Abstract
Background
Few studies have assessed physical functioning in glioma patients with grade II, III, and IV glioma prior to undergoing adjuvant radiation with or without chemotherapy. The aim of this study was to describe the baseline physical functioning capacity of patients with glioma prior to adjuvant therapy compared to validated cutoffs required to maintain independence.
Methods
This study is a cross-sectional study that recruited patients with grade II, III, and IV glioma (n = 33) undergoing adjuvant radiation with or without chemotherapy. The six-minute walk, thirty-second sit-to-stand, and timed “Up & Go” assessments were used to describe baseline physical functioning. Perceived quality of life from the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30) version 3.0 was used to quantify the quality of life.
Results
Mean distance walked in the six-minute walk test was 416.2 m (SD 137.6 m) with a mean of 12.2 stands (SD 3.4 stands) achieved during the thirty-second sit-to-stand. Median time to complete the timed “Up & Go” assessment was 7 s (interquartile range: 3 s). One-sample t tests suggest walking distance and chair stands were significantly lower than cutoff criterions to maintain independent living, t(32) = −5.96, P < .001, bias-corrected accelerated 95% CI [370.7-460.4], and t(32) = −4.60, P < .01, bias-corrected accelerated 95% CI [11.0-13.4], respectively. Wilcoxon signed-rank test identified significantly shorter median time taken to complete the timed “Up & Go” test compared to the cutoff criterion (z = −4.43, n = 33, P < .01).
Conclusion
This study suggests glioma patient’s aerobic endurance and lower limb strength are below criterion cutoffs recommended to maintain independent living. Timed “Up & Go” scores did not exceed the criterion cutoff, indicating respectable levels of mobility.
Keywords: assessments, functional capacity, glioma, independence, quality of life
Primary brain tumors and subsequent treatment can impact physical functioning. In 2017, in Australia, older adults (defined as the general population over 65 years of age) account for 46% of all glioma diagnoses.1 Current treatment for gliomas varies with histological grade and is tailored to age, diagnosis, the presence of comorbidities, and performance status.2 As a result, goals of treatment may vary, but broadly aims are to prolong survival, preserve neurological function, and maintain quality of life.
Physical, cognitive, and emotional changes are common and vary according to various treatment-related and patient-related factors. These may manifest as declines in executive function, strength, and aerobic capacity, with increased levels of fatigue and depression.3,4 Declines in physical capacity are comparable to the deconditioning observed in the aging population and can impact physical capacity to perform activities of daily living, independence, and quality of life.5 For these reasons, there is a pressing need to explore management options to address side effects affecting physical capacity, independence, and quality of life. Clinical research indicates exercise has a potential role in this regard.
In the last decade, the therapeutic benefits of exercise throughout the cancer care continuum have been investigated. A recent systematic review concluded that structured exercise following cancer diagnoses has a significant protective effect against cancer-specific mortality and recurrence.6 Other therapeutic benefits include improvements in body composition, strength and aerobic fitness, physical capacity, and quality of life.7–10 Much of this research was conducted in more common cancers such as breast, prostate, and colon. Research investigating the benefits of individualized exercise within the glioma population is scarce. General exercise guidelines may not apply to the unique pathophysiology, therapeutic management, and symptom-burden experienced in the glioma population.
As the exercise-oncology literature evolves, it is shifting away from generic guidelines in favor of a more focused approach, matching each patient’s goals to their current physical capacity.11 Limited data are available to describe the “baseline” physical capacity of patients with glioma before receiving adjuvant cancer therapy.5,12 However, some existing research indicates that exercise behavior may be associated with improved median survival.13 From a rehabilitative perspective, the assessment of physical capacity is fundamental to identify those at risk of mobility difficulties and falls and is a guiding pre-requisite for an individualized exercise prescription.
To evaluate baseline physical capacity, standardized performance-based assessments of aerobic endurance, lower body strength, and mobility (the six-minute walk, thirty-second sit-to-stand, and timed “Up & Go” tests, respectively) were implemented. Assessments of physical capacity were selected as they represent both clinical and research relevant areas of focus for health care professionals involved in the prescription and delivery of exercise interventions. Although application of these assessments remains novel within the glioma population, they are frequently applied within the exercise-oncology practice and literature. In addition, normative values for these assessments have been published within both healthy and clinical populations allowing health care professionals to quantitatively interpret and compare each patient’s results relative to others of a similar age or medical condition. In turn, these results may provide information required for individualized goal development, exercise prescription, intervention effectiveness, monitoring, and patient care throughout cancer treatment.
The six-minute walk test is a submaximal assessment of aerobic endurance commonly used in clinical practice and research as it does not require any specialized equipment or training to implement, providing a more feasible assessment of aerobic endurance compared to other maximal or submaximal assessments. It has been used in patients with varying cardiopulmonary diseases,14 neurological conditions including stroke,15 Parkinson’s,16 and Alzheimer’s,17 as well as within cancers of the breast,18 prostate,19 and high-grade gliomas.20,13 The six-minute walk test has reported a high test-retest reliability within the cancer population (interclass correlation coefficient [ICC] = 0.93, 95% confidence interval [CI] 0.86-0.97).21
The delivery of the six-minute walk test is standardized using protocols published by the American Thoracic Society.22 At their own pace, patients are to cover as much distance as possible within 6 min by continuously walking over a hard surface between two cones set 30 m apart. The test score is the distance covered in meters, termed the six-minute walk distance. Walk distances have demonstrated moderate to strong correlations with peak oxygen uptake,23 physical capacity and mobility components,24 levels of physical activity,25 and is a robust predictor of mortality26 in a wide range of clinical settings. To facilitate direct comparisons of walk distance, a normative value was selected from the published literature that followed the American Thoracic Society six-minute walk test protocol. As a result, a normative value of 559 m27 was used in this study. Although other published literature on the elderly has reported similar normative values,28 the use of different protocols limits direct comparison.
The thirty-second sit-to-stand test is used to assess lower body strength and was performed according to the test manual described by Jones et al.29 Patients are asked to rise from a seated position and stand in a fully extended standing position as many times possible in 30 s. The score is the total number of correctly executed stands completed within the allocated timeframe. Due to its simplicity and role in assessing common everyday activities including getting in and out of a chair, stair climbing, and maintaining balance, it is implemented within a range of populations including the elderly,29 stroke,30 and cancer patients.31 The thirty-second sit-to-stand test has a high test-retest reliability (ICC = 0.84), correlating strongly with one-repetition maximum leg press scores and is able to discriminate between high-active and low-active adults.29 Older adults that demonstrated lower sit-to-stand performances were associated with nearly twice the likelihood of experiencing a fall-related injury32 and report higher need of assistance with activities of daily living.33 Rikli and Jones34 proposed a cutoff value of 15 stands for women and 16 stands for men as a criterion score for maintaining independent functioning in later life. For the purposes of this study, we used the proposed cutoff for women as a more conservative measure for comparison against our sample of glioma patients.
The timed “Up & Go” test is a measure of functional mobility, balance, and falls risk developed by Podsiadlo and Richardson.35 The test takes only seconds to perform, requires no special equipment or training, and captures important mobility components, including transfer ability, gait, and turning movements. It has been studied in the elderly,36 patients with neurological conditions such as Parkinson’s disease37 and cancer patients.38 At their own pace, patients are asked to rise from a seated position without using armrests, walk to a cone 3 m away, turn, walk back, and sit down again. The test score is the time in seconds to complete the test. Within the literature, however, there is a variation in cutoff values reported to identify patients with functional impairments.39 For example, in community-dwelling older adults, the timed “Up & Go” test has been shown to strongly predict disabilities in activities of daily living, including the ability to get in and out of bed and walk around the house. 40 Further, difficulties in higher level tasks such as money management have been demonstrated in older adults who required more than 13 s to complete the timed “Up & Go” test with older adults that completed the test in more than 20 s demonstrating further disability and difficulty.40 While a cutoff point of 13.5 s has been identified as having 87% sensitivity and specificity in correctly identifying those with increased falls risk.41 Further Bischoff et al.42 promote 12 s as a clinical cutoff point for normal mobility, functioning, and reduced falls risk.
The timed “Up & Go” test has a high intra-rater and inter-rater reliability (ICC = 0.92-0.96)43 with a reported moderate (ICC = 0.56)44 to high (ICC = 0.99)35 test-retest reliability, possibly caused by a change in sample stability or potential learning effects between trails.45 However, construct validity has been supported through a range of moderate to strong correlations with essential functional measurements including gait speed, postural sway, step length, and stair test.43 For the purpose of this study, the 13.5-s cutoff41 was selected due it its ability to identify the risk of mobility issues and falls and being a conservative estimate between two opposing values.
The purpose of this study was to describe the baseline physical functioning capacity of patients with glioma prior to adjuvant therapy. We also explored the correlations between physical capacity and quality of life. We consider the following hypotheses: (1) Patients with glioma after surgery will have physical functioning different from the physical functioning normative values of the older adult population; (2) Better physical functioning will be positively associated with greater perceptions of quality of life.
Methods
Descriptive data reported are part of a larger pilot study exploring the feasibility of implementing an exercise intervention in patients with glioma undergoing adjuvant radiation with or without chemotherapy. Inclusion criteria were: (a) histologically confirmed World Health Organization (WHO) grade II, III, and IV glioma; (b) age greater than 17 years old; (c) Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2; (d) intention to receive radiation therapy; (e) treating oncologist approval; and (f) ability to communicate in English. The study followed institutional guidelines provided by each participating hospital and was approved by their respective Ethics Committees HREC/17/QPEC/43 and HREC/14/LPOOL/408. All participants provided written informed consent.
Procedures
Participants were identified and screened for eligibility during outpatient clinic consultations at their primary hospital. Following oncologist approval, the research team discussed and provided participants an overview of the study. Consenting patients completed physical functional assessments to examine aerobic capacity, lower limb strength, and balance followed by subjective quality of life assessment. Relevant clinicopathological data were also recorded.
Assessments
Three tests were used to assess physical capacity and were selected for ease of administration and scoring requiring minimal space and equipment requirements within a clinical environment. These tests represented both the key physical parameters and functions of independent living including mobility (eg, walking) and transferring (eg, rising from a chair). The six-minute walk test is a reliable and valid submaximal assessment of aerobic endurance; the distance walked in 6 min was recorded to the nearest meter.22 The thirty-second sit-to-stand test, shown to be a valid and reliable measure of lower body strength test, was administered.29 The number of stands achieved in 30 s was counted. The timed “Up & Go” test has demonstrated high construct validity with log-transformed assessments of balance and was administered accordingly.35 Time to complete the test was recorded in seconds. Normative value cutoffs represent the value required to maintain functions of daily living within an adult population independently, safely, and without fatigue.34 For comparisons, cutoffs were 559 m, 15 stands, and 13.5 s, respectively.27,34,41
Health-related quality of life was measured using the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30) version 3.0 and scored according to guidelines.46 The QLQ-C30 contains 30 items across five functional scales (physical, role, emotional, cognitive, and social), nine symptom scales (fatigue, nausea and vomiting, and pain), and one global health status/quality of life scale. After linear transformation, the global health status is scored from 0 to 100. A higher global health status score represents a better perception of quality of life.
Statistical analysis
Baseline physical capacity and quality of life are reported as means and standard deviations if assumptions of normality were met, or as median and interquartile range if violated. Where missing data occurred, Little’s missing completely at random (MCAR) test was used to determine the pattern of missing data. Little’s MCAR test was not significant (χ 2(4) = 2.45, P = .65), suggesting that missing data pattern was non-systematic. Given the non-systematic pattern of missing data, listwise deletion was implemented. When assumptions of normality were met, a one-sample t test was used to compare physical capacity to criterion cutoff values. The Wilcoxon signed-rank test for one sample was used when assumptions of normality were not met. Similarly, Pearson’s correlation coefficient or Spearman’s rank correlation coefficient were used to assess the relationship between physical capacity and quality of life with bias-corrected accelerated 95% CI estimated using 1000 bootstrapped samples. An alpha value (accepted as α = 0.05, two-tailed) was used to determine the significance. Statistical analyses were conducted using SPSS (IBM SPSS Statistics Version 25.0; IBM) for Macintosh.
Results
A total of 33 participants aged 29–72 years were recruited from April 2015 to July 2019. Patients’ baseline demographics and clinical characteristics are shown in Table 1.
Table 1.
Baseline Patient Demographic and Clinical Characteristics (n = 33).
| Characteristics | Mean (SD) | n (%) |
|---|---|---|
| Age, y | 49.0 (13.4) | |
| Height, cm | 175.6 (11.0) | |
| Body mass, kg | 91.1 (21.7) | |
| Body mass index, kg/m2 | 29.4 (5.9) | |
| Time since diagnosis, days | 26.0 (15.0)a | |
| Gender | ||
| Female | 12 (36) | |
| Male | 21 (64) | |
| ECOG | ||
| 0 | 20 (61) | |
| 1 | 12 (36) | |
| 2 | 1 (3) | |
| WHO tumor grade | ||
| II | 6 (18) | |
| III | 5 (15) | |
| IV | 22 (67) | |
| Tumor histology | ||
| Astrocytoma | 10 (30) | |
| Oligodendroglioma | 1 (3) | |
| Glioblastoma | 22 (67) | |
| Surgery (extent of resection) | ||
| Biopsy | 4 (12) | |
| Sub-total resection | 21 (64) | |
| Gross-total resection | 8 (24) | |
| Radiation protocol | ||
| Receiving radiation | 33 (100) | |
| Radiation with concurrent chemotherapy | 25 (76) | |
| Radiation with sequential chemotherapy | 2 (6) | |
| Radiation without chemotherapy | 6 (18) | |
| Supportive agents | ||
| Receiving anti-epileptic medication (levetiracetam) | ||
| Yes (mg/daily) | 500.0 (375.0)a | 15 (46) |
| No | 18 (55) | |
| Receiving corticosteroids (dexamethasone) | ||
| Yes (mg/daily) | 4.0 (1.0)a | 9 (27) |
| No | 24 (73) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization.
Data are presented as mean and SD or as n (%) except where indicated.
aData reported as median and interquartile range.
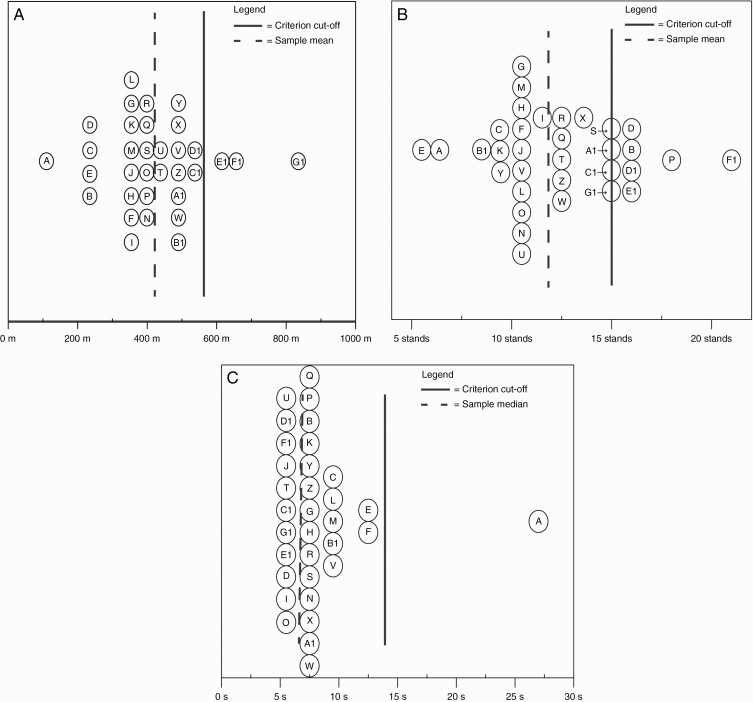
Overall, 33 (100%) patients completed the six-minute walk, thirty-second sit-to-stand, and timed “Up & Go” tests. However, only 29 (88%) of patients completed the QLQ-C30 questionnaire. The mean distance walked in the six-minute walk test was 416.2 m (SD 137.6 m) with a mean of 12.2 stands (SD 3.4 stands) achieved during the thirty-second sit-to-stand were (Figure 2a, b). Median time to complete the timed “Up & Go” assessment was 7 s (interquartile range: 3 s) (see Figure 2c). The mean quality of life score was 60.6 (SD 21.2). Quality of life data were missing for four cases. Of these, three cases were female, two had a performance status of “0,” and two had a performance status of “1.” Causes of missing data include withdrawal from the study due to disease progression (n = 2), patient losing the questionnaire (n = 1), and questionnaires not returned to study researchers (n = 1).
Figure 2.
(a) Dot plot of mean score and criterion cutoff for the six-minute walk test with cutoff of 559 m. As a descriptive tool, patient data are de-identified and labeled from A to G1 facilitating cross-assessment comparisons. For example, “Case D” scored below the cutoff for the six-minute walk test, but above for the thirty-second sit-to-stand test, indicating a possible deficit in aerobic endurance, but respectable lower limb strength. Data for all cases, sample mean, and criterion cutoff are provided in Supplementary Table 2a. (b) Dot plot of mean score and criterion cutoff for the thirty-second sit-to-stand test with criterion cutoff of 15 stands. Data for all cases, sample mean, and criterion cutoff are provided in Supplementary Table 2b. (c) Dot plot of median score for the timed “Up & Go” test with criterion cutoff of 13.5 s. Data for all cases, sample median, and criterion cutoff are provided in Supplementary Table 2c.
The mean distance covered during the six-minute walk test in this sample was significantly lower than the 559-m cutoff, t(32) = −5.96, P < .001, bias-corrected accelerated 95% CI [370.7-460.4], with an absolute mean difference of 142.9 m. Likewise, the mean number of stands achieved during the thirty-second sit-to-stand in this sample were significantly lower from cutoff criterion of 15 stands, t(32) = −4.60, P < .01, bias-corrected accelerated 95% CI [11.0-13.4] with an absolute mean difference of 2.8 stands. Wilcoxon signed-rank test identified significantly shorter median time taken to complete the timed “Up & Go” test compared to the cutoff criterion of 13.5 s, z = −4.43, P < .01. There was no meaningful relationship between meters walked and perceived quality of life (r = −0.02, bias-corrected accelerated 95% CI [−0.56 to 0.66], P = .90, n = 29). There was a positive moderate relationship between the thirty-second sit-to-stand and perceived quality of life (r = 0.42, bias-corrected accelerated 95% CI [−0.05 to 0.75], P = .02, n = 29). The Spearman correlation coefficient between the timed “Up & Go” and perceived quality of life score was negative and weak (rs = −0.15, bias-corrected accelerated 95% CI [−0.53 to 0.27], P = .45, n = 29).
Discussion
The primary aim of this study was to describe physical functioning in a sample of patients with glioma after surgical intervention and before radiation therapy. The results of this study indicate patient’s aerobic endurance and lower limb strength were below criterion cutoff recommended to maintain independent living. Importantly, timed “Up & Go” scores did not exceed the criterion cutoff, indicating respectable levels of mobility within the current sample.
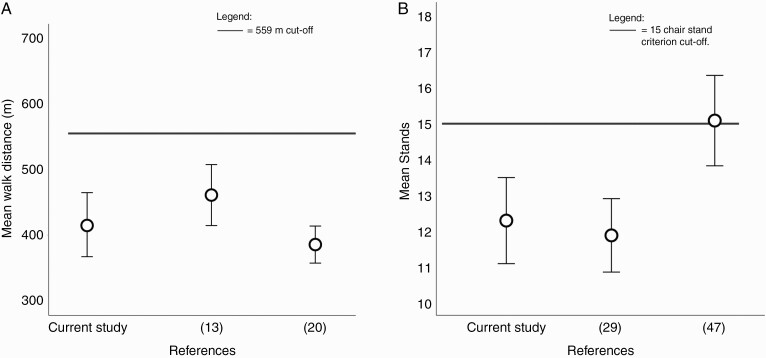
In our sample, the mean distance walked during the six-minute walk test was comparable to findings of previous studies investigating functional capacity in similarly aged patients with glioma (Figure 1a).13,20 Furthermore, performance of our sample was significantly below the 559-m cutoff reported for community-dwelling 60- to 69 years old and cutoffs for maintaining independent living.27,34 In fact, 30 (90.9%) participants were below the cutoff (Figure 2a). Based on these results, our sample presented with an aerobic endurance similar to those of community-dwelling adults aged 80- to 89 years old34 prior to undergoing adjuvant therapy, a value approximately 40 years older than our sample mean.
Figure 1.
(a) Error plot illustrating the distribution of mean and 95% confidence intervals for six-minute walk test distance in our sample of glioma patients compared with walk distances in two other published glioma studies.20,13 Despite recruiting glioma patients of similar age, all studies report a mean walk distance below the 559-m cutoff. Jones et al.20 reported a mean walk distance of 390.0 m (SD 93.0 m). Ruden et al.13 reported a mean walk distance of 448.0 m (SD 135.0 m), while this study reported a mean walk distance of 416.2 m (SD 137.6 m). Further study characteristics are provided in Supplementary Table 1. (b) Error plot illustrating the distribution of mean and 95% confidence intervals for stands completed within the thirty-second sit-to-stand in our sample of glioma patients compared to healthy community-dwelling 80- to 89 years old.29,47 Jones et al.29 reported a mean of 11.9 stands (SD 3.2 stands) and Tveter et al.47 reported a mean of 15.4 stands (SD 3.3 stands), while this study reported a mean of 12.2 stands (SD 3.3 stands). Further study characteristics are provided in Supplementary Table 1.
Similarly, we report a significant difference between the chair stands achieved in our sample and the 15-chair-stand criterion cutoff. In fact, 23 (69.7%) participants were below the cutoff (Figure 2b), suggesting possible deficits in lower limb strength. Although declines in strength reflect normal age-related changes, our data suggest these participants have lower extremity strength equivalent to elderly 80-89 years of age (Figure 1b).29,47 Clinically, the thirty-second sit-to-stand test is a good indicator of lower body strength in older adults, demonstrating a strong association with knee extensor and flexor strength, walking speed, stair climbing ability, and balance.34,48 Our results suggest that glioma patients are at a greater risk of impaired lower limb strength.
The timed “Up & Go” test is a tool used to assess motor function, postural control, and risk of falls in the geriatric population. The criterion cutoff of 13.5 s is used where a completion time greater than the cutoff indicates increased risk of mobility issues and falls. In this study, 32 (97%) of the participants were below the cutoff, with only one participant (3%) completing the assessment in 27 s (Figure 2c). These results suggest participants were not at risk for mobility or falls and are comparable to a previously published descriptive meta-analysis on apparently healthy elders.49 However, caution is warranted when interpreting this result as several factors may at play. Firstly, there is no consensus within the literature as to which “cutoff” times are the most appropriate to use. Some authors set “greater than 10 s” as the cutoff for mobility and falls, whereas others suggest that a time “greater than 20 s” to be the more appropriate cutoff.39,41 For the purposes of this study, the 13.5-s cutoff was selected due it its ability to identify the risk of mobility and falls and being a conservative estimate between two opposing values. Secondly, the mean age in our sample was below the target age range for this assessment, possibly lending to the shorter completion times.50 Thirdly, given our study cohort predominantly had ECOG performance status of 0-1, this may not be representative of more frail patients who would otherwise be at risk of mobility deficits and falls.
A second objective was to examine whether functional measures correlated with perceived quality of life. Here, we found that in general, being able to complete more stands was associated with better quality of life. These results suggest that muscular strength in the lower limbs is associated with the ability to perform activities which are reflective of independent daily living, including stair climbing and rising from a chair. Ultimately, these transfer skills may be important factors impacting quality of life. There were no associations of importance between the six-minute walk and the timed “Up & Go” assessments and quality of life. The lack of association between aerobic endurance and timed “Up & Go” assessments may be due to the questionnaire chosen to measure the quality of life. Previous studies reporting association between the six-minute walk test and quality of life have used the FACT (Functional Assessment of Cancer Therapy) Brain Cancer questionnaire.7,20 Consistent with our primary hypothesis, our assessments indicate a lack of lower limb strength and poor aerobic endurance overall in this group of patients; however, mobility appears to be functional. Our secondary hypothesis was not supported as only the thirty-second sit-to-stand showed any association with quality of life.
Study Limitations
This study has a number of limitations. Firstly, recruitment bias may affect the generalizability of the results. Secondly, given our selection criteria and the performance status of our population, the generalizability of these results is limited to participants with good performance status. Finally, correlations generated in this study are based on cross-sectional analysis, providing insight into correlations between physical capacity and quality of life; however, it is not possible to establish a causal relationship using this approach. Further caution is required when interpreting the comparisons within the study due to the number of univariate statistical analyses undertaken within a small sample size and no adjustments to the significance level used (eg, no Bonferroni adjustment). This analysis was intended as a preliminary description to identify possible deficits in functional capacity that may impact long-term functioning and quality of life in glioma patients. Future research should build on this analysis by using a sufficiently powered sample size and multivariate analysis undertaken over a longer duration to confirm and expand the results of this study. Additionally, investigations should continue to focus on clinically relevant outcomes and their correlates with poorer functional capacity to identify outcomes that may contribute to poorer quality of life.
In summary, our results indicate that even in a group of relatively young patients with good performance status able to participate in an exercise intervention after surgery for glioma, poor aerobic endurance, and reductions in lower limb strength were apparent. These deficits are similar to those observed in community-dwelling 80- to 89 years old. Although the timed “Up & Go” test was below criterion cutoff, these results should be interpreted with caution as our sample age was below the average age used to test the timed “Up & Go” test. Our study also reports a moderate association between lower body strength and quality of life. There is a need to provide rehabilitation and management services including exercise to improve functional capacity and other important end points in glioma patients. As a result, our research group is investigating the feasibility and safety of individualized exercise during adjuvant therapy. Quantitative assessments of functional capacity and supervised tailored exercise interventions may complement existing management therapies, possibly minimize the strength and cardiovascular deficits, and assist with the shift toward person-centered care.
Supplementary Material
Acknowledgments
Clinical trial registration number: ACTRN12616000874415. Study number: 369 599.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. Not applicable/none declared.
References
- 1. Australian Institute of Health and Welfare. Brain and Other Central Nervous System Cancers. Canberra: AIWH; 2017. [Google Scholar]
- 2. Hojan K. Challenges of rehabilitation for patients with primary malignant glioma – a review of recent literature. J Med Sci. 2016;85(2):131–137. [Google Scholar]
- 3. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong TS, Cron SG, Bolanos EV, et al. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. [DOI] [PubMed] [Google Scholar]
- 5. Jones LW, Mourtzakis M, Peters KB, et al. Changes in functional performance measures in adults undergoing chemoradiation for primary malignant glioma: a feasibility study. Oncologist. 2010;15(6):636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cormie P, Zopf EM, Zhang X, et al. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 2017;39(1):71–92. [DOI] [PubMed] [Google Scholar]
- 7. Huang ME, Wartella JE, Kreutzer JS. Functional outcomes and quality of life in patients with brain tumors: a preliminary report. Arch Phys Med Rehabil. 2001;82(11):1540–1546. [DOI] [PubMed] [Google Scholar]
- 8. Knobf MT, Musanti R, Dorward J. Exercise and quality of life outcomes in patients with cancer. Semin Oncol Nurs. 2007;23(4):285–296. [DOI] [PubMed] [Google Scholar]
- 9. Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayes SC, Newton RU, Spence RR, et al. The Exercise and Sports Science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–1199. [DOI] [PubMed] [Google Scholar]
- 12. Jones LW, Friedman AH, West MJ, et al. Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer. 2010;116(3):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruden E, Reardon DA, Coan AD, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29(21):2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solway S, Brooks D, Lacasse Y, et al. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. [DOI] [PubMed] [Google Scholar]
- 15. Bassile CC, Dean C, Boden-Albala B, et al. Obstacle training programme for individuals post stroke: feasibility study. Clin Rehabil. 2003;17(2):130–136. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi E, Himuro N, Takahashi M. Clinical utility of the 6-min walk test for patients with moderate Parkinson’s disease. Int J Rehabil Res. 2017;40(1):66–70. [DOI] [PubMed] [Google Scholar]
- 17. Ries JD, Echternach JL, Nof L, et al. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569–579. [DOI] [PubMed] [Google Scholar]
- 18. Ying L, Yahng JJ, Fisher M, et al. Walking the boundaries: using the 6-min walk test for accurate assessment of the level of fitness in breast clinic outpatients. ANZ J Surg. 2020;90(6):1141–1145. [DOI] [PubMed] [Google Scholar]
- 19. Villumsen BR, Jorgensen MG, Frystyk J, Hørdam B, Borre M. Home-based ‘exergaming’ was safe and significantly improved 6-min walking distance in patients with prostate cancer: a single-blinded randomised controlled trial [published online ahead of print April 22, 2019]. BJU Int. doi: 10.1111/bju.14782. [DOI] [PubMed] [Google Scholar]
- 20. Jones LW, Cohen RR, Mabe SK, et al. Assessment of physical functioning in recurrent glioma: preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009;94(1):79–85. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34(7):631–636. [DOI] [PubMed] [Google Scholar]
- 22. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 23. Cahalin LP, Mathier MA, Semigran MJ, et al. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110(2):325–332. [DOI] [PubMed] [Google Scholar]
- 24. Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80(7):837–841. [DOI] [PubMed] [Google Scholar]
- 25. Wallaert B, Monge E, Le Rouzic O, et al. Physical activity in daily life of patients with fibrotic idiopathic interstitial pneumonia. Chest. 2013;144(5):1652–1658. [DOI] [PubMed] [Google Scholar]
- 26. Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance in COPD. Chest. 2007;132(6):1778–1785. [DOI] [PubMed] [Google Scholar]
- 27. Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–156. [DOI] [PubMed] [Google Scholar]
- 28. Rickli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2), 129–161. [Google Scholar]
- 29. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. [DOI] [PubMed] [Google Scholar]
- 30. Johansen KL, Stistrup RD, Madsen J, Schjott CS, Vinther A. The timed up and go test and 30 second Chair-Stand test are reliable for hospitalized patients with stroke. Physiotherapy. 2015;101(1):e918. [Google Scholar]
- 31. Østergren P, Ragle AM, Jakobsen H, et al. Group-based exercise in daily clinical practice to improve physical fitness in men with prostate cancer undergoing androgen deprivation therapy: study protocol. BMJ Open. 2016;6(6):e011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shea CA, Ward RE, Welch SA, et al. Inability to perform the repeated chair stand task predicts fall-related injury in older primary care patients. Am J Phys Med Rehabil. 2018;97(6):426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. [DOI] [PubMed] [Google Scholar]
- 34. Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53(2):255–267. [DOI] [PubMed] [Google Scholar]
- 35. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 36. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 37. Matinolli M, Korpelainen JT, Korpelainen R, et al. Mobility and balance in Parkinson’s disease: a population-based study. Eur J Neurol. 2009;16(1):105–111. [DOI] [PubMed] [Google Scholar]
- 38. Huisman MG, van Leeuwen BL, Ugolini G, et al. “Timed Up & Go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One. 2014;9(1):e86863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beauchet O, Fantino B, Allali G, et al. Timed Up and Go test and risk of falls in older adults: a systematic review. J Nutr Health Aging. 2011;15(10):933–938. [DOI] [PubMed] [Google Scholar]
- 40. Donoghue OA, Savva GM, Cronin H, et al. Using timed up and go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch Phys Med Rehabil. 2014;95(10):1954–1961. [DOI] [PubMed] [Google Scholar]
- 41. Nnodim JO, Yung RL. Balance and its clinical assessment in older adults – a review. J Geriatr Med Gerontol. 2015;1(1):003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bischoff HA, Stähelin HB, Monsch AU, et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. [DOI] [PubMed] [Google Scholar]
- 43. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Phys Ther. 2002;82(2):128–137. [DOI] [PubMed] [Google Scholar]
- 44. Rockwood K, Awalt E, Carver D, et al. Feasibility and measurement properties of the functional reach and the timed up and go tests in the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2000;55(2):M70–M73. [DOI] [PubMed] [Google Scholar]
- 45. Bloch ML, Jønsson LR, Kristensen MT. Introducing a third timed up & go test trial improves performances of hospitalized and community-dwelling older individuals. J Geriatr Phys Ther. 2017;40(3):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A.. The EORTC QLQ-C30 Scoring Manual. 3rd ed.Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 47. Tveter AT, Dagfinrud H, Moseng T, et al. Health-related physical fitness measures: reference values and reference equations for use in clinical practice. Arch Phys Med Rehabil. 2014;95(7):1366–1373. [DOI] [PubMed] [Google Scholar]
- 48. Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills. 1995;80(1):163–166. [DOI] [PubMed] [Google Scholar]
- 49. Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–68. [DOI] [PubMed] [Google Scholar]
- 50. Ibrahim A, Singh DKA, Shahar S. ‘Timed Up and Go’ test: age, gender and cognitive impairment stratified normative values of older adults. PLoS One. 2017;12(10):e0185641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.