Abstract
Adoptive cell therapies are a group of cancer immunotherapies that involve the infusion of engineered immune cells targeting specific tumor antigens, with chimeric antigen receptor (CAR) T cells at the vanguard of this revolution in cancer therapy. Several CAR T-cell products have been approved for the treatment of leukemia and lymphoma and many more are currently undergoing evaluation in clinical trials for the treatment of other liquid and solid malignancies. Despite their remarkable effectiveness, as with other immunotherapies, CAR T cells are frequently associated with systemic and neurologic toxicity. There has been a major effort by many institutions to develop specific protocols to guide the management of treatment-associated toxicities (eg, cytokine release syndrome [CRS]). However, neurotoxic effects of CAR T-cell therapies are more difficult to evaluate and treat, not easily lending themselves to an algorithmic approach to diagnosis and management. Given the steadily expanding use of CAR T-cell therapies for various malignancies, it is of critical importance for neuro-oncologists to be familiar with the clinical presentation and management principles of CAR T-cell-associated neurotoxicity. Here, we present key principles for the evaluation and management of patients affected by CAR T-cell-associated neurotoxicity based on the most recent evidence.
Keywords: CAR T-cell-related encephalopathy (CRES), CAR T-cell neurotoxicity, CAR T-cell neurotoxicity EEG findings, immune effector cell-associated neurotoxicity syndrome (ICANS)
Clinical Indications of CAR T-cell Therapy and Pathophysiology of Their Adverse Events
The mainstay of adoptive cell therapy relies on the infusion of chimeric antigen receptor (CAR) T cells targeting specific tumor antigens.1 Three CAR T-cell products have recently been approved by the FDA with indications including acute lymphoblastic leukemia, large B-cell lymphoma, and mantle cell lymphoma (Table 1). Two additional products have reported survival benefit in large clinical trials and are awaiting FDA approval, one for the treatment of large B-cell lymphoma and the other one for the novel indication of multiple myeloma.
Table 1.
Clinical Indications of CAR T-Cell Therapy
| CAR T-cell therapy product | Indications | Lymphodepleting chemotherapy | Incidence of CRS | Incidence of neurotoxicity | Key trials |
|---|---|---|---|---|---|
| Axicabtagene ciloleucel | Large B-cell lymphoma (including refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma) | Fludarabine + Cyclophosphamide | 93%2 | 64%2 | Neelapu et al., NEJM 20172 |
| Brexucabtagene ciloleucel | Mantle cell lymphoma | Fludarabine + Cyclophosphamide | 91%3 | 63%3 | Wang et al., NEJM 20203 |
| Tisagenlecleucel | Large B-cell lymphoma, acute lymphoblastic leukemia | Fludarabine + Cyclophosphamide (alternatively, Bendamustine + Cyclophosphamide) | 77-584,5 | 40-214,5 | Maude et al., NEJM 20184; Schuster et al., NEJM 20195 |
| Idecabtagene vicleleucel | Multiple myeloma (pending FDA approval) | Fludarabine + Cyclophosphamide | 76%6 | 42%6 | Raje et al., NEJM 20196 |
| Lisocabtagene maraleucel | Large B-cell lymphoma (pending FDA approval) | Fludarabine + Cyclophosphamide | 42%7 | 30%7 | Abramson et al., Lancet 20207 |
Patients treated with CAR T-cell therapies first undergo T-cell collection via apheresis, followed by T-cell engineering and expansion. When the CAR T cells are ready to be infused, patients undergo a conditioning chemotherapy regimen, in most cases involving a combination of fludarabine and cyclophosphamide, starting approximately 5 days before the infusion. CARs are genetically engineered hybrid molecules that can replace major functions of the T-cell receptor, including antigen recognition, T-cell activation, and co-stimulation via intracellular domains (eg, CD28, 4-1BB).8 Prior to the infusion, baseline chemistries, blood cell counts, and inflammatory markers (C-reactive protein [CRP], ferritin, and lactate dehydrogenase [LDH]) are drawn. A bedside physical examination is also performed. The proposed guidelines for the management of immune effector cell-related adverse events from the Society of Immunotherapy in Cancer recommend that all patients undergo a standardized neurological evaluation, and also recommend consultation with a neurologist prior to CAR T-cell infusion in patients with neoplastic CNS disease involvement or a history of inflammatory neurologic conditions.9
After their infusion, CAR T cells rapidly expand, thereby producing an inflammatory state with associated macrophage activation and subsequent elevated levels of multiple cytokines—IL-1, IL-2, IL-6, IL-8, IL-10, IL-15, IFN-γ—which in concert can cause endothelial activation, intravascular coagulation, increased blood-brain barrier permeability, and meningeal inflammation,10–13 findings that have been established in animal models and corroborated on autopsy samples of patients affected by neurotoxicity.11–14 The resulting alterations in cerebral blood flow and breakdown of the blood-brain barrier provide mechanisms for explaining the frequently observed encephalopathy, seizures, focal ischemia or hemorrhage, as well as cerebral edema.12–15
Clinical Presentation
Within minutes to hours after the infusion of CAR T cells, approximately 42%-93% of patients experience the onset of fever, myalgias, arthralgias, and in some cases, hypoxia and hypotension, accompanied by a rise in inflammatory markers (eg, CRP and ferritin). The above constellation of symptoms and laboratory findings defines the cytokine release syndrome (CRS).
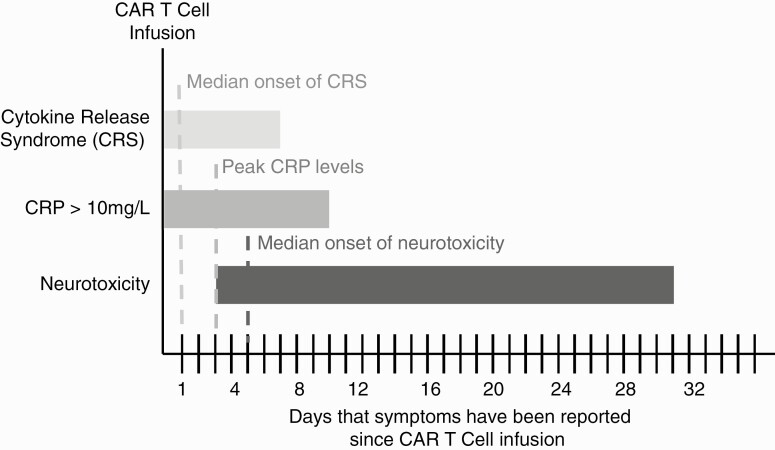
Treatment of CRS most commonly involves supportive therapy with antipyretics (usually acetaminophen) and, when warranted, supplemental oxygen and intravenous (IV) fluids. For severe CRS (characterized by fever, hypoxia, and hypotension-necessitating vasopressors), treatment with tocilizumab (an anti-IL-6R antibody) and high-dose corticosteroids (dexamethasone, methylprednisolone) is routinely instituted.10 Inflammatory markers usually peak by post-infusion day 3 (PID-3) and the symptoms of CRS begin to resolve (Figure 1). It is around this time that many patients begin to exhibit early signs of neurologic toxicity (Figure 1). The initial clinical presentation varies, but it is often characterized by inattention and language deficits,14–18 frequently prompting a consultation with neurology or neuro-oncology to assist in patient evaluation and management.
Figure 1.
Temporal course of cytokine release syndrome, serum C-reactive protein (CRP) elevation, and neurotoxicity after CAR T-cell infusion based on previously published series.14,15 Dashed lines represent the median onset of CRS, peak CRP levels, and median onset of neurotoxicity (ICANS). Note that the horizontal bars depict post-infusion days in which symptoms have been reported and do not represent the typical course of a given patient.
Although different names and acronyms have been used to describe the neurotoxic effects of CAR T-cell therapy (eg, CAR T-cell-related encephalopathy syndrome [CRES]16), the American Society for Transplantation and Cellular Therapy (ASTCT) has proposed the term “immune effector cell-associated neurotoxicity syndrome (ICANS)” as a consensus term.19 In their guideline for grading CRS and ICANS, the ASTCT writes that ICANS “may manifest as delirium, encephalopathy, aphasia, lethargy, difficulty concentrating, agitation, tremor, seizures, and, rarely, cerebral edema.” 19 Given the diverse clinical presentation CAR T-cell neurotoxicity, multiple clinical scales have been proposed to grade severity of neurotoxicity. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), currently on version 5.0, has an extensive list of neurological symptoms with severity scales, but these are mainly of relevance for adverse event reporting during clinical trials.20 The CARTOX grading instrument proposed by a group of investigators at MD Anderson Cancer Center relies on a neurological assessment scale—CARTOX10 (In this scale one point is assigned for each of ten following correctly performed (normal cognitive function is defined by an overall score of 10): orientation to year, month, city, hospital, and head of state (total of 5 points); naming three objects (maximum of 3 points); write a standard sentence, (1 point); count backwards from 100 in tens (1 point).)—as well as on the identification of evidence of raised intracranial pressure (ICP), seizures, or motor weakness.16
In their consensus guideline, the ASTCT proposes a new screening instrument for encephalopathy, the Immune Effector Cell-Associated Encephalopathy (ICE) scale. This 10-point scale is a modified CARTOX-10 (see footnote) scale that reassigns one point for orientation and reassigns it to the ability to follow commands (Table 2). The score from the ICE scale is one of the components of an ASTCT consensus grading scale for ICANS, which also incorporates other neurologic symptoms and signs (Table 3). The proposed guidelines for the management of immune effector cell-related adverse events from the Society of Immunotherapy in Cancer recommend that all patients undergo an evaluation with the ICE instrument prior to CAR T-cell infusion, and the use of ICE to monitor the neurologic status of patients treated with axicabtagene ciloleucel at least daily during the first week after infusion.9
Table 2.
Immune Effector Cell-Associated Encephalopathy (ICE)19
| Orientation | Orientation to year, month, city, hospital (4 points) |
|---|---|
| Naming | Ability to name 3 objects (eg, watch, pen, button; 3 points) |
| Following commands | Ability to follow simple commands (eg, “Show 2 fingers”; 1 point) |
| Writing | Ability to write a standard sentence (eg, “Today is a sunny day”; 1 point) |
| Attention | Ability to count backwards from 100 by 10 s (1 point) |
Table 3.
ASTCT ICANS Consensus Grading for Adults19
| Neurotoxicity domain | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| ICE score | 7-9 | 3-6 | 0-2 | 0 (patient is unarousable or unable to perform ICE scale) |
| Level of consciousness | Awakens spontaneously | Awakens to voice | Awakens only to tactile stimulus | Patient is unarousable or requires vigorous or repetitive tactile stimuli to arouse. Stupor or coma |
| Seizure | N/A | N/A | Any clinical seizure focal or generalized that resolves rapidly or nonconvulsive seizures on EEG that resolve with intervention | Life-threatening prolonged seizure (>5 min); repetitive clinical or electrical seizures without return to baseline in between |
| Motor findings | N/A | N/A | N/A | Deep focal motor weakness such as hemiparesis or paraparesis |
| Elevated ICP/cerebral edema | N/A | N/A | Focal/local edema on neuroimaging | Diffuse cerebral edema on neuroimaging; decerebrate or decorticate posturing; cranial nerve VI palsy; papilledema; or Cushing’s triad |
Although conceived to identify and grade CAR T-cell neurotoxicity, the scales above can be insensitive in the early phase of neurotoxicity, particularly in patients with subtle signs or symptoms.17 The expert eye and careful examination by a neurologist reigns supreme over these instruments, and we strongly recommend that all patients experiencing neurological symptoms after receiving CAR T-cell therapies are evaluated by a neurologist as symptoms listed in Table 2 such as “deep focal motor weakness such as hemiparesis” (central weakness for a neurologist) are more likely to be caused by a stroke than by CAR T-cell neurotoxicity.
A full neurologic examination is paramount. In the mental status exam, particular attention should be devoted to subtle deficits in attention and language. It is important to discuss findings with family at bedside or with the patient’s nurse to establish if there has been a deviation from the patient’s baseline. Cranial nerve exam should include fundoscopy to assess for blurring of optic disk margins as a sign of increased ICP due to increased cerebrospinal fluid (CSF) pressure or cerebral edema. On the general examination, tachycardia, tachypnea, and fever can sometimes be present in the setting of CRS, but as noted previously, the onset of CRS and neurotoxicity is generally not concurrent (most CRS cases are present by PID-1, while most neurotoxicity cases are noted on or after PID-514,15). While the presence of CRS may pose a higher risk for a patient to develop neurotoxicity, changes in vital signs and fever should prompt evaluation for other causes of systemic illness that can cause encephalopathy, such as infection.
It is important to note that the clinical patterns and severity of neurotoxicity vary according to the anti-CD19 CAR T-cell product used, with increased frequency and severity of neurologic symptoms reported for axicabtagene ciloleucel (Yescarta™) compared to tisagenlecleucel (Kymriah™).19 This difference has at least in part be attributed to different co-stimulatory domains—CD28 for axicabtagene ciloleucel and 4-1BB (CD137) for tisagenlecleucel (CD28 is thought to be a more potent albeit shorter-acting costimulatory molecule).8 Another contributor to the difference in reported neurologic adverse events might be the different patient populations treated with these 2 therapies, as tisagenlecleucel is approved for B-cell acute lymphocytic leukemia (B-ALL) in patients of age 25 or younger as well as for large B-cell lymphoma, while axicabtagene ciloleucel is approved only for large B-cell lymphoma, a condition that is more likely to affect older patients with a median age of 65. Comorbidities (eg, mild cognitive impairment) are also more likely to be present in the elderly population, increasing their risk to develop encephalopathy or other neurologic toxicities of the treatment.8 High LDH levels, as a marker of disease burden, and thrombocytopenia, as a marker for heavily pre-treated patients, prior to CAR T-cell infusion have been associated with higher incidence and severity of neurotoxicity.13,15 A recently proposed predictive model of CAR T-cell neurotoxicity identified age, histological subtype, maximum temperature, maximum CRP, maximum ferritin, minimum WBC, CRS severity, CRS onset day, and a number of doses of tocilizumab as predictive factors.21 In this model, a score of 6 or higher (out of a total of 14) is predicted the occurrence of neurotoxicity with 77% accuracy.21
Diagnostic Evaluation
As outlined in the previous section, the cornerstone in the evaluation of neurologic symptoms in patients receiving CAR T-cell therapies is the neurologic exam, which should be repeated at least daily but often more frequently in the setting of high-grade neurotoxicity. Diagnostic studies, although routinely obtained, often do not explain the clinical picture and are of limited utility in guiding management. Diagnostic studies are, however, of critical importance in the evaluation of conditions that must be considered in the differential diagnosis of CAR T-cell neurotoxicity, which is vast and varies with the clinical presentation of neurotoxicity. The differential diagnosis of encephalopathy should include infection, metabolic derailments, medication side effects (eg, opioids or benzodiazepines), seizures, and prolonged hospitalization. Seizures in cancer patients can result from multiple etiologies,22 but it is particularly important to determine if there is neoplastic CNS involvement by neuroimaging and/or CSF cytology studies. Stroke, particularly in the presence of cardiovascular risk factors, should prompt evaluation for common causes (arrhythmia, large vessel atherosclerotic disease, small vessel disease, etc.) before settling on CAR T-cell-related vasculopathy as a mechanism. It is also important to consider neurotoxicity from conventional chemotherapy to which the patient might have been exposed to, with fludarabine, causing acute and delayed leukoencephalopathy,15,23–25 and cyclophosphamide, causing posterior reversible encephalopathy syndrome (PRES),26 as common culprits as components of conditioning regimens. Of note, fludarabine encephalopathy is of slower onset than CAR T-cell neurotoxicity and occurs within weeks and months and does not typically respond to corticosteroid therapy. Cyclophosphamide-associated PRES tends to occur within 24 hours of the infusion, which helps differentiate from CAR T-cell neurotoxicity as the last cyclophosphamide infusion is given at least 2 days prior to CAR T-cell infusion. However, other drugs that the patient may have received also need to be considered, such as rituximab, which has been linked to progressive multifocal leukoencephalopathy.27
Neuroimaging
Computed tomography (CT) without contrast does not usually reveal acute findings in patients with CAR T-cell neurotoxicity,14,15 but should be the first study to obtain in patients with rapidly decreasing level of arousal in order to assess for cerebral edema. There is not a particular constellation of CT findings associated with neurotoxicity. In rare instances (<20%), CT without contrast has revealed infarcts as well as subarachnoid or subdural hemorrhage, and CT angiography has revealed focal intracranial artery narrowing.14,15 Magnetic resonance imaging (MRI) is equally nonspecific compared to CT but has higher sensitivity for small strokes and can at times help reveal other pathologic processes like fludarabine-induced leukoencephalopathy in this patient population.14,15.
Electroencephalography (EEG)
EEG is routinely obtained in CAR T-cell patients after a clinical seizure or in the setting of persistent encephalopathy to evaluate for and rule out nonconvulsive status epilepticus. EEG studies obtained in the setting of acute neurotoxicity have revealed most commonly frontal or diffuse theta-delta slowing (consistent with encephalopathy), but also generalized periodic discharges (GPDs), generalized rhythmic delta activity (GRDA), bilateral independent periodic discharges (BiPEDs), electrographic seizures, and status epilepticus.14,15,18,28 Long-term EEG monitoring should be considered in patients with profound encephalopathy or fluctuating neurologic exam findings.
Serum and CSF Studies
Inflammatory markers—CRP and ferritin—correlate well with the clinical course of CRS but not so with the course of neurotoxicity (Figure 1). However, rising levels of ferritin and CRP may put a patient at higher risk to develop neurotoxicity.14,15,21 CSF in patients with acute neurotoxicity shows mild pleocytosis (median of 3 nucleated cells in a recent series14) as well as median protein in the normal range.14 Animal studies have shown elevated levels of inflammatory cytokines—IL-2, IL-6—but these are not routinely tested. As discussed above, despite not having a defined role in the diagnosis of CAR T-cell neurotoxicity, CSF studies are of major importance in the evaluation of relevant conditions in the differential diagnosis, such as bacterial meningitis (Gram stain and cultures) or neoplastic CNS involvement (cytology and flow cytometry).
Management
There are currently no consensus guidelines for the management of CAR T-cell neurotoxicity. Once a patient develops signs or symptoms of CAR T-cell neurotoxicity, the most critical management decision is to determine the most appropriate care setting for the patient. In patients with high-grade—grades 3 or 4 (see Table 2)—neurotoxicity, prompt arrangements should be made for the patient to be transferred to an intensive care unit (ICU), ideally, a neurologic ICU, so that both his cardiorespiratory and neurologic status could be monitored and supported appropriately. Patients with grade 2 neurotoxicity would also benefit from receiving care in a neurology or neuro-oncology inpatient unit, so that their neurologic exam can be followed closely, and initial measures can be quickly implemented (escalation of antiepileptic therapy for status epilepticus, initiation of hyperosmolar therapy for diffuse cerebral edema) before ICU transfer is initiated in the setting of rapid deterioration. Patients with grade 1 neurotoxicity can usually remain in an oncology unit with close monitoring by a neurology or neuro-oncology consultation service.
Patients with grades 2, 3, or 4 neurotoxicity are treated with corticosteroids to decrease systemic and CNS inflammation. Initial doses of dexamethasone range between 10 and 60 mg, followed by 4-20 mg every 6-12 hours.10 However, there are presently no consensus guidelines on the exact dose, timing, and duration of steroids. Neurologic symptoms begin to improve within hours to days of corticosteroid administration, and there is no established role for continuation of treatment or a prolonged taper once patients have returned to baseline. In a recent series, corticosteroid use for greater than 10 days was associated with worse overall survival, but survival or disease response did not seem to be affected by shorter courses of treatment <10 days.15 If there are clinical grounds to treat patients with high-dose corticosteroids (greater than 4 mg of dexamethasone daily) for 4 weeks or more, it is recommended to initiate pneumocystis prophylaxis.29
Antiepileptic therapy, usually with levetiracetam, is frequently initiated on the day of CAR T-cell infusion as a prophylactic measure against neurotoxicity, although there are no definitive data to support their use in patients without seizures. In the presence of seizures, the dosing should be escalated and in patients with status epilepticus benzodiazepines should be used acutely while additional agents are added.22 EEG should be obtained after a seizure in patients that do not return to their pre-seizure baseline to assess for nonconvulsive status epilepticus. EEG along with clinical exam can help guide the titration of antiepileptic medications.
In patients with neurotoxicity and clinical or laboratory evidence of CRS, the anti-IL-6R monoclonal antibody tocilizumab can be used at a dose of 8 mg/kg IV every 8-12 hours can be given for up to 4 doses.16 Note that there is no role for tocilizumab in patients with neurotoxicity that do not have evidence of concurrent CRS. Blockade of other cytokines—IL-1, GM-CSF, IFN-γ—is currently being investigated in clinical trials for the treatment of CAR T-cell neurotoxicity.
Prognosis and Long-Term Follow-Up
When identified and treated promptly, the short-term prognosis of CAR T-cell neurotoxicity is favorable, with most patients experiencing improvement of symptoms shortly after initiation of corticosteroid therapy.15 A subset of patients, however, report ongoing symptoms affecting cognition and behavior that have not been well characterized. It is unclear how this so-called “delayed” neurotoxicity, relates to the initial neurotoxicity. It is important for patients with neurotoxicity to continue to follow up with a neurologist or neuro-oncologist, at least initially or longitudinally if they have ongoing or progressive cognitive symptoms.
Summary and Key Points
As with other advances in cancer immunotherapy, adoptive cell therapies have proven very effective for the management of different malignancies but have also been associated with a new spectrum of serious toxicities. The mechanisms and clinical patterns of neurotoxicity associated with CAR T-cell therapies are not well understood and subject of ongoing investigations. However, some important general principles are beginning to emerge. Recent large studies stress the importance of closely monitoring CAR T-cell patients for neurologic toxicity as this has been documented in 21%-65% of patients undergoing anti-CD19 CAR T-cell therapies10 (a recent study investigating anti-B cell maturation antigen CAR T cells in multiple myeloma reports neurotoxicity in 42% of patients6). Despite the existence of multiple screening and grading scales for neurotoxicity, the neurologic exam and the experienced eyes of a neurologist remain the most useful diagnostic tools of this condition. Most patients respond readily to treatment with high-dose corticosteroids. This treatment should be instituted early and initial concerns regarding the limited effectiveness of CAR T-cell therapies in the presence of steroids have not been substantiated as long as treatment is not continued beyond the resolution of neurologic symptoms.
CAR T-cell therapy-associated toxicities are a rapidly emerging field of investigation with various new products being developed and likely to be introduced to the market. As a result, a steadily increased use of these therapies is expected in hematologic malignancies as well as in some solid tumors. Neurologists and neuro-oncologists will continue to be called to manage the neurotoxicity of adoptive cell therapies, playing a significant role in the success of these novel therapies.
Funding
No funding sources to report.
Conflict of interest statement: No conflict of interest to report.
References
- 1. June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. [DOI] [PubMed] [Google Scholar]
- 2. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 6. Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. [DOI] [PubMed] [Google Scholar]
- 8. Boyiadzis MM, Dhodapkar MV, Brentjens RJ, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer. 2018;6(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grupp S, Bishop MR, Brudno JN, et al. The society for immunotherapy of cancer consensus statement on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019;37(Suppl 1):48–52. [DOI] [PubMed] [Google Scholar]
- 11. Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8(6):750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. [DOI] [PubMed] [Google Scholar]
- 14. Rubin DB, Danish HH, Ali AB, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. 2019;142(5):1334–1348. [DOI] [PubMed] [Google Scholar]
- 15. Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T-cells. Blood. 2019;133(20):2212–2221. [DOI] [PubMed] [Google Scholar]
- 16. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzalez Castro LN, Dietrich J, Forst DA. Stuttering as the first sign of CAR-T-cell-related encephalopathy syndrome (CRES). J Cancer Res Clin Oncol. 2018;145(7):1917–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sokolov E, Karschnia P, Benjamin R, et al. Language dysfunction-associated EEG findings in patients with CAR-T related neurotoxicity. BMJ Neurol Open. 2020;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4): 625– 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) common terminology criteria for adverse events (CTCAE) v5.0. 2017. https://www.meddra.org/.
- 21. Rubin DB, Jarrah A Al, Li K, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77(12):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez Castro LN, Milligan TA. Seizures in patients with cancer. Cancer. 2020;126(7):1379–1389. [DOI] [PubMed] [Google Scholar]
- 23. Lowe KL, Mackall CL, Norry E, et al. Fludarabine and neurotoxicity in engineered T-cell therapy. Gene Ther. 2018;25(3):176–191. [DOI] [PubMed] [Google Scholar]
- 24. Beitinjaneh A, McKinney AM, Cao Q, et al. Toxic leukoencephalopathy following fludarabine-associated hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(3):300–308. [DOI] [PubMed] [Google Scholar]
- 25. Annaloro C, Costa A, Fracchiolla NS, et al. Severe fludarabine neurotoxicity after reduced intensity conditioning regimen to allogeneic hematopoietic stem cell transplantation: a case report. Clin Case Reports. 2015;3(7)::650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone JB, Deangelis LM. Cancer treatment-induced neurotoxicity: a focus on newer treatments. Nat Rev Clin Oncol. 2016;13(2):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maffini E, Festuccia M, Brunello L, et al. Neurologic complications after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(3):388–397. [DOI] [PubMed] [Google Scholar]
- 28. Herlopian A, Dietrich J, Abramson JS, et al. EEG findings in CAR T-cell therapy-related encephalopathy. Neurology. 2018;91(5):227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44(12b):1350–1363. [DOI] [PubMed] [Google Scholar]