Abstract

Herein, we describe for the first time, the design and fabrication of a novel nicotine paper-based sensor, in which a miniaturized paper reference electrode is integrated for potentiometric measurements. The paper-based sensors were designed using printed wax barriers to define the electrochemical cell and the sample zones. The electrodes were based on the use of the ion association complexes of the nicotinium cation (Nic) with either tetraphenylborate (TPB) or 5-nitrobarbiturate (NB) counter anions as sensing materials for nicotine recognition. A poly (3,4 ethylenedioxythiophene)/poly-(styrene sulfonate) (PEDOT/PSS) conducting polymer was used as an ion-to-electron transducer. The performance characteristics of the proposed sensors were evaluated and it revealed a rapid and stable response with a Nernstian slope of 55.2 ± 0.3 and 51.2 ± 0.6 mV/decade over the linear range of 1.0 × 10–5 to 1.0 × 10–2 M and detection limits of 6.0 and 8.0 μM for [Nic/TPB] and [Nic/NB], respectively. The sensors revealed a constant response over the pH range 3.5–6.5. The designed sensors provided a portable, inexpensive, and disposable way of measuring trace levels of nicotine coming from different cigarettes and in the collected human sweat of heavy smokers. All results were compared favorably with those obtained by the standard gas chromatographic method.
1. Introduction
Nicotine is a bioactive pyridine derivative alkaloid that is found in the leaves of tobacco. It covers about 95% of the total content of alkaloids, which are used in the production of cigarettes, cigars, or flake tobaccos. The content of nicotine in such products varies from 1 to 30 mg/g.1 There are more than 1.2 billion people in the world consuming different tobacco products that can cause nicotine addiction.2 After smoking, nicotine is absorbed by the lungs and immediately enters into the blood stream. Then, it will be metabolized in the liver into cotinine and other metabolites.3 A broad spectrum of diseases such as cardiovascular, respiratory, and central nervous diseases and even cancer can be caused in the human body due to the frequent intake of nicotine.4 According to different studies, the fatal dose of nicotine in adults is limited to be 12.33 μM in blood and 24.66 μM in plasma.5 In addition, there were different reported studies for monitoring nicotine in urine, sweat, and saliva as different physiological fluids rather than in blood and plasma.6 These reports were based on liquid chromatography/mass spectrometry7 immunoassay methods based on colorimetric measurements8 and voltammetry.9 The level of nicotine in sweat was found to be 150–2498 ng/batch.6
Careful monitoring of nicotine levels is of utmost importance in areas as diverse as toxicology and medicine and in the tobacco industry. Therefore, it is necessary to devise or develop analytical methods that are accurate, simple, and inexpensive and characterized by high sensitivity and good selectivity. Numerous analytical methods have been reported for the quantification of nicotine. These methods are based on gas chromatography (GC),10−12 high-performance liquid chromatography,13,14 capillary electrophoresis,15,16 spectroscopy,17 and radio-immunoassay.18 These methods have several practical limitations. They require many steps to prepare the sample, which takes a long time to perform the analysis, as they depend on expensive equipment and require highly skilled analysts. These drawbacks make these methods not meet the requirements for estimating the amount of nicotine in bio-fluids.
Electrochemical methods are considered one of the recommended analytical methods due to their many advantages such as low cost of measuring devices, simple, robust, sensitive, and fast.19,20 Several reports based on voltammetric transduction were provided for nicotine determination.1,2,9,21−32 These methods suffer from poor selectivity, low precision, and limited applicability. Potentiometry as an electrochemical method can offer good advantages over the other electrochemical methods and can overcome the limitations of these methods. It is exciting to notice that sensing methods based on potentiometric transduction are growing strongly. There are a wide variety of conventional ISEs that are commercially available, and many are reported in the literature. These electrodes permit selective determination of numerous cations and anions by direct potentiometric measurements.33−44
Nowadays, the current research is focused on the removal of interdisciplinary boundaries and explores new principles for new sensors’ readout. The researchers of the material science branch offered now different nanomaterials that can be used as new ion-to-electron transducers that advance new trends in sensors’ miniaturization. These trends opened now new chances for wide applications for potentiometric sensor use. In particular, wearable potentiometric sensors have gained wide interest especially in the field of medical applications. One of these is the paper-based sensors, which are easy to dispose of and highly active and brings potentiometric-based transduction closer to the point-of-care and in-field applications.45 There were different reported paper-based electrochemical sensors that were capable of quantifying the concentrations of a number of analytes.46−49 Electrochemical paper-based potentiometric sensors are able to provide high accuracy in the measurements that are referenced by an electrode with a constant, well-defined potential.50 The devices are easy to fabricate, portable, and inexpensive.
Herein, we present a design of a well-referenced electrochemical paper-based analytical device, defining a reliable paper Ag/AgCl reference electrode with a small, ion-selective paper electrode. This device provides a stable and well-defined reference potential for direct and accurate measurements of nicotine levels in sweats of heavy and light cigarette smokers. The simplicity of the presented electrochemical design includes fast, mediator-, and pretreatment-free features for the sweat analysis. In addition, the device is made from paper, which is inexpensive, lightweight, portable, less fragile than glass, and easily disposable (for single use). The presented device has no need for reference electrode storage. Moreover, the planar design is appropriate for mass production with role-to-role printing. The presented electrodes revealed a detection limit of 6.0 μM or 311.5 ng/patch that is lower than the permitted level of nicotine in blood (12.33 μM) and sweat (150–2498 ng/patch).6
2. Results and Discussion
2.1. Design of the Electrochemical Paper-Based Device for Nicotine Sensing
The electrochemical paper-based analytical device has wax-patterned sample and reference zones. Each zone contains a stencil-printed Ag/AgCl electrode, as explained in detail in the Experimental Section. The fabricated electrochemical paper-based device has a planar configuration that is very suitable for mass production. About 30 devices can be fabricated from one page of chromatographic paper (20 × 20 cm2) in less than 1/2 h. Through the microfluidic channels present in the central zone in the paper-based device, the measurement zones were connected. According to the geometry of the designed electrochemical paper-based device, the ionic conductivity between the sample and reference solutions is allowed when they were added into their appropriate zones. The slow transfer of the ions and solvent into the liquid-filled paper channels prevents large-scale convective mixing. This shifts the reference electrode potential or alters the analyte concentration and thus affects the potential measurement accuracy.51 The interfacial line at the contact between the sample and reference solutions acts as a salt bridge, similar to that present in the conventional reference electrodes. The concentration of nicotine hydrochloride in the sample can be obtained by measuring the difference in potential between the two Ag/AgCl electrodes that are connected together in the sample zone through the sample solution and the reference zone through 1 M KCl as a reference solution. The ion-sensing membrane (<500 μm thickness) is added to separate the indicator electrode (i.e., additional wax-printed paper layer with a Ag/AgCl electrode printed on top) from the sample solution. Both the sensing membrane and the indicator electrode were attached sequentially to the sample zone of the electrochemical paper-based device. The wax barriers act as the plastic body in the conventional ion-selective electrode based on polymeric sensing membranes.
2.2. Potentiometric Measurements of Nicotine
Ion-sensing membranes based on [Nic/TPB] and [Nic/NB] ion pairs were incorporated into the paper devices and used for nicotine measurements. A total of 10 μL of both 10–3 M nicotinium hydrochloride (i.e., inner reference solution) and 1 M KCl (i.e., reference solution) was spotted on their respective zones in the electrochemical paper-based device, as mentioned in Figure 1. The two solutions under the capillary forces were wicked through the device and connected together in <20 s. The device is then sandwiched between two PVC sheets using binder clips, and the potential difference of the constructed electrochemical cell is then recorded. The PVC sheets and binder clips were chosen because of the following: (1) They allow reversible attachment and easy disassembly between the sensing membrane and the paper layers. This enables a single ion-sensing membrane to be calibrated using more than one-point calibration. Multiple paper devices impregnated with calibrators or sample solutions can be also used according to this concept. (2) The PVC sheets slow down the rate of evaporation of the solvent and keep the electrochemical paper-based device in a flat horizontal position, which reduces the liquid flow caused by gravity, which may cause contamination of the sample or reference areas.
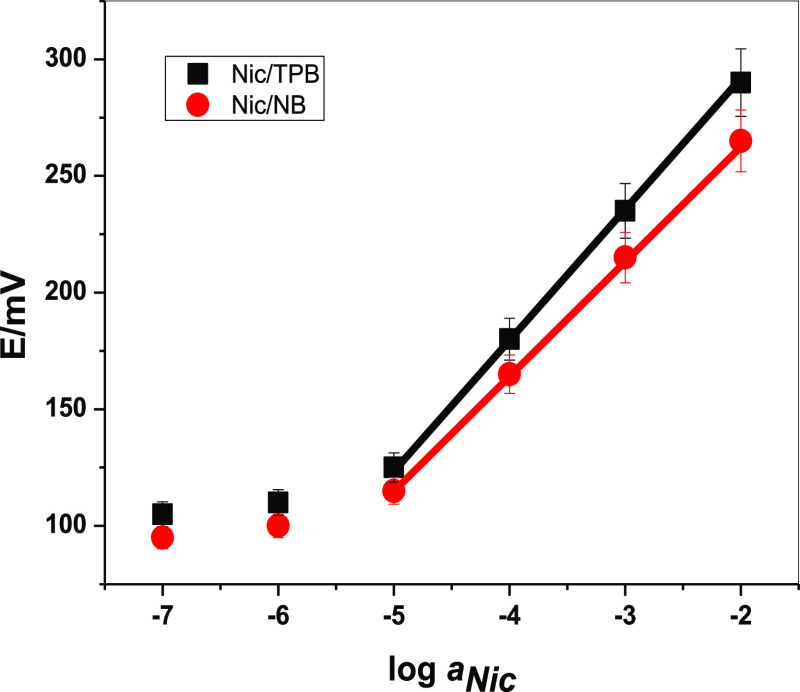
Figure 1.
Potentiometric response behavior of the presented nicotine-sensing electrochemical paper-based device.
The potentiometric response of the presented electrochemical paper-based devices for different nicotine concentrations is shown in Figure 1. A linear relationship is observed between the measured potential and log anicotine. The devices revealed a linear range from 10–5 to 10–2 M, with slopes of 55.2 ± 0.3 and 51.2 ± 0.6 mV/decade and detection limits of 6.0 and 8.0 μM for membranes based on [Nic/TPB] and [Nic/NB], respectively. Sensors’ repeatability was checked by repeating the experiments (n = 5) under the same conditions. These repeated experiments revealed similar slopes with standard deviations ±0.3 and ±0.8 for devices based on [Nic/TPB] and [Nic/NB], respectively. The obtained performance characteristics of the presented devices cover medically the relevant nicotine levels in the physiological fluids.5,6
Method precision and accuracy were examined after different measurements of a fixed nicotine concentration (10.0 μg/mL, n = 6) acting as an internal quality control sample. The relative standard deviation were found to be 0.9 ± 0.07–1.4 ± 0.2% and 1.3 ± 0.2–0.7 ± 0.04% for [Nic/TPB] and [Nic/NB], respectively. Accuracy of the presented method was examined by spiking a known nicotine amount and found to be 97.3 ± 0.4–98.2 ± 0.4%.
For the pH, over the working pH range 3.5–6.5, the predominant species is the monoprotonated nicotine [NicH+]
At pH above pH7, the potential declined sharply due to the formation of the non-sensed nicotine base. At pH < 3, the potential was decreased due to the formation of the diprotonated nicotinium ion [NicH22+], which is not sensed by the sensor.
2.3. Interfering Ion Study
The response of the presented nicotine paper-based device toward nicotine and in the presence of other nitrogenous compounds and inorganic cations was investigated. The selectivity coefficient values were calculated using the modified separate solution method suggested by Bakker.52 In brief, a new ISM is inserted without conditioning and then subjected to 10–4–10–2 M aqueous solutions of the interfering ion starting with the less-diverting ion and ending with nicotine hydrochloride solutions. The potential was recorded for each concentration and plotted versus log aM. The potentiometric selectivity coefficient values were calculated as
| 1 |
where EBO and ENic are the potential at activity a = 1 M for the interfering ions and nicotinium ion, respectively.
The potential plot for all tested interfering ions is mentioned in Figure 2. The calculated selectivity coefficient values are presented in Table 1. As seen, nicotinic acid (NA), caffeine (CA), ascorbic acid (AA), glutamic acid (GA), glycine (Gly), urea (UA), Na+, and K+ ions showed the less-interfering effect at concentration levels as high as 1000-fold molar excess over nicotine. Dopamine hydrochloride (DA) and cotinine (Ct) revealed a higher interfering effect than the abovementioned ions. This is attributed to the formation of water-insoluble ion–pair complexes with the counter anions in the membrane, which are extractable and interfere. Generally, sensors based on Nic/TPB and Nic/NB displayed a comparable selectivity behavior. The former one was used for all analytical applications.
Figure 2.
Selectivity pattern for the paper-based electrochemical device based on (a) Nic/TBP and (b) Nic/NB membrane-based sensors.
Table 1. Selectivity Coefficient Values for Nic/TPB and Nic/NB Membrane-Based Sensors.
| interfering ion, B | Nic/TPB | Nic/NB |
|---|---|---|
| dopamine (DA) | –1.95 ± 0.3 | –2.04 ± 0.4 |
| cotinine (Ct) | –3.53 ± 0.4 | –3.64 ± 0.6 |
| CA | –3.98 ± 0.5 | –4.12 ± 0.4 |
| NA | –4.45 ± 0.4 | –4.61 ± 0.3 |
| AA | –4.91 ± 0.1 | –4.88 ± 0.2 |
| GA | –5.1 ± 0.2 | –5.07 ± 0.4 |
| glycine (Gly) | –5.21 ± 0.3 | –5.24 ± 0.2 |
| urea (UA) | –5.32 ± 0.2 | –5.35 ± 0.1 |
| K+ | –5.61 ± 0.2 | –5.60 ± 0.1 |
| Na+ | –5.65 ± 0.1 | –5.61 ± 0.1 |
2.4. Nicotine Assessment in Sweat Samples
The presented electrochemical paper-based devices were tested at first for nicotine determination in artificial sweat samples and then in real human sweat samples collected on sweat patches. The samples were analyzed in triplicate, and the presented results were the mean of three measurements. Different nicotine concentrations were spiked into the artificial sweat samples covering the concentration range of 10.0–100 μM. As shown in Table 2, the results revealed a recovery range of 91.4–103.6%. This reflects the neglected impact of the matrix of the artificial sweat samples on the accuracy of the results.
Table 2. Nicotine Determination in Spiked Artificial Sweat Samples.
| sample no. | added, μM | afound, μM | recovery, % |
|---|---|---|---|
| 1 | 10.0 | 9.4 ± 0.3 | 94 |
| 2 | 25.0 | 25.9 ± 0.6 | 103.6 |
| 3 | 50.0 | 45.7 ± 1.2 | 91.4 |
| 4 | 100.0 | 93.2 ± 0.7 | 93.2 |
Average of three measurements.
To test the applicability of the presented device toward nicotine determination in real samples, nicotine is collected on a sweat patch from heavy smokers during their physical activity and then analyzed. As shown in Table 3, the amount of nicotine absorbed on the patch of the heavy smokers was found to be in the range of 18.3–23.4 μM. These results reflect a promising feasibility of the presented electrochemical paper-based devices for nicotine determination in sweat samples using the sweat patches.
Table 3. Nicotine Determination in Real Sweat Samples.
| sample no. | content
of nicotine, μMa |
|
|---|---|---|
| potentiometry | GC | |
| volunteer 1 (25–30 cigarette/day) | 18.3 ± 1.3 | 16.8 ± 0.2 |
| volunteer 2 (30–40 cigarette/day) | 21.6 ± 2.2 | 20.8 ± 0.4 |
| volunteer 3 (40–50 cigarette/day) | 23.4 ± 1.1 | 21.4 ± 0.5 |
Average of five measurements.
2.5. Nicotine Determination in Cigarette Smoke
The nicotine levels of the smoke of different cigarette brands were assessed after adsorption of the smoke in a solution containing 1 M HCl. The pH of the solution containing nicotine is adjusted with 50 mM PBS to pH 5. An aliquot of each solution is introduced to the electrochemical paper-based device, and the potential reading was recorded and compared with a calibration plot prepared from pure nicotine hydrochloride standard solutions. For comparison, the smoke from cigarettes collected on a 44 mm cellulose filter disc and followed by nicotine extraction was analyzed using gas/liquid chromatography. The results are shown in Table 4. The F-test revealed no significant difference between the means and variances of the two sets of results.
Table 4. Determination of Nicotine in the Smoke of Different Cigarette Brands.
| cigarette brand | content
of nicotine, mg/cigarettea |
F-test | |
|---|---|---|---|
| potentiometry | GC | ||
| Cleopatra (Egypt) | 0.93 ± 0.07 | 0.89 ± 0.05 | 1.42 |
| Marlboro Red (USA) | 1.11 ± 0.02 | 1.07 ± 0.02 | 2.34 |
| Marlboro White (USA) | 1.13 ± 0.01 | 1.08 ± 0.05 | 3.32 |
| LM (Egypt) | 0.89 ± 0.06 | 0.86 ± 0.03 | 1.66 |
| Winston (USA) | 0.93 ± 0.02 | 0.97 ± 0.03 | 2.56 |
Mean of three measurements ± standard deviation (SD); F-test at a 95% confidence level value.
2.6. Comparative Study between Nicotine-ISEs
Advantages and limitations of previously reported potentiometric sensors53 in comparison with the presented sensors are presented in Table 5. The presented analytical device is characterized by its ease of design and fabrication, in which a miniaturized paper reference electrode is integrated for potentiometric measurements. In particular, these types of potentiometric sensors have gained great attention and wide interest in the field of medical applications. Different potentiometric electrodes were reported for nicotine determination by Hassan et al.53 The electrodes were of liquid-membrane type. These electrodes suffer from different limitations such as difficulty of miniaturization, very limited application use, handling difficulty, high detection limit, and non-availability for mass production. The presented paper-based sensors are easy to dispose of and highly active and bring potentiometric-based transduction closer to the point-of-care and in-field applications.
Table 5. Comparison of Some Potentiometric Sensors Used for the Determination of Nicotine.
| sensing material | sensor Type | slope (mV/decade) | lower limit of linear range, M | detection limit, M | pH range | ref. |
|---|---|---|---|---|---|---|
| nicotinium tetraphenyl borate | liquid membrane electrode | 55 | 4.0 × 10–5 | 1.7 × 10–5 | 4.5–7 | (53) |
| nicotinium nitrobarbaturate | 54 | 1.0 × 10–5 | 1.1 × 10–5 | 3.5–7 | ||
| nicotinium flavinate | 50 | 5.0 × 10–5 | 3.0 × 10–5 | 3.5–7 | ||
| nicotinium reineckate | 55 | 5.0 × 10–5 | 2.5 × 10–5 | 3.5–7 | ||
| nicotinium picrolonate | 52 | 1.0 × 10–3 | 3.0 × 10–4 | 3.7–6.7 | ||
| nicotine hydrogen tetra (m-chlorophenyl) borate | liquid membrane electrode | 58 | 1.0 × 10–5 | 1.0 × 10–5 | 4.0–7.0 | (54) |
| nicotinium tetraphenyl borate | paper-based analytical devices | 55.2 ± 0.3 | 1.0 × 10–5 | 6.0 × 10–6 | 3.5–6.5 | this work |
| nicotinium nitrobarbaturate | 51.2 ± 0.6 | 1.0 × 10–5 | 8.0 × 10–6 |
3. Conclusions
Electrochemical devices based on paper substrates can be considered as an attractive tool for reliable and trace analysis after controlling the mass transfer within the channels in the paper substrate. In this work, a new paper-based analytical device based on potentiometric transduction was designed and characterized for nicotine tracing in sweat specimen and in the smoke of cigarettes. An all-solid-state ISE and a reference electrode were successfully designed and integrated on the paper substrate. The presented device is characterized by its suitability for future mass production because it is realized on commercially available and relatively low-cost off-the-shelf components. No sensor pre-conditioning or any further liquid handling is required. The presented devices are applicable to trace nicotine in aqueous solutions over concentration ranges that are biologically and environmentally relevant (1.0 × 10–5 to 1.0 × 10–2 M) with a Nernstian slope of 55.2 ± 0.3 and 51.2 ± 0.6 mV/decade and detection limits of 6.0 and 8.0 μM for [Nic/TPB] and [Nic/NB], respectively. The designed sensors were successfully applied for nicotine determination in the smoke of cigarettes and in collected human smoker sweat. The results were compared with those obtained by the standard gas chromatographic method.
4. Experimental Section
4.1. Chemicals and Reagents
Nicotine from Acros Organics TM (purity ≥99%) was obtained from Fisher Scientific. AA, dopamine hydrochloride, cotinine, GA, tetradodecylammonium tetrakis (4-chlorophenyl) borate (ETH500), CaCl2, o-nitrophenyl octyl ether (o-NPOE), MgCl2, Zn (NO3)2·6H2O, Pb(NO3)2, NH4Cl, poly (3,4-ethylenedioxythiophene)/poly-(styrenesulfonate) (PEDOT/PSS), NH2CONH2, CH3COOH, NaCl, NaH2PO4, and Na2HPO4 were obtained from Sigma-Aldrich (St. Lois, MO, USA). Ag/AgCl ink (E2414) was purchased from Ercon (Wareham, MA). Tetrahydrofuran (THF) was purchased from Fluka. Sodium tetraphenylborate and 5-nitrobarbituric acid were purchased from BDH (Poole, Dorset, UK).
Deionized pure water (18.2 MΩ cm, Millipore Milli-Q system) was used for all solution preparations throughout this work. Phosphate buffer solution (PBS, 50 mM, pH 5.0) was used as a buffer solution, and the ionic strength of the solutions was adjusted using 0.01 M NaCl as an ionic strength adjustor. A 10mM nicotine stock solution was prepared by dissolving the corresponding amount of nicotine in water and stored at 4 °C in a dark bottle. All working solutions (10–8–10–3 M) were prepared from the stock solution daily prior to the measurements by appropriate dilution with PBS at pH 5.
An artificial sweat solution was prepared as described elsewhere.55 In brief, 327 mM NH4Cl was mixed with 166 mM lactic acid, 83 mM urea, 42 mM CH3COOH, and 34 mM NaCl. All were dissolved in deionized water, and the pH of the solution was adjusted to pH 7.4 by adding aliquots of 2 M NaOH.
4.2. Instrumentations
A PXSJ-216 pH/mV meter (INESA Scientific Instrument Co., Ltd, Shanghai, China) was used for all potentiometric measurements. All pH measurements were carried out using an Orion combination pH electrode (model 91–01).
4.3. Fabrication of the Paper Devices
Wax printing on a chromatographic paper (Whatman 1-Chr) was used to pattern all paper-based zones and microfluidic channels. Each printed wax line has 2 mm width. First, the paper was placed in an oven at 80 °C to melt the wax and left to fill all pores in the paper. The electrodes were designed with stencil printing Ag/AgCl ink on the wax-printed paper. The stencil for printing was designed using the AutoCAD 2012 program. The pattern was cut into a frisket film (Grafix, low tack) using a laser cutter. The stencil was placed over the paper, and then, all spaces were filled with the ink. The paper after adding the ink was placed in an oven at 100 °C for 10 min until the ink adhered to the paper surface. All electrode zones were treated with 1 μL of 3-aminopropyldimethylethoxysilane (APDES, 2 wt %) in each zone to enhance the hydrophilicity of these zones. A schematic representation for the presented electrochemical paper-based device is shown in Figure 3.
Figure 3.
Schematic illustrations of a referenced electrochemical paper-based electrochemical device.
4.4. Membrane Preparation
The sensing membrane typically contains the lipophilic ion receptor, the lipophilic additive ETH500, PVC, o-NPOE, and the solid-contact transducer PEDOT/PSS. The sensing membrane contained 1.5 wt % of the lipophilic ion-receptor, 0.5 wt % of ETH, 10 μL of PEDOT/PSS, 33.0 wt % of PVC, and 65.0 wt % of o-NPOE and was dissolved in 2 mL of THF. The membrane cocktail was poured into a Petri dish (3 cm diameter) and allowed for solvent evaporation over 24 h. The produced membrane was peeled off and cut into small circular pieces (10 mm diameter) and then conditioned in 10–3 M nicotine solution.
4.5. Electrode Fabrication and Potential Measurements
The designed paper device includes three different zones. They are classified as sample, central, and reference zones. The inner reference, sample, and external reference solutions were spotted onto the sample, central, and reference zones, respectively. All measurements were carried out at 25 °C. Activity coefficient values were estimated according to the Debye-Hückel equation.56
The mV/pH meter was used to measure the potential difference between the two paper electrodes for aqueous nicotine hydrochloride samples with different concentrations of nicotinium ions. The reference solution used for the external paper-based reference electrode was aqueous 1 M KCl. A total of 10 μL of solutions was applied to their respective zones. The solutions wicked into the central mixing zone in <20 s.
4.6. Human Sweat Collection and Extraction
For sweat collection, a special adsorbent pad that is commercially available (PharmCheck Sweat Patches) was purchased from PharmChem, Inc. Three heavy smoker volunteers (i.e., smoking ∼25–50 cigarettes/day) were chosen for nicotine determination in their sweat. The sweat patches were applied to the volunteers and were put at the upper arm area of each volunteer after cleaning the subjected area with alcohol wipes. Then, the volunteers were let to perform physical exercises for 1 h. The patch was then removed and placed in a 50 mL tube for analysis. A total of 5 mL of methanol is added with the patch followed by shaking for 1/2 h. The extract was quantitatively transferred into a 25 mL beaker and heated to evaporate the solvent. The remaining residue was dissolved in 2 mL of 50 mM PBS at pH 5.0 and transferred to the electrochemical paper-based device for analysis. Then, the samples were spiked with different nicotine concentrations as recovery experiments. All spiked samples were used in triplicate measurements.
4.7. Nicotine Assessment in Cigarette Smoke
To collect nicotine from cigarette smoke, a simple device was used, consisting of a 100 mL Quickfit Buchner flask with a ground neck (size 19/26) and a bubbler of 0.8 cm diameter. The bubbler was terminated with a micron filter (Acculab, Norwood, NJ, USA), reaching the bottom of the flask. The top of the bubbler was connected to a 3 cm × 7 mm ID PVC tube. A 10 mL aliquot of 1 M HCl was added to the flask with constant stirring. The flask was attached to a water pump running at a flow rate 1 L/min. This rate is equivalent to 200 to 300 bubbles/min as the rate of air flow through the cigarette and the absorbing solution. The cigarette was connected to the PVC tube, which was connected to the bubbler and then burned. After complete burning, another cigarette was connected, and its smoke was collected in the same absorption solution by the same way. The content of the flask was quantitatively transferred into a 50 mL measuring flask, and the pH of this solution was adjusted to 5 by adding few drops of 0.1 M NaOH solution. Aliquots of this solution were introduced to the electrochemical paper-based device for analysis. The reading was recorded after potential stabilization. The analysis was repeated in triplicate (two cigarettes/run), and the average potential reading was recorded. Two standard nicotine solutions were treated as the cigarette smoke solution for comparison. In brief, two 5 mL aliquots of standard 1.0 and 10 mM aqueous nicotine hydrochloride solutions were mixed with 10 mL of 1 M HCI, and the solution pH was adjusted to 5 after adding small aliquots of 0.1 M NaOH solution. Aliquots of these solutions were inserted to the sample zone in the electrochemical paper-based device for analysis.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this work through Vice Deanship of Scientific Research Chairs.
Author Contributions
The listed authors contributed to this work as described in the following: H.S.M.A.-R. and A.H.K. gave the concepts of the work, interpretation of the results and the experimental part and prepared the manuscript, A.H.K., H.S.M.A.-R., and A.E.-G.E.A. cooperated in the preparation of the manuscript, and A.H.K. and H.S.M.A.-R. performed the revision before submission. A.A.A., E.A.E., and A.Y.A.S. obtained the financial support for the work. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Stočes M.; Švancara I. Electrochemical behavior of nicotine at unmodified carbon paste electrode and its determination in a set of refilling liquids for electronic cigarettes. Electroanalysis 2014, 26, 2655–2663. 10.1002/elan.201400403. [DOI] [Google Scholar]
- Wu C.-T.; Chen P.-Y.; Chen J.-G.; Suryanarayanan V.; Ho K.-C. Detection of nicotine based on molecularly imprinted TiO2-modified electrodes. Anal. Chim. Acta 2009, 633, 119–126. 10.1016/j.aca.2008.11.038. [DOI] [PubMed] [Google Scholar]
- Lunell E.; Molander L.; Ekberg K.; Wahren J. Site of nicotine absorption from a vapour inhaler—comparison with cigarette smoking. Eur. J. Clin. Pharmacol. 2000, 55, 737–741. 10.1007/s002280050007. [DOI] [PubMed] [Google Scholar]
- Karaconji I. B. Facts about nicotine toxicity. Arh. Hig. Rada Toksikol. 2005, 56, 363–371. [PubMed] [Google Scholar]
- Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch. Toxicol. 2014, 88, 5–7. 10.1007/s00204-013-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintz P.; Henrich A.; Cirimele V.; Ludes B. Nicotine monitoring in sweat with a sweat patch. J. Chromatogr. B: Biomed. Sci. Appl. 1998, 705, 357–361. 10.1016/s0378-4347(97)00551-3. [DOI] [PubMed] [Google Scholar]
- Concheiro M.; Shakleya D. M.; Huestis M. A. Simultaneous analysis of buprenorphine, methadone, cocaine, opiates and nicotine metabolites in sweat by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 69–78. 10.1007/s00216-010-4392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar P. Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J. Pharm. Biomed. Anal. 2004, 35, 155–168. 10.1016/j.jpba.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Mehmeti E.; Kilic T.; Laur C.; Carrara S. Electrochemical determination of nicotine in smokers’ sweat. Microchem. J. 2020, 158, 105155. 10.1016/j.microc.2020.105155. [DOI] [Google Scholar]
- Shrivas K.; Patel D. K. Liquid-phase microextraction combined with gas chromatography mass spectrometry for rapid determination of nicotine in one-drop of nightshades vegetables and commercial food products. Food Chem. 2010, 122, 314–318. 10.1016/j.foodchem.2010.02.035. [DOI] [Google Scholar]
- Man C. N.; Ismail S.; Harn G. L.; Lajis R.; Awang R. Determination of hair nicotine by gas chromatography-mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2009, 877, 339–342. 10.1016/j.jchromb.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Cai J.; Liu B.; Lin P.; Su Q. Fast analysis of nicotine related alkaloids in tobacco and cigarette smoke by megabore capillary gas chromatography. J. Chromatogr. A 2003, 1017, 187–193. 10.1016/j.chroma.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Page-Sharp M. P.; Hale T. W.; Hackett L. P.; Kristensen J. H.; Ilett K. F. Measurement of nicotine and cotinine in human milk by high-performance liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2003, 796, 173–180. 10.1016/j.jchromb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Tambwekar K. R.; Kakariya R. B.; Garg S. A validated high performance liquid chromatographic method for analysis of nicotine in pure form and from formulations. J. Pharm. Biomed. Anal. 2003, 32, 441–450. 10.1016/s0731-7085(03)00236-x. [DOI] [PubMed] [Google Scholar]
- Baidoo E. E. K.; Clench M. R.; Smith R. F.; Tetler L. W. Determination of nicotine and its metabolites in urine by solid-phase extraction and sample stacking capillary electrophoresis-mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2003, 796, 303–313. 10.1016/j.jchromb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Sun J.; Du H.; You T. Determination of nicotine and its metabolite cotinine in urine and cigarette samples by capillary electrophoresis coupled with electrochemiluminescence. Electrophoresis 2011, 32, 2148–2154. 10.1002/elps.201100075. [DOI] [PubMed] [Google Scholar]
- Figueiredo E. C.; de Oliveira D. M.; de Siqueira M. E. P. B.; Arruda M. A. Z. On-line molecularly imprinted solid-phase extraction for the selective spectrophotometric determination of nicotine in the urine of smokers. Anal. Chim. Acta 2009, 635, 102–107. 10.1016/j.aca.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Langone J. J.; Gjika H. B.; Van Vunakis H. Nicotine and its metabolites. Radioimmunoassays for nicotine and cotinine. Biochemistry 1973, 12, 5025–5030. 10.1021/bi00748a032. [DOI] [PubMed] [Google Scholar]
- Mehmeti E.; Stanković D. M.; Chaiyo S.; Zavasnik J.; Žagar K.; Kalcher K. Wiring of glucose oxidase with graphene nanoribbons: an electrochemical third generation glucose biosensor. Microchim. Acta 2017, 184, 1127–1134. 10.1007/s00604-017-2115-5. [DOI] [Google Scholar]
- Chaiyo S.; Mehmeti E.; Žagar K.; Siangproh W.; Chailapakul O.; Kalcher K. Electrochemical sensors for the simultaneous determination of zinc, cadmium and lead using a Nafion/ionic liquid/graphene composite modified screen-printed carbon electrode. Anal. Chim. Acta 2016, 918, 26–34. 10.1016/j.aca.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Levent A.; Yardim Y.; Senturk Z. Voltammetric behavior of nicotine at pencil graphite electrode and its enhancement determination in the presence of anionic surfactant. Electrochim. Acta 2009, 55, 190–195. 10.1016/j.electacta.2009.08.035. [DOI] [Google Scholar]
- Suffredini H. B.; Santos M. C.; de Souza D.; Codognoto L.; Homem-de-Mello P.; Honório K. M.; da Silva A. B. F.; Machado S. A. S.; Avaca L. A. Electrochemical behavior of nicotine studied by voltammetric techniques at boron-doped diamond electrodes. Anal. Lett. 2005, 38, 1587–1599. 10.1081/al-200065801. [DOI] [Google Scholar]
- Švorc L.; Stanković D. M.; Kalcher K. Boron-doped diamond electrochemical sensor for sensitive determination of nicotine in tobacco products and anti-smoking pharmaceuticals. Diamond Relat. Mater. 2014, 42, 1–7. 10.1016/j.diamond.2013.11.012. [DOI] [Google Scholar]
- Geto A.; Amare M.; Tessema M.; Admassie S. Voltammetric Determination of Nicotine at Poly(4-Amino-3-Hydroxynaphthalene Sulfonic Acid)-Modified Glassy Carbon Electrode. Electroanalysis 2012, 24, 659–665. 10.1002/elan.201100653. [DOI] [Google Scholar]
- Li X.; Zhao H.; Shi L.; Zhu X.; Lan M.; Zhang Q.; Hugh Fan Z. Electrochemical sensing of nicotine using screen-printed carbon electrodes modified with nitrogen-doped graphene sheets. J. Electroanal. Chem. 2017, 784, 77–84. 10.1016/j.jelechem.2016.12.009. [DOI] [Google Scholar]
- Kassa H.; Geto A.; Admassie S. Voltammetric determination of nicotine in cigarette tobacco at electrochemically activated glassy carbon electrode. Bull. Chem. Soc. Ethiop. 2013, 27, 321–328. 10.4314/bcse.v27i3.1. [DOI] [Google Scholar]
- Xiong H.; Zhao Y.; Liu P.; Zhang X.; Wang S. Electrochemical properties and the determination of nicotine at a multi-walled carbon nanotubes modified glassy carbon electrode. Microchim. Acta 2010, 168, 31–36. 10.1007/s00604-009-0258-8. [DOI] [Google Scholar]
- Jing Y.; Yu B.; Li P.; Xiong B.; Cheng Y.; Li Y.; Li C.; Xiao X.; Chen M.; Chen L.; Zhang Y.; Zhao M.; Cheng C. Synthesis of graphene/DPA composite for determination of nicotine in tobacco products. Sci. Rep. 2017, 7, 14332. 10.1038/s41598-017-13716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T. W. B.; Aldous L.; Compton R. G. The use of nano-carbon as an alternative to multi-walled carbon nanotubes in modified electrodes for adsorptive stripping voltammetry. Sens. Actuators, B 2012, 162, 361–368. 10.1016/j.snb.2011.12.104. [DOI] [Google Scholar]
- Highton L.; Kadara R. O.; Jenkinson N.; Logan Riehl B.; Banks C. E. Metallic free carbon nanotube cluster modified screen printed electrodes for the sensing of nicotine in artificial saliva. Electroanalysis 2009, 21, 2387–2389. 10.1002/elan.200904683. [DOI] [Google Scholar]
- Wang S.-J.; Liaw H.-W.; Tsai Y.-C. Low potential detection of nicotine at multiwalled carbon nanotube–alumina-coated silica nanocomposite. Electrochem. Commun. 2009, 11, 733–735. 10.1016/j.elecom.2009.01.026. [DOI] [Google Scholar]
- Karthika A.; Karuppasamy P.; Selvarajan S.; Suganthi A.; Rajarajan M. Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode. Ultrason. Sonochem. 2019, 55, 196–206. 10.1016/j.ultsonch.2019.01.038. [DOI] [PubMed] [Google Scholar]
- Ashmawy N. H.; Almehizia A. A.; Youssef T. A.; El-Galil E. A. A.; Al-Omar M. A.; Kamel A. H. Novel Carbon/PEDOT/PSS-Based screen-printed biosensors for acetylcholine neurotransmitter and acetylcholinesterase detection in human serum. Molecules 2019, 24, 1539. 10.3390/molecules24081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S. S. M.; Elnemma E. M.; Mohamed A. H. K. Novel Biomedical Sensors for Flow Injection Potentiometric Determination of Creatinine in Human Serum. Electroanalysis 2005, 17, 2246–2253. 10.1002/elan.200503363. [DOI] [Google Scholar]
- Kamel A. H.; Amr A. E.; Galal H. R.; Al-Omar M. A.; Almehizia A. A. Screen-Printed Sensor Based on Potentiometric Transduction for Free Bilirubin Detection as a Biomarker for Hyperbilirubinemia Diagnosis. Chemosensors 2020, 8, 86. 10.3390/chemosensors8030086. [DOI] [Google Scholar]
- Abd-Rabboh H. S. M.; Kamel A. H.; Amr A. E.-G. E. Article All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors 2020, 8, 93. 10.3390/chemosensors8040093. [DOI] [Google Scholar]
- Eldin A. G.; Amr A. E.-G. E.; Kamel A. H.; Hassan S. S. M. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer as Solid Transducer for Ultratrace Determination of Azides. Molecules 2019, 24, 1392. 10.3390/molecules24071392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel A. H.; Hassan A. M. E. Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. 10.20964/2016.11.40. [DOI] [Google Scholar]
- El-Naby E. H.; Kamel A. H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng., C 2015, 54, 217–224. 10.1016/j.msec.2015.05.044. [DOI] [PubMed] [Google Scholar]
- El-Kosasy A. M.; Kamel A. H.; Hussin L. A.; Ayad M. F.; Fares N. V. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food Taint. Food Chem. 2018, 250, 188–196. 10.1016/j.foodchem.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Kamel A. H.; Jiang X.; Li P.; Liang R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. 10.1039/c8ay01229f. [DOI] [Google Scholar]
- Kamel A. H.; Soror T. Y.; Al Romian F. M. Flow through potentiometric sensors based on molecularly imprinted polymers for selective monitoring of mepiquat residue, a quaternary ammonium herbicide. Anal. Methods 2012, 4, 3007–3012. 10.1039/c2ay25317h. [DOI] [Google Scholar]
- Hassan S. S. M.; Badr I. H. A.; Kamel A. H.; Mohamed M. S. A Novel Poly (Vinyl Chloride) Matrix Membrane Sensor for Batch and Flow-injection Determination of Thiocyanate, Cyanide and Some Metal Ions. Anal. Sci. 2009, 25, 911–917. 10.2116/analsci.25.911. [DOI] [PubMed] [Google Scholar]
- Kamel A. H.; Sayour H. E. M. Flow-Through Assay of Quinine Using Solid Contact Potentiometric Sensors Based on Molecularly Imprinted Polymers. Electroanalysis 2009, 21, 2701–2708. 10.1002/elan.200904699. [DOI] [Google Scholar]
- Zdrachek E.; Bakker E. Potentiometric sensing. Anal. Chem. 2021, 93, 72–102. 10.1021/acs.analchem.0c04249. [DOI] [PubMed] [Google Scholar]
- Maxwell E. J.; Mazzeo A. D.; Whitesides G. M. Paper-based electroanalytical devices for accessible diagnostic testing. MRS Bull. 2013, 38, 309–314. 10.1557/mrs.2013.56. [DOI] [Google Scholar]
- Cunningham J. C.; Brenes N. J.; Crooks R. M. Paper Electrochemical Device for Detection of DNA and Thrombin by Target-Induced Conformational Switching. Anal. Chem. 2014, 86, 6166–6170. 10.1021/ac501438y. [DOI] [PubMed] [Google Scholar]
- Zang D.; Ge L.; Yan M.; Song X.; Yu J. Electrochemical immunoassay on a 3D microfluidic paper-based device. Chem. Commun. 2012, 48, 4683–4685. 10.1039/c2cc16958d. [DOI] [PubMed] [Google Scholar]
- Lan W.-J.; Zou X. U.; Hamedi M. M.; Hu J.; Parolo C.; Maxwell E. J.; Bühlmann P.; Whitesides G. M. Paper-Based Potentiometric Ion Sensing. Anal. Chem. 2014, 86, 9548–9553. 10.1021/ac5018088. [DOI] [PubMed] [Google Scholar]
- Lan W.-J.; Maxwell E. J.; Parolo C.; Bwambok D. K.; Subramaniam A. B.; Whitesides G. M. Paper-based electroanalytical devices with an integrated, stable reference electrode. Lab Chip 2013, 13, 4103–4108. 10.1039/c3lc50771h. [DOI] [PubMed] [Google Scholar]
- Lan W.-J.; Maxwell E. J.; Parolo C.; Bwambok D. K.; Subramaniam A. B.; Whitesides G. M. Paper-based electroanalytical devices with an integrated, stable reference electrode. Lab Chip 2013, 13, 4103–4108. 10.1039/c3lc50771h. [DOI] [PubMed] [Google Scholar]
- Bakker E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. 10.1149/1.1836608. [DOI] [Google Scholar]
- Hassan S. S. M.; Elnemma E. M. Liquid Membrane Electrodes for the Selective Nicotine in Cigarette Smoke. Analyst 1989, 114, 1033–1037. 10.1039/an9891401033. [DOI] [PubMed] [Google Scholar]
- Efstathiou C. E.; Diamandis E. P.; Hadjiioannou T. P. Potentiometric determination of nicotine in tobacco products with a nicotine-sensitive liquid membrane electrode. Anal. Chim. Acta 1981, 127, 173–180. 10.1016/s0003-2670(01)83973-8. [DOI] [Google Scholar]
- Brunet B. R.; Barnes A. J.; Scheidweiler K. B.; Mura P.; Huestis M. A. Development and validation of a solid-phase extraction gas chromatography-mass spectrometry method for the simultaneous quantification of methadone, heroin, cocaine and metabolites in sweat. Anal. Bioanal. Chem. 2008, 392, 115–127. 10.1007/s00216-008-2228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P. C. Two-parameter debye-hückel approximation for the evaluation of mean activity coefficients of 109 electrolytes. Anal. Chim. Acta 1982, 136, 363–368. 10.1016/s0003-2670(01)95397-8. [DOI] [Google Scholar]