Abstract
Mycobacterium leprae was detected by optical microscopy, fluorescent in situ hybridization, and molecular detection in feces collected for the diagnosis of Entamoeba coli enteritis in a leprosy patient in Burkina Faso. This observation raises questions about the role of fecal excretion of M. leprae in the natural history and diagnosis of leprosy.
Keywords: DDD, FISH, Mycobacterium, Mycobacterium leprae, bacteria, bacterial infections, leprosy, fecal excretion, stool specimens, Burkina Faso, tuberculosis and other mycobacteria
Leprosy caused by Mycobacterium leprae remains endemic in Burkina Faso, a West Africa country with a level of disability 2 of 31.2% among new patient cases (1). Laboratory diagnosis of leprosy is determined by observation of acid-fast bacilli after microscopic examination of a Ziehl-Neelsen–stained nasal smears and cutaneous lesions (1). Recently, fluorescence in situ hybridization (FISH) was introduced as a complementary approach to increase the specificity of microscopic observations (1,2). We report on the specific microscopic detection of M. leprae in the stool specimen of a patient in Burkina Faso.
A 20-year-old man originating from the village of Bama in Burkina Faso sought care at the dermatology department at the Centre Hospitalier Universitaire Souro Sanou (Bobo-Dioulasso, Burkina Faso) for multiple infiltrated papules and nodules on his face and ear pavilions. These symptoms were accompanied by rhinitis and nosebleeds, which had been evolving for >2 months. Clinical examination further showed nasal enlargement (papulonodular), ulcerative-crusted lesions on the limbs, ulnar nerve hypertrophy, and a sausage-like appearance of the fingers, all of which suggested a lepromatous form of leprosy. A nasal smear and skin biopsy were performed on an infiltrative lesion (right arm), and 3 swab specimens were collected from a skin wound (left forearm), crusted lesions (elbow of right arm), and ulcerative papules (left arm). All samples were microscopically examined after Ziehl-Neelsen staining and revealed acid-fast bacilli in all 5 samples. Acid-fast bacilli were further identified as M. leprae by partial PCR amplification sequencing of the rpoB gene using a validated protocol (1).
The patient also had abdominal pain, and stool samples were collected to check for parasites. Microscopic examination (at 400× magnification) of fresh stool specimens mixed with Lugol’s solution revealed cysts containing >6 nuclei, suggesting cysts of Entamoeba coli. Microscopic examination of the stool specimens filtrate after Ziehl-Neelsen staining (at 60× magnification) revealed 2 acid-fast bacilli per 300 microscopic fields (Figure).
Figure.
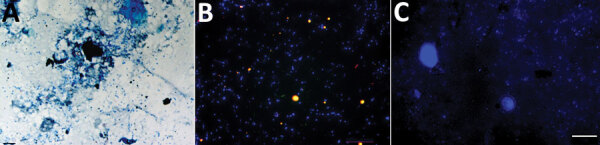
Optical microscopy observation of Mycobacterium leprae in the stool specimens of a leprosy patient in Burkina Faso. A) Ziehl-Neelsen staining; B) fluorescence in situ hybridization. No mycobacteria were observed inside the Entamoeba coli cysts (C). Scale bars represent 10 (A), 20 (B), and 20 (C) microns.
Identification of the pathogens was confirmed by a PCR-based method and FISH for M. leprae (Appendix). Because M. leprae has been identified as an intra-amoebal pathogen (3), we tested the intracystic location of M. leprae by FISH in clarified stool specimens using sucrose-density gradients. In brief, the cyst wall was permeabilized by incubating stool specimen in 1 mL of cellulase (Sigma Aldrich, https://www.sigmaaldrich.com) for 48 hours at 45°C (4). After cellulase activity was stopped by washing with physiologic water and 5 minutes of centrifugation at 3,000 g, the pellet was incorporated into 4’,6-diamidino-2-phenylindole-FISH staining. Observation of 8 Escherichia coli cysts disclosed nuclei stained with 4’,6-diamidino-2-phenylindole and an absence of any detectable M. leprae by FISH (Figure). Dynamic, dormant, and dead staining to identify the viability of mycobacteria (5) revealed dead mycobacteria in the skin biopsy, the 3 cutaneous swab specimens, and stool specimens, whereas 8 bacilli out of a total of 22 observed in a series of 6 microscopic fields in the nasal smear were dynamic (Appendix Figure).
Previous reports relied only on Ziehl-Neelsen staining to assess the presence of acid-fast bacilli in stool specimens collected from patients in whom leprosy was diagnosed, without any further formal identification (6,7). In the patient we report, stoolborne acid-fast bacilli were identified as M. leprae by 2 independent methods in the presence of negative controls. These M. leprae organisms were possibly swallowed by the patient along with blood or upper respiratory secretions during leprosy rhinitis and epistaxis (7). This observation correlates with a study in armadillos, an M. leprae host in some leprosy-endemic regions, in which experimental infection results in the extensive involvement of the intestine and the presence of M. leprae in stools (8). In the stool specimens of the patient described in this study, only dead M. leprae cultures were observed using dynamic, dormant, dead staining, whereas dynamic mycobacteria were detected in the nasal smear (9).
On the basis of this research, further studies are required to confirm the prevalence of fecal excretion of M. leprae in various leprosy populations. Because stools are a noninvasive specimen, they could be collected for the positive diagnosis of leprosy using appropriate laboratory methods, as reported for the positive diagnosis of pulmonary tuberculosis (10).This diagnostic approach is easy to implement, including in children, in contrast to the current biopsy procedure, which requires a qualified staff and postsurgical management.
Additional information about fecal excretion of Mycobacterium leprae, Burkina Faso.
Acknowledgments
This work was supported by the government of France under the Investments for the Future Program managed by France’s National Research Agency (reference: Méditerranée Infection [project no. 10-IAHU-03]). This work also was supported by the Région Le Sud, Provence Alpes Côte d’Azur, and European 95 funding (grant no. FEDER PA 0000320 PRIMMI).
Biography
Mr. Millogo is a biology doctoral student at the University of Montpellier, France, and Institut Hospitalo-Universitaire Méditerranée Infection, Marseille, France, and a biologist at the Souro Sanou University Hospital in Bobo-Dioulasso, Burkina Faso. His primary research interests include cutaneous mycobacterioses.
Footnotes
Suggested citation for this article: Millogo A, Loukil A, L’Ollivier C, Djibougou DA, Godreuil S, Drancourt M. Fecal excretion of Mycobacterium leprae, Burkina Faso. Emerg Infect Dis. 2021 Jun [date cited]. https://doi.org/10.3201/eid2706.200748
References
- 1.Millogo A, Loukil A, Fellag M, Diallo B, Ouedraogo AS, Godreuil S, et al. Fluorescent hybridization of Mycobacterium leprae in skin samples collected in Burkina Faso. J Clin Microbiol. 2020;58:e02130–19. 10.1128/JCM.02130-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musso D, Rovery C, Loukil A, Vialette V, Nguyen NL. Leprosy in French Polynesia. New Microbes New Infect. 2019;29:100514. 10.1016/j.nmni.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahiri R, Krahenbuhl JL. The role of free-living pathogenic amoeba in the transmission of leprosy: a proof of principle. Lepr Rev. 2008;79:401–9. 10.47276/lr.79.4.401 [DOI] [PubMed] [Google Scholar]
- 4.Brahim Belhaouari D, Baudoin JP, Gnankou F, Di Pinto F, Colson P, Aherfi S, et al. Evidence of a cellulosic layer in Pandoravirus massiliensis tegument and the mystery of the genetic support of its biosynthesis. Front Microbiol. 2019;10:2932. 10.3389/fmicb.2019.02932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loukil A, Darriet-Giudicelli F, Eldin C, Drancourt M. Pulmonary tuberculosis conversion documented by microscopic staining for detection of dynamic, dormant, and dead mycobacteria (DDD staining). J Clin Microbiol. 2018;56:e01108–18. 10.1128/JCM.01108-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzullo A, Manzi RO, Lefevre A, Oteiza ML. Investigation of acid-fast bacilli in the digestive tract of leprosy patients. Leprologia. 1965;10:14–6. [Google Scholar]
- 7.Koshy A, Karat ABA. A study of acid-fast bacilli in the urine, gastric juice and feces of patients with lepromatous leprosy. Lepr India. 1971;43:3–7. [Google Scholar]
- 8.Kirchheimer WF, Storrs EE, Binford CH. Attempts to establish the Armadillo (Dasypus novemcinctus linn.) as a model for the study of leprosy. II. Histopathologic and bacteriologic post-mortem findings in lepromatoid leprosy in the Armadillo. Int J Lepr Other Mycobact Dis. 1972;40:229–42. [PubMed] [Google Scholar]
- 9.Palomino JC, Falconi E, Marin D, Guerra H. Assessing the viability of Mycobacterium leprae by the fluorescein diacetate/ethidium bromide staining technique. Indian J Lepr. 1991;63:203–8. [PubMed] [Google Scholar]
- 10.Drancourt M. Culturing stools to detect Mycobacterium tuberculosis. J Clin Microbiol. 2018;56:e02033–17. 10.1128/JCM.02033-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about fecal excretion of Mycobacterium leprae, Burkina Faso.


