Abstract
Anopheles stephensi mosquitoes share urban breeding sites with Aedes aegypti and Culex quinquefasciatus mosquitoes in the Republic of Djibouti. We present evidence that A. stephensi mosquitoes might be responsible for an increase in malaria incidence in this country. We also document resistance of Plasmodium falciparum to dihydroartemisinin/piperaquine.
Keywords: malaria, Anopheles stephensi, urban malaria, outbreak, resistance, antimalarial drug, Plasmodium falciparum, Plasmodium vivax, Djibouti, mosquitoes, vector-borne infections, parasitic infections, mosquito-borne infections, parasites, antimicrobial resistance
The Republic of Djibouti, bordered by Eritrea, Ethiopia, and Somalia, is a semiarid country in the Horn of Africa. The population comprises <900,000 persons, 70% of whom live in Djibouti, the capital city. Before 2013, malaria was hypoendemic to the country, with low levels of transmission in periruban and rural areas during December–May. Localized outbreaks occurred regularly, possibly caused by migration from surrounding countries. Most cases were caused by infection with Plasmodium falciparum (>80%) or P. vivax. Before 2013, researchers considered the Anopheles arabiensis mosquito to be the primary vector (1).
The incidence of malaria had drastically decreased in the country since 2008; by 2012, this transmission level was compatible with preelimination goals (2,3). In 2013, an autochthonous outbreak of malaria occurred in Djibouti; field entomologic investigations identified An. stephensi mosquitoes as a new malaria vector (4). This species, a known vector of urban malaria in India and the Arabian Peninsula, has changed the epidemiologic profile of malaria in Djibouti (5). In 2018, malaria incidence increased to 25,319 confirmed cases (64% caused by P. falciparum and 36% by P. vivax) and >100,000 suspected cases (Appendix Figure 1).
The French Armed Forces (FAF) have served in Djibouti for decades. Service members and their families (≈2,700 persons) live in the capital. Despite malaria prevention and treatment measures described elsewhere (6), an outbreak among French military personnel occurred in February 2019; failure of early artemisinin combined therapy was documented in 1 patient.
The Study
We collated FAF epidemiologic surveillance data on malaria cases among service members in Djibouti during 1993–2019; the 2019 data included cases among family members. We defined a malaria case as an illness resulting in a positive result on a rapid diagnostic test or thin blood smear.
We conducted the field investigation in the capital during February 28–March 22, 2019. We obtained a dried blood spot on filter paper from each patient and stored the samples in a sealed plastic pouch until processing. We extracted DNA from the samples and confirmed diagnosis using PCR. We sequenced the antimalarial drug resistance molecular markers Pfdhfr, Pfmdr1, Pfcrt, and the propeller domain of PfK13 as described elsewhere (7). We treated patients with a 3-day regimen of dihydroartemisinin/piperaquine and measured levels of parasitemia on days 0 and 3; this treatment failed in 1 patient with malaria caused by P. falciparum. As follow-up for this patient, we collected blood samples from that patient on day 8 to determine piperaquine concentration using liquid chromatographic-tandem mass spectrometry.
We collected adult mosquitoes using human landing catches, CDC light traps, and BG-Sentinel and Suna traps (Biogents, https://www.biogents.com) (Table). We conducted larval prospecting in pools of water in French military camps, Djiboutian military police locations, and the Ambouli Gardens (a public area with a garden market and cattle breeding range). We reared larvae until imago emergence, then identified adult mosquitoes using a morphologic key (Walter Reed Biosystematics Unit, http://vectormap.si.edu/downloads/VHazardReports/VHR_Anopheles_stephensi_2018.pdf). We extracted DNA from the legs of 103 An. stephensi mosquitoes and sequenced the cytochrome oxidase C subunit I gene to confirm morphologic identification. In addition, we conducted a phylogenetic analysis (Appendix Figure 2).
Table. Adult and larval mosquitoes collected by human landing catches and traps, Djibouti, Republic of Djibouti, 2019*.
| Species and sampling method† | No. (% female) | Resources/time expended |
|---|---|---|
| Anopheles stephensi | ||
| HLC | 1 (100.0) | 2 persons/7 h |
| BG Sentinel Trap | 1 (100.0) | 2 traps/120 h |
| Larval emergence | 190 (56.8) | |
| Subtotal |
192 (57.3) |
|
| Aedes aegypti | ||
| HLC | 11 (100.0) | 2 persons/7 h |
| BG Sentinel Trap | 88 (56.8) | 2 traps/120 h |
| Suna Trap | 10 (90.0) | 2 traps/96 h |
| CDC Light Trap | 2 (100.0) | 2 traps/120 h |
| Larval emergence | 32 (46.9) | |
| Subtotal |
143 (60.8) |
|
| Culex quinquefasciatus | ||
| HLC | 113 (100.0) | 2 persons/7 h |
| BG Sentinel Trap | 573 (68.2) | 2 traps/120 h |
| Suna Trap | 221 (57.5) | 2 traps/96 h |
| CDC Light Trap | 408 (66.2) | 2 traps/120 h |
| Larval emergence | 26 (92.3) | |
| Subtotal |
1,341 (69.0) |
|
| Other Culex sp. | ||
| HLC | 43 (100.0) | 2 persons/7 h |
| BG Sentinel Trap | 5 (40.0) | 2 traps/120 h |
| Suna Trap | 2 (100.0) | 2 traps/96 h |
| CDC Light Trap | 10 (100.0) | 2 traps/120 h |
| Larval emergence | 99 (71.7) | |
| Subtotal |
159 (80.5) |
|
| Total | 1,835 (68.1) |
*HLC, human landing catch. †Sampling methods were CDC Light traps, BG-Sentinel and Suna traps (Biogents, https://www.biogents.com), as well as HLC and larval emergence in laboratory. Each BG-Sentinel trap was baited with BG-MB5 attractant (Biogents) and a CO2 production system (>50,000 ppm) based on the fermentation of sugar, yeast, and agar. For larval emergence method, larvae were collected and reared in laboratory until imago emergence.
In the early 2000s, malaria incidence in the FAF was only 1–4 cases per year; during 2011–2013, no cases were documented (Figure 1). Malaria reemerged in 2014 and reached an incidence of 5.9 cases/1,000 persons in 2018 and 8.1 cases/1,000 persons in 2019. In the 2018–19 season, P. falciparum and P. vivax cocirculated (P. falciparum caused 20/38 [53%] cases, P. vivax caused 17/38 [45%] cases, and P. ovale caused 1 [2%] case). Among the country’s population, incidence increased from 25.5 cases/1,000 persons in 2018 to 49.8 cases/1,000 persons in 2019 (Appendix Figure 1) (8). In 2019, we documented 1 instance of treatment failure in a FAF service member with P. falciparum infection; this patient had a thin blood smear showing a parasitemia level of 2.0%. After 3 days of treatment with dihydroartemisinin/piperaquine, the patient still had a fever and 2.0% parasitemia level. The piperaquine plasma concentration on day 8 was 77.7 ng/mL, above the therapeutic threshold (38.1 ng/mL [95% CI 25.8–59.3] expected on day 7), confirming good regimen adherence and absorption (9). This case met the definition for early treatment failure of an artemisinin derivative according to criteria from the World Health Organization (https://apps.who.int/iris/handle/10665/162441). We sequenced molecular markers of resistance to antimalarial drugs for 9 P. falciparum isolates (Appendix Table 3). All isolates had molecular markers associated with resistance to mefloquine. In addition, 89% had resistance markers against chloroquine and pyrimethamine or proguanil. We did not observe any mutations in the K13 propeller region (which sometimes contains mutations associated with artemisinin resistance), including the isolate from the patient in whom treatment failed (10). In Africa, failures of artemisinin combined therapy potentially caused by K13 mutations observed in Southeast Asia remain rarely described (11).
Figure 1.
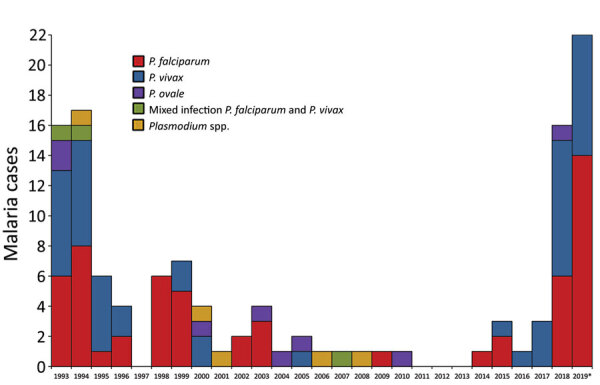
Distribution of 120 malaria cases caused by Plasmodium species among French Armed Force members, Djibouti, Republic of Djibouti, 1993–2019. *Data for 2019 include 2 P. falciparum infections among service members’ families, 1 P. vivax relapse, and 3 P. vivax infections in France imported from Djibouti.
We conducted entomologic investigations during a dry period (i.e., February–March). We collected 1,835 adult mosquitoes and larvae: 1,500 Culex, 143 Aedes aegypti, and 192 An. stephensi (Table). We caught 2 adult An. stephensi mosquitoes using the human landing catch and BG-Sentinel trap. We identified 25 breeding sites, 15 of which contained An. stephensi larvae. All the An. stephensi breeding sites were artificial and located in urban or suburban areas; 9/15 also contained Ae. aegypti larvae, Cx. quinquefasciatus larvae, or both (Appendix Table 2). Examples of An. stephensi breeding sites included manholes, ditches, plastic drums, and water tanks (Figure 2). In military camps, standing water was related to leaks and stagnation caused by faulty maintenance of the water distribution and drainage network. The most productive breeding sites (≈800 water tanks with thousands of An. stephensi larvae) were near livestock areas, mainly in the Ambouli Gardens district. We confirmed morphologic identification of adult An. stephensi mosquitoes by cytochrome oxidase C subunit I sequencing, which identified 8 haplotypes. Phylogenetic trees did not clearly indicate the origin of An. stephensi mosquitoes in Djibouti (Appendix Figure 2).
Figure 2.

Anopheles stephensi breeding sites, Djibouti, Republic of Djibouti, 2019. A) Manhole. B) Ditch. C) Plastic drum. D) Water tank.
Conclusions
In the Republic of Djibouti, malaria transmission has increased since 2013. Even populations with strong malaria control programs, such as the FAF, are now affected. In 2018, the country notified the World Health Organization of ≈100,000 suspected cases, mainly among febrile patients with negative results on a rapid diagnostic test (Appendix Figure 1). Considering these suspected cases, we believe the true incidence could be 5 times higher than the 25,319 cases confirmed that year. A recent study (12) found a high prevalence (86.5%) of pfhrp2 and pfhrp3 gene deletion among P. falciparum parasites in the city of Djibouti.
We documented an early treatment failure of dihydroartemisinin/piperaquine in an isolate lacking a K13 mutation. This finding could signal the emergence of P. falciparum resistance to artemisinin derivatives in Djibouti.
An. stephensi mosquitoes are well-established in Djibouti and have been observed in Sudan and Ethiopia (13). Our study shows that this species shares breeding sites with Ae. aegypti and Cx. quinquefasciatus mosquitoes, highlighting its adaptation to urban areas. Models predict broad expansion of An. stephensi mosquito distribution into major cities in Africa, where large malaria outbreaks could occur among growing resident populations susceptible to the disease (14). Furthermore, a high level of resistance among mosquitoes to all insecticide families (e.g., organochlorates, pyrethroids, carbamates, and organophosphates) has been described in Djibouti and Ethiopia (8,15). In semiarid regions such as the Republic of Djibouti, residents often store water in plastic drums that act as breeding sites for An. stephensi mosquitoes. To control malaria and limit the spread of this anopheline species, communities and governments should prioritize larval control and access to the water distribution network.
Additional information on role of Anopheles stephensi in malaria outbreak, Djibouti, 2019.
Biography
Dr. Pommier de Santi is a military physician and specialist in public health and epidemiology at the French Armed Forces Center for Epidemiology and Public Health, Marseille, France. His research interests include vectorborne diseases and other tropical diseases affecting the French Armed Forces and travelers.
Footnotes
Suggested citation for this article: Pommier de Santi V, Khaireh BA, Chiniard T, Pradines V, Taudon N, Larréché S, et al. Role of Anopheles stephensi mosquitoes in malaria outbreak, Djibouti, 2019. Emerg Infect Dis. 2021 June [date cited]. https://doi.org/10.3201/eid2706.204557
References
- 1.Khaireh BA, Assefa A, Guessod HH, Basco LK, Khaireh MA, Pascual A, et al. Population genetics analysis during the elimination process of Plasmodium falciparum in Djibouti. Malar J. 2013;12:201. 10.1186/1475-2875-12-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World malaria report 2019. 2019 [cited 2020 Oct 21]. https://apps.who.int/iris/rest/bitstreams/1262394/retrieve
- 3.Ollivier L, Nevin RL, Darar HY, Bougère J, Saleh M, Gidenne S, et al. Malaria in the Republic of Djibouti, 1998–2009. Am J Trop Med Hyg. 2011;85:554–9. 10.4269/ajtmh.2011.11-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014;139:39–43. 10.1016/j.actatropica.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 5.Seyfarth M, Khaireh BA, Abdi AA, Bouh SM, Faulde MK. Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established—malaria emerging. Parasitol Res. 2019;118:725–32. 10.1007/s00436-019-06213-0 [DOI] [PubMed] [Google Scholar]
- 6.Migliani R, Pradines B, Michel R, Aoun O, Dia A, Deparis X, et al. Malaria control strategies in French Armed Forces. Travel Med Infect Dis. 2014;12:307–17. 10.1016/j.tmaid.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Voumbo-Matoumona DF, Akiana J, Madamet M, Kouna LC, Lekana-Douki JB, Pradines B. High prevalence of Plasmodium falciparum antimalarial drug resistance markers in isolates from asymptomatic patients from the Republic of the Congo between 2010 and 2015. J Glob Antimicrob Resist. 2018;14:277–83. 10.1016/j.jgar.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 8.Djibouti Ministry of Health. National strategic plan to fight malaria, 2020–2024 [in French]. 2020. [cited 2020 Oct 22]. https://erc.undp.org/evaluation/managementresponses/keyaction/documents/download/3685
- 9.Hoglund RM, Workman L, Edstein MD, Thanh NX, Quang NN, Zongo I, et al. Population pharmacokinetic properties of piperaquine in Falciparum malaria: an individual participant data meta-analysis. PLoS Med. 2017;14:e1002212. 10.1371/journal.pmed.1002212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5. 10.1038/nature12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foguim Tsombeng F, Gendrot M, Robert MG, Madamet M, Pradines B. Are k13 and plasmepsin II genes, involved in Plasmodium falciparum resistance to artemisinin derivatives and piperaquine in Southeast Asia, reliable to monitor resistance surveillance in Africa? Malar J. 2019;18:285. 10.1186/s12936-019-2916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iriart X, Menard S, Chauvin P, Mohamed HS, Charpentier E, Mohamed MA, et al. Misdiagnosis of imported falciparum malaria from African areas due to an increased prevalence of pfhrp2/pfhrp3 gene deletion: the Djibouti case. Emerg Microbes Infect. 2020;9:1984–7. 10.1080/22221751.2020.1815590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkew M, Mumba P, Dengela D, Yohannes G, Getachew D, Yared S, et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 2020;13:35. 10.1186/s13071-020-3904-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci U S A. 2020;117:24900–8. 10.1073/pnas.2003976117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yared S, Gebressielasie A, Damodaran L, Bonnell V, Lopez K, Janies D, et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar J. 2020;19:180. 10.1186/s12936-020-03252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on role of Anopheles stephensi in malaria outbreak, Djibouti, 2019.


