Abstract

Chitosan/poly(vinyl alcohol)/amino-functionalized montmorillonite nanocomposite electrospun membranes with enhanced adsorption capacity and thermomechanical properties were fabricated and utilized for the removal of a model cationic dye (Basic Blue 41). Effects of nanofiller concentrations (up to 3.0 wt %) on the morphology and size of the nanofibers as well as the porosity and thermomechanical properties of the nanocomposite membranes are studied. It is shown that the incorporation of the nanoclay particles with ∼10 nm lateral sizes into the polymer increases the size of the pores by about 80%. To demonstrate the efficiency of the adsorbents, the dye removal rate is investigated as a function of pH, adsorbent dosage, dye concentration, and nanofiller loading. The highest and fastest dye removal occurs for the nanofibrous membranes containing 2 wt % nanofiller, where about 80% of the cationic dye is removed after 15 min. This performance is at least 20% better than the pristine chitosan/poly(vinyl alcohol) membrane. The thermal stability and compression resistance of the nanocomposite membranes are found to be higher than those of the pristine membrane. In addition, reusability studies show that the dye removal performance of this nanocomposite membrane reduces by only about 5% over four cycles. The adsorption kinetics is explained by the Langmuir isotherm model and is expressed by a pseudo-second-order kinetic mechanism that determines a spontaneous chemisorption process. The results of this study provide a valuable perspective on the fabrication of high-performance, reusable, and efficient electrospun fibrous nanocomposite adsorbents.
1. Introduction
Nowadays, one of the most critical challenges for all countries is the remediation of water contaminated with artificial dyes because of their carcinogenic and mutagenic properties and their harmful impacts on the environment, ecosystem, and human health. The treatment of dye-containing wastewater is challenging because of the complex chemical structure, high molecular weight, and low biological degradation potential of the dyes.1−3 There are various physical, chemical, and biological dye removal methods such as coagulation,4 photocatalytic degradation,5−7 and enzyme degradation.8,9 Among various methods, adsorption has received considerable attention due to its cost-effective and easy operation nature.10
Electrospun nanofibrous membranes (ENMs) are prominent
candidates
for use as adsorbents in wastewater treatment due to their high recoverability,
high adsorption capacity, and relatively high production rate.11−15 These advantages result from large effective area, fine fibrous,
porous and interconnected structure, flexible surface functionalization,
surface modification ability, and pore size tailorability.16−18 Chitosan is a nontoxic, biocompatible polymer that was widely utilized
in nanofiber preparation and presents excellent fiber-forming properties.19,20 The high adsorption capability of chitosan and the ease of electrospinning
make it a suitable candidate for the preparation of nanofibrous adsorbents.
However, because of the soft nature of the electrospun membranes and
their low solidity, they show a high tendency to compaction at high
pressures, which reduces their permeability significantly. Hence,
the low compaction resistance of electrospun membranes is a significant
drawback of the ENMs and has hindered their full deployment in water
treatment applications.18,21,22 For example, Choong (2015) reported that the permeability of a polysulfone
nanofibrous membrane decreased from 0.0015 to 0.007 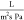 by increasing the pressure from 5 to 14
kPa.22
by increasing the pressure from 5 to 14
kPa.22
Recently, research studies showed that utilizing advanced nanoparticles can greatly improve adsorbents and membranes’ performance in wastewater treatment. For instance, Dadashi Firouzjaei et al. (2020) synthesized a graphene oxide–copper metal–organic framework (MOF) nanocomposite as an adsorbent and achieved a maximum adsorption capacity of 262 mg g–1 for methylene blue.23 In another study, Parkerson et al. prepared Cu-MOF-polydopamine-coated nanofiltration membranes. They reported a decrease in water flux (e.g., 43 LMH compared to 53 LMH for filtration of methylene blue) and an increase in dye rejections (46% compared to 25%).24 Even though using advanced nanoparticles may boost the removal efficiency of the adsorbents and membranes, nanomaterials like nanoclay are preferred due to their environmentally friendly nature.
Clay-based adsorbents have recently gained considerable attention as adsorbents in the treatment of effluents that are contaminated with dyes and heavy metals.25 Chitosan/montmorillonite (MMT) nanocomposites were prepared by Wang and Wang (2007) for Congo Red dye adsorption.26 They studied the effect of various chitosan/MMT molar ratios as well as temperature and pH of the dye solution on the adsorption capacity. It was shown that the chitosan to MMT molar ratio of 5 resulted in a higher adsorption capacity than other prepared nanocomposite adsorbents and pristine chitosan adsorbents at 50 °C and pH 4. Wan Ngah et al. (2010) synthesized epichlorohydrin cross-linked chitosan/bentonite adsorbents with improved performance in azo group-containing dye adsorption. They also reported that cross-linked chitosan-coated bentonite beads provided fast adsorption and excellent adsorption capacity (435.0 mg g–1). Also, they reported that the maximum adsorption capacity happened at a pH of 6.27 Mokhtar et al. (2020) prepared chitosan-magadiite hydrogel beads and evaluated their absorption capacity for both anionic and cationic dye molecule elimination from colored wastewater.28 The beads provided a high adsorption capacity for anionic dyes compared to the cationic ones. The maximum adsorbed values for cationic methylene blue and Congo red dyes were determined to be 45.25 and 135.77 mg g–1, respectively. Hosseini et al. (2019) fabricated chitosan/PVA/montmorillonite electrospun affinity membranes for cationic dye removal.19 The synthesized ENMs with 2 wt % nanoclay demonstrated 95% removal of Basic Blue 41 (BB41). Other investigations have also affirmed dye adsorption improvement by incorporating clay-based nanofillers in the chitosan matrix.29−31 Wang et al. (2014) synthesized a chitosan/PVA/bentonite nanocomposite with high selectivity and adsorption capacity for Hg2+.32 Liu et al. (2015) prepared cross-linked chitosan/bentonite ENMs for the removal of Cr6+. They studied the effect of pH of the effluent, initial concentration of Cr6+ in the effluent, adsorbent dosage, and operating time on dye removal efficiency. The maximum removal of Cr6+ (99.8%) was attained at lower initial Cr6+ ion concentrations, higher adsorbent dosage, and highly acidic conditions (pH = 2).33 In another research, Wang et al. (2019) prepared magnetic field-responsive bentonite–chitosan–Fe3+/Fe2+ beads as an adsorbent to remove Cs+. They optimized the bentonite/chitosan ratio, solution pH, and operating time to reach a maximum adsorption capacity of 57.1 mg g–1.34 Feng et al. (2019) showed that magnetic Fe3O4-chitosan@bentonite composite was an outstanding adsorbent for removing Cr6+.35 The maximum adsorption capacity was 62.1 mg g–1 at pH = 2. Also, they used the prepared adsorbent in the remediation of acid mine drainage containing Cr, Cd, Cu, Zn, Fe, Pb, and Ni. They reported the removal of more than 84% for each element. The enhanced efficiency of chitosan/clay composites for other heavy metals removal such as arsenic,36 selenium,37 copper,38 and lead39 was also reported. Ali et al. (2019) reported the preparation of phytic acid-doped polyaniline-MMT nanofibers for heavy metal adsorption. They studied the effect of MMT concentration in the structure of the synthesized nanocomposite on its adsorption performance. The results showed that the adsorption capacity of the prepared adsorbent under optimized experimental conditions was 87 mg g–1 for the removal of Cu2+. They reported that an increase in the amount of MMT in the nanocomposite structure decreased the adsorption capacity substantially, which could be due to the tendency of MMT particles to aggregate in the polyaniline matrix and therefore diminish the availability of active adsorption sites.40 Dela Peña et al. (2021) prepared polycaprolactone nanofibers with iron-intercalated MMT fillers for arsenic removal from wastewater in a bench-scale adsorbent column. They reported that a low flow rate of wastewater, low initial influent concentration, and thick nanofiber membrane resulted in a longer breakthrough time. The results showed that the breakthrough time was 201.50 min for an adsorption test with a 20 mL/min flow rate and an initial arsenic concentration of 2 ppm.41 Bansal and Purwar (2021) fabricated polyacrylonitrile/MMT and polyacrylonitrile/ZnO-MMT electrospun nanofibrous nanocomposites as adsorbents. The performance of the prepared ENMs in adsorption was studied in Cr6+ ion adsorption. They investigated the effect of solution pH, initial concentration of Cr6+, time, and adsorbent dosage on the removal of Cr6+ from wastewater. The maximum adsorption capacity was reported to be 416.67 and 476.19 mg g–1 for polyacrylonitrile/MMT and polyacrylonitrile/ZnO-MMT, respectively.42
The research so far has focused on the performance of ENMs in batch adsorption tests rather than their robustness and reusability for longer-term operations. Also, the low mechanical stability of these ENMs against compaction is a major drawback for their scale-up, which is rarely investigated in previous studies.
The primary objective of this research is to prepare compaction-resistant nanofibrous membranes with high adsorption characteristics and reusability using bio-inspired materials. In this study, we attempted to modify the thermomechanical stability and adsorption characteristics of chitosan/PVA ENMs using functional clay nanoparticles. To improve the adsorption capacity of the bio-inspired membranes, surface-modified clay nanoparticles by amine functional groups were incorporated in the electrospinning dope to functionalize the surface of the prepared nanofibers. The prepared ENMs were employed as efficient adsorbents for dye removal from colored wastewater. Basic Blue 41 was used as a model cationic dye. The effect of nanoclay loading and environmental conditions, such as adsorption time, pH of the colored wastewater, dye concentration, and temperature, on the adsorption capacity of ENMs was studied. In order to reach a comprehensive understanding of the mechanism of adsorption, the kinetics and thermodynamics of the process were investigated. Finally, the reusability and mechanical stability of the synthesized ENMs were investigated.
2. Results and Discussion
2.1. Effect of Nanoclay Particles on the Nanofiber Characteristics
SEM images of chitosan/PVA ENM (M1) and chitosan/PVA/OCT-MMT nanocomposite ENM (M4 with 3 wt % OCT-MMT) are illustrated in Figure 1a,b, respectively. As can be observed, uniform and crack- and bead-free fibers are formed. The fibers have a random orientation with a Gaussian size distribution. The mean diameter of the fibers is 115 and 130 nm for the M1 and M4 membranes, respectively. AFM determines that the fibers have a uniform cylindrical shape with a 3D interconnected structure (Figures 1c,d). A representative back-scattered SEM image of the M4 membrane (Figure 1e) indicates a uniform distribution of aggregate-free OCT-MMT nanoparticles with ∼10 nm sizes in the nanofiber structure. The size of the OCT-MMT nanoparticles was determined by image analysis using Image J 1.51 software.
Figure 1.
Morphology and size distribution of fibers in the adsorbent membranes. SEM and AFM images show the morphology, diameter distribution, and 3D structure of (a, c) chitosan/PVA and (b, d) chitosan/PVA/OCT-MMT (3 wt %) membranes. (e) Back-scattered SEM image shows the uniform incorporation of MMT nanoparticles in nanocomposite fibers’ structure (M4 specimen).
Further SEM studies have shown that the mean fiber diameter increases with the incorporation of OCT-MMT nanoparticles (Figure 2a). The membranes also become more porous with a larger average pore size as a higher amount of OCT-MMT particles is embedded (Figure 2b). The more porous structure renders a higher surface area and provides a more effective platform for adsorption.18,43 The incorporation of metallic or ceramic nanoparticles in the electrospinning solutions increases the electrospinning dope viscosity.17,44 Higher viscoelastic forces of more viscous spinning solution resist the axial stretching during whipping; hence, larger fiber diameters are formed.45,46 As shown in eq 14, the larger fiber diameter leads to larger pore sizes and slightly bigger pore volumes.47,48 Based on the studies in the literature49 and eq 14, the pore size of the ENMs depends on both fiber diameter and porosity. However, the fiber diameter is the more dominant factor compared to the porosity of the ENMs. Nakamura et al. investigated the effect of porosity on the pore size of the fibrous filter media with fixed fiber diameter and reported that the effect of porosity on the pore size is insignificant compared to the fiber diameter.49 This result can be attributed to the three-dimensional, highly interconnected, and tortuous structure of the nanofibrous membranes, resulting in high porosity values.50,51 Kiani et al. reported the same observation for the effect of porosity on the pore size of ENMs.52
Figure 2.
Effect of OCT-MMT nanoparticles on the physical properties of the ENMs. (a) Mean fiber diameter and (b) porosity and mean pore sizes.
2.2. Chemical Composition of ENMs
FTIR analysis was performed to examine the chemical composition of ENMs. Figure 3 affirms the presence of MMT nanoparticles in the structure of ENMs as the peak located at 3625 cm–1 is attributed to vibration of the hydroxyl group of aluminol and silanol.53,54 The peak at 3250 cm–1 is ascribed to N–H stretching of alkylammonium55 that shows the presence of octadecylamine in ENMs, which was used in surface modification of MMT nanoparticles. The chemical reaction between the aldehyde groups of GA and the amino groups of chitosan leads to the formation of the imine (C≡N) groups at 1628 cm–1 through a Schiff base reaction.56 The position of this peak is slightly shifted to 1640 cm–1 in the presence of OCT-MMT particles, likely due to the electrostatic interaction of the negative sites of nanoclay mineral with the amine functional groups of the chitosan.57 On the other hand, the reaction between GA and the hydroxyl group of PVA forms the acetal bond at 1059 cm–1.17,58 The amino group stretching vibration of chitosan is noticed at 1575 cm–1,57 which is also slightly shifted to 1560 cm–1 in the presence of OCT-MMT nanoparticles.57
Figure 3.

FTIR spectroscopy of the fabricated ENMs.
2.3. Thermomechanical Stability of the Fibrous Membranes
Generally, textile effluents’ temperature can be as high as 65 °C,59 which is far below the PVA/chitosan degradation temperature. However, it has been reported in the literature that the incorporation of clay into the polymer matrix may accelerate the thermal degradation, which can be due to the catalysis effect of water in MMT and hydroxyl groups on the clay platelets.60 Also, in this study, nanoclay has been modified using an organic agent, which may accelerate the thermal degradation of the nanocomposite.60 Therefore, the thermal stability of the prepared adsorbents is investigated. To examine the thermal stability of the prepared nanocomposites, TGA was performed. As shown in Figure 4a, thermal degradation occurs in three steps. The first weight loss is attributed to the loosely bound moisture evaporation. The samples lose about 8% of their weight up to 100 °C. The second step of thermal degradation happens in the range of 200–400 °C. Dehydration reactions and the evaporation of volatile components lead to eliminating hydroxyl and amine functional groups of chitosan and PVA degradation.61 The third step is related to the polymer cleavage above 400 °C.61−64 The TGA results indicate that the presence of OCT-MMT nanoparticles improves the thermal stability of the polymer matrix. The effect of OCT-MMT is more significant in the last stage of degradation, where polymer cleavage occurs. The more OCT-MMT nanoparticles, the higher the thermal stability of the ENMs. The presence of a nanoclay structure and their good compatibility with the polymer network decrease the polymer’s chain mobility, therewith enhancing the thermal stability of ENMs.65,66
Figure 4.

Thermal stability and mechanical durability of fibrous membranes. (a) TGA curves showing the effect of OCT-MMT nanoparticles on the thermal degradation of the polymer matrix. (b) Typical compressive stress–strain curves of the examined membranes. (c) Elastic modulus of fibrous membranes as a function of OCT-MMT concentration.
The mechanical characteristic of the ENMs was investigated via the compaction test. Representative stress–strain responses for the chitosan/PVA ENMs containing different amounts of OCT-MMT nanoparticles are shown in Figure 4b. All ENMs exhibit typical stress–strain curves under compression loading.21 The incorporation of the OCT-MMT nanoparticles enhances the stiffness and strength of the polymeric membranes. Other studies in the literature have reported similar results.18,67,68 For instance, the elastic modulus of the porous membrane almost linearly increases by increasing the OCT-MMT concentration (Figure 4c).
The improved mechanical stability is ascribed to (i) the load-bearing capacity of the clay nanoparticles and (ii) increased fiber diameters.21 The superior thermomechanical properties of nanocomposite ENMs provide a more robust platform during the filtration process to resist compaction and degradation at high hydraulic pressures and solution temperatures.17,18,69
2.4. Dye Removal Performance
The effect of nanoparticle loading, solution pH, dye concentration, and adsorbent dosage on the dye removal performance of ENMs is evaluated, and the results are provided in this subsection.
2.4.1. Effect of pH
Figure 5a,b shows that the most effective adsorption of chitosan/PVA ENM occurs using neutral solutions (pH = 7). The dye removal rate is rapid at the early stage (up to 20 min) and then reaches a plateau because of the saturation of the adsorbent active sites.10 The adsorption mechanism is the electrostatic attraction between the positive surface charge of the cationic dye molecule and the lone electron pair on the nitrogen atom of amine functional groups of chitosan.17,70,71 This electrostatic interaction leads to the formation of a coordination complex via sharing of the electron pair70−72 as follows:
| 1 |
Figure 5.
Adsorption removal rate (left-hand side) and adsorption capacity (right-hand side) of the nanofibrous membranes. (a, b) Effect of solution pH (dye concentration = 10 mg L–1 and adsorbent dosage = 0.04 g) and (c, d) effect of the OCT-MMT concentration (pH = 7, adsorbent dosage = 0.04 g and dye concentration = 10 mg L–1).
Chitosan is a cationic polymer, which is protonated in acidic solutions according to the following equation:17,73
| 2 |
As the surface charge of chitosan becomes more positive in acidic solutions, the electrostatic repellence between the adsorbent active sites and the dye molecules reduces the adsorption efficiency. Hence, pH affects the protonation of the adsorbent amine groups and thus alters the electrostatic charge of the ENMs and the number of active adsorption sites on their surface.74
In an acidic condition, although the number of active sites for the adsorption of cationic dye molecules decreases due to the protonation of amine groups, some active adsorption sites still exist. In fact, there is a competition between H+ and cationic dye molecules (CD+) to react with the amine functional groups of the ENMs (eqs 1 and 2). In an acidic solution, the concentration of H+ is high, so the equilibrium of eq 2 shifts to the right, and the cationic dye removal capacity decreases. However, by increasing the pH, the competition reduces, and the adsorption efficiency of the ENMs increases gradually.17,75−77 It should be mentioned that the electrostatic attraction is the primary adsorption mechanism; however, other interactions, such as hydrophobic interaction, may be partially involved in the dye removal process.78
2.4.2. Effect of Nanoclay Particles
The effect of OCT-MMT nanoparticles on the dye removal rate and adsorption capacity of chitosan/PVA/OCT-MMT ENMs is shown in Figure 5c,d. As can be seen, nanocomposite ENMs performed significantly better than the pristine chitosan/PVA ENM. For instance, after 15 min, the amount of dye removal for the membrane containing 2 wt % nanoclay is ∼80% at pH = 7, which is almost 30% more than that of the chitosan/PVA membrane. Based on the literature, MMT particles are negatively charged in a wide range of pH.79−81 Therefore, the nanoclay particles effectively contribute to the adsorption process through electrostatic interactions.17,82 In addition, −NH2 groups on amino-functionalized nanoclay promote its adsorption efficiency as the amine groups provide more active adsorption sites for removal of cationic dye. As mentioned before, the donation of the lone pair of electrons on the nitrogen in amine groups of octadecylamine and interaction with the positively charged cationic dye molecules leads to the formation of a coordination complex (Lewis acid/base reaction, eq 1) via sharing of the electron pair.70−72 Furthermore, the more porous structure of the nanocomposite membranes (Figure 2b) provides a higher surface area for adsorption, increasing the selectivity of the nanocomposite ENMs compared to the pristine chitosan/PVA membrane. Taking a closer look at Figure 5c,d, the dye removal and adsorption capacity increase with the OCT-MMT concentration up to 2 wt % but decrease with further increasing OCT-MMT loading. The decrease in the dye removal performance of the ENMs with an increase in OCT-MMT loading may be attributed to the following reasons. The number of active sites on the polymer chain is higher than the nanoparticles. Therefore, the addition of nanoclay decreases the amount of chitosan, or the number of active sites, in a unit mass of the adsorbent that cannot be likely compensated for by the nanoparticles.83 In addition, the interactions among OCT-MMT, chitosan, and PVA become more intensive gradually by increasing the nanoparticle loading, resulting in reduced elasticity of the polymer chains, which decreases dye removal performance and adsorption capacity of the ENMs.83−85 Finally, by increasing OCT-MMT concentration, the aggregation tendency of nanoparticles in the polymer matrix increases, which may cause a decrease in the adsorption capacity.40 Hence, there should be an optimum value for the loading of OCT-MMT in nanocomposite membranes. Pal et al. (2012) observed a resembling response for the adsorption of Methylene Blue on carboxymethyl tamarind-g-poly(acrylamide)/silica. They reported that the dye removal performance of the nanocomposite improved with the SiO2 nanoparticle loading up to 1.5 wt % and then reduced at a higher concentration.86 Therefore, the concentration of OCT-MMT must be optimized for the fabrication of robust ENMs with high dye removal efficiency, adsorption capacity, and thermomechanical strength.
2.4.3. Effect of Adsorbent Dosage and Dye Concentration
The number of active sites increases by increasing the weight of the adsorbent in the solution. Therefore, the dye removal and adsorption capacity improve by adding more adsorbent to the solution. For instance, at the constant dye concentration of 10 mgL–1, the amount of adsorbed dye on the nanofibrous composite membrane containing 2 wt % OCT-MMT nanoparticles increases (Figure S1a). The results of the adsorption capacity in Figure S1b are in agreement with the dye removal efficiency results in Figure S1a. Also, an increase in the dye concentration in the solution affects the adsorption efficiency as the active sites eventually become saturated by cationic dye molecules (Figure S1c). Figure S1d shows that increasing the dye concentration up to 30 mg L–1 increases the adsorption capacity. Dye removal percentage increases by decreasing the initial dye concentration due to the availability of unoccupied active sites on the electrospun membrane surface. However, a further increase in initial dye concentration decreases the dye removal percentage due to the almost complete coverage of the adsorption active sites by dye molecules at high dye concentrations. On the other hand, the number of dye molecules adsorbed onto the surface of the adsorbent increases with an increase in initial dye concentration while the amount of adsorbent in the solution is constant.87 Therefore, according to eq 16, the adsorption capacity increases with an increase in initial dye concentration, which can be attributed to the enhanced concentration gradient driving force and the increased mass transfer rate.17,48,88−90 Sun et al. (2013) reported the same results for the effect of initial dye concentration on adsorption efficiency.87 On the other hand, rapid adsorption of the organic molecules on the active sites causes electrostatic hindrances between the adsorbed molecules and those in the solution.
Taking a closer look at Figure 5, it is found that the dye removal efficiency of our developed ENMs never exceeds 90%, which is less than that of common carbon-based adsorbents. This slight reduction in removal efficiency occurred at the expense of developing green adsorbents using an environmentally friendly method, which is the main purpose of this study. Chitosan and PVA are biodegradable materials, and acetic acid solution can be replaced by vinegar for future deployment of the technology that makes the production process even greener. It should be noted that the reusability characteristic of our fabricated ENMs (see Section 2.5) is also in an acceptable range.
2.5. Reusability of Nanocomposite Membranes
From a practical point of view, the reusability and durability of adsorbents are crucial factors.91 To study the reusability of adsorbents, the used ENMs were recovered using a stripping solution (HCl with pH = 2.1) for 15 min to leach out the adsorbed dye molecules. After rinsing with double-distilled water for 10 min, the regenerated ENMs were reused for dye adsorption experiments. Figure 6a shows that the removal efficiency of reused electrospun adsorbents is reduced by about 5 to 15% (dependent on the composition). The minimum decline is noticed for the nanocomposite membrane containing 3 wt % OCT-MMT, likely due to the larger pore diameter and more porous structure of the M4 membrane, causing the dye molecules to be leached out by the stripping solution easily.92 The reusability of the ENM containing 2 wt % OCT-MMT was evaluated over 4 cycles. As shown in Figure 6b, the nanocomposite membrane’s performance remains almost unchanged after the first cycle, implying its viability for dye removal.
Figure 6.

Reusability assessment of nanocomposite membranes. (a) Dye removal performance of ENMs over two cycles of operation. (b) Adsorption capacity of the membrane containing 2 wt % OCT-MMT over four adsorption cycles at pH = 7 and initial dye concentration of 10 mg L–1.
2.6. Adsorption Kinetics
In order to determine the kinetics of the adsorption process on ENMs, non-linear pseudo-first-order (eq 3), non-linear pseudo-second-order (eq 4), and intraparticle diffusion models (eq 5) are tried to fit the experimental data:93,94
| 3 |
| 4 |
| 5 |
where qe (mg g–1) and qt (mg g–1) are the adsorption capacity values at equilibrium and time t (min), respectively. k1(min–1) and k2 (g mg–1 min–1) are the equilibrium rate constants of pseudo-first-order and pseudo-second-order models, respectively, and kid (mg g–1 min-1/2) is the intraparticle diffusion rate constant. I (mg g–1) in eq 5 is the intraparticle diffusion model intercept. To determine the applicability of these kinetics models for the BB41 adsorption at different OCT-MMT concentrations, the values of the reaction rate constants are calculated and presented in Table 1. The calculated qe and R2 values indicate the suitability of the pseudo-second-order model to explain the adsorption process. This finding shows that the predominant adsorption mechanism is chemisorption.93,95
Table 1. Kinetic Parameters of Dye Adsorption for Different Adsorbent Dosages.
| pseudo-first-order |
pseudo-second-order |
intraparticle diffusion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| membrane | (qe)exp | (qe)cal | k1 | R2 | (qe)cal | k2 | R2 | kid | I | R2 |
| M1 | 35.62 | 36.12 | 0.047442 | 0.9379 | 35.78 | 0.005135 | 0.9982 | 3.021 | 13.68 | 0.7876 |
| M2 | 39.51 | 40.67 | 0.396120 | 0.9102 | 39.93 | 0.00430 | 0.9972 | 3.33 | 14.37 | 0.7757 |
| M3 | 44.72 | 45.90 | 0.040533 | 0.9298 | 44.30 | 0.007681 | 0.9995 | 2.725 | 25.68 | 0.7014 |
| M4 | 42.85 | 43.28 | 0.050896 | 0.9482 | 43.06 | 0.004295 | 0.9982 | 3.649 | 16.58 | 0.7938 |
2.7. Adsorption Isotherm
The BB41 adsorption isotherm for the chitosan/PVA/OCT-MMT nanocomposites is investigated by Langmuir, Freundlich, and Temkin models. The Langmuir model assumes monolayer adsorption of the dye molecules on specific sites of the adsorbent without chemical interactions between the adsorbate molecules:93,94
| 6 |
where Ce (mg L–1), KL (L mg–1), and qmax (mg g–1) are the equilibrium concentration of the dye in the solution, Langmuir isotherm constant, and maximum adsorption capacity, respectively. The Freundlich model is generally used to explain the multilayer and heterogeneous adsorption to the adsorbent surface. The Freundlich isotherm linear model is written as follows:93,94
| 7 |
where Kf (L g–1) is
the adsorption capacity or the
Freundlich constant and 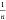 is the adsorption intensity. The Temkin
isotherm considers the effect of adsorption heat, which linearly reduces
with the coverage of adsorbed molecules. Such a decrease in the adsorption
heat is because of the interaction between the adsorbed molecules.
The Temkin isotherm is illustrated as follows:93
is the adsorption intensity. The Temkin
isotherm considers the effect of adsorption heat, which linearly reduces
with the coverage of adsorbed molecules. Such a decrease in the adsorption
heat is because of the interaction between the adsorbed molecules.
The Temkin isotherm is illustrated as follows:93
| 8 |
where KT (L mol–1) and B1 are the equilibrium binding constant and Temkin constant, respectively. Table 2 summarizes the fitting parameters of these equations along with the regression coefficient (R2) for the nanocomposite membrane containing 2 wt % OCT-MMT. It appears that the Langmuir isotherm sufficiently explains the adsorption process based on monolayer coverage.
Table 2. Evaluations of the Isotherm Constants for the Adsorption of BB41 on Nanocomposite Membranes at 298 K.
| Langmuir constants |
Freundlich constants |
Temkin constants |
||||||
|---|---|---|---|---|---|---|---|---|
| KL | qmax | R2 | n | Kf | R2 | B1 | KT | R2 |
| 0.3489 | 121.95 | 0.971 | 3.74 | 47.068 | 0.928 | 19.663 | 9.952 | 0.923 |
2.8. Thermodynamics of Adsorption
To study the adsorption process thermodynamics, the values of thermodynamic parameters including enthalpy (ΔH0), entropy (ΔS0), and Gibbs free energy (ΔG0) were calculated by eqs 9–11 and van’t Hoff plots.94,96,97
| 9 |
| 10 |
| 11 |
Ke0 (dimensionless) and KL (L mol–1) are the adsorption equilibrium and Langmuir equilibrium constants, respectively. γ is the activity coefficient, [adsorbate]0 (mol L–1) is the standard molar concentration of adsorbate, T (K) is the dye solution temperature, and R (8.314 J mol–1 K–1) is the gas constant. It is assumed that the dye solution is very diluted, so the activity coefficient is considered unitary.96 The Langmuir equilibrium constant was employed in the van’t Hoff model to determine the thermodynamics of the adsorption process. According to eq 11, the values of enthalpy and entropy were obtained from the plot of ln(Ke0) versus 1/T (Figure S2). The values of ΔH0, ΔS0, and ΔG0 are reported in Table 3. The negative value of ΔG0 at various temperatures determines the spontaneous nature of the adsorption process of BB41 on the chitosan/PVA/OCT-MMT nanocomposite membrane. The value of ΔG0 increases from 12.7 to 15.5 kJ mol–1 by increasing the solution temperature, which confirms that the adsorption process can be more desirable at higher temperatures. This result can also be inferred from the positive value of enthalpy change (ΔH0 > 0) that affirms that the endothermic nature of the adsorption process commonly occurs in chemical adsorption. The positive value of ΔH0 shows the presence of an energy barrier in the dye adsorption process, which is due to the excited form of an activated complex in the transition state in eq 1.98,99 The low value of the ΔH0 in the temperature range of 298–318 K suggests that the adsorption of BB41 occurs by the cation-exchange mechanism due to the electrostatic adsorption,98 which can be reversible by a change in the solution pH. In addition, the positive value of entropy (ΔS0 > 0) indicates the affinity of the adsorbent for cationic dyes and the randomness at the solid/solution interface throughout the dye removal process.100−102 In conclusion, the kinetics and thermodynamics of the adsorption process indicate a spontaneous, endothermic, and chemical adsorption of the cationic dye by the nanocomposite membranes.
Table 3. Thermodynamic Parameters for the Adsorption of BB41 on Chitosan/PVA/OCT-MMT (2 wt %) Nanocomposite at an Adsorbent Dosage of 0.04 g, Contact Time of 90 min, and Dye Concentration of 10 mg/L.
| temperature (K) | ΔH0 (kJ mol–1) | ΔS0(kJ mol–1 K–1) | ΔG0(kJ mol–1) |
|---|---|---|---|
| 298 | 29.37 | 0.14 | –12.7 |
| 308 | –14.4 | ||
| 313 | –14.8 | ||
| 318 | –15.5 |
3. Conclusions
Electrospun nanofibrous membranes were fabricated based on chitosan/PVA and used for colored wastewater treatment. To improve the cationic dye removal of the membranes, amino-functionalized silicate nanoparticles were incorporated into ENMs. SEM and AFM studies indicated that the incorporation of OCT-MMT nanoparticles increased the porosity and the average pore size of the membranes significantly. The nanoparticles decorated the surface of the nanofibers and enhanced the dye adsorption capacity. The optimum concentration of OCT-MMT particles was determined to be 2 wt %, at which the highest removal capacity and the fastest removal rate were attained. Importantly, this nanocomposite membrane was reused many times without a significant loss in its dye removal performance. The results showed that the incorporation of amino-functionalized nanoclay improved the thermal stability of the ENM structure. In addition, Young’s modulus of the chitosan/PVA membrane was enhanced from 0.9 to 2.4 MPa by the addition of 2.0 wt % OCT-MMT. Experimental analysis along with model fitting determined that the Langmuir isotherm and pseudo-second-order model could describe the adsorption process with high correlation to experimental data. Evaluation of thermodynamics and kinetics of the adsorption process indicated that the adsorption was spontaneous and endothermic and carried out by a chemisorption process.
4. Experimental Section
4.1. Materials
Chitosan (Mw ≈ 75–85% deacetylated), aluminum silicate flat hexagonal crystals (H2Al2O6Si, Mw ≈ 180.1 g.mol–1), and octadecylamine (97%) were purchased from Sigma-Aldrich (USA). PVA (Mw ≈ 145,000), hydrochloric acid, ethanol, acetic acid, glutaraldehyde (GA), and sodium hydroxide were bought from Merck (Germany). Double-distilled water was used in this study. Basic Blue 41 (BB41) dye (C20H26N4O6S2, Mw = 482.57 g mol–1) was supplied by Alvan Sabet Co (Iran).
4.2. Surface Modification of MMT
Surface modification of MMT nanoparticles was performed using octadecylamine according to the method used by Han et al. (2003).103 First, 6.5 mL of octadecylamine was acidified by adding 1.2 mL of HCl followed by dispersion in 200 mL of ethanol. Next, 10 g of MMT was dispersed into 250 mL of double-distilled water using stirring at 80 °C. Afterward, the octadecylamine-acidified solution was mixed with nanoparticle dispersion and strongly stirred at 80 °C for 10 h. Finally, the mixture was centrifuged, and the surface-modified MMT (OCT-MMT) was recovered and rinsed several times with ethanol and double-distilled water until the absence of chloride (Ag+ test).104,105 The nanoparticles were heated at 60 °C for 24 h to be dried completely.
4.3. Preparation of Nanofibrous Nanocomposite Membranes
PVA/chitosan with the ratio of 80/20 was dissolved in a water/acetic acid solution with the same volume percent to prepare a polymer blend solution (7.0 wt %). The electrospinning dopes were stirred for 24 h at 150 rpm. Afterward, a certain amount of OCT-MMT was added to the polymer solution and stirred for 24 h to produce four spinning dopes containing 0, 1, 2, and 3 wt % OCT-MMT, named M1 to M4. Since the chitosan/PVA ratio was constant in the prepared electrospun membrane composition, the hydrophilicity of the ENMs was not altered by the polymer loading.
The electrospinning process was conducted at ambient temperature under an injection rate of 5 μL min–1 using a syringe having a 0.7 mm inner diameter needle. An aluminum foil-wrapped rotating drum, which was placed 150 mm from the needle tip, collected the nanofibers. The rotating speed of the collector and voltage between the collector and the needle tip was 300 rpm and 25 kV, respectively. Cross-linking of the prepared ENMs was performed via GA at 90 °C for 12 h, followed by drying at 60 °C for 12 h.
4.4. Materials Characterizations
Morphological observations were carried out by field emission scanning electron microscopy (FE-SEM, TESCAN model MIRA3 LM). The fiber size distribution was assessed using image analysis software (Image J 1.51). FTIR spectroscopy (ThermoNicolet NEXUS 870 FTIR from Nicolet Instrument Corp., USA) was utilized to examine the surface chemistry of the ENMs. Atomic force microscopy (AFM, Dual- Scope C-26, Denmark) was utilized to study the morphology and surface topography of ENMs. Thermogravimetric analysis (TGA) was carried out using a Q50 TGA (TA Instruments, USA) in a temperature range of 25–550 °C with a heating rate of 10 °C min–1 under a nitrogen atmosphere. A mechanical testing apparatus (STM-20, Iran) with a 1.0 N load cell was employed to conduct compression tests according to the ASTM-D575 standard.18
4.5. Porosity and Pore Size Evaluation
The porosity of the fibrous membranes was determined by the following equations:46,47
| 12 |
| 13 |
where ε and ρ are the porosity and apparent density, respectively. ρ0 is the mean density of the polymers and MMT, and φ is the mass fraction of the materials (Chitosan, PVA, and MMT). The amounts of ρCS, ρPVA, and ρMMT are 0.3, 1.3, and 2.15 g cm–3, respectively. To calculate ρ, a piece of ENM with a certain surface area was precisely weighed, and its dimensions were measured using a micrometer. Then, ρ was determined by the mass and the computed volume.
The average pore radius (r) of the ENMs was estimated from the mean fiber diameter (d) as follows:18,51
 |
14 |
4.6. Evaluation of ENM Performance in Dye Removal
To examine the removal efficiency and adsorption capacity of the membranes, aqueous solutions with various concentrations of BB41 (10, 20, and 30 mg L–1) were prepared. The solution pH was tuned by NaOH and HCl to the desired values. Afterward, ENMs were placed into the dye solutions, followed by stirring on a magnetic stirrer for a certain period at a constant temperature. The changes in the dye concentration were determined over time by employing a CECIL2021 UV/Vis spectrophotometer (Cecil, UK) at the dye’s specific absorbance wavelength (617 nm). Each adsorption test was repeated three times, and the average values were used to obtain calibration curves. The dye removal percentage and adsorption capacity (q, mg g–1) were determined using the following equations:48,82
| 15 |
| 16 |
where C0 and Ct are the dye concentration in the colored wastewater (g L–1) at time t = 0 and time t, m is the dry mass of the ENM (g), and V is the solution volume (L).
Acknowledgments
The financial support for this work by the Natural Science and Engineering Reserach Council of Canada (NSERC RGPIN-2016-04690), Iran’s National Elites Foundation and Iran National Science Foundation is gratefully acknowledged. A.S. acknowledges the financial support of the Iran National Science Foundation (INSF, grant nos. 95-S-48740 and 96016364) and Sharif University of Technology (grant no. QA970816).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00480.
Adsorption removal rate and adsorption capacity of the nanofibrous membranes and plots of ln(Ke0) – 1/T for adsorption of BB41on ENM containing 2 wt % OCT-MMT (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wang Q.; Luan Z.; Wei N.; Li J.; Liu C. The Color Removal of Dye Wastewater by Magnesium Chloride/Red Mud (MRM) from Aqueous Solution. J. Hazard. Mater. 2009, 170, 690–698. 10.1016/j.jhazmat.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Hokkanen S.; Bhatnagar A.; Sillanpää M. A Review on Modification Methods to Cellulose-Based Adsorbents to Improve Adsorption Capacity. Water Res. 2016, 91, 156–173. 10.1016/j.watres.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Ma X.; Chen P.; Zhou M.; Zhong Z.; Zhang F.; Xing W. Tight Ultrafiltration Ceramic Membrane for Separation of Dyes and Mixed Salts (Both NaCl/Na2SO4) in Textile Wastewater Treatment. Ind. Eng. Chem. Res. 2017, 56, 7070–7079. 10.1021/acs.iecr.7b01440. [DOI] [Google Scholar]
- Wei Y.; Ding A.; Dong L.; Tang Y.; Yu F.; Dong X. Characterisation and Coagulation Performance of an Inorganic Coagulant—Poly-Magnesium-Silicate-Chloride in Treatment of Simulated Dyeing Wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 137–141. 10.1016/j.colsurfa.2015.01.066. [DOI] [Google Scholar]
- Mahmoodi N. M. Photocatalytic Ozonation of Dyes Using Multiwalled Carbon Nanotube. J. Mol. Catal. A: Chem. 2013, 366, 254–260. 10.1016/j.molcata.2012.10.002. [DOI] [Google Scholar]
- Karimi L.; Zohoori S.; Yazdanshenas M. E. Photocatalytic Degradation of Azo Dyes in Aqueous Solutions under UV Irradiation Using Nano-Strontium Titanate as the Nanophotocatalyst. J. Saudi Chem. Soc. 2014, 18, 581–588. 10.1016/j.jscs.2011.11.010. [DOI] [Google Scholar]
- Daneshvar e Asl S.; Sadrnezhaad S. K. Photocatalytic Activity of Rutile/Anatase TiO 2 Nanorod/Nanobranch Thin Film Loaded with Au@Ag@Au Core Double Shell Nanoparticles. J. Photochem. Photobiol., A 2019, 111843. 10.1016/j.jphotochem.2019.05.006. [DOI] [Google Scholar]
- Bento R. M. F.; Almeida M. R.; Bharmoria P.; Freire M. G.; Tavares A. P. M. Improvements in the Enzymatic Degradation of Textile Dyes Using Ionic-Liquid-Based Surfactants. Sep. Purif. Technol. 2020, 235, 116191. 10.1016/j.seppur.2019.116191. [DOI] [Google Scholar]
- Lopes L. S.; Vieira N.; da Luz J. M. R.; Silva M. D. C. S.; Cardoso W. S.; Kasuya M. C. M. Production of Fungal Enzymes in Macaúba Coconut and Enzymatic Degradation of Textile Dye. Biocatal. Agric. Biotechnol. 2020, 26, 101651. 10.1016/j.bcab.2020.101651. [DOI] [Google Scholar]
- Mahmoodi N. M.; Mokhtari-Shourijeh Z.; Abdi J. Preparation of Mesoporous Polyvinyl Alcohol/Chitosan/Silica Composite Nanofiber and Dye Removal from Wastewater. Environ. Prog. Sustainable Energy 2019, 38, S100–S109. 10.1002/ep.12933. [DOI] [Google Scholar]
- Ding B.; Yu J.. Electrospun Nanofibers for Energy and Environmental Applications; Springer:New York, 2014, 10.1007/978-3-642-54160-5. [DOI] [Google Scholar]
- Bjorge D.; Daels N.; De Vrieze S.; Dejans P.; Van Camp T.; Audenaert W.; Hogie J.; Westbroek P.; De Clerck K.; Van Hulle S. W. H. Performance Assessment of Electrospun Nanofibers for Filter Applications. Desalination 2009, 249, 942–948. 10.1016/j.desal.2009.06.064. [DOI] [Google Scholar]
- Teng M.; Li F.; Zhang B.; Taha A. A. Electrospun Cyclodextrin-Functionalized Mesoporous Polyvinyl Alcohol/SiO2 Nanofiber Membranes as a Highly Efficient Adsorbent for Indigo Carmine Dye. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 229–234. 10.1016/j.colsurfa.2011.06.020. [DOI] [Google Scholar]
- Kaur S.; Kotaki M.; Ma Z.; Gopal R.; Ramakrishna S.; Ng S. C. Oligosaccharide Functionalized Nanofibrous Membrane. Int. J. Nanosci. 2006, 05, 1–11. 10.1142/S0219581X06004206. [DOI] [Google Scholar]
- Si Y.; Ren T.; Li Y.; Ding B.; Yu J. Fabrication of Magnetic Polybenzoxazine-Based Carbon Nanofibers with Fe3O4 Inclusions with a Hierarchical Porous Structure for Water Treatment. Carbon N. Y. 2012, 50, 5176–5185. 10.1016/j.carbon.2012.06.059. [DOI] [Google Scholar]
- Lin T.Nanofibers-Production, Properties and Functional Applications; InTech: Croatia, 2011, 10.5772/916. [DOI] [Google Scholar]
- Hosseini S. A.; Vossoughi M.; Mahmoodi N. M.; Sadrzadeh M. Clay-Based Electrospun Nanofibrous Membranes for Colored Wastewater Treatment. Appl. Clay Sci. 2019, 168, 77–86. 10.1016/j.clay.2018.11.003. [DOI] [Google Scholar]
- Hosseini S. A.; Vossoughi M.; Mahmoodi N. M.; Sadrzadeh M. Efficient Dye Removal from Aqueous Solution by High-Performance Electrospun Nanofibrous Membranes through Incorporation of SiO2 Nanoparticles. J. Cleaner Prod. 2018, 183, 1197–1206. 10.1016/j.jclepro.2018.02.168. [DOI] [Google Scholar]
- Jia Y. T.; Gong J.; Gu X. H.; Kim H. Y.; Dong J.; Shen X. Y. Fabrication and Characterization of Poly (Vinyl Alcohol)/Chitosan Blend Nanofibers Produced by Electrospinning Method. Carbohydr. Polym. 2007, 67, 403–409. 10.1016/j.carbpol.2006.06.010. [DOI] [Google Scholar]
- Cheaburu-Yilmaz C. N.; Yilmaz O.; Vasile C.. Eco-Friendly Chitosan-Based Nanocomposites: Chemistry and Applications. In Eco-friendly Polymer Nanocomposites. Advanced Structured Materials; Thakur V.; Thakur M., Eds.; Springer: New Delhi, 2015; Vol. 74, pp. 341–386, 10.1007/978-81-322-2473-0_11. [DOI] [Google Scholar]
- Choong L. T.; Mannarino M. M.; Basu S.; Rutledge G. C. Compressibility of Electrospun Fiber Mats. J. Mater. Sci. 2013, 48, 7827–7836. 10.1007/s10853-013-7528-x. [DOI] [Google Scholar]
- Choong L. T. (Simon). Application of Electrospun Fiber Membranes in Water Purification, Massachusetts Institute of Technology, 2015.
- Dadashi Firouzjaei M.; Akbari Afkhami F.; Rabbani Esfahani M.; Turner C. H.; Nejati S. Experimental and Molecular Dynamics Study on Dye Removal from Water by a Graphene Oxide-Copper-Metal Organic Framework Nanocomposite. J. Water Process Eng. 2020, 34, 101180. 10.1016/j.jwpe.2020.101180. [DOI] [Google Scholar]
- Parkerson Z. J.; Le T.; Das P.; Mahmoodi S. N.; Esfahani M. R. Cu-MOF-Polydopamine-Incorporated Functionalized Nanofiltration Membranes for Water Treatment: Effect of Surficial Adhesive Modification Techniques. ACS ES&T Water 2021, 1, 430–439. 10.1021/acsestwater.0c00173. [DOI] [Google Scholar]
- Unuabonah E. I.; Taubert A. Clay-Polymer Nanocomposites (CPNs): Adsorbents of the Future for Water Treatment. Appl. Clay Sci. 2014, 83–92. 10.1016/j.clay.2014.06.016. [DOI] [Google Scholar]
- Wang L.; Wang A. Adsorption Characteristics of Congo Red onto the Chitosan/Montmorillonite Nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. 10.1016/j.jhazmat.2007.01.145. [DOI] [PubMed] [Google Scholar]
- Wan Ngah W. S.; Ariff N. F. M.; Hanafiah M. A. K. M. Preparation, Characterization, and Environmental Application of Crosslinked Chitosan-Coated Bentonite for Tartrazine Adsorption from Aqueous Solutions. Water, Air, Soil Pollut. 2010, 206, 225–236. 10.1007/s11270-009-0098-5. [DOI] [Google Scholar]
- Mokhtar A.; Abdelkrim S.; Djelad A.; Sardi A.; Boukoussa B.; Sassi M.; Bengueddach A. Adsorption Behavior of Cationic and Anionic Dyes on Magadiite-Chitosan Composite Beads. Carbohydr. Polym. 2020, 229, 115399. 10.1016/j.carbpol.2019.115399. [DOI] [PubMed] [Google Scholar]
- Monvisade P.; Siriphannon P. Chitosan Intercalated Montmorillonite: Preparation, Characterization and Cationic Dye Adsorption. Appl. Clay Sci. 2009, 42, 427–431. 10.1016/j.clay.2008.04.013. [DOI] [Google Scholar]
- Nesic A. R.; Velickovic S. J.; Antonovic D. G. Characterization of Chitosan/Montmorillonite Membranes as Adsorbents for Bezactiv Orange V-3R Dye. J. Hazard. Mater. 2012, 209-210, 256–263. 10.1016/j.jhazmat.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Wang X.; Du Y.; Luo J. Biopolymer/Montmorillonite Nanocomposite: Preparation, Drug-Controlled Release Property and Cytotoxicity. Nanotechnology 2008, 19, 065707. 10.1088/0957-4484/19/6/065707. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yang L.; Zhang J.; Wang C.; Li Q. Preparation and Characterization of Chitosan-Poly(Vinyl Alcohol)/Bentonite Nanocomposites for Adsorption of Hg(II) Ions. Chem. Eng. J. 2014, 251, 404–412. 10.1016/j.cej.2014.04.089. [DOI] [Google Scholar]
- Liu Q.; Yang B.; Zhang L.; Huang R. Adsorptive Removal of Cr(VI) from Aqueous Solutions by Cross-Linked Chitosan/Bentonite Composite. Korean J. Chem. Eng. 2015, 32, 1314–1322. 10.1007/s11814-014-0339-1. [DOI] [Google Scholar]
- Wang K.; Ma H.; Pu S.; Yan C.; Wang M.; Yu J.; Wang X.; Chu W.; Zinchenko A. Hybrid Porous Magnetic Bentonite-Chitosan Beads for Selective Removal of Radioactive Cesium in Water. J. Hazard. Mater. 2019, 362, 160–169. 10.1016/j.jhazmat.2018.08.067. [DOI] [PubMed] [Google Scholar]
- Feng G.; Ma J.; Zhang X.; Zhang Q.; Xiao Y.; Ma Q.; Wang S. Magnetic Natural Composite Fe3O4-Chitosan@bentonite for Removal of Heavy Metals from Acid Mine Drainage. J. Colloid Interface Sci. 2019, 538, 132–141. 10.1016/j.jcis.2018.11.087. [DOI] [PubMed] [Google Scholar]
- Anjum A.; Seth C. K.; Datta M. Removal of As3+ Using Chitosan-Montmorillonite Composite: Sorptive Equilibrium and Kinetics. Adsorpt. Sci. Technol. 2013, 31, 303–323. 10.1260/0263-6174.31.4.303. [DOI] [Google Scholar]
- Bleiman N.; Mishael Y. G. Selenium Removal from Drinking Water by Adsorption to Chitosan-Clay Composites and Oxides: Batch and Columns Tests. J. Hazard. Mater. 2010, 183, 590–595. 10.1016/j.jhazmat.2010.07.065. [DOI] [PubMed] [Google Scholar]
- Azzam E. M. S.; Eshaq G.; Rabie A. M.; Bakr A. A.; Abd-Elaal A. A.; El Metwally A. E.; Tawfik S. M. Preparation and Characterization of Chitosan-Clay Nanocomposites for the Removal of Cu(II) from Aqueous Solution. Int. J. Biol. Macromol. 2016, 89, 507–517. 10.1016/j.ijbiomac.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Tirtom V. N.; Dinçer A.; Becerik S.; Aydemir T.; Çelik A. Removal of Lead (II) Ions from Aqueous Solution by Using Crosslinked Chitosan-Clay Beads. Desalin. Water Treat. 2012, 39, 76–82. 10.1080/19443994.2012.669161. [DOI] [Google Scholar]
- Ben Ali M.; Wang F.; Boukherroub R.; Lei W.; Xia M. Phytic Acid-Doped Polyaniline Nanofibers-Clay Mineral for Efficient Adsorption of Copper (II) Ions. J. Colloid Interface Sci. 2019, 553, 688–698. 10.1016/j.jcis.2019.06.065. [DOI] [PubMed] [Google Scholar]
- Dela Peña E. M. B.; Araño K.; Dela Cruz M. L.; Yro P. A.; Diaz L. J. L. The Design of a Bench-scale Adsorbent Column Based on Nanoclay-loaded Electrospun Fiber Membrane for the Removal of Arsenic in Wastewater. Water Environ. J. 2021, wej.12683. 10.1111/wej.12683. [DOI] [Google Scholar]
- Bansal P.; Purwar R. Polyacrylonitrile/Clay Nanofibrous Nanocomposites for Efficient Adsorption of Cr (VI) Ions. J. Polym. Res. 2021, 28, 7. 10.1007/s10965-020-02362-4. [DOI] [Google Scholar]
- Suresh Kumar P.; Korving L.; Keesman K. J.; van Loosdrecht M. C. M.; Witkamp G. J. Effect of Pore Size Distribution and Particle Size of Porous Metal Oxides on Phosphate Adsorption Capacity and Kinetics. Chem. Eng. J. 2019, 358, 160–169. 10.1016/j.cej.2018.09.202. [DOI] [Google Scholar]
- Prince J. A.; Singh G.; Rana D.; Matsuura T.; Anbharasi V.; Shanmugasundaram T. S. Preparation and Characterization of Highly Hydrophobic Poly(Vinylidene Fluoride) - Clay Nanocomposite Nanofiber Membranes (PVDF-Clay NNMs) for Desalination Using Direct Contact Membrane Distillation. J. Memb. Sci. 2012, 397-398, 80–86. 10.1016/j.memsci.2012.01.012. [DOI] [Google Scholar]
- Ramakrishna S.; Fujihara K.; Teo W.-E.; Lim T.-C.; Ma Z.. An Introduction to Electrospinning and Nanofibers; WORLD SCIENTIFIC: Singapore, 2005, 10.1142/5894. [DOI] [Google Scholar]
- Hosseini S. A.; Soltanieh M.; Mousavi S. M. Investigating Morphology and Performance of Cellulose Acetate Butyrate Electrospun Nanofiber Membranes for Tomato Industry Wastewater Treatment. Desalin. Water Treat. 2017, 64, 127–135. 10.5004/dwt.2017.20141. [DOI] [Google Scholar]
- Homaeigohar S. S.; Elbahri M. Novel Compaction Resistant and Ductile Nanocomposite Nanofibrous Microfiltration Membranes. J. Colloid Interface Sci. 2012, 372, 6–15. 10.1016/j.jcis.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Hosseini S. A.; Vossoughi M.; Mahmoodi N. M. Preparation of Electrospun Affinity Membrane and Cross Flow System for Dynamic Removal of Anionic Dye from Colored Wastewater. Fibers Polym. 2017, 18, 2387–2399. 10.1007/s12221-017-7530-z. [DOI] [Google Scholar]
- Nakamura K.; Suda T.; Matsumoto K. Characterization of Pore Size Distribution of Non-Woven Fibrous Filter by Inscribed Sphere within 3D Filter Model. Sep. Purif. Technol. 2018, 197, 289–294. 10.1016/j.seppur.2018.01.012. [DOI] [Google Scholar]
- Wang R.; Liu Y.; Li B.; Hsiao B. S.; Chu B. Electrospun Nanofibrous Membranes for High Flux Microfiltration. J. Memb. Sci. 2012, 392-393, 167–174. 10.1016/j.memsci.2011.12.019. [DOI] [Google Scholar]
- Eichhorn S. J.; Sampson W. W. Statistical Geometry of Pores and Statistics of Porous Nanofibrous Assemblies. J. R. Soc., Interface 2005, 2, 309–318. 10.1098/rsif.2005.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani S.; Mousavi S. M.; Shahtahmassebi N.; Saljoughi E. Hydrophilicity Improvement in Polyphenylsulfone Nanofibrous Filtration Membranes through Addition of Polyethylene Glycol. Appl. Surf. Sci. 2015, 359, 252–258. 10.1016/j.apsusc.2015.10.107. [DOI] [Google Scholar]
- Vanamudan A.; Pamidimukkala P. Chitosan, Nanoclay and Chitosan-Nanoclay Composite as Adsorbents for Rhodamine-6G and the Resulting Optical Properties. Int. J. Biol. Macromol. 2015, 74, 127–135. 10.1016/j.ijbiomac.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Oh J.-A.; Zhuge Y.; Araby S.; Wang R.; Yu H.; Fan W.; Liu M.; Lee S.-H.; Alam M. J.; Ma J. Cement Nanocomposites Containing Montmorillonite Nanosheets Modified with Surfactants of Various Chain Lengths. Cem. Concr. Compos. 2021, 116, 103894. 10.1016/j.cemconcomp.2020.103894. [DOI] [Google Scholar]
- Chuayjuljit S.; Thongraar R.; Saravari O. Preparation and Properties of PVC/EVA/ Organomodified Montmorillonite Nanocomposites. J. Reinf. Plast. Compos. 2008, 27, 431–442. 10.1177/0731684407084124. [DOI] [Google Scholar]
- Mohammadi S.; Mohammadi S.; Salimi A. A 3D Hydrogel Based on Chitosan and Carbon Dots for Sensitive Fluorescence Detection of MicroRNA-21 in Breast Cancer Cells. Talanta 2021, 224, 121895. 10.1016/j.talanta.2020.121895. [DOI] [PubMed] [Google Scholar]
- Hu C.; Deng Y.; Hu H.; Duan Y.; Zhai K. Adsorption and Intercalation of Low and Medium Molar Mass Chitosans on/in the Sodium Montmorillonite. Int. J. Biol. Macromol. 2016, 92, 1191–1196. 10.1016/j.ijbiomac.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Dudek G.; Turczyn R.; Konieczny K. Robust Poly(Vinyl Alcohol) Membranes Containing Chitosan/Chitosan Derivatives Microparticles for Pervaporative Dehydration of Ethanol. Sep. Purif. Technol. 2020, 234, 116094. 10.1016/j.seppur.2019.116094. [DOI] [Google Scholar]
- Hossain L.; Sarker S. K.; Khan M. S. Evaluation of Present and Future Wastewater Impacts of Textile Dyeing Industries in Bangladesh. Environ. Dev. 2018, 26, 23–33. 10.1016/j.envdev.2018.03.005. [DOI] [Google Scholar]
- Bikiaris D. Can Nanoparticles Really Enhance Thermal Stability of Polymers? Part II: An Overview on Thermal Decomposition of Polycondensation Polymers. Thermochim. Acta 2011, 523, 25–45. 10.1016/j.tca.2011.06.012. [DOI] [Google Scholar]
- Garnica-Palafox I. M.; Estrella-Monroy H. O.; Vázquez-Torres N. A.; Álvarez-Camacho M.; Castell-Rodríguez A. E.; Sánchez-Arévalo F. M. Influence of Multi-Walled Carbon Nanotubes on the Physico-Chemical and Biological Responses of Chitosan-Based Hybrid Hydrogels. Carbohydr. Polym. 2020, 236, 115971. 10.1016/j.carbpol.2020.115971. [DOI] [PubMed] [Google Scholar]
- Liu L.; Liang H.; Zhang J.; Zhang P.; Xu Q.; Lu Q.; Zhang C. Poly(Vinyl Alcohol)/Chitosan Composites: Physically Transient Materials for Sustainable and Transient Bioelectronics. J. Cleaner Prod. 2018, 195, 786–795. 10.1016/j.jclepro.2018.05.216. [DOI] [Google Scholar]
- Lewandowska K. Miscibility and Thermal Stability of Poly(Vinyl Alcohol)/Chitosan Mixtures. Thermochim. Acta 2009, 493, 42–48. 10.1016/j.tca.2009.04.003. [DOI] [Google Scholar]
- Han Y.; Chen S.; Yang M.; Zou H.; Zhang Y. Inorganic Matter Modified Water-Based Copolymer Prepared by Chitosan-Starch-CMC-Na-PVAL as an Environment-Friendly Coating Material. Carbohydr. Polym. 2020, 234, 115925. 10.1016/j.carbpol.2020.115925. [DOI] [PubMed] [Google Scholar]
- Khorshidi B.; Hosseini S. A.; Ma G.; McGregor M.; Sadrzadeh M. Novel Nanocomposite Polyethersulfone- Antimony Tin Oxide Membrane with Enhanced Thermal, Electrical and Antifouling Properties. Polymer 2019, 163, 48–56. 10.1016/j.polymer.2018.12.058. [DOI] [Google Scholar]
- Poonpipat Y.; Leelachai K.; Pearson R. A.; Dittanet P. Fracture Behavior of Silica Nanoparticles Reinforced Rubber/Epoxy Composite. J. Reinf. Plast. Compos. 2017, 1156. 10.1177/0731684417709952. [DOI] [Google Scholar]
- Lv Y.; Du Y.; Qiu W.-Z.; Xu Z.-K. Nanocomposite Membranes via the Codeposition of Polydopamine/Polyethylenimine with Silica Nanoparticles for Enhanced Mechanical Strength and High Water Permeability. ACS Appl. Mater. Interfaces 2017, 9, 2966–2972. 10.1021/acsami.6b13761. [DOI] [PubMed] [Google Scholar]
- Chen L.; Schadler L. S.; Ozisik R. An Experimental and Theoretical Investigation of the Compressive Properties of Multi-Walled Carbon Nanotube/Poly(Methyl Methacrylate) Nanocomposite Foams. Polymer 2011, 52, 2899–2909. 10.1016/j.polymer.2011.04.050. [DOI] [Google Scholar]
- Choong L. T. S.; Lin Y.-M.; Rutledge G. C. Separation of Oil-in-Water Emulsions Using Electrospun Fiber Membranes and Modeling of the Fouling Mechanism. J. Membr. Sci. 2015, 486, 229–238. 10.1016/j.memsci.2015.03.027. [DOI] [Google Scholar]
- Gutha Y.; Zhang Y.; Zhang W.; Jiao X. Magnetic-Epichlorohydrin Crosslinked Chitosan Schiff’s Base (m-ECCSB) as a Novel Adsorbent for the Removal of Cu(II) Ions from Aqueous Environment. Int. J. Biol. Macromol. 2017, 97, 85–98. 10.1016/j.ijbiomac.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Jiao X.; Gutha Y.; Zhang W. Application of Chitosan/Poly(Vinyl Alcohol)/CuO (CS/PVA/CuO) Beads as an Adsorbent Material for the Removal of Pb(II) from Aqueous Environment. Colloids Surf. B Biointerfaces 2017, 149, 184–195. 10.1016/j.colsurfb.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Wan Ngah W. S.; Teong L. C.; Hanafiah M. A. K. M. Adsorption of Dyes and Heavy Metal Ions by Chitosan Composites: A Review. Carbohydr. Polym. 2011, 83, 1446–1456. 10.1016/j.carbpol.2010.11.004. [DOI] [Google Scholar]
- Jin L.; Bai R. Mechanisms of Lead Adsorption on Chitosan/PVA Hydrogel Beads. Langmuir 2002, 18, 9765–9770. 10.1021/la025917l. [DOI] [Google Scholar]
- Annadurai G.; Ling L. Y.; Lee J.-F. Adsorption of Reactive Dye from an Aqueous Solution by Chitosan: Isotherm, Kinetic and Thermodynamic Analysis. J. Hazard. Mater. 2008, 152, 337–346. 10.1016/j.jhazmat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Wu H.-X.; Wang T.-J.; Chen L.; Jin Y.; Zhang Y.; Dou X.-M. The Roles of the Surface Charge and Hydroxyl Group on a Fe–Al–Ce Adsorbent in Fluoride Adsorption. Ind. Eng. Chem. Res. 2009, 48, 4530–4534. 10.1021/ie800890q. [DOI] [Google Scholar]
- Farrah H.; Pickering W. F. PH Effects in the Adsorption of Heavy Metal Ions by Clays. Chem. Geol. 1979, 25, 317–326. 10.1016/0009-2541(79)90063-9. [DOI] [Google Scholar]
- Farrah H.; Hatton D.; Pickering W. F. The Affinity of Metal Ions for Clay Surfaces. Chem. Geol. 1980, 28, 55–68. 10.1016/0009-2541(80)90035-2. [DOI] [Google Scholar]
- Chatterjee S.; Lee D. S.; Lee M. W.; Woo S. H. Congo Red Adsorption from Aqueous Solutions by Using Chitosan Hydrogel Beads Impregnated with Nonionic or Anionic Surfactant. Bioresour. Technol. 2009, 100, 3862–3868. 10.1016/j.biortech.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Kosmulski M. PH-Dependent Surface Charging and Points of Zero Charge III. Update. J. Colloid Interface Sci. 2006, 298, 730–741. 10.1016/j.jcis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Fan M.; Boonfueng T.; Xu Y.; Axe L.; Tyson T. A. Modeling Pb Sorption to Microporous Amorphous Oxides as Discrete Particles and Coatings. J. Colloid Interface Sci. 2005, 281, 39–48. 10.1016/j.jcis.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Almeida C. A. P.; Debacher N. A.; Downs A. J.; Cottet L.; Mello C. A. D. Removal of Methylene Blue from Colored Effluents by Adsorption on Montmorillonite Clay. J. Colloid Interface Sci. 2009, 332, 46–53. 10.1016/j.jcis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Salam M. A.; Kosa S. A.; Al-Beladi A. A. Application of Nanoclay for the Adsorptive Removal of Orange G Dye from Aqueous Solution. J. Mol. Liq. 2017, 241, 469–477. 10.1016/j.molliq.2017.06.055. [DOI] [Google Scholar]
- Wei C.; Xu Z.; Han F.; Xu W.; Gu J.; Ou M.; Xu X. Preparation and Characterization of Poly(Acrylic Acid-Co-Acrylamide)/Montmorillonite Composite and Its Application for Methylene Blue Adsorption. Colloid Polym. Sci. 2018, 296, 653–667. 10.1007/s00396-018-4277-z. [DOI] [Google Scholar]
- Wang L.; Zhang J.; Wang A. Removal of Methylene Blue from Aqueous Solution Using Chitosan-g-Poly(Acrylic Acid)/Montmorillonite Superadsorbent Nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 47–53. 10.1016/j.colsurfa.2008.02.019. [DOI] [Google Scholar]
- Wang L.; Zhang J.; Wang A. Fast Removal of Methylene Blue from Aqueous Solution by Adsorption onto Chitosan-g-Poly (Acrylic Acid)/Attapulgite Composite. Desalination 2011, 266, 33–39. 10.1016/j.desal.2010.07.065. [DOI] [Google Scholar]
- Pal S.; Ghorai S.; Das C.; Samrat S.; Ghosh A.; Panda A. B. Carboxymethyl Tamarind-g-Poly(Acrylamide)/Silica: A High Performance Hybrid Nanocomposite for Adsorption of Methylene Blue Dye. Ind. Eng. Chem. Res. 2012, 51, 15546–15556. 10.1021/ie301134a. [DOI] [Google Scholar]
- Sun D.; Zhang X.; Wu Y.; Liu X. Adsorption of Anionic Dyes from Aqueous Solution on Fly Ash. J. Hazard. Mater. 2010, 181, 335–342. 10.1016/j.jhazmat.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Chung W.-J.; Torrejos R. E. C.; Park M. J.; Vivas E. L.; Limjuco L. A.; Lawagon C. P.; Parohinog K. J.; Lee S.-P.; Shon H. K.; Kim H.; Nisola G. M. Continuous Lithium Mining from Aqueous Resources by an Adsorbent Filter with a 3D Polymeric Nanofiber Network Infused with Ion Sieves. Chem. Eng. J. 2017, 309, 49–62. 10.1016/j.cej.2016.09.133. [DOI] [Google Scholar]
- Mahmoodi N. M.; Mokhtari-Shourijeh Z.; Ghane-Karade A. Synthesis of the Modified Nanofiber as a Nanoadsorbent and Its Dye Removal Ability from Water: Isotherm, Kinetic and Thermodynamic. Water Sci. Technol. 2017, 75, 2475–2487. 10.2166/wst.2017.022. [DOI] [PubMed] [Google Scholar]
- Li L.; Li Y.; Yang C. Chemical Filtration of Cr (VI) with Electrospun Chitosan Nanofiber Membranes. Carbohydr. Polym. 2016, 140, 299–307. 10.1016/j.carbpol.2015.12.067. [DOI] [PubMed] [Google Scholar]
- Liu C.; Cheng L.; Zhao Y.; Zhu L. Interfacially Crosslinked Composite Porous Membranes for Ultrafast Removal of Anionic Dyes from Water through Permeating Adsorption. J. Hazard. Mater. 2017, 337, 217–225. 10.1016/j.jhazmat.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Li A.; Zhou Q.; Shuang C.; Zhou W.; Wang M. Effect of Pore Size Distribution on Tetracycline Adsorption Using Magnetic Hypercrosslinked Resins. Microporous Mesoporous Mater. 2014, 184, 105–111. 10.1016/j.micromeso.2013.10.010. [DOI] [Google Scholar]
- Mahmoodi N. M.; Hosseinabadi-Farahani Z.; Chamani H. Dye Adsorption from Single and Binary Systems Using NiO-MnO2 Nanocomposite and Artificial Neural Network Modeling. Environ. Prog. Sustainable Energy 2017, 36, 111–119. 10.1002/ep.12452. [DOI] [Google Scholar]
- Hasanzadeh M.; Simchi A.; Shahriyari Far H. Nanoporous Composites of Activated Carbon-Metal Organic Frameworks for Organic Dye Adsorption: Synthesis, Adsorption Mechanism and Kinetics Studies. J. Ind. Eng. Chem. 2020, 81, 405–414. 10.1016/j.jiec.2019.09.031. [DOI] [Google Scholar]
- Venkatesha T. G.; Viswanatha R.; Arthoba Nayaka Y.; Chethana B. K. Kinetics and Thermodynamics of Reactive and Vat Dyes Adsorption on MgO Nanoparticles. Chem. Eng. J. 2012, 198-199, 1–10. 10.1016/j.cej.2012.05.071. [DOI] [Google Scholar]
- Lima E. C.; Hosseini-Bandegharaei A.; Moreno-Piraján J. C.; Anastopoulos I. A Critical Review of the Estimation of the Thermodynamic Parameters on Adsorption Equilibria. Wrong Use of Equilibrium Constant in the Van’t Hoof Equation for Calculation of Thermodynamic Parameters of Adsorption. J. Mol. Liq. 2019, 273, 425–434. 10.1016/j.molliq.2018.10.048. [DOI] [Google Scholar]
- Liu Y.; Liu Y. J. Biosorption Isotherms, Kinetics and Thermodynamics. Sep. Purif. Technol. 2008, 229–242. 10.1016/j.seppur.2007.10.002. [DOI] [Google Scholar]
- Özcan A. S.; Özcan A. Adsorption of Acid Dyes from Aqueous Solutions onto Acid-Activated Bentonite. J. Colloid Interface Sci. 2004, 276, 39–46. 10.1016/j.jcis.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Kooli F.; Liu Y.; Al-Faze R.; Al Suhaimi A. Effect of Acid Activation of Saudi Local Clay Mineral on Removal Properties of Basic Blue 41 from an Aqueous Solution. Appl. Clay Sci. 2015, 116-117, 23–30. 10.1016/j.clay.2015.07.044. [DOI] [Google Scholar]
- Jain C.; Singhal D.; Sharma M. Adsorption of Zinc on Bed Sediment of River Hindon: Adsorption Models and Kinetics. J. Hazard. Mater. 2004, 114, 231–239. 10.1016/j.jhazmat.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bulut Y.; Aydin H. A Kinetics and Thermodynamics Study of Methylene Blue Adsorption on Wheat Shells. Desalination 2006, 194, 259–267. 10.1016/j.desal.2005.10.032. [DOI] [Google Scholar]
- Iqbal M. J.; Ashiq M. N. Adsorption of Dyes from Aqueous Solutions on Activated Charcoal. J. Hazard. Mater. 2007, 139, 57–66. 10.1016/j.jhazmat.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Han B.; Ji G.; Wu S.; Shen J. Preparation and Characterization of Nylon 66/Montmorillonite Nanocomposites with Co-Treated Montmorillonites. Eur. Polym. J. 2003, 39, 1641–1646. 10.1016/S0014-3057(03)00075-2. [DOI] [Google Scholar]
- Sharma S. K.; Nayak S. K. Surface Modified Clay/Polypropylene (PP) Nanocomposites: Effect on Physico-Mechanical, Thermal and Morphological Properties. Polym. Degrad. Stab. 2009, 94, 132–138. 10.1016/j.polymdegradstab.2008.09.004. [DOI] [Google Scholar]
- Pérez-Santano A.; Trujillano R.; Belver C.; Gil A.; Vicente M. A. Effect of the Intercalation Conditions of a Montmorillonite with Octadecylamine. J. Colloid Interface Sci. 2005, 284, 239–244. 10.1016/j.jcis.2004.09.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





