Abstract
Background
Adjuvant stereotactic radiosurgery (SRS) improves the local control of resected brain metastases (BrM). However, the dependency of long-term outcomes on SRS timing relative to surgery remains unclear.
Methods
Retrospective analysis of patients treated with metastasectomy-plus-adjuvant SRS at Memorial Sloan Kettering Cancer Center (MSK) between 2013 and 2016 was conducted. Kaplan-Meier methodology was used to describe overall survival (OS) and cumulative incidence rates were estimated by type of recurrence, accounting for death as a competing event. Recursive partitioning analysis (RPA) and competing risks regression modeling assessed prognostic variables and associated events of interest.
Results
Two hundred and eighty-two patients with BrM had a median OS of 1.5 years (95% CI: 1.2-2.1) from adjuvant SRS with median follow-up of 49.8 months for survivors. Local surgical recurrence, other simultaneously SRS-irradiated site recurrence, and distant central nervous system (CNS) progression rates were 14.3% (95% CI: 10.1-18.5), 4.9% (95% CI: 2.3-7.5), and 47.5% (95% CI: 41.4-53.6) at 5 years, respectively. Median time-to-adjuvant SRS (TT-SRS) was 34 days (IQR: 27-39). TT-SRS was significantly associated with surgical site recurrence rate (P = 0.0008). SRS delivered within 1 month resulted in surgical site recurrence rate of 6.1% (95% CI: 1.3-10.9) at 1-year, compared to 9.2% (95% CI: 4.9-13.6) if delivered between 1 and 2 months, or 27.3% (95% CI: 0.0-55.5) if delivered >2 months after surgery. OS was significantly lower for patients with TT-SRS >~2 months. Postoperative length of stay, discharge to a rehabilitation facility, urgent care visits, and/or disease recurrence between surgery and adjuvant SRS associated with increased TT-SRS.
Conclusions
Adjuvant SRS provides durable local control. However, delays in initiation of postoperative SRS can decrease its efficacy.
Keywords: adjuvant therapy, brain metastasis, postoperative, radiation, recurrence
Brain metastases (BrM) are common central nervous system (CNS) tumors in adults.1 The current management paradigm for single, large, dominant, and/or symptomatic BrM involves surgical metastasectomy followed by postoperative radiation.2 Due to residual microscopic tumor, even in the common case of gross total resection, surgery provides a local control rate of approximately 50% at 1 year.2,3 The addition of adjuvant postoperative radiation has repeatedly demonstrated significant improvement in local control rates compared to surgery alone.3–5 Postoperative adjuvant stereotactic radiosurgery (SRS) is increasingly utilized instead of whole-brain radiation therapy (WBRT) due to fewer cognitive side effects, which manifest with WBRT as early as 3 months following irradiation, and better quality of life.6,7
Unlike WBRT, which is a dose-standardized treatment, SRS is patient-specific and requires simulation of the patient in an SRS-style immobilization system, and generation of a patient-specific radiation plan. Delays can occur throughout this process and thus the time from surgery to SRS treatment (time-to-adjuvant SRS [TT-SRS]) can vary greatly between patients. Furthermore, rehabilitation needs and institutional variation due to differences in equipment and physics planning methodologies can also contribute to differences in TT-SRS on the order of weeks. While it is posited that shorter time intervals from surgery to adjuvant SRS will benefit local control, the effects of TT-SRS on outcomes remain unclear, with only small case series examining this issue.8,9 Furthermore, the initiation of radiation is often limited by removal of staples which can deter scar healing if left in place during treatment. In this large retrospective, single-institution study, we assessed the relationship of adjuvant SRS timing on the local control rate for surgically resected BrM and any simultaneously irradiated, un-resected BrM, in order to make recommendations on the optimal timing of adjuvant SRS. We further analyzed the effect of TT-SRS and other contributing factors on radiation necrosis (RN), wound complications, and leptomeningeal dissemination.
Methods
Study Cohort
This retrospective study of patients treated at Memorial Sloan Kettering Cancer Center (MSK) between 2013 and 2016 was approved by the MSK Institutional Review Board. Patients diagnosed with BrM and treated with surgical resection followed by postoperative SRS within 90 days were included (n = 282). Patients were excluded if they had been treated with brain radiation prior to resection (n = 68) or had alternative forms of postoperative radiation such as WBRT or fractionated radiation with >10 fractions (n = 91) as their next adjuvant treatment. Patients with all histologic subtypes were included.
Radiosurgical Techniques
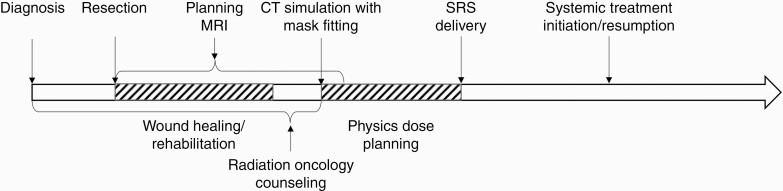
Figure 1 demonstrates the timeline of events from BrM diagnosis through adjuvant radiation. The patients were immobilized for simulation and radiation either in an invasive frame (single-fraction cases only) or in the cranial Freedom SystemTM (CDR Systems, Alberta, Canada) utilizing a custom head mold and an open face mask. A simulation computed tomography (CT) scan reconstructed at 1 mm slices was acquired, typically with administration of iodine-based intravenous contrast. An MRI with 1 mm slice thickness performed within 1 week prior to the simulation was fused to the CT acquired at simulation and was used for contouring guidance of both the postoperative cavity as well as any intact BrM. If the patient had undergone prior SRS, the previously treated BrM were contoured as objects of avoidance. iPlan (RT Image, Brainlab, Munich, Germany) or MIM (MIM Software Inc., Cleveland, OH) was used to fuse contrast-enhanced spoiled gradient recalled acquisition (1 mm) and T1-weighted (3 mm) magnetic resonance (MR) images to the CT and also for auto-segmentation of normal structures. Postoperative cavities were defined based on the enhancing changes of the MRI and/or CT and a 2-3 mm expansion margin was produced to define the planning target volume (PTV). If the cavity was superficial, the cavity was typically defined to the border of the craniotomy site. Radiation treatment plans were generated in iPlan (RT Dose, Brainlab, Munchen, Germany) for the patients immobilized in the invasive frame and receiving a single-fraction treatment. Treatment plans for patients in the Freedom System treated with 1-5 fractions were planned using Eclipse (Varian Medical Systems, Palo Alto, CA). Smaller lesions treated with a single fraction had a 125%-140% hotspot and a steep dose gradient outside the PTV. The larger lesions were treated with hypo-fractionated volumetric modulated arc therapy (VMAT) or intensity-modulated radiation therapy (IMRT) with a hotspot in the range 110%-140% depending on the size of the PTV. All patients were treated on a linear accelerator (LINAC) with 6 MV photons and a multileaf collimator (MLC) with 2.5 mm or 5 mm leaves. The invasive frame patients were positioned for treatment with a stereotactic technique and the frameless patients were positioned using image guidance with cone beam CT (CBCT) and an optical surface monitoring system for motion monitoring.
Figure 1.
Components of a brain metastasis treatment episode from surgery to resumption of systemic therapy.
Study Endpoints
Retrospective chart review was conducted to identify demographics including age at surgery for BrM, Karnofsky Performance Status score (KPS), location of the operative BrM, maximal diameter of the operative BrM, CNS presenting symptoms, postoperative length-of-stay (LOS), new postoperative deficits, disposition to home vs inpatient rehabilitation facility, urgent care/emergency room visits between surgery and SRS, number of BrM undergoing simultaneous postoperative SRS, stable vs progressive systemic/extracranial disease on most contemporaneous cross-sectional staging (body positron emission tomography [PET] or CT), recurrence after SRS, and survival. Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria were used to appraise for recurrence.10 Many patients underwent SRS treatment not just to the surgically resected “index” lesion, but also had simultaneous SRS to additional un-resected BMs. As such, local recurrence was separated and recorded as date of first surgical site recurrence (ie, at site treated with surgical resection plus adjuvant SRS) as well as first date of recurrence at any of the simultaneously SRS-only treated lesions. Distant CNS recurrence was denoted as evidence of a new BrM outside of a treated field or development of leptomeningeal disease (LMD). RN was determined based on pathologic review, when available. If no pathology samples were obtained but RN was suspected, diagnosis was based on radiographic and clinical features including increased volume of enhancement on imaging fulfilling RANO-BM criteria for recurrence, but without evidence of hyperperfusion on MR-perfusion imaging or without hypermetabolism on brain PET scan, or spontaneous subsequent regression without CNS-directed therapy, for example, reirradiation or use of a CNS-active systemic agent. Radiographic findings were based on board-certified neuroradiologist interpretations of MRI and CT studies; these were further reviewed when quantitative features of interest were not commented upon.
Statistical Analysis
Descriptive statistics such as medians, interquartile ranges (IQRs), ranges, and proportions were used to characterize the cohort under study. Variables were associated with time to SRS in days using linear regression modeling. For overall survival (OS) analyses, follow-up was calculated from date of SRS until death or last follow-up, whichever occurred first. Kaplan-Meier methodology was used to describe OS. There were three types of recurrence events of interest: (1) local surgical site recurrence defined as recurrence at the surgical site, (2) other site recurrence defined as recurrence to other sites simultaneously treated with SRS, and (3) distant recurrence defined as recurrence to distant CNS sites. For analyses of each type of recurrence, follow-up was calculated from date of SRS until recurrence event of interest, death without the recurrence event of interest (competing event), or last follow-up, whichever occurred first. Cumulative incidence in a competing risks setting was used to individually describe recurrence events. Recursive partitioning analysis (RPA) was performed to identify the time to SRS cutpoint most statistically significantly associated with outcomes including OS, local site recurrence, other site recurrence, and distant recurrence. Competing risks regression modeling was used to associate time to SRS and other variables with recurrence events of interest. Cox proportional hazards regression was used to associated time to SRS and other variables with OS. Known and suspected risk factors for outcomes of interest were included in multivariable models. All tests were two-sided with an alpha level of statistical significance set at <0.05. All analyses were performed in SAS v9.4 (The SAS Institute, Cary, NC) and R v3.6.0.
Results
Demographics and Treatment
Two hundred and eighty-two patients were treated with transcranial metastasectomy followed by postoperative SRS within 90 days as their next adjuvant CNS-directed therapy at MSK between the years 2013 and 2016 (Table 1). Median OS from SRS was 1.5 years (95% CI: 1.2-2.1) with median follow-up of 49.8 months for survivors. Mean age was 62 years with a female predominance (60% female, 40% male). The most common histology was non-small cell lung cancer (NSCLC) (34%), breast (16%), melanoma (13%), and gastrointestinal (GI) (13%) cancer. KPS score was ≥80 in a majority of cases (79%, n = 224). Index surgical lesions were primarily located in the frontal (35%), parietal (22%), or cerebellar (21%) regions. Only 10% of patients were asymptomatic at presentation. Fifty percent of the patients (n = 142) had progressive systemic (extracranial) disease at the time of surgery.
Table 1.
Cohort Characteristics
| Variable | Level | % (N) | Mean ± SD (Range) |
|---|---|---|---|
| Age at surgery | Continuous | 100 (282) | 62 ± 12.9 (19.8-93.1) |
| TT-SRS (days) | Continuous | 100 (282) | 34.6 ± 11.5 (10-82) |
| No. of fractions | Continuous | 100 (282) | 4.5 ± 1.3 (1-6) |
| Radiation dose (cGy) | Continuous | 100 (282) | 2783.7 ± 379.1 (1500-3000) |
| Dose per fraction | Continuous | 100 (282) | 722 ± 402.3 (500-2100) |
| No. of lesions irradiated simultaneously | Continuous | 100 (282) | 1.9 ± 1.3 (1-10) |
| Max diameter | Continuous | 100 (282) | 3.2 ± 1.2 (0.9-6.8) |
| Sex | Female | 60 (170) | |
| Male | 40 (112) | ||
| KPS | 40 | 0 (1) | |
| 50 | 2 (6) | ||
| 60 | 4 (10) | ||
| 70 | 15 (41) | ||
| 80 | 34 (96) | ||
| 90 | 44 (124) | ||
| 100 | 1 (4) | ||
| Primary site histology | NSCLC | 34 (97) | |
| Breast | 16 (44) | ||
| Melanoma | 13 (36) | ||
| RCC | 3 (8) | ||
| GI | 13 (38) | ||
| Ovarian | 5 (14) | ||
| Endometrial | 3 (9) | ||
| Sarcoma | 3 (8) | ||
| Thyroid | 3 (8) | ||
| Prostate | 2 (5) | ||
| Urothelial | 1 (3) | ||
| Squamous cell | 1 (3) | ||
| Cholangiocarcinoma | 0 (1) | ||
| Other/unknown | 3 (8) | ||
| Index lesion lobe | Cerebellar | 21 (58) | |
| Frontal | 35 (98) | ||
| Occipital | 12 (33) | ||
| Parietal | 22 (62) | ||
| Temporal | 11 (31) | ||
| Index lesion laterality | Left | 46 (131) | |
| Right | 54 (151) | ||
| Presenting symptom | Asymptomatic | 10 (28) | |
| Headache/nausea | 18 (50) | ||
| Weakness | 17 (49) | ||
| Speech | 7 (20) | ||
| Seizures | 12 (33) | ||
| Parietal/sensory | 9 (26) | ||
| AMS | 10 (27) | ||
| Unsteadiness/dizziness | 13 (37) | ||
| Visual changes | 3 (8) | ||
| Other | 1 (4) | ||
| Systemic disease at DOS | Stable | 50 (140) | |
| Progressive | 50 (142) | ||
| Recurrence between surgery and SRS | No | 94 (264) | |
| Yes | 6 (18) | ||
| Wound complication | No | 99 (278) | |
| Yes | 1 (4) | ||
| Extent of resection | GTR | 94 (266) | |
| STR | 6 (16) |
Abbreviations: KPS, Karnofsky Performance Status; NSCLC, non-small cell lung cancer; GI, gastrointestinal; AMS, altered mental status; DOS, date of surgery; RCC, Renal Cell Carcinoma; SRS, stereotactic radiosurgery; GTR, Gross total resection; STR, Subtotal resection.
Gross total resection of the index surgical lesions was achieved in 94% of cases (n = 266), and 6% (n = 16) underwent subtotal resection. The maximum diameter of the index lesion was on average 3.2 ± 1.2 cm. Postoperative SRS included treatment of additional targets beyond the surgical bed in 40% of cases (n = 113). The mean number of simultaneously irradiated masses was 1.9 ± 1.3 (range: 1-10). Thirty-one patients (11%) were treated with single-fraction SRS and 251 (89%) were treated with 2-6 fractions; overall median fractionation was 600 cGy/fraction × 5 fractions.
Factors Associated With Time to SRS
The median time from surgery to SRS (TT-SRS) was 34 days (IQR: 27-39) and the mean was 34.6 ± 11.5 days (Figure 2). There was no significant difference in median TT-SRS between histologic types (NSCLC: 32 days; breast: 34 days; melanoma: 35 days; GI: 34 days; other/unknown: 33 days; Kruskal-Wallis test P = .96). Factors associated with TT-SRS were examined in Table 2. On initial analysis, LOS did not associate with TT-SRS, however, after excluding three patients who were treated with inpatient SRS prior to discharge, a significant association with LOS was demonstrated (1.27, 95% CI [0.71-1.84], P < .0001). Longer TT-SRS was also associated with postoperative discharge to inpatient subacute or acute rehabilitation facility with an estimated increase of ~6 or 13 days, respectively (subacute: 6.31, 95% CI [0.42–12.21], P = .04; acute: 13.36, 95% CI [5.40-21.33], P = .001). Unplanned visits to the urgent care center after discharge were also associated with an estimated 9-day increase in TT-SRS (9.09, 95% CI [6.05-12.13], P < .0001). Patients with fewer number of irradiated lesions demonstrated longer TT-SRS (1.62, 95% CI [0.63-2.61], P = .002). For each increase in the number of irradiated lesions, there was a 1.6-day decrease in TT-SRS, suggesting there may have been greater urgency in facilitating SRS for patients with more lesions. On the other hand, patients who experienced distant CNS progression (ie, new site of CNS disease not present on prior preoperative or postoperative imaging), local progression of sub-totally resected lesion, or recurrence of gross totally resected lesion between surgery and adjuvant SRS underwent SRS treatment approximately 6 days later (6.17, 95% CI [0.68-11.66], P = .03). KPS, the presence/absence of presenting symptoms, systemic disease, new postoperative deficit, and gross total vs subtotal resection did not associate with TT-SRS.
Figure 2.
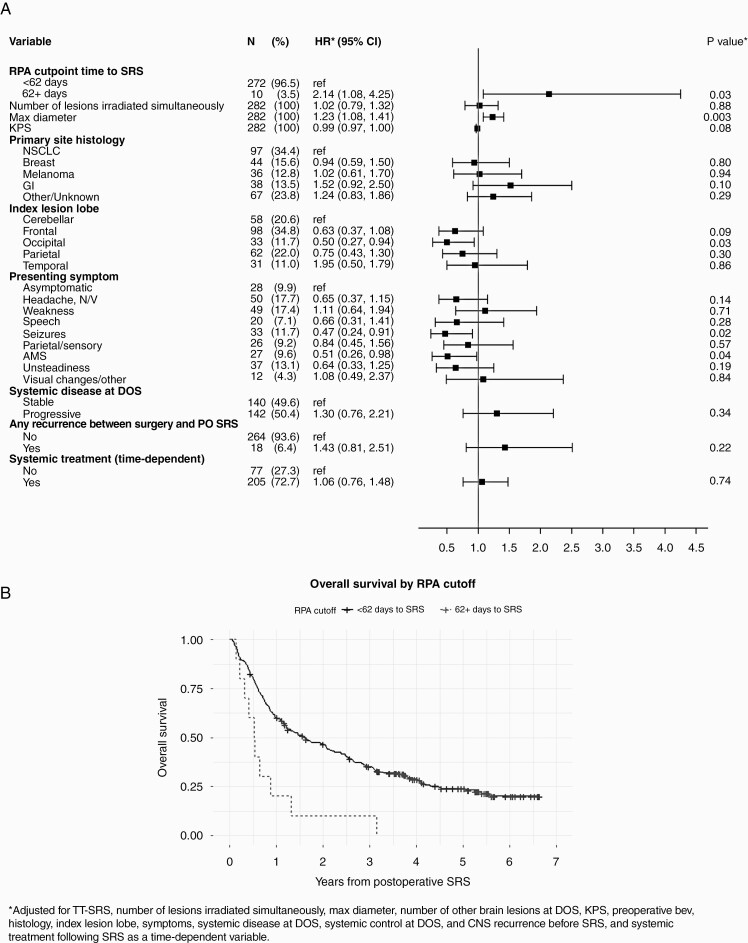
(A) Forest plot demonstrating hazard ratios for multivariable analysis of patient factors with overall survival (OS). (B) Kaplan-Meier curve of OS stratified by time to SRS using the RPA cutpoint of 62 days. Abbreviations: TT-SRS, time to SRS; RPA, recursive partitioning analysis; KPS, Karnofsky Performance Status; NSCLC, non-small cell lung cancer; GI, gastrointestinal; N/V, nausea/vomiting; AMS, altered mental status; DOS, date of surgery; PO, postoperative.
Table 2.
Association of Variables With TT-SRS
| Unadjusted | ||||||
|---|---|---|---|---|---|---|
| Level | N | % | Estimate | 95% CI | P Value | |
| Age at SRS | Continuous | 282 | 100 | 0.06 | −0.04, 0.17 | .23 |
| KPS | Continuous | 282 | 100 | −0.008 | −0.14, 0.13 | .91 |
| Max diameter | Continuous | 282 | 100 | 0.72 | −0.43, 1.86 | .22 |
| No. of lesions irradiated simultaneously | Continuous | 282 | 100 | −1.62 | −2.61, −0.63 | .002 |
| Any recurrence between surgery and postoperative SRS | No | 264 | 94 | ref | ||
| Yes | 18 | 6 | 6.17 | 0.68, 11.66 | .03 | |
| Sex | Female | 170 | 60 | ref | ||
| Male | 112 | 40 | −1.00 | −3.76, 1.77 | .48 | |
| Primary site histology | NSCLC | 97 | 34 | ref | ||
| Breast | 44 | 16 | −1.32 | −5.47, 2.83 | .53 | |
| Melanoma | 36 | 13 | −1.41 | −5.87, 3.04 | .53 | |
| GI | 38 | 13 | 0.44 | −3.93, 4.81 | .84 | |
| Other/unknown | 67 | 24 | 0.47 | −3.16, 4.09 | .80 | |
| Index lesion lobe | Cerebellar | 58 | 21 | ref | ||
| Frontal | 98 | 35 | −1.54 | −5.31, 2.24 | .42 | |
| Occipital | 33 | 12 | −2.40 | −7.37, 2.57 | .34 | |
| Parietal | 62 | 22 | −1.03 | −5.19, 3.14 | .63 | |
| Temporal | 31 | 11 | −2.88 | −7.96, 2.19 | .26 | |
| Index lesion laterality | Left | 131 | 46 | ref | ||
| Right | 151 | 54 | −0.67 | −3.39, 2.04 | .63 | |
| Presenting symptom | Asymptomatic | 28 | 10 | ref | ||
| Headache, N/V | 50 | 18 | −0.79 | −6.18,4.60 | .77 | |
| Weakness | 49 | 17 | 1.17 | −4.24, 6.58 | .67 | |
| Speech | 20 | 7 | 0.70 | −5.99, 7.39 | .84 | |
| Seizures | 33 | 12 | 1.22 | −4.65, 7.09 | .68 | |
| Parietal/sensory | 26 | 9 | −3.90 | −10.12, 2.32 | .22 | |
| AMS | 27 | 10 | 1.06 | −5.09, 7.22 | .73 | |
| Unsteadiness | 37 | 13 | −0.64 | −6.36, 5.08 | .83 | |
| Visual changes/other | 12 | 4 | −2.50 | −10.38, 5.38 | .53 | |
| Systemic disease at DOS | Stable | 140 | 50 | ref | ||
| Progressive | 142 | 50 | −1.87 | −4.57, 0.83 | .17 | |
| Systemic control at DOS | Stable | 115 | 41 | ref | ||
| Progressive | 104 | 37 | −2.36 | −5.41, 0.68 | .13 | |
| Slow | 63 | 22 | 2.54 | −0.99, 6.06 | .16 | |
| GTR vs STR | GTR | 266 | 94 | ref | ||
| STR | 16 | 6 | −3.64 | −9.48, 2.20 | .22 | |
| LOSa | Continuous | 279 | 100 | 1.27 | 0.71, 1.84 | <.0001 |
| Urgent care visits | Continuous | 282 | 100 | 9.09 | 6.05, 12.13 | <.0001 |
| Disposition | Home | 258 | 91 | ref | ||
| Acute rehabilitation | 8 | 3 | 13.36 | 5.40, 21.33 | .001 | |
| Subacute rehabilitation | 15 | 5 | 6.31 | 0.42, 12.21 | .04 | |
| Inpatient hospice | 1 | 0 | −12.89 | −35.12, 9.35 | .25 | |
| New postoperative deficit | No | 250 | 89 | ref | ||
| Yes | 32 | 11 | 1.70 | −2.57, 5.97 | .43 |
Abbreviations: SRS, stereotactic radiosurgery; KPS, Karnofsky Performance Status; NSCLC, non-small cell lung cancer; GI, gastrointestinal; N/V, nausea/vomiting; AMS, altered mental status; DOS, date of surgery LOS, length-of-stay; GTR, gross total resection; STR, subtotal resection.
aExcludes three patients treated with inpatient SRS prior to discharge.
OS and Recurrence
Forest plots demonstrate hazard ratios from multivariable analysis of patient-specific factors with OS (Figure 2), as well as local surgical site recurrence (Figure 3) in this cohort. Supplemental Tables 1 and 2 provide further detailed univariable and multivariable analysis. A RPA cutpoint analysis demonstrated that a TT-SRS of 62-90 days was associated with worse survival outcomes (HR = 2.14, 95% CI [1.08-4.25], P = 0.03, Figure 2). Larger diameter index lesions were associated with worse OS outcomes (HR = 1.23, 95% CI [1.08-1.41], P = .0027), while occipital index lesion location (HR = 0.50, 95% CI [0.27-0.94], P = .03), or presenting symptoms of altered mental status (AMS, HR = 0.51, 95% CI [0.26-0.98], P = .04) or seizure (HR 0.47, 95% CI [0.24-0.91], P = .02) associated with improved OS on multivariable analysis. KPS, histology, progressive systemic disease, subtotal resection, and recurrence of surgical lesion prior to adjuvant SRS initiation were not independently associated with worse survival in this cohort.
Figure 3.
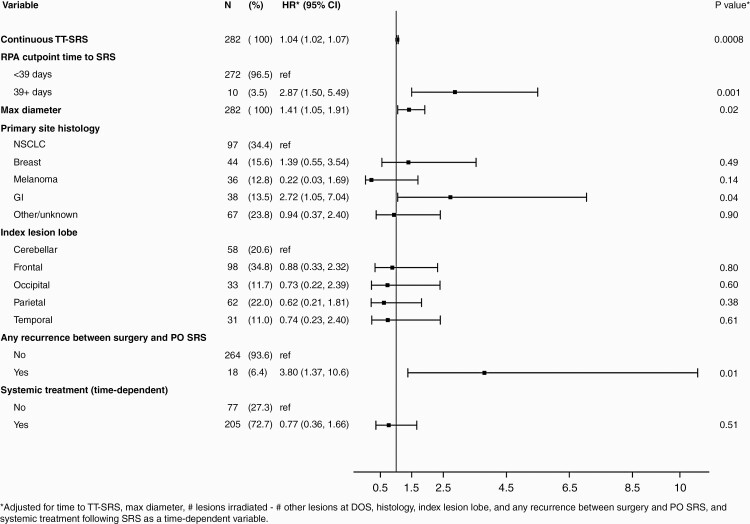
Forest plot demonstrating hazard ratios for multivariable analysis of patient factors with local surgical site recurrence. Abbreviations: TT-SRS, time to SRS; RPA, recursive partitioning analysis; NSCLC, non-small cell lung cancer; GI, gastrointestinal; DOS, date of surgery; PO, postoperative.
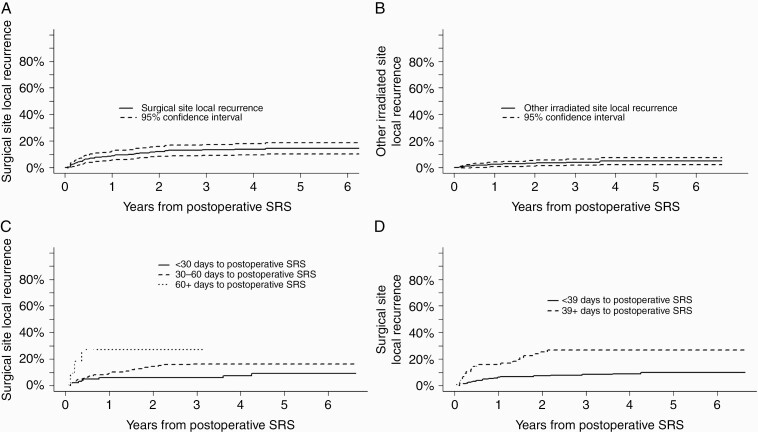
Each category of recurrence event was evaluated separately, with local surgical site, other irradiated site, and distant CNS recurrence rates of 14.3% (95% CI: 10.1-18.5), 4.9% (95% CI: 2.3-7.5), and 47.5% (95% CI: 41.4-53.6), respectively, at 5 years (Figure 4). TT-SRS was significantly associated only with recurrence rate at the surgically resected site (P = .0008, Figure 3). Patients irradiated within 1 month of surgery had a 1-year surgical site recurrence rate of 6.1% [95% CI 1.3-10.9], SRS between 1 and 2 months after surgery resulted in recurrence rate of 9.2% [95% CI 4.9-13.6] and patients with SRS >2 months after surgery had a recurrence rate of 27.3% [95% CI 0.0-55.5]. An RPA cutpoint of TT-SRS ≥39 days associated most significantly with increased surgical site recurrence risk (Figure 3, HR for ≥39 days = 2.87, 95% CI [1.50-5.49], P = .0014). For patients with simultaneously irradiated (non-resected) BrM, RPA cutpoint analysis demonstrated significantly increased risk of recurrence with TT-SRS ≥57 days (HR = 11.06, [2.25-54.32], P = .003). TT-SRS demonstrated no association with distant recurrence in any analysis.
Figure 4.
Cumulative incidence of recurrence after postoperative SRS over time at the (A) local surgical site and (B) other irradiated site. Panels (C) and (D) demonstrate cumulative incidence of recurrence at the surgical site as stratified by time to SRS. Abbreviation: SRS, stereotactic radiosurgery.
Maximum diameter of the surgical tumor associated with increased risk of local surgical site recurrence (HR = 1.41, 95% CI [1.05-1.91], P = .02). Histology also demonstrated a significant association with recurrence risks. Risk of surgical site recurrence was higher with GI malignancy (HR = 2.72, 95% CI [1.05-7.04], P = .04), and risk of recurrence at other irradiated sites was higher for GI (HR = 8.36, 95% CI [1.12-62.24], P = .04) and breast (HR = 6.71, 95% CI [1.26-35.91], P = .03) malignancies. Greater risk of distant CNS recurrence was associated only with breast malignancy relative to other histologic types (HR = 1.88, 95% CI [1.11-3.20], P = .02). Given the statistical association of histology with recurrence risks, we assessed whether certain histologic types may demonstrate greater associations between recurrence and TT-SRS than others. While TT-SRS was particularly important for patients with primary GI (HR = 1.08, 95% CI [1.04-1.12], P = .0003) and breast (HR = 1.07, 95% CI [1.01-1.13], P = .03) malignancies, there was no statistically significant heterogeneity across primary sites (p-Het = 0.42).
Early recurrence at the surgical site or distant CNS progression elsewhere in the brain prior to initiating adjuvant SRS was associated with higher risk of postirradiation local surgical site (HR = 3.80, 95% CI [1.37-10.57], P = .01) and simultaneously irradiated site recurrence (HR = 6.43, 95% CI [1.06-38.99], P = .04) after SRS, but did not associate with worse OS in the multivariable setting. Risk of distant progression was lower in patients presenting with speech difficulty (HR = 0.31, 95% CI [0.10-1.00], P = .05) or seizure (HR = 0.39, 95% CI [0.17-0.92], P = .03).
Systemic Therapy
Two hundred and five patients received systemic therapy following adjuvant SRS. Of the 151 patients with TT-SRS <39 days and who had subsequent systemic therapy, the median time from surgery to systemic therapy was 61 days (IQR: 43-146). Of the 54 patients with time to SRS 39+ days and who had subsequent systemic therapy, the median time from surgery to systemic therapy was 77 days (IQR: 63-178). The time from surgery to systemic therapy did not demonstrate an association with OS. Receipt of systemic therapy after adjuvant SRS did not associate with OS or recurrence of any type on multivariable analysis.
Complications
Wound complications were rare, occurring in only 1% of patients. RN occurred in 13.7% (95% CI: 9.3-18.1) of patients and leptomeningeal dissemination (LMD) in 14.9 % (95% CI: 10.7-19.1) at 5 years. TT-SRS did not associate with risk of any of these complications. Increased rate of RN was associated only with recurrence/progression prior to adjuvant SRS (HR = 3.28, 95% CI [1.13-9.53], P = .03). Increased risk of LMD was associated with subtotal resection (HR = 2.71, 95% CI [1.10-6.73], P = .03), treatment with preoperative bevacizumab (HR = 2.95, 95% CI [1.03-8.45], P = .04) and index surgical lesion location in the cerebellum (HR = 4.14, 95% CI [1.88-9.09], P = .0004).
Discussion
Adjuvant SRS has become an integral part of the treatment paradigm to improve local control of resected BrM.3,4 This large, retrospective cohort demonstrated a local surgical site control rate of 86% at 5 years for surgery plus adjuvant SRS, highlighting a durability in CNS response that compares favorably relative to previously published reports of 50%-87% at 1 year of follow-up.3,4,9,11–13 However, our data also reveal an important time-dependent corollary to the paradigm-changing studies that established the efficacy of adjuvant SRS. Local control rates significantly associated with time from surgery to SRS (TT-SRS). The timing of adjuvant radiation must balance maximal microscopic disease control with theoretical risks of wound or infectious complications that can affect quality of life and survival. While studies have found inferior survival with early initiation of chemoradiation therapy in glioblastoma (GBM) across RPA prognostic groups,14,15 small case series in BrM populations had demonstrated inconsistent results. BrM patients demonstrated either worse survival and no improvement in local control for SRS within 3 weeks of surgery, or improved local control but no concurrent survival benefit for SRS delivered within 30 days.8,9 As such, there are limited guidelines on the optimal timing of adjuvant SRS after metastasectomy. Our study identified significant improvement in local control rates when SRS was delivered within approximately 1 month of surgery. Furthermore, adjuvant SRS administered beyond 2 months after surgery associated with worse OS. The benefits associated with early adjuvant SRS highlight the need for coordinated efforts in treatment planning and consideration of TT-SRS in future outcome analyses and recommendations. The 18-month median OS of this heterogeneous, oligometastatic BrM cohort suggests that advances in systemic therapy have improved survival relative to historical cohorts.16,17 While randomized controlled trials have not shown survival benefits with adjuvant radiation,2,3 durable CNS control and palliation become increasingly vital as patients survive longer with improved systemic therapy. Shorter TT-SRS allows for earlier resumption or initiation of systemic therapy for extracranial disease control. This has recently been recognized as an important survival determinant for these patients given that extracranial disease is often more life-limiting than CNS control, making reduced systemic treatment breaks potentially more important from a survival perspective than CNS-directed treatments themselves.18–20
TT-SRS is potentially modifiable, and thus factors associated with increased TT-SRS warrant heightened clinical awareness and forethought. Our data corroborate prior studies that found as association between discharge to acute/subacute rehabilitation and increased TT-SRS.9 Rehabilitation centers are often unable to arrange for transport to adjuvant radiation treatments, and therefore can delay initiation of SRS. Interestingly, patients in this cohort with larger numbers of BrMs were associated with shorter TT-SRS, suggesting that they were shuttled to adjuvant treatment more quickly. Institutional efforts such as Multidisciplinary Brain Metastasis Clinics including one developed at our Center, allow for preoperative outpatient radiation oncology counseling, consenting, and treatment simulation performed the same day as neurosurgery visits, in an attempt to increase efficiency and avoid potential delays for BrM patients. Furthermore, inpatient SRS simulation and treatment have been facilitated in cases of higher clinical concern. Consistent with that effort, three patients in this cohort underwent inpatient SRS prior to hospital discharge. Unsurprisingly, unplanned visits to the urgent care center after surgery, longer postoperative LOS, and CNS disease progression/recurrence prior to initiating SRS were all associated with increased TT-SRS.
Timing of adjuvant SRS is just one of the many variables that can affect efficacy/local control. A superior local control rate and tractability of longer TT-SRS was found for un-resected, simultaneously irradiated lesions relative to resected lesions in our study. The excellent 95.1% local control rate at 5 years likely represents improved control of smaller volume lesions that did not require surgical intervention.4,13,21–24 Postoperative changes in the surgical cavity volume over time may be important in regards to optimal timing of radiation planning scans.25–27 The time of planning MRI relative to treatment was not evaluated in this study, although it could represent a confounding variable for TT-SRS. Given the lack of consistent data on cavity dynamics, inclusion of a 2-5 mm margin dose in institution protocols, and CT simulations that would have detected significant changes relative to planning MRI, we do not expect that this was a significant factor in this study. However, determining optimal time for planning MRIs remains an important question for future dedicated studies. While SRS delivers a high biologically effective dose of radiation that is thought to be radio-ablative and less susceptible to cancer-specific radio-resistance,28 our data do also support differences in local failure risk based on histology. Radiation resistance of GI tumors has been previously described.29–31 Alternatively, differences in response to systemic therapy may underlie the association with histology.19 Specific institutional protocols such as the inclusion of a 2-5 mm expansion to define the planning target volume dose and/or early administration of targeted and immunotherapies could also account for differences in control rates. While KPS, the presence of progressive systemic disease, or receipt of systemic therapy after adjuvant radiation typically associate with OS and prognosis for patients with BrM,32–34 they were not found to be significant in the multivariable setting in this cohort. This is likely related to the relatively high KPS of patients selected for surgery and wide range of heterogeneous primary tumors included in the cohort.
Complications
RN rates in this cohort were consistent with the literature, which are generally reported in the range of 5%-17%.11,12,23,35,36 No factors assessed including TT-SRS, radiation dose, fractions, or index tumor maximal diameter were associated with RN risk within this study. While concern for pachymeningeal and LMD seeding associated with surgery has grown, we did not find any association between LMD and TT-SRS.5,37–40 The wound complication rate was too low to be statistically evaluated for risk factors or possible association with bevacizumab, however, of the 4 patients with wound complications, the median TT-SRS was 33 days (range: 27-51). Often adjuvant SRS is held for 2-3 weeks following surgery to theoretically allow for better wound healing and to reduce radiation beam scatter, however, to our knowledge no data has demonstrated this as truly necessary.41
Limitations
As with any retrospective series, this study’s conclusions are limited based on the selection biases inherent in the design. However, given the sample size, it is well positioned compared to prior attempts to answer the question at hand. Ultimately, the TT-SRS is affected by a number of variables, many of which we have assessed and controlled for in our multivariable analysis, but others which may remain unaccounted for could confound some of the differences identified in this study. Further evaluation of causes of long TT-SRS is warranted for better understanding and design of pathways to decrease or avoid logistical barriers and consideration of neoadjuvant, preoperative SRS42–44 or intraoperative brachytherapy45 may be warranted for patients where extended TT-SRS is a significant concern.
Conclusions
Ultimately, our data support durable, long-term local BrM control with adjuvant post-metastasectomy SRS. However, delays in initiation of postoperative SRS can decrease its efficacy. Time-to-adjuvant SRS greater than 38 days, only 7 days longer than the historical median at our large Cancer Center, can significantly decrease local control rates, and beyond 61 days can be associated with worse OS. Ongoing efforts must be made to identify and minimize logistical, procedural, and socioeconomic factors responsible for these delays.
Supplementary Material
Funding
This research was funded in part through the NIH/NCI cancer center grant, number P30 CA008748.
Conflicts of interest statement. At the time of research execution and publication, all authors are affiliated with Memorial Sloan Kettering Cancer Center. The authors of this research deny any conflicts of interest regarding this study and make the following disclosures: N.S.M.: Consulting fees for advisory board participation: AstraZeneca.
Authorship. Conception and design: E.D.B., M.Y., N.S.M., V.T., K.S.P., A.S.R.; Acquisition of data: E.D.B., M.Y.; Analysis and interpretation: E.D.B., A.S.R., K.S.P., V.T., N.S.M.; Drafting or revising of article: E.D.B., M.Y., A.S.R., K.S.P., K.B., A.M.B., C.W.B., V.T., N.S.M.; All authors approved the final manuscript.
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 2. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. [DOI] [PubMed] [Google Scholar]
- 3. Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennan C, Yang TJ, Hilden P, et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 8. Scharl S, Kirstein A, Kessel KA, et al. Stereotactic irradiation of the resection cavity after surgical resection of brain metastases - when is the right timing? Acta Oncol. 2019;58(12):1714–1719. [DOI] [PubMed] [Google Scholar]
- 9. Yusuf MB, Amsbaugh MJ, Burton E, et al. Increasing time to postoperative stereotactic radiation therapy for patients with resected brain metastases: investigating clinical outcomes and identifying predictors associated with time to initiation. J Neurooncol. 2018;136(3):545–553. [DOI] [PubMed] [Google Scholar]
- 10. Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–278. [DOI] [PubMed] [Google Scholar]
- 11. Mengue L, Bertaut A, Ngo Mbus L, et al. Brain metastases treated with hypofractionated stereotactic radiotherapy: 8 years experience after Cyberknife installation. Radiat Oncol. 2020;15(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathieu D, Kondziolka D, Flickinger JC, et al. Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 2008;62(4):817–23; discussion 823. [DOI] [PubMed] [Google Scholar]
- 13. Vogelbaum MA, Angelov L, Lee SY, et al. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104(6):907–912. [DOI] [PubMed] [Google Scholar]
- 14. Press RH, Shafer SL, Jiang R, et al. Optimal timing of chemoradiotherapy after surgical resection of glioblastoma: stratification by validated prognostic classification. Cancer. 2020;126(14):3255–3264. [DOI] [PubMed] [Google Scholar]
- 15. Blumenthal DT, Won M, Mehta MP, et al. Short delay in initiation of radiotherapy for patients with glioblastoma-effect of concurrent chemotherapy: a secondary analysis from the NRG Oncology/Radiation Therapy Oncology Group database. Neuro Oncol. 2018;20(7):966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medikonda R, Jackson CM, Feghali J, Lim M. The effects of postoperative neurological deficits on survival in patients with single brain metastasis. Oper Neurosurg. 2020;19(6):628–634. [DOI] [PubMed] [Google Scholar]
- 17. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. [DOI] [PubMed] [Google Scholar]
- 18. McTyre ER, Johnson AG, Ruiz J, et al. Predictors of neurologic and nonneurologic death in patients with brain metastasis initially treated with upfront stereotactic radiosurgery without whole-brain radiation therapy. Neuro Oncol. 2017;19(4):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shenker RF, Hughes RT, McTyre ER, et al. Potential prognostic markers for survival and neurologic death in patients with breast cancer brain metastases who receive upfront SRS alone. J Radiosurg SBRT. 2018;5(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 20. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elliott RE, Rush SC, Morsi A, et al. Local control of newly diagnosed and distally recurrent, low-volume brain metastases with fixed-dose (20 Gy) gamma knife radiosurgery. Neurosurgery. 2011;68(4):921–31; discussion 931. [DOI] [PubMed] [Google Scholar]
- 22. Petrovich Z, Yu C, Giannotta SL, O’Day S, Apuzzo MLJ. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg. 2002;97(Supplement 5):499–506. [DOI] [PubMed] [Google Scholar]
- 23. Mohammadi AM, Schroeder JL, Angelov L, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. 2017;126(3):735–743. [DOI] [PubMed] [Google Scholar]
- 24. Hughes RT, Black PJ, Page BR, et al. Local control of brain metastases after stereotactic radiosurgery: the impact of whole brain radiotherapy and treatment paradigm. J Radiosurg SBRT. 2016;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 25. Atalar B, Choi CYH, Harsh GR, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72(2):180–185; discussion 185. [DOI] [PubMed] [Google Scholar]
- 26. Patel RA, Lock D, Helenowski IB, et al. Postsurgical cavity evolution after brain metastasis resection: how soon should postoperative radiosurgery follow? World Neurosurg. 2018;110:e310–e314. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed S, Hamilton J, Colen R, et al. Change in post-surgical cavity size within first 30 days correlates with extent of surrounding edema: consequences for postoperative radiosurgery. J Comput Assist Tomogr. 2014;38(3):457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanghvi SM, Lischalk JW, Cai L, et al. Clinical outcomes of gastrointestinal brain metastases treated with radiotherapy. Radiat Oncol. 2017;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Go PH, Klaassen Z, Meadows MC, et al. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117(16):3630–3640. [DOI] [PubMed] [Google Scholar]
- 31. Farnell GF, Buckner JC, Cascino TL, et al. Brain metastases from colorectal carcinoma. The long term survivors. Cancer. 1996;78(4):711–716. [DOI] [PubMed] [Google Scholar]
- 32. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 33. Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017;99(4):812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ebner D, Rava P, Gorovets D, et al. Stereotactic radiosurgery for large brain metastases. J Clin Neurosci. 2015;22(10):1650–1654. [DOI] [PubMed] [Google Scholar]
- 36. Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 38. Atalar B, Modlin LA, Choi CY, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713–718. [DOI] [PubMed] [Google Scholar]
- 39. Susko M, Yu Y, Ma L, et al. Preoperative dural contact and recurrence risk after surgical cavity stereotactic Radiosurgery for brain metastases: new evidence in support of consensus guidelines. Adv Radiat Oncol. 2019;4(3):458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cagney DN, Lamba N, Sinha S, et al. Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019;5(5):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sankey EW, Tsvankin V, Grabowski MM, et al. Operative and peri-operative considerations in the management of brain metastasis. Cancer Med. 2019;8(16):6809–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asher AL, Burri SH, Wiggins WF, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88(4):899–906. [DOI] [PubMed] [Google Scholar]
- 43. Patel KR, Burri SH, Boselli D, et al. Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: a multi-institutional analysis. J Neurooncol. 2017;131(3):611–618. [DOI] [PubMed] [Google Scholar]
- 44. Takami H, Nassiri F, Moraes FY, et al. A phase II study of neoadjuvant stereotactic radiosurgery for large brain metastases: clinical trial protocol [published online ahead of print November 1, 2019]. Neurosurgery. [DOI] [PubMed] [Google Scholar]
- 45. Wernicke AG, Yondorf MZ, Peng L, et al. Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J Neurosurg. 2014;121(2):338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.