Abstract
The chemical and alignment structures of coal impacts coalbed methane behavior: adsorption, desorption, and diffusion. Recently, the research on accurate characterization techniques for coal structure has received widespread attention. In particular, spatial alignment is critical for the molecular modeling of coal. However, due to the great challenges of quantification, spatial alignment has often been ignored in previous studies. In this study, high-resolution transmission electron microscopy (HRTEM) was employed to quantitatively characterize the fringe length, orientation, and stacking distributions of these five coal samples with different ranks. Raman spectroscopy was utilized to investigate the overall structural disorder of the coal molecules. 13C nuclear magnetic resonance (13C NMR) was conducted to characterize the chemical structures of coals, and XRD experiments recorded the transition of the microcrystallite structure. The results show that in the range of %Ro = 0.39–2.07%, the distributions of the aromatic structural units were similar: mainly composed of fringes of size equivalent to naphthalene and 2 × 2 and 3 × 3 rings. When %Ro > 2.07%, the distribution shifted to longer fringes. Moreover, all the samples showed a regional orientation, and when %Ro > 2.07%, there was significantly higher alignment. The degree of stacking of fringes were limited, most of which appeared in the form of a single layer. When %Ro < 2.07%, the stacking appeared in the form of two or three layers. However, five-layer stacking merely appeared in the sample with %Ro = 2.47%. In addition, based on the Raman data, the evolution of carbon disorder was divided into three stages: %Ro = 0.39–1.23%, 1.23–2.07%, and 2.07–2.47%, and aromatization caused the overall disorder to decrease. The 13C NMR data indicated that the chemical structure also transitioned in stages, with aliphatic carbon and oxygen-containing groups gradually decreasing and aromatic carbon increasing. Meanwhile, the XRD data supported increased organization (lower d002 values) with maturities. Thus, this study provides quantitative information about the spatial alignment and the size of aromatic rings, which helps to improve a comprehensive understanding of the chemical structure of coal and coalbed methane behaviors.
1. Introduction
The complex and strongly heterogeneous nature of coal make its structural characterization very challenging.1−3 Coal holds a considerable volume of coalbed methane, and the structural variations have a significant influence on gas storage and transport.4−7 The micropores inside and between the macromolecules of coal are the significant gas storage locations.8−11 The chemical and alignment structures will impact these micropores, indirectly affecting the interaction with gas. In fact, many previous studies have found that the micropore variations of coal are directly controlled by the coal chemical structure.4,12−14 However, most studies merely focused on the influence of chemical and microcrystalline structures on micropores, and a few studies have recognized that the spatial alignment of polycyclic aromatic hydrocarbons (PHA) is also a crucial factor affecting the structure of the micropores.15,16 It has been revealed that the micropore structure in coal is closely related to the spatial alignment of the aromatic fringes.15 Therefore, quantifying the variation of the spatial alignment and structure of coal will be beneficial for improving the understanding of coal structure characterization and the interaction between gas molecules.
With software development and technological progress, numerous modern analytical techniques have been applied to investigate new information on complicated chemical structural changes in recent years,17 for instance, elemental analysis, Raman spectroscopy, solid-state 13C nuclear magnetic resonance(NMR)spectroscopy,1,18,19 high-resolution transmission electron microscopy (HRTEM),20−22 X-ray diffraction (XRD),2,23−25 and atomic force microscopy (AFM).26 It is well accepted that the fraction of aliphatic carbon is high and structural ordering is low in low-rank coals, while the structural order is significantly enhanced in high-rank coals.25,27 This factor affects the pore evolution and CBM enrichment properties. Therefore, the topic of coal structure variation has been the core of research in recent years. Many researchers have integrated multiple analytical methods to examine the variation of the chemical structures. From the Raman data, He et al.28 studied the carbon disorder degree transition of a series of coals varying in rank from peat to anthracite and found that the degree of carbon disorder gradually decreased and led to an increase in the degree of graphitization during the process of coalification. Guedes et al.29 reached a similar conclusion. In addition, Erdenetsogt et al.30 studied the chemical structure transition via the 13C NMR technique from lignite to sub-bituminous coal. They also concluded that oxygen-containing functional groups were gradually replaced by carbon substituents, and the carbonyl and oxidized aliphatic carbon decreased during the coalification process.
Apart from the chemical structure, research regarding the variation of the crystallite structure has also achieved successfully unanimous conclusions, among which the structural parameters, such as interlayer spacing (d002) and crystallite sizes La and Lc, have been considered31 for evaluating the stacking structure of coal from X-ray diffractograms. Baysal et al.17 characterized the degree of ordered structure of Turkish lignite via XRD experiments, and the results demonstrated that the crystal structure of the lignite has a slight alignment and low structural order. Similarly, Okolo et al.32 determined that low-rank coals are structurally less orderly than high-rank coals. In addition, Sharma et al. reported that the XRD analysis results and the improved HRTEM technology have achieved good consistency in characterizing the carbon stacking number and stacking layers.
Raman, 13C NMR, XRD, and other spectroscopic techniques have enhanced the knowledge of the average parameter transitions in the coal chemical structure during coalification. However, HRTEM micrograph analysis can directly obtain the distribution and structural organization of aromatic fringes. Previous studies have studied carbons,33−35 char,36−38 and kerogen39,40 via HRTEM regarding the parameter information of the aromatic fringe length, orientation, and stacking distribution. Sharma et al.41 developed a new filtration technique for HRTEM micrographs and new computer algorithms to quantitatively analyze the size of the aromatic layer, interlayer spacing, and stacking in the coal char and proposed that there are more stacking layers and higher fringe lengths in high-rank coals than in low-rank coals. Assuming that the aromatic fringes are long and deep (i.e., parallelograms), the distribution of the aromatic molecular mass can be estimated. Based on the new approach coupling HRTEM lattice fringe image data, Mathews et al.42 estimated the molecular weight distribution of Pocahontas No. 3 low-volatile bituminous coal, which was in good agreement with the laser desorption ionization mass spectra (LDIMS) technique. Thus, the molecular weight distribution estimated by HRTEM micrographs was reliable. Meanwhile, Okolo et al.32 also investigated the chemical structure of four South African bituminous coals based on HRTEM micrographs and found that high-rank coals have higher molecular weights. In addition, this approach has been widely used in the construction of coal molecular models. For instance, Narkiewicz and Matthews43 established the largest Pocahontas No. 3 low-volatile bituminous coal model, and Niekerk and Mathews44 successfully established Permian South African coal and Illinois No. 6 Arogonne Premium coal models. These constructed models determined molecular weights that compared well with the data from NMR, indicating that HRTEM micrographs analysis provides a new insight for coal model construction. Moreover, Louw et al.45 quantified the stacking of aromatic fringes via HRTEM micrographs analysis for coal and found that there are more stacking layers in high-rank coals.
The application of HRTEM provides a new insight into the chemical and spatial structure transitions of the coal structure during coalification. This study primarily aimed at the chemical and spatial alignment of coal structures with five different ranks. Owing to the complexity of the coal structure, it was necessary to integrate multiple advanced techniques to characterize the structure. HRTEM was applied to quantitatively examine the distribution of the fringe length, orientation, and stacking layers. Moreover, Raman spectroscopy was applied to investigate the degree of ordering and crystallinity. Specific functional groups and aromatic and aliphatic components were determined using the 13C NMR cross-polarization magic angle spinning technique. The crystallite parameters were characterized using XRD. The results of the different advanced techniques were compared to explore whether better consistency exists. Because of the limited number of five coal samples with different ranks, which not enough to determine the evolution of coal structure, the study mainly aimed to investigate the chemical and spatial alignment in the different maturation stages. These data will not only help us gain an in-depth and comprehensive understanding of the chemical and physical properties of coal but also can provide a theoretical basis for further studies, including atomistic representations of coal, coal utilization, and even more appropriately obtain coalbed gas simulations.
2. Materials and Methods
2.1. Samples Preparation and Pyrolysis Experiment
The five different maturity samples from well-known coalfields in China were collected: Dananhu coal sample from Xinjiang Autonomous Region (XJ-1), Carboniferous Permian Coal Seam from Guizhou province (GZ-2), Xinjing and Xinyuan Coalfield samples from Shanxi Province (SX-3 and SX-4), and Laochang Coalfield sample from Yunnan Province (LC-5). To avoid experimental differences caused by microscopic components in the coal, in this study, we carefully selected vitrain parts from the raw coal by hand before all the experiments. After separation, the vitrinite content was greater than 95% in the five samples.
Pyrolysis experiments have been considered as an effective method to investigate the coalification process and determine the coal ranks.19,46−48 In this study, we used this experiment to determine the coal ranks and obtain the elemental C and O contents. Before each experiment, the vitrinite samples were crushed with an agate mortar to less than 80 mesh, resulting in approximately 30 g. All the pyrolysis experiments were performed at the Jiangsu Geology and Mineral Design and Research Institute in China. The results for the five coal vitrinite samples are shown in Table 1.
Table 1. Proximate Analysis and Element Analysis of Five Coal Samplesa.
| sample name | Ro% | Mad | Ad% | St,d % | Odaf% | Cdaf% | Hdaf% | Ndaf% |
|---|---|---|---|---|---|---|---|---|
| XJ-1 | 0.39 | 9.54 | 7.03 | 0.72 | 20.56 | 72.60 | 4.80 | 1.26 |
| GZ-2 | 1.23 | 1.02 | 30.39 | 0.91 | 6.94 | 85.30 | 5.05 | 1.41 |
| SX-3 | 2.07 | 0.92 | 22.00 | 0.32 | 6.87 | 87.37 | 4.11 | 1.23 |
| SX-4 | 2.43 | 1.14 | 6.98 | 0.47 | 4.71 | 89.70 | 3.78 | 1.30 |
| LC-5 | 2.47 | 1.92 | 6.25 | 1.05 | 4.22 | 90.60 | 2.96 | 1.10 |
Ro: vitrinite reflectance; Mad: moisture, air dry; Ad: ash, dry; St,d: total sulfur; Odaf: oxygen, dry and ash free; Cdaf: carbon, dry and ash free; Hdaf: hydrogen, dry and ash free; Ndaf: nitrogen, dry and ash free.
2.2. Raman Spectroscopy Analysis
Previous studies have shown that Raman spectroscopy can not only provide information on the maturity of organic matter but also reflect the degree of ordering and crystallinity.49 The Raman experiments in this work were acquired using a Senterra Raman spectrometers produced by Bruker (Germany). Monochromatic excitation was performed with a 532 nm laser with a data acquisition time of 2 s. The spectral resolution of the recorded Stokes Raman spectra was set to 9–18 cm–1 at the Raman shift of 45–4500 cm–1. Extended scans shifting from 1000 to 1800 cm–1 for the first-order and from 2400 to 3000 cm–1 for the second-order were performed on each sample. Here, we focus on the latter. In order to determine the precise band position, width, and relative intensity, the built-in curve fitting analysis tool of the Origin software was used to deconvolute and fit the obtained Raman spectra. For all spectra, a baseline correction was applied to the spectra in the shift range of 1000–2000 cm–1. Finally, the Raman spectrum was divided into five Gaussian band combinations D1, D2, D3, D4, and G, in which the shift of the D1 peak was fixed at approximately 1350 cm–1, and the position of the D2-D5 band was adjusted to obtain the best fitting effect. Furthermore, various Raman parameters were determined: the band position difference of the G and D1 bands (G-D1) and the peak area ratio of the D1 and G bands (AD1/AG).
2.3. Solid-State Cross-Polarization Magic-Angle Spinning (CP/MAS) 13C NMR
After more than three decades of the development of the NMR theory, 13C solid-state NMR technology is considered to be the most advantageous tool for the structural characterization of natural organic matter.19,50−53 The 13C NMR spectra of the five coal samples were measured using a Bruker Advance III 600 spectrometer produced by Bruker Company (Germany) at the Institute of Coal Chemistry (Chinese Academy of Sciences). The samples were characterized at a contact time condition of 3 ms and a pulse repetition delay of 3 s under a rotation frequency of 4 kHz. Moreover, we combined the total sideband suppression technology to obtain the semi-quantitative compositional information.54 To better quantitatively understand the relative content of the different carbon types, we deconvoluted the 13C NMR spectra and calculated the NMR parameters (Table 3).
Table 3. 13C NMR Parameters of Coal Samples and Carbon Assignmenta.
| sample | fa | fac | fa’ | faN | faH | faP | faS | faB | fal | fal* | falH | falO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XJ-1 | 0.50 | 0.15 | 0.35 | 0.23 | 0.13 | 0.12 | 0.05 | 0.06 | 0.49 | 0.13 | 0.36 | 0.11 |
| GZ-2 | 0.69 | 0.11 | 0.58 | 0.34 | 0.24 | 0.10 | 0.10 | 0.14 | 0.31 | 0.10 | 0.21 | 0.08 |
| SX-3 | 0.74 | 0.07 | 0.67 | 0.29 | 0.38 | 0.04 | 0.09 | 0.16 | 0.26 | 0.08 | 0.18 | 0.07 |
| SX-4 | 0.75 | 0.06 | 0.69 | 0.26 | 0.43 | 0.02 | 0.05 | 0.19 | 0.25 | 0.11 | 0.14 | 0.06 |
| LC-5 | 0.78 | 0.04 | 0.74 | 0.25 | 0.49 | 0.01 | 0.03 | 0.21 | 0.22 | 0.12 | 0.10 | 0.07 |
fa : total aromatic carbon; fac: carbonyl; fa’:,aromatic ring; faN: non-protonated and aromatic; faH: protonated and aromatic; faP: hydroxyl or ether oxygen; faS: alkylation aromatic; faB: aromatic bridgehead; fal: total aliphatic carbon; fal*: CH3 or non- protonated; falH: CH or CH2; falO: bonded to oxygen.
2.4. X-ray Diffraction Analysis
The XRD analysis involving the determination of the carbonaceous material crystallite properties in the different ranks of coal was carried out in the Advanced Analysis and Computation Center of the China University of Mining & Technology using the D8 ADVANCE, produced by Bruker Company (Germany). The operating conditions of the X-ray tube were U = 40 kV and I = 30 mA, and the XRD scans were conducted on a Cu target diffractometer using K radiation. The XRD curve consists of 002 and 100 peaks, in which the 002 peak is closely related to the aromatic layer stacking at approximately 15–30°.55 The shift of the 100 peak in the range of 40–50°.17 Information regarding the significant crystallite structure of the interlayer spacing (d002), average crystallite diameter (La), and crystallite height (Lc) was obtained through curve fitting using the Origin7.5 software and then could be determined using the Bragg’s and Scherrer equations (eqs 1–3).17,23,36,54,56,57
| 1 |
| 2 |
| 3 |
where λ is the wavelength of the X-ray (Å), θ002 and θ100 are the peak positions of the 002 and 100 bands (°), and β002 and β100 are the peak widths at half height of 002 and 100 bands, respectively.
2.5. High-Resolution Transmission Electron Microscopy (HRTEM)
High-resolution transmission electron microscopy (HTREM) has been considered an analytical method that was employed to quantitatively characterize the multiscale spatial and structural organization of the coal.20,21,36,41,44 HRTEM images were obtained in the State Laboratory for Coal Conversion (Chinese Academy of Sciences) using an FEI F20 Field Emission Electron Microscope produced in the USA. The acceleration voltage was 200 kV, and the point resolution and crystal lattice resolution were 0.23 and 0.14 nm, respectively. Before the experiment, the samples were pulverized to less than 200 mesh (0.074–0.2 mm) using a mortar. Then, these finely ground samples were in full contact with ethanol for 10–30 min using ultrasonic vibration. Next, approximately 2–3 drops of the translucent suspension were placed on a TEM copper grid with holey amorphous carbon, and the sample particles adhered to the hole edges for observation. The samples were first examined at moderate magnification to find the sharp thin layer at the edge of the coal particles under TEM. Several images of the black and white lattice fringes were taken from different spots to obtain a fine view and analyzed using Adobe Photoshop and Auto CAD software.
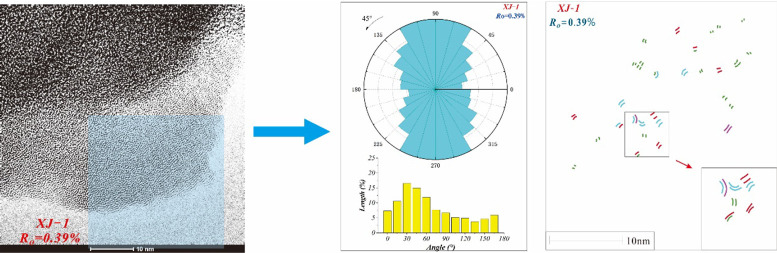
The data determined through HRTEM image analysis were the fringe length distribution, the orientation, and relative position. According to the principle of the parallelogram-shaped aromatic rings introduced by Mathews et al.42 the fringe length was converted into the molecular size. In addition, the orientation and stacking degree determined the spatial position of the aromatic fringes. Therefore, we quantified the angular distribution of the fringes by counting the coordinates of the endpoints of the fringes and recorded the angular distribution in the form of eight 15° angle bins. Each rose angle statistical chart was rotated so that the fringe angles were roughly concentrated in the same direction. Moreover, based on the method of stacking of aromatic fringes determined by Louw et al.45 and Sharma et al.,21 the stacking was primarily determined by the parallel aromatic fringes and the vertical distance between the midpoints of the aromatic fringes. Here, the direct distance between the midpoints and the fringe angle difference was <5 Å and < 20°, respectively.
3. Results
3.1. Raman Spectroscopy
It is generally accepted that the complex structure of coal is closely related to coal maturity. Figure 1 displays the measured Raman spectra of the five coal samples, stacked in order of their vitrinite reflectance. All five samples produced clean Raman spectra with two clearly identifiable bands at approximately 1580 and 1350 cm–1, which are assigned to the G and D bands, respectively. The D band is caused by Raman-active A1g symmetry associated with lattice defects and discontinuities of the sp2 carbon network, and the G band is caused by the breathing of aromatic rings.58−60 The intensity of the G band is clearly larger than that of the D1 band. The curve-fitting procedure for the five samples is shown in Figure 2. The detailed Raman spectra derived from the curve-fitted are listed in Table 2. From Table 2, the center shift position of the G band is obtained from 1578 to 1591 cm–1 and from 1365 to 1346 cm–1 for the D band. With the increase in maturity, the bands of G and D become narrower, and the band spacing increases. In addition to the D1 and G bands, the D3 band is the third largest area and intensity of amorphous carbon. As the maturity increases, the proportion of the shoulder band of the G and D2 components gradually decreases. The gradual increase in the dominance of the D1 band over the G band is consistent with the generation of defects in the aromatic plane, but the latter does not increase significantly, which indicates that graphitization has not yet occurred.
Figure 1.

Raman spectra of coal samples.
Figure 2.
Curve-fitting Raman spectra of the five coal samples (red dotted line experimental Raman spectra; green line fitting peaks; purple shaded area final results of generated curve-fitting).
Table 2. Raman Structural Parameters Determined from the Spectruma.
| peak
position(cm–1) |
||||
|---|---|---|---|---|
| sample | D1 | G | G-D1(cm–1) | AD1/AG |
| XJ-1 | 1363.57 | 1578.81 | 215.24 | 0.98 |
| GZ-2 | 1352.86 | 1580.47 | 227.61 | 0.69 |
| SX-3 | 1365.35 | 1591.63 | 226.28 | 0.40 |
| SX-4 | 1348.43 | 1586.64 | 238.22 | 0.41 |
| LC-5 | 1346.06 | 1581.75 | 235.69 | 0.46 |
D1: C–C of aromatic rings at about 1350 cm–1; G: graphite E22g at about1590 cm–1; G-D1:band position difference of the G and D1 bands; AD1/AG: the band area ratio of the D1and G bands.
3.2. 13C NMR Spectroscopy
13C NMR experiments were performed on five coal samples to characterize the chemical structures of the coals. Figure 3 displays the 13C NMR spectra of coals with different ranks. Also, these 13C NMR spectra clearly consist of three distinct bands: aliphatic carbon (shift: 0–90 ppm), aromatic carbon (shift: 100–156 ppm), and carbonyl carbon (shift: 165–220 ppm). In these five coal samples, only the XJ-1 sample had an aliphatic carbon peak area greater than that in the aromatic carbon peak, indicating that there are more aliphatic carbon structures within the coal structure in the sample. However, in the other four samples (GZ-2, SX-3, SX-4, and LC-5), the peak area of aliphatic carbon is significantly smaller than that of aromatic carbon. Overall, the aliphatic carbon showed a decreasing trend with increasing maturity, while the aromatic carbon exhibited an increasing trend. However, during the early stage of coalification, especially from the sample of XJ-1 (%Ro = 0.39%) to GZ-2 (%Ro = 1.23%), the aliphatic peak intensity decreased significantly. In contrast, the intensity of the aromatic peak increased significantly with %Ro ranging from GZ-2 (%Ro = 2.07%) to LC-5(%Ro = 2.47%). In addition, the peak area of carbonyl carbon showed a downward trend.
Figure 3.

13C NMR curves of coal samples of different ranks.
13C NMR parameters were also calculated using the curve-fitting method, and the results are shown in Table 3. It is obvious that the value of faP (proportion of aromatic carbon bonded to hydroxyl or ether oxygen) and fac (proportion of carbonyl) show a decreasing trend as the maturity increases, indicating that the oxygen-containing functional groups was tailored down from the chemical structure of coal during the process of coalification. faH (proportion of protonated and aromatic carbon) and faB (proportion of aromatic bridgehead) are attributed to aromatic carbons, which tend to increase gradually during coalification, and the proportion of faH in the LC-5 sample is nearly half of the total, suggesting an increase of protonated aromatic carbons. However, the other aromatic parameter of faS (proportion of alkylation aromatic) increases first and then decreases, reaching the maximum (0.12) in the SX-2 sample. In contrast, the aliphatic carbon parameter of falH (proportion of CH or CH2) and fal* (proportion of CH3 or non-protonated) decreased significantly with an increase in maturation, suggesting that the aliphatic structure fell off during the coalification process.
3.3. XRD Structural Parameters
The XRD spectra for different ranks of coal samples are depicted in Figure 4, where two distinct peaks of 002 and 100 can be seen. The peak of 002 (2θ = 15–30°) is obvious and corresponds to the stacking structure of the aromatic layers. The peak of 100 in the high-angle region (2θ = 40°–50°) is relatively poorly defined. Small spikes on the peaks are also observed for the samples of SX-3, SX-4, and especially for GZ-2, which demonstrates the residual minerals in the coal samples. However, the spikes did not interfere with the intensities of the 100 and 002 peaks of the three coal samples. As the maturity increases, the background intensity of 002 increases and the shape of the 002 peak becomes sharper and shifts to larger angles. Although the change is not significant, the peak of 100 tends to increase slightly, suggesting a higher order for high-rank samples. Furthermore, the peaks move to larger angles as the carbon content increases.25 The calculated crystallite structural parameters are presented in Table 4. The d002 is an important indicator of the perfection in the stacking structure periodicity. All five coals demonstrate a d002 in close proximity to graphite (d002 for graphite is 3.36–3.37 Å).26,61 During the process of coalification, d002 exhibits a gradual decreasing trend, except for the LC-5sample. On the contrary, the value of La increases significantly for all five coal samples. Significant differences can be observed in the Lc value of XJ-1with respect to the other four samples. The lower La value of XJ-1supports the low-rank coals with a less-ordered crystallite structure compared with the other four samples. In general, the value of Lc presents an increasing trend first and begins to show a decreasing trend in the sample of SX-3 (%Ro = 2.07%).
Figure 4.

XRD profiles of the five coal samples of different ranks.
Table 4. Crystallite Structural Parameters of Coal Samples.
| sample | 2θ002 (°) | 2θ100 (°) | β002 (rad) | β100 (rad) | d002 (Å) | Lc (Å) | La (Å) |
|---|---|---|---|---|---|---|---|
| XJ-1 | 23.71 | 41.23 | 0.24 | 15.83 | 3.75 | 5.81 | 10.96 |
| GZ-2 | 24.29 | 42.90 | 0.12 | 16.04 | 3.66 | 11.40 | 10.88 |
| SX-3 | 25.56 | 44.16 | 0.07 | 14.21 | 3.48 | 20.90 | 12.34 |
| SX-4 | 25.70 | 43.69 | 0.08 | 12.35 | 3.46 | 18.18 | 14.17 |
| LC-5 | 25.38 | 42.18 | 0.08 | 8.85 | 3.50 | 17.09 | 19.67 |
3.4. HRTEM Micrographs
Figure 5 shows the HRTEM micrographs of the five coal samples. By comparing the HRTEM micrographs of coal samples with different ranks, it can be clearly seen that the fringes of the first three samples (%Ro values of 0.39–2.07%) were short and aligned to a certain extent. However, for the high-rank coal samples, the lattice fringes were longer with better alignment. The false-colored fringes of the five coal samples were processed to intuitively observe, as shown in Figure 5. In order to quantitatively investigate the characteristics of the lattice fringes, based on the method proposed by the Mathews group,20,44 the aromatic fringes are assigned to different-sized aromatic rings. From Figure 5, the different rank coal samples are all composed of 1 × 1 to 8 × 8 aromatic rings. In addition, the distribution, orientation, and stacking of the lattice fringes of different sizes for the different rank samples exist. These differences are quantified in the following sections.
Figure 5.
HRTEM micrographs and false-colored fringes of five coal samples: (a1) XJ-1 HRTEM image; (b1) GZ-2 HRTEM image; (c1) SX-3 HRTEM image; (d1) SX-4 HRTEM image; (e1) LC-5 HRTEM image; (a2) XJ-1 false-colored fringes; (b2) GZ-2 false-colored fringes; (c2) SX-3 false colored; (d2) SX-4 false-colored fringes; (e2) LC-5 false-colored fringes; the blue box is the selected thinner edge area.
4. Discussion
4.1. Raman Characterization
Figure 6a demonstrates that the band position difference of the G and D1 bands is positively related to %Ro. The growth of the difference (G-D1) is remarkable, up to approximately 25 cm–1. However, the peak area ratio (AD1/AG) was negatively correlated with %Ro (Figure 6b). Therefore, the variation of Raman parameters during the coalification process is not linear, and it is composed of three stages at %Ro = 0.39–1.23%, %Ro = 1.23–2.07%, and %Ro = 2.07–2.47%, respectively. This reflects the complex coalification mechanisms. As shown in Figure 6, when %Ro = 0.39–1.23%, there is a sharp linear increase and reduction in G-D1 and AD1/AG, respectively. When %Ro = 1.23–2.07%, the value of G-D1 slightly decreases but is still linearly reduced for AD1/AG. In addition, when %Ro = 2.07–2.47%, the value of G-D1 increases, but the value of AD1/AG tends to remain steady.
Figure 6.
Raman parameters and Ro correlation: (a) the relationship between Ro and (G-D1) band positions; (b) the relationship between Ro and AD1/AG.
In the early stage of coalification (%Ro = 0.39–1.23%), the sharp linear changes of both AD1/AG and G-D1 are observed in Figure 6. This illustrates the proceeding of aromatization. However, when %Ro = 1.23–2.07%, AD1/AG continues to show a clear downward trend in Figure 6b, indicating a further increase in aromatization. However, this change in aromatization is a relatively gradual process compared with that the stage of %Ro = 0.39–1.23%, which also can be proven by the slight decrease in the value of G-D1 as shown in Figure 6a. Meanwhile, the aromatic rings and degree of graphitization are significantly enhanced. When %Ro = 2.07–2.47%, as Ro rises, the band position difference of G-D1continues to increase, while AD1/AG tends to be relatively stable, which proves the enhancement of condensation of aromatic rings, and confirming that the amorphous carbon structures have disappeared to a large extent and kept in a relatively stable stage. A similar finding was also obtained by He et al.28 during the coalification process, there is a tendency for defects and disorder in the coal structure to decrease.
4.2. Chemical Structures Characteristics
Figure 7 shows that the 13C NMR parameters all change as the vitrinite reflectance changes. Consistent with the Raman parameters, the evolution of the chemical structure is also divided into three stages. Overall, the aromatic ring (fa’), aromatic carbon (fa), protonated aromatic carbon (faH), and aromatic bridgehead (faB) increase with %Ro ranging from 0.39 to 2.47%. In contrast, the total aliphatic carbon (fal), CH or CH2 (falH), hydroxyl or ether oxygen (faP), and carbonyl (fac) tend to decrease. At the stage of %Ro = 0.39–1.23%, the growth rate of aromatic carbon (fa) and aromatic ring (fa’) are fast (Figure 7a,d). Similarly, in this stage, the total aliphatic carbon (fal) and CH or CH2 (falH) decrease at a fast rate (Figure 7e,f), suggesting that this stage is in the proceeding of aromatization. In addition, the rapid decline in aliphatic side chains (falH) is the main characteristic. However, at the stage of %Ro = 1.23–2.07%, the growth rate of aromatic carbon (fa) and aromatic ring (fa’) slow down compared with the stage of %Ro = 0.39–1.23%. In the meantime, the decline rate of the total aliphatic carbon (fal) and CH or CH2 (falH) become low. However, the decline rate of the hydroxyl or ether oxygen (faP) and carbonyl (fac) becomes high (Figure 7g,h) and enables the increase in the atomic ratio of aromatic carbon. The degree of aromatization is enhanced at this stage, and its main feature is the rapid decline of oxygen. When %Ro = 2.07–2.47%, the aromatic ring (fa’) and aromatic carbon (fa) continue to increase. In contrast, CH or CH2 (falH) still decreases, and protonated aromatic carbon (faH) (Figure 7 b) and aromatic bridgehead (faB) (Figure 7 c) still increase, indicating the enhancement of condensation of aromatic rings. This is consistent with the results of Raman spectroscopy that the aliphatic structure has largely tailored off from the coal structure at this stage.
Figure 7.
13C NMR parameters as a function of %Ro: (a) the relationship between fa and %Ro; (b) the relationship between faH and %Ro; (c) the relationship between faB and %Ro; (d) the relationship between fa’ and %Ro; (e) the relationship between fal and %Ro; (f) the relationship between falH and %Ro; (g) the relationship between faP and %Ro; (h) the relationship between fac and %Ro.
4.3. Crystallite Structural Characteristics
The parameter d002 is an important indicator for the evolution of degree of the coal BSUs. Figure 8 shows the values of d002, La, and Lc of the coal crystallite structures as a function of the vitrinite reflectivity (%Ro). The trend of the d002 values with increasing %Ro was divided into three distinct stages (Figure 8a). The first stage occurs in the range of %Ro = 0.39–1.23%. The d002 value decreased significantly in this stage. A similar decrease was observed in the pyrolysis coal samples.40 In the second stage of %Ro = 1.23–2.07%, the downward trend of the d002 value was more significant than in the previous stage, which is consistent with the decreasing trend of the AD1/AG in the Raman parameter during this stage. It has been proven that aromatization can increase the degree of stacking of the coal crystallite structure. However, in the third stage (%Ro = 2.07–2.47%), the d002 value decreased slightly for SX-4 coal and remained stable.
Figure 8.
Relationship between %Ro and XRD parameters: (a) the relationship between %Ro and d002; (b) the relationship between %Ro and La; (c) the relationship between %Ro and Lc.
As seen in Figure 8b, with an increase of %Ro, the variation of La for all the coal samples is also characterized by three stages. In the stage of %Ro = 0.39–1.23%, the La value decreased slightly. On the contrary, in the second stage of %Ro = 1.23–2.07%, the La value of the five coal samples increased gradually. In the third stage, %Ro = 2.07–2.47%, the value of La increased significantly.
Figure 8c shows the variation of the Lc values of coals with different ranks. The Lc value of %Ro = 0.39–1.23% significantly increased. When %Ro ranged from 1.23 to 2.07%, the growth of the Lc values was more significant. However, when %Ro = 2.07%–2.47%, Lc showed a slight downward trend, with a maximum value of 20.90 obtained at %Ro = 2.07%. Similar findings can also be found in other papers.19,25,54
In the stage of %Ro = 0.39–1.23%, the d002 value decreases, La slightly decreases, and the increase of Lc may be due to the small molecules swelling in the layer spacing and the hydrogen bonds breaking.23 Significant changes in the XRD parameters in the second stage demonstrated the development of a graphitized structure.23 During the last stage, small changes in d002, La, and Lc, reflect the crystallite structure stability. Overall, during the coalification process, the crystallite structures vary with decreasing layer spacing and increasing crystal diameter and height.25
It is known that graphite-like structures are more likely to occur in high-rank coals.25 The lowest distance between two adjacent crystal layers was found to be 3.46 Å for the high-rank coal sample of SX-4 (%Ro = 2.07%) in this work, which is closer to a perfect graphite single crystal with d002 in the range of 3.36–3.37 Å than other samples. Therefore, it can be found that graphite-like parameters also have significant effects on the crystallite structure. The disorder parameter of the D1 band area to the G band area was found in the different ranks of coal depending on d002 and Lc distinctly. Figure 9 illustrates the relationship between the Raman parameters AD1/AG and the crystallite structure parameters (d002, Lc). Generally, the AD1/AG parameter is proportional to d002 in Figure 9a (R2 = 0.95), indicating that as the coal rank increases, the degree of disorder decreases, and the interlayer spacing also decreases. On the contrary, the AD1/AG parameter is inversely proportional to Lc in Figure 9b (R2 = 0.95). This means that the decrease in disorder is the result of the increase in crystallite height. Furthermore, it also demonstrates that as the coal rank increases, the degree of disorder decreases, the crystal height increases, and the interlayer spacing decreases, indicating more aromatic layers in the stacking structure.
Figure 9.
Correlation between Raman parameter (AD1/AG) and crystallite parameters: (a) the relationship between AD1/AG and d002; (b) the relationship between AD1/AG and Lc.
4.4. Spatial Alignment of Coal Samples Based on HRTEM
4.4.1. Lattice Fringe Distribution
The length of aromatic fringe was converted to the corresponding molecular size after the approach of Mathews et al.42 using the central hypothesis that the fringe was as deep as it was wide assuming a parallelogram–ring catenation. Thus, lattice fringes could be attributed to aromatic parallelograms according to their length. And the fringe length is counted according to the assignment of the aromatic fringe length by the Mathews group.44
The lattice fringe length distributions are shown in Figure 10. For the samples with the %Ro range (0.39–2.07%), the aromatic structural unit distributions are similar: mainly composed of naphthalene (30–47%), 2 × 2 rings (15–35%), and 3 × 3 rings (7–33%). The frequency of naphthalene decreases distinctly as the maturity increases. The frequency of the 2 × 2 rings increases in the range of %Ro (0.39–2.07%) and then decreases slightly in the range of %Ro (2.07–2.47%). A positive correlation between the 3 × 3 rings and %Ro is observed in Figure 10 (R2 = 0.91). In addition, 4 × 4 rings and 5 × 5 rings are partly distributed in the high rank samples of SX-4 and LC-5 but are less distributed in the other low-rank samples. In the low-rank coal sample of XJ-1, naphthalene contributes to nearly half of the total aromatic rings. Moreover, all the aromatic fringes are smaller than the 5 × 5 rings. Compared with the XJ-1 sample, 8 × 8 rings appear in the samples of SX-4 and LC-5. When % Ro > 2.07%, there is a shift toward the size of 3 × 3 rings. Therefore, as the maturity increases, the size of the aromatic ring increases, which is similar to the maturityresponsive kerogen39,40 and other coal types.20,62 Moreover, this result agrees with the results from the XRD experiment parameter La. Compared with other coal samples,21,63 the five coal samples in this work had a higher frequency of 3 × 3 rings. It may be due to the differences in organic matter or the deposition environment.
Figure 10.

Distributions of the lattice fringe length of five coal samples (there is a positive correlation between the frequency of 3 × 3 rings and R2 = 0.91).
PHA mainly exists in the form of aromatic layers in the macromolecular structure of the coal.20,64 The aromatic layers are mainly controlled by the temperature and pressure of the deposition environment. The value of %Ro is a direct indicator of temperature and pressure. Therefore, the increase in temperature and pressure promotes the growth of aromatic rings.
4.4.2. Fringe Orientation
The orientation bar charts and rose diagrams of the lattice fringe micrographs for the five different rank coal samples are shown in Figure 11. For a clear demonstration, all the statistical rose diagrams have been rotated, and all the fringes are concentrated in the vertical direction. It can be observed from the rose diagrams (in Figure 11) that there is regional heterogeneity in the orientation distribution. The aromatic fringes of the coal samples all have their own preferential alignment. When %Ro < 2.07%, the fringe distribution is scattered, and the degree of orientation is weak. However, at %Ro > 2.07%, as the length of the aromatic fringes increases, the degree of orientation increases (similar to a rank series of kerogen41). As shown in the rose diagrams, the trend of fringe angle distribution increases as the %Ro increases but with a significant increase at %Ro = 2.07% (sample SX-3). Here, the orientation is quantified by the proportion of aromatic fringes in the primary direction (75–120°) of the total fringes. In the low-rank sample of XJ-1, aromatic fringes have the lowest orientation, contributing only to 35%. A previous work has concluded that the fringes are oriented randomly in low-rank coal.27 Compared with the previous study, this study found that even at the stage of lignite, aromatic fringes have partial orientation. The orientation degree increases slightly with %Ro in the range of 0.39–2.07% (35–45%), but when %Ro > 2.07%, the orientation degree changes significantly, especially in the sample of LC-5, reaching 81% of the total fringe. Based on the above Raman results, it can be assumed that in the low-rank coal samples, there are more heterocyclic compounds or amorphous carbon structures on the chain side of aromatic clusters, leading to a weak degree of orientation.65 However, according to the Raman data at this stage, the aromatization and rearrangement are enhanced. A majority of heterocyclic compounds and amorphous carbon have tapered down, resulting in a significant increase in the size of the single aromatic fringe. Moreover, the alignment and orientation of the aromatic fringes become strong. Liu et al.18 also established the relationship between the H/C ratio and fringe orientation and concluded that aliphatic structures have a negative impact on the fringe orientation alignment.67 Overall, the aromatization promotes the orientation of the aromatic fringes.
Figure 11.
Angle distribution of the aromatic fringe from the HRTEM micrographs.
4.4.3. Fringe Stacking
The stacking of aromatic fringes has been widely investigated. There are mainly face-to-face,66 stepped stacking,67 herringbone,66 and T-shaped68 stacking aromatic fringe stacking styles based on the aromatic ring sizes. Different stacking styles occur in certain aromatic structures, such as face-to-face stacking, which occurs in pure crystals of larger polyaromatic structures. Aliphatics, heteroatoms and polyaromatic aromatic structures will affect the interlayer stacking. Here, all the samples are present in the form of face-to-face. The stacking ratio, as measured by the ratio of layers in a stack, is much more extensive for the high-rank coals. Also, according to the method proposed by Louw et al.,45 it was suitable for stacking recognition. Here, we define the stacking ratio to be calculated by the ratio of the number of stacked fringes to the counted number of the false color fringes. As shown in Figure 12, it can be seen clearly that all five coal samples have a low stacking ratio (3.15–14.38%). Most of the fringes (85.62–96.85%) exist as a single layer, similar to previous research.20,41,67 For samples from XJ-1 to SX-3 (Ro = 0.39–2.07%), the fringe stacking ratio is between 3.15 and 7.16% and significantly increases in samples from SX-4 to LC-5, between 9.15 and 14.38%. The fringe stacking change trend increases as the fringe length increases with %Ro, and both have a significant transition at %Ro = 2.07%. Figure 13 illustrates the frequency distribution of the aromatic fringe stacking layers. Clearly, there are only two layer stacks in sample XJ-1, which are close to the SHE and PLQ.68 The GZ-2 and SX-3 samples had contributions from two or three layers. However, only five layers were found in sample LC-5.These results are in good agreement with those obtained from the XRD technique.
Figure 12.
Distribution of lattice fringe stacking.
Figure 13.

Frequency distribution of stacked layers for samples of lattice fringe.
PHA stacking units refer to the fact that the aromatic fringes form stiff linkages in the form of short aliphatic side chains and cycloalkanes under the π–π interaction, which prevent the folding of aromatic fringes to a certain extent and form a parallel or quasi-parallel structure.69 It is well-known that the vitrinite reflectivity (%Ro) jumps at 0.5–0.6, 1.1–1.2, 1.2–1.5, 2.2–2.3, 2.8–2.9, and 3.7–3.9% during the coalification process.70 Sample SX-3 (Ro = 2.07%) approaches the fourth coalification jumping point. At this stage, as the aromatization and ring condensation increase, resulting in the rearrangement of the crystallite structure and increasing the number of staked layers.20 Moreover, there is another view that the degree of crystallinity in low-rank samples is low, and part of the low-crystalline carbon disappears or is converted to high-crystalline carbon during the coalification process, resulting in increased stacking layers.21
5. Conclusions
Here, the spatial alignment and structural characteristics of coal samples were investigated using HRTEM micrograph quantitative analysis. Raman spectroscopy was utilized to analyze the overall structural disorder of the structure. In addition, 13C NMR spectroscopy was employed to study the chemical structure, and the XRD spectrum also recorded the microcrystalline structure of different rank coal samples. Also, these following conclusions can be drawn from this study:
-
(i)
The variation of ordering during the coalification process is nonlinear, and the overall evolutionary paths have stage characteristics with three stages at %Ro = 0.39–1.23%, %Ro = 1.23–2.07%, and %Ro = 2.07–2.47%. Aromatization occurs in the stage of %Ro = 0.39–1.23%. As %Ro increases, the growth rate of aromatization at the stage of %Ro = 1.23–2.07% is a relatively gradual process compared with that the stage of %Ro = 0.39–1.23%. For %Ro = 2.07–2.47%, the amorphous carbon structures disappeared substantially and the coal structure disorder decreased.
-
(ii)
When %Ro = 0.39–1.23%, the rapid decline in aliphatic side chains (falH) is the main characteristic. However, at the stage of %Ro = 1.23–2.07%, the oxygen-containing groups that fall off from the coal molecular structure are more significant. In addition, at the stage of %Ro = 2.07–2.47%, the aliphatic structure largely tailored and condensation of aromatic rings enhanced.
-
(iii)
The variation of the crystallite structure was also divided into three stages. In the first stage, the d002 value decreases, La slightly decreases, and Lc increases. When %Ro = 1.23%–2.07%, significant changes occurred in the XRD parameters, suggesting the rapid development toward the graphitized crystallite structure. In the last stage, the crystallite parameters remain stable. Meanwhile, there was a good linear relationship between the Raman disorder parameter (AD1/AG) and XRD crystallite structure parameters (d002, Lc).
-
(iv)
Through HRTEM micrographs, quantitative analysis concluded that the aromatic fringes were similar in distribution for different rank coal samples and mainly composed by naphthalene (30–47%), 2 × 2 rings (15–35%), and 3 × 3 rings (7–33%). The 3 × 3 rings have a good positive correlation with %Ro. When %Ro > 2.07%, there was a shift toward longer fringes. For all samples, the aromatic fringes showed regional orientation, and the quantitative orientation was characterized by fringes contributing to the major direction (75–120°). The lowest orientation degree was only 35% in sample XJ-1, and a higher orientation degree occurred when %Ro > 2.07% (35–81%). Moreover, there was a limited degree of stacking for all samples (3.15–14.38%). When %Ro < 2.07%, most stacks are in the form of only two or three layers. However, when %Ro > 2.07%, the stacking layers increase, and even five-layer stacking appears in the sample of LC-5.
Acknowledgments
This study was supported by Fundamental Research Funds for the Central Universities (no. 2017CXNL03).
The authors declare no competing financial interest.
References
- Fletcher T. H.; Kerstein A. R.; Pugmire R. J.; Solum M. S.; Grant D. M. Chemical Percolation Model for Devolatilization. 3. Direct Use of 13C NMR Data To Predict Effects of Coal Type. Energy Fuels 1992, 6, 414–431. 10.1021/ef00034a011. [DOI] [Google Scholar]
- Saikia B. K.; Boruah R. K.; Gogoi P. K. A X-ray diffraction analysis on graphene layers of Assam coal. J. Chem. Sci. 2009, 121, 103–106. 10.1007/s12039-009-0012-0. [DOI] [Google Scholar]
- Li X.; Cao D.; Liu D. Structure of different types of coal metamorphism by HTEM. Min. Sci. Technol. 2010, 20, 835–838. 10.1016/S1674-5264(09)60291-X. [DOI] [Google Scholar]
- Liu X.; He X. Effect of pore characteristics on coalbed methane adsorption in middle-high rank coals. Adsorption 2017, 23, 3–12. 10.1007/s10450-016-9811-z. [DOI] [Google Scholar]
- Okolo G. N.; Everson R. C.; Neomagus H. W. J. P.; Roberts M. J.; Sakurovs R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. 10.1016/j.fuel.2014.10.046. [DOI] [Google Scholar]
- An F.-H.; Cheng Y.-P.; Wu D.-M.; Wang L. The effect of small micropores on methane adsorption of coals from Northern China. Adsorption 2013, 19, 83–90. 10.1007/s10450-012-9421-3. [DOI] [Google Scholar]
- Fan L.; Liu S. Numerical prediction of in situ horizontal stress evolution in coalbed methane reservoirs by considering both poroelastic and sorption induced strain effects. Int. J. Rock Mech. Min. Sci. 2018, 104, 156–164. 10.1016/j.ijrmms.2018.02.012. [DOI] [Google Scholar]
- Ji H.; Li Z.; Yang Y.; Hu S.; Peng Y. Effects of Organic Micromolecules in coal on its Pore Structure and Gas Diffusion Characteristics. Transp. Porous Media 2015, 107, 419–433. 10.1007/s11242-014-0446-9. [DOI] [Google Scholar]
- Liu H.; Mou J.; Cheng Y. Impact of pore structure on gas adsorption and diffusion dynamics for long-flame coal. J. Nat. Gas Sci. Eng. 2015, 22, 203–213. 10.1016/j.jngse.2014.11.030. [DOI] [Google Scholar]
- Zhao Y.; Feng Y.; Zhang X. Selective Adsorption and Selective Transport Diffusion of CO2 −CH4 Binary Mixture in Coal Ultramicropores. Environ. Sci. Technol. 2016, 50, 9380–9389. 10.1021/acs.est.6b01294. [DOI] [PubMed] [Google Scholar]
- Clarkson C. R.; Bustin R. M. The effect of pore structure and gas pressure upon the transport properties of coal: a laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. 10.1016/S0016-2361(99)00056-3. [DOI] [Google Scholar]
- Faulon J.-L.; Mathews J. P.; Carlson G. A.; Hatcher P. G. Correlation between Microporosity and Fractal Dimension of Bituminous Coal Based on Computer-Generated Models. Energy Fuels 1994, 8, 408–414. 10.1021/ef00044a019. [DOI] [Google Scholar]
- Teng J.; Mastalerz M.; Hampton L. Maceral controls on porosity characteristics of lithotypes of Pennsylvanian high volatile bituminous coal: Example from the Illinois Basin. Int. J. Coal Geol. 2017, 172, 80–94. 10.1016/j.coal.2017.02.001. [DOI] [Google Scholar]
- Feng B.; Bhatia S. K. Variation of the pore structure of coal chars during gasification. Carbon 2003, 41, 507–523. 10.1016/S0008-6223(02)00357-3. [DOI] [Google Scholar]
- Romero-Sarmiento M.-F.; Rouzaud J.-N.; Bernard S.; Deldicque D.; Thomas M.; Littke R. Evolution of Barnett Shale organic carbon structure and nanostructure with increasing maturation. Org. Geochem. 2014, 71, 7–16. 10.1016/j.orggeochem.2014.03.008. [DOI] [Google Scholar]
- Charon E.; Rouzaud J. N.; Aléon J. Graphitization at low temperatures (600-1200°C) in the presence of iron implications in planetology. Carbon 2014, 66, 178–190. 10.1016/j.carbon.2013.08.056. [DOI] [Google Scholar]
- Baysal M.; Yürüm A.; Yıldız B.; Yürüm Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. 10.1016/j.coal.2016.07.009. [DOI] [Google Scholar]
- Liu Y.; Zhu Y.; Liu S.; Chen S.; Li W.; Wang Y. Molecular structure controls on micropore evolution in coal vitrinite during coalification. Int. J. Coal Geol. 2018, 199, 19–30. 10.1016/j.coal.2018.09.012. [DOI] [Google Scholar]
- Solum M. S.; Pugmire R. J.; Grant D. M. 13C Solid-State NMR of Argonne Premium Coals. Energy Fuels 1989, 3, 187–193. 10.1021/ef00014a012. [DOI] [Google Scholar]
- Mathews J. P.; Chaffee A. L. The molecular representations of coal - A review. Fuel 2012, 96, 1–14. 10.1016/j.fuel.2011.11.025. [DOI] [Google Scholar]
- Sharma A.; Kyotani T.; Tomita A. A new quantitative approach for microstructural analysis of coal char using HRTEM images. Fuel 1999, 78, 1203–1212. 10.1016/S0016-2361(99)00046-0. [DOI] [Google Scholar]
- Obeelin A.; Boulmier J. L.; Durnand B. Electron microscope investigation of the structure of naturally and artificially metamorphosed kerogen. Geochim. Cosmochim. Acta 1974, 38, 647–650. 10.1016/0016-7037(74)90048-9. [DOI] [Google Scholar]
- Li M.; Zeng F.; Chang H.; Xu B.; Wang W. Aggregate structure evolution of low-rank coals during pyrolysis by in-situ X-ray diffraction. Int. J. Coal Geol. 2013, 116-117, 262–269. 10.1016/j.coal.2013.07.008. [DOI] [Google Scholar]
- Takagi H.; Maruyama K.; Yoshizawa N.; Yamada Y.; Sato Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. 10.1016/j.fuel.2004.06.019. [DOI] [Google Scholar]
- Lu L.; Sahajwalla V.; Kong C.; Harris D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. 10.1016/S0008-6223(00)00318-3. [DOI] [Google Scholar]
- Pan J.; Zhu H.; Hou Q.; Wang H.; Wang S. Macromolecular and pore structures of Chinese tectonically deformed coal studied by atomic force microscopy. Fuel 2015, 139, 94–101. 10.1016/j.fuel.2014.08.039. [DOI] [Google Scholar]
- Hirsch P. B.X-ray scttering from coals; Proc Royal Society: London, UK, 1954, p 143–175. [Google Scholar]
- He X.; Liu X.; Nie B.; Song D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. 10.1016/j.fuel.2017.05.101. [DOI] [Google Scholar]
- Guedes A.; Valentim B.; Prieto A. C.; Noronha F. Raman spectroscopy of coal macerals and fluidized bed char morphotypes. Fuel 2012, 97, 443–449. 10.1016/j.fuel.2012.02.054. [DOI] [Google Scholar]
- Erdenetsogt B.-O.; Lee I.; Lee S. K.; Ko Y.-J.; Bat-Erdene D. Solid-state C-13 CP/MAS NMR study of Baganuur coal, Mongolia: Oxygen-loss during coalification from lignite to subbituminous rank. Int. J. Coal Geol. 2010, 82, 37–44. 10.1016/j.coal.2010.02.005. [DOI] [Google Scholar]
- Sonibare O. O.; Haeger T.; Foley S. F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. 10.1016/j.energy.2010.07.025. [DOI] [Google Scholar]
- Okolo G. N.; Neomagus H. W. J. P.; Everson R. C.; Roberts M. J.; Bunt J. R.; Sakurovs R.; Mathews J. P. Chemical-structural properties of South African bituminous coals: Insights from wide angle XRD-carbon fraction analysis, ATR-FTIR, solid state 13 C NMR, and HRTEM techniques. Fuel 2015, 158, 779–792. 10.1016/j.fuel.2015.06.027. [DOI] [Google Scholar]
- Vander Wal R. L.; Tomasek A. J.; Pamphlet M. I.; Taylor C. D.; Thompson W. K. Analysis of HRTEM images for carbon nanostructure quantification. J. Nanopart. Res. 2004, 6, 555–568. 10.1007/s11051-004-3724-6. [DOI] [Google Scholar]
- Huang Y.; Cannon F. S.; Watson J. K.; Reznik B.; Mathews J. P. Activated carbon efficient atomistic model construction that depicts experimentally-determined characteristics. Carbon 2015, 83, 1–14. 10.1016/j.carbon.2014.11.012. [DOI] [Google Scholar]
- Pré P.; Huchet G.; Jeulin D.; Rouzaud J.-N.; Sennour M.; Thorel A. A new approach to characterize the nanostructure of activated carbons from mathematical morphology applied to high resolution transmission electron microscopy images. Carbon 2013, 52, 239–258. 10.1016/j.carbon.2012.09.026. [DOI] [Google Scholar]
- Roberts M. J.; Everson R. C.; Neomagus H. W. J. P.; Van Niekerk D.; Mathews J. P.; Branken D. J. Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals. Fuel 2015, 142, 9–20. 10.1016/j.fuel.2014.10.033. [DOI] [Google Scholar]
- Zhong Q.; Mao Q.; Zhang L.; Xiang J.; Xiao J.; Mathews J. P. Structural features of Qingdao petroleum coke from HRTEM lattice fringes: Distributions of length, orientation, stacking, curvature, and a large-scale image-guided 3D atomistic representation. Carbon 2018, 129, 790–802. 10.1016/j.carbon.2017.12.106. [DOI] [Google Scholar]
- Wang C.; Huddle T.; Huang C.-H.; Zhu W.; Vander Wal R. L.; Lester E. H.; Mathews J. P. Improved quantification of curvature in high-resolution transmission electron microscopy lattice fringe micrographs of soots. Carbon 2017, 117, 174–181. 10.1016/j.carbon.2017.02.059. [DOI] [Google Scholar]
- Wang X.; Zhu Y.; Song Y.; Mathews J. P. Structure and partial ordering of terrestrial kerogen: Insight from high-resolution transmission electron microscopy. Fuel 2020, 281, 118759. 10.1016/j.fuel.2020.118759. [DOI] [Google Scholar]
- Liu Y.; Zhu Y.; Chen S.; Wang Y.; Song Y. Evaluation of Spatial Alignment of Kerogen in Shale Using High-Resolution Transmission Electron Microscopy, Raman Spectroscopy, and Fourier Transform Infrared. Energy Fuels 2018, 32, 10616–10627. 10.1021/acs.energyfuels.8b02591. [DOI] [Google Scholar]
- Sharma A.; Kyotani T.; Tomita A. Comparison of structural parameters of PF carbon from XRD and HRTEM techniques. Carbon 2000, 38, 1977–1984. 10.1016/S0008-6223(00)00045-2. [DOI] [Google Scholar]
- Mathews J. P.; Fernandez-Also V.; Daniel Jones A.; Schobert H. H. Determining the molecular weight distribution of Pocahontas No.3 low-volatile bituminous coal utilizing HRTEM and laser desorption ionization mass spectra data. Fuel 2010, 89, 1461–1469. 10.1016/j.fuel.2009.10.014. [DOI] [Google Scholar]
- Narkiewicz M. R.; Mathews J. P. Improved Low-Volatile Bituminous Coal Representation: Incorporating the Molecular-Weight Distribution. Energy Fuels 2008, 22, 3104–3111. 10.1021/ef700779j. [DOI] [Google Scholar]
- Niekerk D. V.; Mathews J. P. Molecular representations of Permian-aged vitrinite-rich and inertinite-rich South African coals. Fuel 2010, 89, 73–82. 10.1016/j.fuel.2009.07.020. [DOI] [Google Scholar]
- Wang C.; Watson J. K.; Louw E.; Mathews J. P. Construction Strategy for Atomistic Models of Coal Chars Capturing Stacking Diversity and Pore Size Distribution. Energy Fuels 2015, 29, 4814–4826. 10.1021/acs.energyfuels.5b00816. [DOI] [Google Scholar]
- Bailey A. M.; Cohen A. D. ABSTRACT: Stepwise Compositional Variations in Solutions Released from Peats During Laboratory Coalification Experiments. Critica 1993, 27, 3–48. [Google Scholar]
- Orem W. H.; Neuzil S. G.; Lerch H. E.; Cecil C. B. Experimental early-stage coalification of a peat sample and a peatified wood sample from Indonesia. Org. Geochem. 1996, 24, 111–125. 10.1016/0146-6380(96)00012-5. [DOI] [Google Scholar]
- Yao S.; Xue C.; Hu W.; Cao J.; Zhang C. A comparative study of experimental maturation of peat, brown coal and subbituminous coal: Implications for coalification. Int. J. Coal Geol. 2006, 66, 108–118. 10.1016/j.coal.2005.07.007. [DOI] [Google Scholar]
- Sadezky A.; Muckenhuber H.; Grothe H.; Niessner R.; Pöschl U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. 10.1016/j.carbon.2005.02.018. [DOI] [Google Scholar]
- Mao J. D.; Schimmelmann A.; Mastalerz M.; Hatcher P. G.; Li Y. Structural Features of a Bituminous Coal and Their Changes during Low-Temperature Oxidation and Loss of Volatiles Investigated by Advanced Solid-State NMR Spectroscopy. Energy Fuels 2010, 24, 2536–2544. 10.1021/ef9015069. [DOI] [Google Scholar]
- Genetti D.; Fletcher T. H.; Pugmire R. J. Development and Application of a Correlation of13C NMR Chemical Structural Analyses of Coal Based on Elemental Composition and Volatile Matter Content. Energy Fuels 1999, 13, 60–68. 10.1021/ef980074k. [DOI] [Google Scholar]
- Tong J.; Han X.; Wang S.; Jiang X. Evaluation of Structural Characteristics of Huadian Oil Shale Kerogen Using Direct Techniques (Solid-State 13C NMR, XPS, FT-IR, and XRD). Energy Fuels 2011, 25, 4006–4013. 10.1021/ef200738p. [DOI] [Google Scholar]
- Cook R. L. Coupling NMR to NOM. Anal. Bioanal. Chem. 2004, 378, 1484–1503. 10.1007/s00216-003-2422-z. [DOI] [PubMed] [Google Scholar]
- Li W.; Zhu Y. Structural Characteristics of Coal Vitrinite during Pyrolysis. Energy Fuels 2014, 28, 3645–3654. 10.1021/ef500300r. [DOI] [Google Scholar]
- Smȩdowski Ł.; Krzesińska M.; Kwaśny W.; Kozanecki M. Development of Ordered Structures in the High-Temperature (HT) Cokes from Binary and Ternary Coal Blends Studied by Means of X-ray Diffraction and Raman Spectroscopy. Energy Fuels 2011, 25, 3142–3149. 10.1021/ef200609t. [DOI] [Google Scholar]
- Hattingh B. B.; Everson R. C.; Neomagus H. W. J. P.; Bunt J. R.; van Niekerk D.; Jordaan J. H. L.; Mathews J. P. Elucidation of the Structural and Molecular Properties of Typical South African Coals. Energy Fuels 2013, 27, 3161–3172. 10.1021/ef400633d. [DOI] [Google Scholar]
- Everson R. C.; Okolo G. N.; Neomagus H. W. J. P.; Dos Santos J.-M. X-ray diffraction parameters and reaction rate modeling for gasification and combustion of chars derived from inertinite-rich coals. Fuel 2013, 109, 148–156. 10.1016/j.fuel.2012.12.043. [DOI] [Google Scholar]
- Potgieter-Vermaak S.; Maledi N.; Wagner N.; Van Heerden J. H. P.; Van Grieken R.; Potgieter J. H. Raman spectroscopy for the analysis of coal: a review. J. Raman Spectrosc. 2011, 42, 123–129. 10.1002/jrs.2636. [DOI] [Google Scholar]
- Beyssac O.; Goffé B.; Petitet J.-P.; Froigneux E.; Moreau M.; Rouzaud J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta A. 2003, 59, 2267–2276. 10.1016/S1386-1425(03)00070-2. [DOI] [PubMed] [Google Scholar]
- Xu J.; Su S.; Sun Z.; Qing M.; Xiong Z.; Wang Y.; Jiang L.; Hu S.; Xiang J. Effects of steam and CO2 on the characteristics of chars during devolatilization in oxy-steam combustion process. Appl. Energy 2016, 182, 20–28. 10.1016/j.apenergy.2016.08.121. [DOI] [Google Scholar]
- Iwashita N.; Inagaki M. Relations between structural parameters obtained by X-ray powder diffraction of various carbon materials. Carbon 1993, 31, 1107–1113. 10.1016/0008-6223(93)90063-G. [DOI] [Google Scholar]
- Song Y.; Jiang B.; Li M.; Hou C.; Mathews J. P. Macromolecular transformations for tectonically-deformed high volatile bituminous via HRTEM and XRD analyses. Fuel 2020, 263, 116756. 10.1016/j.fuel.2019.116756. [DOI] [Google Scholar]
- Castro-Marcano F.; Lobodin V. V.; Rodgers R. P.; McKenna A. M.; Marshall A. G.; Mathews J. P. A molecular model for Illinois No. 6 Argonne Premium coal: Moving toward capturing the continuum structure. Fuel 2012, 95, 35–49. 10.1016/j.fuel.2011.12.026. [DOI] [Google Scholar]
- Mathews J. P.; van Duin A. C. T.; Chaffee A. L. The utility of coal molecular models. Fuel Process. Technol. 2011, 92, 718–728. 10.1016/j.fuproc.2010.05.037. [DOI] [Google Scholar]
- Huan X.; Tang Y.; Xu J.; Xu M. Nano-level resolution determination of aromatic nucleus in coal. Fuel 2020, 262, 116532. 10.1016/j.fuel.2019.116532. [DOI] [Google Scholar]
- Desiraju G. R.; Gavezzotti A. From molecular to crystal structure; polynuclear aromatic hydrocarbons. J. Chem. Soc. 1989, 621. 10.1039/C39890000621. [DOI] [Google Scholar]
- Mathews J. P.; Sharma A. The structural alignment of coal and the analogous case of Argonne Upper Freeport coal. Fuel 2012, 95, 19–24. 10.1016/j.fuel.2011.12.046. [DOI] [Google Scholar]
- Zhang Y.; Zhang X.; Zhong Q.; Hu S.; Mathews J. P. Structural Differences of Spontaneous Combustion Prone Inertinite-Rich Chinese Lignite Coals: Insights from XRD, Solid-State 13C NMR, LDIMS, and HRTEM. Energy Fuels 2019, 33, 4575–4584. 10.1021/acs.energyfuels.9b00123. [DOI] [Google Scholar]
- DeLapp R. C.; LeBoeuf E. J.; Chen J.; Gu B. Advanced thermal characterization of fractionated natural organic matter. J. Environ. Qual. 2005, 34, 842–853. 10.2134/jeq2004.0241. [DOI] [PubMed] [Google Scholar]
- Bustin R. M.; Clarkson C. R. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26. 10.1016/S0166-5162(98)00030-5. [DOI] [Google Scholar]