, and (c) TPP-treated CsPbBrI2 NCs.
Figure 6 shows the transmission electron microscopy (TEM) and high-resolution TEM (HR-TEM) images of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs. In Figure 6a, irregular crystalline sizes were observed for the pristine perovskite NCs, with sizes ranging from 7.1 to 21.4 nm. Some NCs were overgrown with sizes up to 20 nm, which is considered as a result of the serious loss of surface ligands.27 In Figure 6b, the DPMP-treated CsPbBrI2 shows more regular NCs with an average crystalline size of 14 nm, but there were still some rectangular NCs to be observed. In Figure 6c, cubic-shaped NCs with an average size of 13.2 nm were found for the TPP-treated CsPbBrI2. It is seen that DPMP- and TPP-treated CsPbBrI2 have more uniform and smaller crystalline sizes compared with the pristine one, which can be explained by different interactions between the perovskite core and ligands. Using DPMP or TPP as the surface ligands, a stronger P–Pb interaction was formed. The formation of P–Pb interaction was examined by X-ray photoelectron spectroscopy (XPS) experiments, and the corresponding XPS spectra are shown in Figure S3 in the Supporting Information. The Pb 4f5/2 and 4f7/2 signals were observed at 143.1 and 138.2 eV, respectively, which were attributed to the formation of Pb–I or Pb–Br bonds for all samples. Moreover, two additional XPS signals were found at 142.1 and 137.1 eV for DPMP- and TPP-treated CsPbBrI2, providing direct observation of P–Pb interaction. Hence, these two phosphine ligands could be anchored onto the perovskite surface more closely compared with OAm through the same purification procedure, preventing NCs from growing to a larger size. The HR-TEM images of different CsPbBrI2 NCs are shown in Figure 6 (inset), revealing a lattice spacing of 0.59 nm that corresponds to the (100) plane of the cubic perovskite structure for all of the three samples.28 Therefore, we realized that introducing the two phosphine ligands would not interfere with the crystalline structure.
Figure 6.
TEM micrographs of the (a) pristine, (b) DPMP-, and (c) TPP-treated CsPbBrI2 NCs. The insets show the corresponding HR-TEM images.
The X-ray diffraction (XRD) patterns of the pristine, TPP-, and DPMP-treated CsPbBrI2 NCs are shown in Figure S4 in the Supporting Information. Several significant diffraction peaks can be found at 2θ = 14.4, 20.9, and 29.4°, indicative of the (100), (110), and (200) planes, respectively.24,39,40 According to the XRD patterns, all of the three samples are well consistent with the cubic phase of CsPbBrI2, while no other observable phase or peak shift can be found. In other words, the lattice spacing of CsPbBrI2 NCs was not influenced by TPP or DPMP ligands. Besides, most diffraction peaks from DMPT- and TPP-treated CsPbBrI2 NCs are stronger than those from the pristine one, implying that the crystallinity of the formed perovskite NCs becomes higher after incorporating DPMP or TPP ligands. This observation is in good accordance with TEM results, revealing that DPMP- and TPP-treated CsPbBrI2 have more uniform and intact crystalline structures.
The absorption, PL emission, and time-resolved photoluminescence (TR-PL) decay curves of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs are depicted in Figure S5 in the Supporting Information. A clear absorption band is located at 600 nm, and the absorption edge is observed at 630 nm for all of the three samples, corresponding to an optical band gap of 1.93 eV. The PL emission spectra of the synthesized CsPbBrI2 NCs without and with DPMP (or TPP) ligands are also depicted in Figure S5a. The pristine, DPMP-, and TPP-treated CsPbBrI2 NCs all exhibit a red emission at around 646 nm and an FWHM value of 46 nm. It is seen that the absorption and PL emission behaviors of CsPbBrI2 NCs were not affected by the incorporation of DPMP or TPP ligands.41,42 The PLQY of the pristine CsPbBrI2 NCs was measured to be 19%; after treating with DPMP or TPP ligands, the PLQY of the corresponding CsPbBrI2 NCs was increased to 34 or 26%, respectively. To verify the effect of the two ligands on the carrier lifetime of CsPbBrI2 NCs, the TR-PL measurement was carried out and the corresponding PL decay curves are illustrated in Figure S5b in the Supporting Information. The TR-PL decay curves were fitted with a biexponential decay model, and the resulting fitted curves are shown in Figure S5c in the Supporting Information. The fast decay originates from the nonradiative quenching of carriers, and the slow decay occurs as a result of radiative recombination.43,44 The parameters of time component (τi) and the corresponding weight (Ai) are listed in Table S1 in the Supporting Information. The DPMP-treated CsPbBrI2 has the longest PL lifetime τ2 of 27.87 ns, implying the strongest PL emission intensity. Meanwhile, the TPP-treated CsPbBrI2 possesses the second longer τ2 of 24.37 ns that is longer than that of the pristine CsPbBrI2 (19.95 ns). The results of TR-PL experiments are in agreement with the PLQY values. We suspect that the increased carrier lifetime is due to the surface passivation of perovskite NCs by DPMP and TPP ligands, which reduces nonradiative recombination at the trap sites.2,45
The PeLEDs using the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs as the active layers were fabricated to evaluate their device performance. The device structure and energy-level diagram of PeLEDs with the configuration of indium-tin oxide (ITO)/poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS)/poly[N-(4-butylphenyl)-N′,N″-diphenylamine] (Poly-TPD)/CsPbBrI2 NCs/1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene (TPBi)/LiF/Al are shown in Figure 7a,b, respectively. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of Poly-TPD are reported to be −5.6 and −2.4 eV, respectively.46 The HOMO and LUMO levels of TPBi are found to be −6.2 and −2.7 eV, respectively.16 Furthermore, the valence band and conduction band of CsPbBrI2 are reported to be −5.9 and −4.0 eV,42 respectively. The cross-sectional SEM image of the device is displayed in Figure 7c, indicating layer thicknesses of 40, 45, 40, and 100 nm for PEDOT:PSS/Poly-TPD, CsPbBrI2 NCs, TPBi, and LiF/Al, respectively. The current density–voltage–luminance and current efficiency–current density characteristics of PeLEDs based on the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs are shown in Figure 8a,b, respectively, while the corresponding device performances are summarized in Table 1. The maximum luminance (Lmax) and current efficiency (ηmax) of the device based on the pristine CsPbBrI2 NCs were measured to be 168 cd/m2 and 0.015 cd/A, respectively. After introducing DPMP and TPP ligands, the device performance of the related PeLEDs was promoted. The optimized device based on the DPMP-treated CsPbBrI2 NCs reached Lmax = 299 cd/m2 and ηmax = 0.046 cd/A. Moreover, the optimized device based on the TPP-treated CsPbBrI2 NCs reached Lmax = 285 cd/m2 and ηmax = 0.11 cd/A.16,24,47 The electroluminescent (EL) spectra of the three PeLEDs are depicted in Figure 8c, indicative of a red emission at 647 nm with an FWHM value of 39 nm. Similar to PL emission, the EL emission behavior of CsPbBrI2 NCs was not affected by the introduction of DPMP or TPP ligands.24 To monitor device stability without and with DPMP/TPP ligands, the luminance decay curves of PeLEDs as a function of time were recorded by applying a constant voltage of 7.0 V, as revealed in Figure 8d. The lifetime of the device is defined as the time when the luminance is decreased to half of its initial luminance, which is determined to be 38, 235, and 380 s, respectively, for the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs. The device stability was improved significantly by introducing DPMP or TPP ligands to passivate surface traps.
Figure 7.
(a) Device structure, (b) energy-level diagram, and (c) cross-sectional SEM image of PeLEDs.
Figure 8.
(a) Current density–voltage–luminance; (b) current efficiency–current density characteristics; (c) EL spectra of PeLEDs; (d) luminance decay curves of PeLEDs as a function of time at a constant driving voltage of 7.0 V.
Table 1. Device Performance of PeLEDs Based on the Pristine, DPMP-, and TPP-Treated CsPbBrI2 NCs.
| perovskite | EL (nm) | Vturn-on (V) | Lmax (cd/m2@V) | ηmax (cd/A@V) |
|---|---|---|---|---|
| pristine | 647 | 5.9 | 168@10.1 | 0.015@7 |
| DPMP-treated | 647 | 4.9 | 299@10.9 | 0.046@10.9 |
| TPP-treated | 647 | 5.6 | 285@11.7 | 0.11@10.6 |
3. Conclusions
In this work, all-inorganic CsPbBrI2 NCs with red emission were synthesized by hot injection. Two phosphine ligands DPMP and TPP containing multiple phenyl rings were introduced onto the perovskite surface to improve air stability and film-forming property. DPMP is a better ligand to stabilize the emission of CsPbBrI2 NCs than TPP after storage for 21 days. The existence of DPMP and TPP in CsPbBrI2 NCs was confirmed by FT-IR and NMR experiments. The DPMP- and TPP-treated NCs have more uniform and smaller crystalline size compared with the pristine one, while the lattice spacing was not influenced. The SEM and AFM results revealed that the DPMP- and TPP-treated CsPbBrI2 films show nearly no cracks or holes. The red emission of CsPbBrI2 NCs was not shifted by introducing DPMP or TPP ligands, while the PLQY was enhanced. The PL lifetime of perovskite NCs was prolonged due to the surface passivation by DPMP and TPP ligands to reduce nonradiative recombination at the trap sites. Red light-emitting PeLEDs based on the DPMP- and TPP-treated CsPbBrI2 NCs were fabricated with enhanced device performance and longer lifetime relative to the pristine one. Our results provide a new possibility for passivation of perovskite NCs by new phosphine ligands.
4. Experimental Section
4.1. Materials
ITO glass substrates (7 Ω/square) were purchased from Merck. Cesium carbonate (Cs2CO3, purity 99.99%), OAm (purity 80–90%), and 1-octadecene (ODE, purity 90%) were purchased from Acros Organics. Lead bromide (PbBr2, purity 99.998%), lead iodide (PbI2, purity 99.9985%), TPP (purity 99%), and DPMP (purity 99%) were purchased from Alfa Aesar. OA (purity 90%) and Poly-TPD were purchased from Sigma-Aldrich. PEDOT:PSS aqueous solution (Clevios P VP AI 4083) was purchased from Heraeus Precious Metals GmbH & Co. KG. TPBi was purchased from Lumtec and Shine Material Technology, respectively. Other solvents were bought from Acros Organics, MACRON, or TEDIA and used without further purification.
4.2. Synthesis of the Pristine and Phosphine-Containing Cs-Oleate Precursors
Cs2CO3 (0.163 g, 0.5 mmol), OA (0.5 mL, 0.4 mmol), and ODE (8 mL) were mixed in a 50 mL two-neck flask. The mixture was then heated to 120 °C and dried under vacuum for 30 min, followed by purging nitrogen gas for 30 min at the same temperature. Afterward, the Cs2CO3 solution was heated to 160–180 °C under a nitrogen atmosphere for 20 min to obtain the clarified Cs-oleate precursor solution.
To prepare the TPP-containing Cs-oleate precursors, Cs2CO3 (0.163 g, 0.5 mmol), OA (0.5 mL, 0.4 mmol), ODE (8 mL), and different weights of TPP (0.125, 0.25, and 0.5 mg) were mixed in a 50 mL two-neck flask. About DPMP-containing Cs-oleate precursors, different weights of DPMP (0.125, 0.25, and 0.5 mg) were added in the mixture. The synthetic procedure was the same as that of the pristine Cs-oleate precursor to obtain eight different TPP- or DPMP-containing Cs-oleate precursors.
4.3. Synthesis and Purification of CsPbBrI2 NCs
PbBr2 (23 mg), PbI2 (58 mg), OA (0.5 mL), OAm (0.5 mL), and ODE (5 mL) were mixed in a 50 mL two-neck flask. The mixture was heated to 120 °C and dried under vacuum for 30 min, followed by purging nitrogen gas for 30 min at the same temperature. Afterward, the mixed solution was heated to 160–180 °C under a nitrogen atmosphere for 20 min to obtain the clarified Pb-oleate precursor solution with a pale yellow color. To obtain CsPbBrI2 NCs, 0.4 mL of the Cs-oleate precursor was quickly injected into the Pb-oleate precursor solution and reacted for 5 s. The flask was immersed in an ice bath rapidly to quench the reaction mixture.
The purification procedure of CsPbBrI2 NCs is described as follows. First, the solution was centrifuged at 10 000 rpm for 10 min and the crude precipitate was collected. Second, the precipitate was dispersed in 0.5 mL of n-hexane and 2 mL of ethyl acetate was poured into the above dispersion to obtain the precipitate again, followed by centrifugation at 10 000 rpm for 10 min. The supernatant was discarded, and the precipitate at the bottom was redispersed at 1 mL of hexane to obtain the final CsPbBrI2 NCs dispersion, which could be kept at room temperature for further characterization and device fabrication.
4.4. Characterization Methods
The morphology and size of CsPbBrI2 NCs were examined with a JEOL 3010 TEM. The top-view and cross-sectional micrographs of the samples were investigated with an ultrahigh-resolution ZEISS crossbeam SEM. The AFM images of the samples were acquired from a Bruker Innova atomic force microscope with a tapping mode. The absorption, PL spectra, and PLQY of the samples were recorded with a Princeton Instruments Acton 2150 spectrophotometer equipped with a xenon lamp as the light source. To perform TR-PL measurements, a 377 nm pulsed laser (PicoQuant) was utilized as the excitation light source. The laser was introduced into a 50× objective lens then focused on the sample. The PL emission was collected from the same objective lens in a Horiba iHR320 spectrometer equipped with a liquid nitrogen-cooled charge-coupled device (CCD) array detector. The TR-PL measurement was recorded by a time-correlated single-photon counting system with a PicoHarp 300 acquisition unit and a photomultiplier tube at room temperature at around 60% relative humidity. The XPS experiments were performed on a Thermo K-Alpha XPS instrument. The XRD patterns of the samples were performed with a Bruker D8 SSS instrument. The FT-IR spectra were measured using a Thermo Scientific Nicolet iS-10 spectrometer. The NMR spectra were recorded on a Bruker Avance III HD 600 MHz NMR spectrometer. The performance and EL spectra of the light-emitting devices were recorded using an Agilent 4155C semiconductor parameter analyzer and an Ocean Optics USB2000+ spectrometer.
4.5. Device Fabrication and Evaluation
The light-emitting devices with a regular configuration of ITO/PEDOT:PSS/Poly-TPD/CsPbBrI2 NCs/TPBi/LiF/Al were fabricated. The ITO substrates were cleaned sequentially with detergent, deionized water, acetone, and isopropanol under ultrasonication for 30 min, followed by nitrogen purging and ultraviolet–ozone treatment for 20 min. The PEDOT:PSS was spin-cast onto the ITO substrate at 3000 rpm for 30 s and baked at 150 °C for 15 min in air. The substrates were then transferred into a nitrogen-filled glovebox. Poly-TPD (in chlorobenzene, 6 mg/mL) was deposited on top of PEDOT:PSS by spin-coating at 3000 rpm for 30 s, followed by drying at 150 °C for 30 min. The CsPbBrI2 NCs without and with DPMP/TPP ligands dispersed in n-hexane were spin-coated onto the Poly-TPD layer at 1500 rpm for 30 s and heated. Finally, 40 nm of TPBi, 0.5 nm of LiF, and 100 nm of aluminum (Al) electrodes were deposited sequentially by thermal evaporation under a base pressure of ∼10–6 Torr. The active area of each device is 1 mm2.
Acknowledgments
The authors thank the Ministry of Science and Technology (MoST) of Republic of China (grant nos. MOST 108-2923-E-009-007-MY2 and MOST 109-2112-M-006-016) and the Higher Education Sprout Project of the Ministry of Education (MOE), Taiwan, for financially supporting this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00910.
Parameters of time component (τi) and corresponding weight (Ai) from TR-PL experiments; FT-IR spectra of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs using 0.0625 mg of phosphine ligands; AFM topographic images of the pristine, DPMP-, and TPP-treated CsPbBrI2 thin films; XPS spectra corresponding to the Pb 4f signals of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs; XRD patterns of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs; absorption; PL emission spectra; and TR-PL decay curves of the pristine, DPMP-, and TPP-treated CsPbBrI2 NCs (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-H.; Wolf C.; Kim Y.-T.; Cho H.; Kwon W.; Do S.; Sadhanala A.; Park C. G.; Rhee S.-W.; Im S. H.; Friend R. H.; Lee T.-W. Highly Efficient Light-Emitting Diodes of Colloidal Metal–Halide Perovskite Nanocrystals beyond Quantum Size. ACS Nano 2017, 11, 6586–6593. 10.1021/acsnano.6b07617. [DOI] [PubMed] [Google Scholar]
- Park J. H.; Lee A.; Yu J. C.; Nam Y. S.; Choi Y.; Park J.; Song M. H. Surface Ligand Engineering for Efficient Perovskite Nanocrystal-Based Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 8428–8435. 10.1021/acsami.8b20808. [DOI] [PubMed] [Google Scholar]
- Dai J.; Xi J.; Zu Y.; Li L.; Xu J.; Shi Y.; Liu X.; Fan Q.; Zhang J.; Wang S.; Yuan F.; Dong H.; Jiao B.; Hou X.; Wu Z. Surface mediated ligands addressing bottleneck of room-temperature synthesized inorganic perovskite nanocrystals toward efficient light-emitting diodes. Nano Energy 2020, 70, 104467 10.1016/j.nanoen.2020.104467. [DOI] [Google Scholar]
- Yu B.; Zhang H.; Wu J.; Li Y.; Li H.; Li Y.; Shi J.; Wu H.; Li D.; Luo Y.; Meng Q. Solvent-engineering toward CsPb(IxBr1-x)3 films for high-performance inorganic perovskite solar cells. J. Mater. Chem. A 2018, 6, 19810–19816. 10.1039/C8TA07968D. [DOI] [Google Scholar]
- Gao L.; Spanopoulos I.; Ke W.; Huang S.; Hadar I.; Chen L.; Li X.; Yang G.; Kanatzidis M. G. Improved Environmental Stability and Solar Cell Efficiency of (MA,FA)PbI3 Perovskite Using a Wide-Band-Gap 1D Thiazolium Lead Iodide Capping Layer Strategy. ACS Energy Lett. 2019, 4, 1763–1769. 10.1021/acsenergylett.9b00930. [DOI] [Google Scholar]
- Lau C. F. J.; Deng X.; Ma Q.; Zheng J.; Yun J. S.; Green M. A.; Huang S.; Ho-Baillie A. W. Y. CsPbIBr2 Perovskite Solar Cell by Spray-Assisted Deposition. ACS Energy Lett. 2016, 1, 573–577. 10.1021/acsenergylett.6b00341. [DOI] [Google Scholar]
- Bai F.; Zhang J.; Yuan Y.; Liu H.; Li X.; Chueh C.-C.; Yan H.; Zhu Z.; Jen A. K.-Y. A 0D/3D Heterostructured All–Inorganic Halide Perovskite Solar Cell with High Performance and Enanced Phase Stability. Adv. Mater. 2019, 31, 1904735 10.1002/adma.201904735. [DOI] [PubMed] [Google Scholar]
- Tao J.; Xiao Z.; Wang J.; Li C.; Sun X.; Li F.; Zou X.; Liao G.; Zou Z. A self-powered, flexible photodetector based on perovskite nanowires with Ni-Al electrodes. J. Alloys Compd. 2020, 845, 155311 10.1016/j.jallcom.2020.155311. [DOI] [Google Scholar]
- Ramasamy P.; Lim D.-H.; Kim B.; Lee S.-H.; Lee M.-S.; Lee J.-S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. 10.1039/C5CC08643D. [DOI] [PubMed] [Google Scholar]
- Lu C.; Li H.; Kolodziejski K.; Dun C.; Huang W.; Carroll D.; Geyer S. M. Enhanced stabilization of inorganic cesium lead triiodide (CsPbI3) perovskite quantum dots with tri–octylphosphine. Nano Res. 2018, 11, 762–768. 10.1007/s12274-017-1685-1. [DOI] [Google Scholar]
- Yao J.-S.; Ge J.; Wang K.-H.; Zhang G.; Zhu B.-S.; Chen C.; Zhang Q.; Luo Y.; Yu S.-H.; Yao H.-B. Few-Nanometer-Sized α-CsPbI3 Quantum Dots Enabled by Strontium Substitution and Iodide Passivation for Efficient Red–Light Emitting Diodes. J. Am. Chem. Soc. 2019, 141, 2069–2079. 10.1021/jacs.8b11447. [DOI] [PubMed] [Google Scholar]
- Ren J.; Dong X.; Zhang G.; Li T.; Wang Y. Air-stable and water-resistant all-inorganic perovskite quantum dot films for white-light-emitting applications. New J. Chem. 2017, 41, 13961–13967. 10.1039/C7NJ03017G. [DOI] [Google Scholar]
- Zhou J.; Hu Z.; Zhang L.; Zhu Y. Perovskite CsPbBr1.2I1.8 quantum dot alloying for application in white light-emitting diodes with excellent color rendering index. J. Alloys Compd. 2017, 708, 517–523. 10.1016/j.jallcom.2017.03.043. [DOI] [Google Scholar]
- Lin Y.-H.; Qiu Z.-H.; Wang S.-H.; Zhang X.-H.; Wu S.-F. All-inorganic RbxCs1-xPbBrI2 perovskite nanocrystals with wavelength-tunable properties for red light–emitting. Inorg. Chem. Commun. 2019, 103, 47–52. 10.1016/j.inoche.2019.03.007. [DOI] [Google Scholar]
- Yang J.-N.; Song Y.; Yao J.-S.; Wang K.-H.; Wang J.-J.; Zhu B.-S.; Yao M.-M.; Rahman S. U.; Lan Y.-F.; Fan F.-J.; Yao H.-B. Potassium Bromide Surface Passivation on CsPbI3-xBrx Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 2956–2967. 10.1021/jacs.9b11719. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Liu X.; Jiang P.; Chen H.; Wang Y.; Ma J.; Zhang R.; Yang F.; Wang M.; Zhang J.; Tu G. Red-emitting CsPbBrI2/PbSe heterojunction nanocrystals with high luminescent efficiency and stability for bright light–emitting diodes. Nano Energy 2019, 66, 104142 10.1016/j.nanoen.2019.104142. [DOI] [Google Scholar]
- Hoffman J. B.; Schleper A. L.; Kamat P. V. Transformation of Sintered CsPbBr3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3–x through Halide Exchange. J. Am. Chem. Soc. 2016, 138, 8603–8611. 10.1021/jacs.6b04661. [DOI] [PubMed] [Google Scholar]
- Pradhan B.; Mushtaq A.; Roy D.; Sain S.; Das B.; Ghorai U. K.; Pal S. K.; Acharya S. Postsynthesis Spontaneous Coalescence of Mixed-Halide Perovskite Nanocubes into Phase-Stable Single-Crystalline Uniform Luminescent Nanowires. J. Phys. Chem. Lett. 2019, 10, 1805–1812. 10.1021/acs.jpclett.9b00832. [DOI] [PubMed] [Google Scholar]
- Trots D. M.; Myagkota S. V. High-temperature structural evolution of caesium and rubidium triiodoplumbates. J. Phys. Chem. Solids 2008, 69, 2520–2526. 10.1016/j.jpcs.2008.05.007. [DOI] [Google Scholar]
- Swarnkar A.; Marshall A. R.; Sanehira E. M.; Chernomordik B. D.; Moore D. T.; Christians J. A.; Chakrabarti T.; Luther J. M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskit for high–efficiency photovoltaics. Science 2016, 354, 92–95. 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Kumar S.; Bär J.; Bertolotti F.; Masciocchi N.; Guagliardi A.; Grotevent M.; Shorubalko I.; Bodnarchuk M. I.; Shih C.-J.; Kovalenko M. V. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. 10.1021/acsnano.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal R. E.; Slotcavage D. J.; Leijtens T.; Bowring A. R.; Belisle R. A.; Nguyen W. H.; Burkhard G. F.; Hoke E. T.; McGehee M. D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. 10.1021/acs.jpclett.6b00002. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhang L.; Cai P.; Xue X.; Wang M.; Zhang J.; Tu G. Enhancing stability of red perovskite nanocrystals through copper substitution for efficient light–emitting diodes. Nano Energy 2019, 62, 434–441. 10.1016/j.nanoen.2019.05.027. [DOI] [Google Scholar]
- Ma J.; Yao Q.; McLeod J. A.; Chang L.-Y.; Pao C.-W.; Chen J.; Sham T.-K.; Liu L. Investigating the luminescence mechanism of Mn-doped CsPb(Br/Cl)3 nanocrystals. Nanoscale 2019, 11, 6182–6191. 10.1039/C9NR00143C. [DOI] [PubMed] [Google Scholar]
- Wang L.; Liu B.; Zhao X.; Demir H. V.; Gu H.; Sun H. Solvent-Assisted Surface Engineering for High–Performance All-Inorganic Perovskite Nanocrystal Light–Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 19828–19835. 10.1021/acsami.8b06105. [DOI] [PubMed] [Google Scholar]
- Li J.; Xu L.; Wang T.; Song J.; Chen J.; Xue J.; Dong Y.; Cai B.; Shan Q.; Han B.; Zeng H. 50–Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885 10.1002/adma.201603885. [DOI] [PubMed] [Google Scholar]
- Li G.; Huang J.; Zhu H.; Li Y.; Tang J.-X.; Jiang Y. Surface Ligand Engineering for Near–Unity Quantum Yield Inorganic Halide Perovskite QDs and High-Performance QLEDs. Chem. Mater. 2018, 30, 6099–6107. 10.1021/acs.chemmater.8b02544. [DOI] [Google Scholar]
- Li G.; Huang J.; Li Y.; Tang J.; Jiang Y. Highly bright and low turn–on voltage CsPbBr3 quantum dot LEDs via conjugation molecular ligand exchange. Nano Res. 2019, 12, 109–114. 10.1007/s12274-018-2187-5. [DOI] [Google Scholar]
- Xie Q.; Wu D.; Wang X.; Li Y.; Fang F.; Wang Z.; Ma Y.; Su M.; Peng S.; Liu H.; Wang K.; Sun X. W. Branched capping ligands improve the stability of cesium lead halide (CsPbBr3) perovskite quantum dots. J. Mater. Chem. C 2019, 7, 11251–11257. 10.1039/C9TC03377G. [DOI] [Google Scholar]
- Shen R.-H.; Yang S.-H.; Lin P.-Y. Morphological and Optoelectronic Investigations of CsPbBr3 Nanocrystals Chelating Diphenylammonium Halide Ligands via Low–Temperature Synthesis. ACS Appl. Electron. Mater. 2020, 2, 1619–1627. 10.1021/acsaelm.0c00212. [DOI] [Google Scholar]
- Nedelcu G.; Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Grotevent M. J.; Kovalenko M. V. Fast Anion–Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. 10.1021/acs.nanolett.5b02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Wang X.; Xue W.; Wang W.; Zhu W.; Zhao L. Highly luminescent and stable CsPbBr3 perovskite quantum dots modified by phosphine ligands. Nano Res. 2019, 12, 785–789. 10.1007/s12274-019-2289-8. [DOI] [Google Scholar]
- Wu L.; Zhong Q.; Yang D.; Chen M.; Hu H.; Pan Q.; Liu H.; Cao M.; Xu Y.; Sun B.; Zhang Q. Improving the Stability and Size Tunability of Cesium Lead Halide Perovskite Nanocrystals Using Trioctylphosphine Oxide as the Capping Ligand. Langmuir 2017, 33, 12689–12696. 10.1021/acs.langmuir.7b02963. [DOI] [PubMed] [Google Scholar]
- Ambroz F.; Xu W.; Gadipelli S.; Brett D. J. L.; Lin C.-T.; Contini C.; McLachlan M. A.; Durrant J. R.; Parkin I. P.; Macdonald T. J. Room Temperature Synthesis of Phosphine-Capped Lead Bromide Perovskite Nanocrystals without Coordinating Solvents. Part. Part. Syst. Charact. 2020, 37, 1900391 10.1002/ppsc.201900391. [DOI] [Google Scholar]
- Sun C.; Zhang Y.; Ruan C.; Yin C.; Wang X.; Wang Y.; Yu W. W. Efficient and Stable White LEDs with Silica-Coated Inorganic Perovskite Quantum Dots. Adv. Mater. 2016, 28, 10088–10094. 10.1002/adma.201603081. [DOI] [PubMed] [Google Scholar]
- Quarta D.; Imran M.; Capodilupo A.-L.; Petralanda U.; van Beek B.; De Angelis F.; Manna L.; Infante I.; De Trizio L.; Giansante C. Stable Ligand Coordination at the Surface of Colloidal CsPbBr3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 3715–3726. 10.1021/acs.jpclett.9b01634. [DOI] [PubMed] [Google Scholar]
- Almeida G.; Ashton O. J.; Goldoni L.; Maggioni D.; Petralanda U.; Mishra N.; Akkerman Q. A.; Infante I.; Snaith H. J.; Manna L. The Phosphine Oxide Route toward Lead Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 14878–14886. 10.1021/jacs.8b08978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Luan W.; Yin Y.; Yang F. PTFE–based microreactor system for the continuous synthesis of full–visible–spectrum emitting cesium lead halide perovskite nanocrystals. Beilstein J. Nanotechnol. 2017, 8, 2521–2529. 10.3762/bjnano.8.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Wu Y.; Zhang S.; Cai B.; Gu Y.; Song J.; Zeng H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. 10.1002/adfm.201600109. [DOI] [Google Scholar]
- Cha J.-H.; Noh K.; Yin W.; Lee Y.; Park Y.; Ahn T. K.; Mayoral A.; Kim J.; Jung D.-Y.; Terasaki O. Formation and Encapsulation of All-Inorganic Lead Halide Perovskites at Room Temperature in Metal–Organic Frameworks. J. Phys. Chem. Lett. 2019, 10, 2270–2277. 10.1021/acs.jpclett.9b00510. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Sun C.; Zhang Y.; Wu H.; Ji C.; Chuai Y.; Wang P.; Wen S.; Zhang C.; Yu W. W. Bright Perovskite Nanocrystal Films for Efficient Light–Emitting Devices. J. Phys. Chem. Lett. 2016, 7, 4602–4610. 10.1021/acs.jpclett.6b02073. [DOI] [PubMed] [Google Scholar]
- Shi D.; Adinolfi V.; Comin K.; Yuan M.; Alarousu E.; Buin A.; Chen Y.; Hoogland S.; Rothenberger A.; Katsiev K.; Losovyj Y.; Zhang X.; Dowben P. A.; Mohammed O. F.; Sargent E. H.; Bakr O. M. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. 10.1126/science.aaa2725. [DOI] [PubMed] [Google Scholar]
- Shin Y. S.; Yoon Y. J.; Lee K. T.; Jeong J.; Park S. Y.; Kim G.-H.; Kim J. Y. Vivid and Fully Saturated Blue Light-Emitting Diodes Based on Ligand-Modified Halide Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 23401–23409. 10.1021/acsami.9b04329. [DOI] [PubMed] [Google Scholar]
- Cho H.; Jeong S.-H.; Park M.-H.; Kim Y.-H.; Wolf C.; Lee C.-L.; Heo J. H.; Sadhanala A.; Myoung N.; Yoo S.; Im S. H.; Friend R. H.; Lee T.-W. Overcoming the electroluminescence efficiency limitations of perovskite light–emitting diodes. Science 2015, 350, 1222–1225. 10.1126/science.aad1818. [DOI] [PubMed] [Google Scholar]
- Xiao Z.; Kerner R. A.; Zhao L.; Tran N. L.; Lee K. M.; Koh T.-W.; Scholes G. D.; Rand B. P. Efficient perovskite light–emitting diodes featuring nanometer-sized crystallites. Nat. Photonics 2017, 11, 108–115. 10.1038/nphoton.2016.269. [DOI] [Google Scholar]
- Vashishtha P.; Halpert J. E. Field-Driven Ion Migration and Color Instability in Red-Emitting Mixed Halide Perovskite Nanocrystal Light-Emitting Diodes. Chem. Mater. 2017, 29, 5965–5973. 10.1021/acs.chemmater.7b01609. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.