Abstract

In this work, the real tar was introduced into the circulating fluidized bed gasifier by pre-mixing tar and char. The effect of steam on the tar reforming characteristics at both 850 and 900 °C was investigated by combining the analysis of the rate of tar conversion, the change of tar content, and char physical structure. The test results indicated that steam could effectively promote the tar conversion. Therefore, the content of tar in the final gas could be reached as low as 32 mg/Nm3. It was found that the effect of steam on the different components of tar was in difference. Among the various components, polycyclic aromatic substances were more inclined to decompose. The results of BET confirmed that the distribution and structure of pore were obviously developed at the presence of steam, and the abundant pore structure further improved the catalytic performance of the char on the tar conversion in turn.
1. Introduction
The syngas (CO, CH4, H2, etc.)1,2 produced from coal/biomass gasification can be widely used in chemical industry, metallurgy, integrated gasification combined cycle power generation and other fields.3,4 In the process of coal/biomass gasification, tar is an inevitable product and has become a key obstacle restricting the development of coal gasification technology. The tar in the syngas can cause a series of problems, such as blockage of pipes and valves, corrosion of metal parts, causing phenol water and other secondary pollution problems.5,6 In industrial processes, physical removal methods are often used for tar removal, such as water washing, filtration, cyclone separation, electric trapping, and absorption.7 According to the reports, the calorific value of tar in syngas is about 3% of the calorific value of coal and about 15% of the total energy of syngas.8 Physical removal methods cannot fully utilize this part of the energy. Therefore, the chemical conversion method of tar is widely favored. The chemical conversion methods of tar mainly include thermal cracking method, partial oxidation method, plasma method, catalytic conversion method, and so forth.9−12 Among them, the catalytic conversion method is considered to be one of the most promising tar removal technologies.13,14 The catalytic conversion method has the advantages of fast removal speed and high efficiency of conversion into effective gas (such as CO, H2, etc.).15,16 Compared with other conventional catalysts, char has more outstanding advantages.17,18 The most prominent advantage is that the problem of catalyst deactivation can be effectively resolved for the recovery of char activity during the gasification process. The used char can be fully utilized through gasification and combustion processes.19 Therefore, the research on char as catalysts has received extensive attention in recent years.
The current study found that steam can significantly improve the catalytic conversion effect of char on the tar reforming in a fixed bed reactor20 and can improve the homogeneous conversion and heterogeneous reforming reaction of tar. The steam reforming reaction of tar is shown in the equation below.21 After feeding steam, tar can be converted more fully at lower temperature.22 The excess steam can also react with CO to produce more H2. On the other hand, the introduction of steam can significantly inhibit the formation of carbon deposits.23
| 1 |
Lots of research studies have been carried out on the catalytic reforming of tar and steam in fixed beds. Miyazawa et al. studied the effects of different Ni-based catalysts on tar reforming with steam at 650 °C.24 Min et al. studied the effect of char-based catalysts on biomass pyrolysis tar reforming and the changes in the structure of the char and the AAEM contents on the char surface during steam reforming were also discussed.20,25 Their results show that the introduction of steam could significantly change the AAEM content on the char surface and the tar reforming was enhanced. Hosokai et al. studied steam reforming of two model compounds (benzene and naphthalene) at 800–900 °C.26 Their research showed that at a residence time of 0.2 s, the conversion rate of naphthalene was 99%, while the conversion rate of benzene was lower than that of naphthalene (84–98%). Abu El-Rub et al. used biomass semicoke to obtain a tar conversion rate of about 97% at 850 °C, while obtaining a syngas tar content of 115 mg/Nm3.27 Mathieu et al. studied the on interactions between char and toluene at 850 °C in a fluidized bed reactor.28 These experiments were mainly carried out on fixed bed, and there is lack of research in fluidized bed, especially in the circulating fluidized bed fully mixed of tar and char.
This study was conducted in a circulating fluidized bed gasifier. The real tar was introduced into the gasifier by premixing of the tar and char. The effect of steam on tar reforming characteristics at 850 and 900 °C were investigated. The tar conversion rate, the changes of the tar content, and the changes of char were also discussed in this research.
2. Results and Discussion
2.1. Effect of Steam on the Coal Gas Content
The contents of coal gas at different temperatures and with different steam contents are shown in Figure 1. At the same temperature, with the injection of steam, the content of CO decreased, whereas the contents of CO2 and H2 increased. The steam promoted the water–gas shift reaction which is shown in reaction 2. According to reaction 3, the CH4 was consumed by steam. The content of CH4 in the coal gas with steam was lower than that without steam. The steam promoted the tar reforming to CO and H2, as shown in reactions 4 and 5. The conversion of C and CO2 was promoted with the increasing of the reaction temperature. The CO content at 900 °C with steam in Figure 2 is small compared to that without steam. The shift reaction is just one of the reasons for this phenomenon. Another important reason is that the amount of oxygen in the gasifying agent was increased during the test at 900 °C in order to increase gasification temperature. The contents of CO and H2 in the coal gas of 900 °C were higher than they were at 850 °C. With the injection of steam, the char and the steam reacted as reaction 7 represented, which has great effect on the gas compositions. The coal gas yield rates are 1.81 Nm3/kg and 1.79 Nm3/kg, respectively.
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
Figure 1.
Coal gas contents at different temperatures and with different steam contents.
Figure 2.
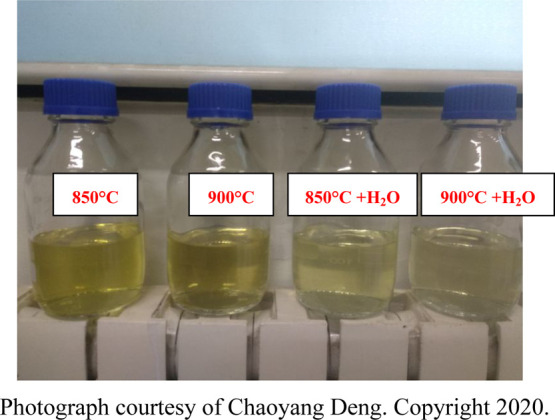
Isopropanol solution after the tar is collected. Photograph courtesy of Chaoyang Deng. Copyright 2020.
2.2. Effect of Steam on the Tar Conversion
2.2.1. Effect of Steam on the Tar Conversion Rate
Figure 2 is the isopropanol solution after the tar is collected during the experiments. The effect of the steam on the content of tar collected in the coal gas can be intuitively seen from the color depth of the solution. It can be seen from the figure that at the same reaction temperature, with the injection of steam, the isopropanol solution after the tar is collected becomes significantly shallower, which shows that steam can effectively reduce the tar content in coal gas. The effects of the steam on the tar conversion rate and the tar content in the produced gas are shown in Figure 4. Considering the amount of char is different in each experiment as the accumulated of the char, the error bar was presented in Figure 3. The error bar was calculated by the measurement error of the portable infrared analyzer and accumulated error of the amount of char in each experiment. The error was kept within ±5%. At 850 °C, the tar conversion rate increased from 91.66 to 97.60% after feeding 2 kg/h of steam (steam/char = 0.14). The tar content in the coal gas decreased from 1474 to 398 mg/Nm3. At 900 °C, after feeding 2 kg/h steam, the tar conversion rate increased from 96.70 to 99.81%, and the tar content was reduced from 530 to 32 mg/Nm3. The improvement effect of steam on the tar conversion rate is significant. After feeding steam at 900 °C, the tar content of 32 mg/Nm3 was obtained, and the improvement was greater than that at 850 °C. There are two reasons for this phenomenon. The research results based on the temperature show that several factors (the BET surface, the char microstructure, the metal content on the char surface, etc.) may affect the catalytic activity of char. These factors are more favorable at 900 °C than those at 850 °C. Feeding steam can also make these factors develop in a more favorable direction. At the same time, steam provides more H/OH and other free radicals, which greatly improves the conversion rate of tar. On the other hand, after steam is fed at 900 °C, the residual tar component becomes lighter. The content of volatile light aromatic hydrocarbons is large, and some volatile components escape during the evaporation and weighing process. The feeding of steam is similar to that of changing the temperature to some extent, and both can affect the homogeneous reaction and the heterogeneous reforming reaction of tar. Free radicals (H/OH) can react with active free tar fragments, so the increase of free radicals such as H/OH will lead to the strengthening of the homogeneous reaction of tar and the heterogeneous reforming reaction.29 At the same time, the reaction of steam and char will lead to changes in its pore structure, microcrystalline structure, functional groups, and surface metal content, which affect the distribution of active sites and further affect the heterogeneous reforming reaction.
Figure 4.
GC/MS chromatograms (total ion current) for different conditions of tar conversion.
Figure 3.
Tar conversion rate and the tar content in the coal gas.
2.2.2. Effect of Steam on Tar Composition Conversion Characteristics
GC–MS was applied to analyze the composition and content of the residual tar and the original tar after the reaction. The total ion chromatogram of the tar collected from the coal gas is shown in Figure 4. As shown in Figure 4, compared with no steam feeding conditions, the total ion chromatogram of the tar has more peaks in the range of residence time 0–10 min with steam feeding and the total ion chromatogram of the tar is flatter when the residence time is greater than 10 min. This indicates that the introduction of steam can further promote the conversion of polycyclic aromatic substances. More specific data can be obtained from Figure 5. After steam is fed into the gasifier, the content of light aromatic hydrocarbons and others increased, while the content of polycyclic aromatic hydrocarbons decreased. At 850 °C, the content of light aromatic hydrocarbons and other hydrocarbons increased from 15 to 23%, and heavy polycyclic aromatic hydrocarbons decreased from 7 to 2%. At 950 °C, the content of light aromatics and other hydrocarbons increased from 16 to 22%. It is worth noting that the improvement of the tar composition after feeding steam at 850 °C is even better than that at 900 °C without steam feeding. Compared with 900 °C, the content of light aromatic hydrocarbons and others increased, and the content of polycyclic aromatic hydrocarbons decreased at 850 °C with steam. During the char gasification of circulating fluidized bed, because of the presence of steam, a variety of free radicals (O, OH, H, etc.) can be generated, and these free radicals can react with tar in a homogeneous phase. The debris reacts, thereby inhibiting the polymerization of tar and producing light aromatic substances. At the same time, the reaction of steam with char changes the tar heterogeneous reforming reaction. It can be predicted that steam can simultaneously promote the homogeneous reaction and the heterogeneous reforming reaction of tar and further promoting the catalytic conversion of tar.
Figure 5.
Relative peak area of main tar species as analyzed by GC/MS.
In order to more intuitively show the conversion rule of each component in tar, the components in tar are arranged according to the relative molecular weight from small to large, as shown in Figure 6. Compared with the result of no steam condition, the large molecular weight range of tar (above 140) becomes smoother and the number of components becomes smaller when the steam was fed. This indicates that the macromolecules have been effectively reduced with the injection of steam.
Figure 6.
Molecular weight distribution of tar.
Separate conversion rate calculations are performed for the main components with a content of more than 3% in the original tar, and the results are shown in Figure 7. The conversion rates were calculated based on the total amount of the tar in the coal gas and the % area of GC/MS. It can be seen that the conversion rate of each main component in tar is significantly improved after the steam is fed. Among them, anthracene and pyrene were completely converted at 850 °C. Fluorene, anthracene, and pyrene were completely converted at 900 °C. The conversion rate of indene, naphthalene, and methylnaphthalene with less ring number and lower molecular weight is lower than that of compounds with more ring number and higher molecular weight. Light polycyclic aromatic hydrocarbons reduce the conversion rate of light polycyclic aromatic hydrocarbons.
Figure 7.
Conversion rates of the main components in the tar.
2.3. Effect of Steam on the Char
2.3.1. Char Structure
The specific surface area analyzer (BET) was used to analyze the gasified char after the reaction at different temperatures and different steam conditions. The results are shown in Figure 8. In Figure 8, the ordinate is the adsorption amount and the abscissa is the relative pressure. With the injection of steam, the equilibrium adsorption capacity of the char is significantly improved. At the same time, there is a hysteresis loop in each adsorption and desorption isotherm, which is a typical type IV isotherm. The hysteresis loop area also increases with the injection of steam, indicating that steam increases the pore volume of the char. The porous structure of char can effectively reduce tar adsorption and increase the residence time of tar on the surface of char.30 The specific values are listed in Table 1. At 850 and 900 °C, the pore volumes of the char after steam injection increase from 0.0345 cc/g and 0.0569 cc/g to 0.1421 cc/g and 0.1353 cc/g, respectively. The pore volume increased by 64.93% at 850 °C and decreased by 5.03% at 900 °C. The starting points of the hysteresis loops at all experimental conditions are at lower relative pressures, indicating that gasified char is a typical microporous material. After the steam was fed, the specific surface area of the char significantly increased. At 850 and 900 °C, the steam increased from 8.64 and 45.84 m2/g to 135.71 m2/g and 138.87 m2/g, respectively. After the steam was fed, the specific surface area did not increase significantly when the temperature was increased from 850 to 900 °C, but the pore distribution was changed. The ratio of micropore to mesoporous surface area was 1.86 and 1.79, respectively, and the ratio of mesopore was increased. The results show that mesoporous has a greater effect on the catalytic activity of char than the micropore because mesopores can absorb macromolecules, so that more tar macromolecules can be adsorbed on the surface of char.31 Higher specific surface area is beneficial to promote the conversion of tar.
Figure 8.
N2-absorption/desorption isotherms of the chars.
Table 1. Pore Structure Parameters of the Chars.
| experimental conditions | BET specific surface area (m2/g) | volume (cc/g) | diameter (nm) | mesoporous surface area (m2/g) | micropore surface area (m2/g) |
|---|---|---|---|---|---|
| 850 °C | 8.64 | 0.0345 | 1.54 | 10.93 | 0.00 |
| 850 °C with steam | 135.71 | 0.1421 | 1.41 | 41.04 | 76.18 |
| 900 °C | 45.84 | 0.0569 | 1.41 | 20.18 | 11.77 |
| 900 °C with steam | 138.87 | 0.1353 | 1.41 | 39.07 | 70.06 |
2.3.2. Metal Distribution
The injection of steam can significantly change the content of alkali metals and alkaline earth metals in semicoke, especially the metal content on the surface of char.32 XRF and EDX analyses were performed to determine the contents of the alkali metals and alkaline earth metals in the char and on the surface of the char. The results are shown in Table 2 and Table 3. As shown in the tables, the Shenmu char used in this experiment mainly consists of Ca. Ca plays an important role in the catalysis of the tar. After injection of steam, the Ca content on the char surface decreased. The steam could strengthen the cross-linking reaction of tar and char and a part of the metal would leave the char surface. However, the metal content measured by EDX is the content of a certain area, and the total content should be calculated in relation to the specific surface area. The specific surface area of steam conditions at 850 and 900 °C had increased by 15 and 3 times, respectively. The relative Ca content on the char surface decreased and the total content of Ca on the surface increased at 850 °C, but not much changed at 900 °C.
Table 2. Contents of the Alkali Metals and Alkaline Earth Metals in the Char, wt %.
| experimental conditions | Ca | Na | Mg | K |
|---|---|---|---|---|
| 850 °C | 18.942 | 1.515 | 0.799 | 0.314 |
| 850 °C with steam | 22.417 | 1.509 | 0.8 | 0.328 |
| 900 °C | 23.537 | 1.489 | 0.754 | 0.318 |
| 900 °C with steam | 25.778 | 1.315 | 0.742 | 0.251 |
Table 3. Contents of the Alkali Metals and Alkaline Earth Metals on the Surface of the Char, wt %.
| experimental conditions | K | Ca |
|---|---|---|
| 850 °C | 0 | 0.19 |
| 850 °C with steam | 0 | 0.11 |
| 900 °C | 0 | 4.02 |
| 900 °C with steam | 0.01 | 1.18 |
3. Conclusions
In this work, the real tar was introduced into the circulating fluidized bed gasifier by pre-mixing the tar and char. The effect of steam on tar reforming characteristics during circulating fluidized bed char gasification at 850 and 900 °C were investigated. The tar conversion rate, the changes of tar content, and the changes of char were also discussed in this research. The main conclusions are as follows:
-
1
In the circulating fluidized bed gasifier, steam can effectively promote the conversion of the tar and reduce the tar content in coal gas. Under the experimental conditions, at 900 °C, the tar conversion rate increased from 96.70 to 99.81% after feeding 2 kg/h of steam, and the tar content in the coal gas decreased from 530 to 32 mg/Nm3.
-
2
The effects of steam on different components of the tar are different. The content of light aromatic hydrocarbons and others increased, while the content of polycyclic aromatic hydrocarbons decreased.
-
3
The steam affected the char structure. After the steam was fed, the pore structure of the char was obviously developed, and the pore distribution of the char was also improved. The steam could improve the catalytic performance of the char.
4. Experimental Section
4.1. Fuel and Bed Material
Shenmu char was chosen as the experimental fuel. The size range of char used in the experiments was from 0.1 to 0.5 mm. The char basis properties are presented in Table 4. The proximate analyses were carried out according to Chinese Standard GB/T212-2008, and ultimate analyses were carried out according to Chinese Standard GB/T476-2001.
Table 4. Proximate and Ultimate Analysis of Shenmu Chara.
| proximate analysis [wt %, ad] | ultimate analysis [wt %, ad] | ||||||||
| fuel | M | A | V | FC | C | H | N | O | St |
| shenmu char | 1.78 | 8.53 | 7.23 | 82.46 | 83.24 | 1.81 | 0.90 | 3.40 | 0.34 |
ad: air-dry basis.
Silica sand was chosen as the bed material. The bed material particle size ranged from 0.18 to 0.71 mm. The d50 of the bed material is 592 μm. The density of the bed material is 1325 kg/m3. The bed material mainly composes of SiO2 (95.64 wt %).
The experimental tar is low-temperature coal tar. The main compositions are shown in Table 5. Among them, heavy polycyclic aromatic hydrocarbons represent polycyclic aromatic hydrocarbons with ring number greater than 3 (such as pyrene, benzofluoranthene, etc.); light polycyclic aromatic hydrocarbons represent polycyclic aromatic hydrocarbons with ring number of 2–3 (such as naphthalene, biphenylene, etc.); light aromatic hydrocarbons and other aromatic hydrocarbons with ring number of 1 and other acyclic chain hydrocarbons (such as benzene, phenol, etc.). The same classification method was used for tar components collected after reaction.
Table 5. Components and Content of the Tar, wt %.
| component | content |
|---|---|
| heavy polycyclic aromatic hydrocarbons | 1.386 |
| light polycyclic aromatic hydrocarbons | 88.778 |
| light aromatic hydrocarbons and others | 9.836 |
4.2. Experimental System and Operating Conditions
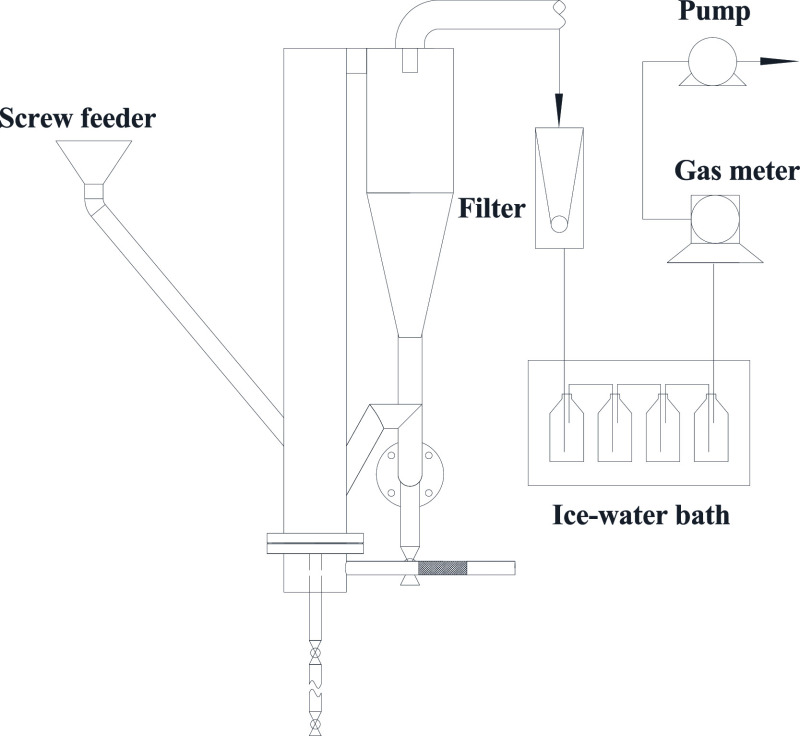
Figure 9 shows the experimental system for tar reforming. The test system was mainly composed of circulating fluidized bed reaction unit, gas cooler, fabric filter, tail flue, and auxiliary equipments. The char was fed in the bottom of the riser by the screw feeder and reacted with the gasification agent fed by the wind caps; the high-temperature gas was discharged from the outlet of the cyclone separator and then entered the gas cooler, fabric filter, and other purification devices. Detailed descriptions could be found in our other work.33 The gasifier is a stainless steel cylindrical tube with an inner diameter of 130 mm and a height of 1000 mm. The wall thickness is 10 mm. The loop seal is a U-valve.
Figure 9.
Circulating fluidized bed gasification system.
Compared with bubbling fluidized bed or fixed bed, CFB gasifier has less particle density in the riser, but has better mixing condition. The char and the tar could mix better and more tar gas can reach the char surface and react with radicals on the char surface. Under mild reaction conditions (e.g., 850 °C with less steam), the char shows an excellent performance on the tar reforming.
The tar was injected into the gasifier by premixing the raw char and the tar. The injection position of the tar is about 230 mm higher than the air distributor. The mass of the tar is 3% by weight of the coal, based on the result of coal gray-king assay. During the experiments, the gasification temperature was kept at 850 and 900 °C, respectively. 2 kg/h steam was injected into the gasifier, and the steam temperature is 170 °C. The ratio of steam to coal is about 0.15 kg/kg, which refers to the operating parameters of industrial gasifier. The tests were conducted at atmospheric pressure.
The detailed operating parameters of each working condition are presented in Table 6. Each working condition was kept for 2 h to analyze the coal gas components and collect the tar maintained in the coal gas.
Table 6. Operating Parameters of Each Working Condition.
| no. | temperature [°C] | steam [kg/h] | air equivalent ratio | tar [g/h] | superficial gas velocity [m/s] | residence time [s] |
|---|---|---|---|---|---|---|
| 1 | 850 | 0 | 0.22 | 423 | 1.70 | 0.45 |
| 2 | 850 | 2 | 0.22 | 423 | 1.91 | 0.40 |
| 3 | 900 | 0 | 0.22 | 423 | 1.82 | 0.42 |
| 4 | 900 | 2 | 0.22 | 423 | 2.05 | 0.38 |
4.3. Sampling and Analysis Methods
The coal gas contents (H2, CO, CO2, and CH4) were analyzed by a portable infrared analyzer (Gasboard-3100P, Siguang, China) at the outlet of the cyclone. Zero adjustments were required before each analysis. The analysis precision of the portable infrared analyzer is 1% FS.
The tar in the coal gas was collected at the outlet of the cyclone. The collecting device consisted of a filter, an ice-water bath, a wet flowmeter, and a vacuum pump. The ice water bath consisted of four gas wash bottles (250 mL) with 100 mL isopropyl alcohol. The tar in 50 L coal gas was collected. To avoid the condensing of gaseous tar, the filter and pipes were heated to 350 °C using a heating cable.
The liquid sample collected with the isopropyl alcohol solvent is allowed to stand and be filtered and then subjected to evaporation treatment to obtain the tar content (mtar-50L, mg) contained in the 50 L coal gas. The content of tar in the coal gas (mtar, mg/Nm3) is obtained through eq 8.
| 8 |
The tar conversion rate is obtained through eq 9
| 9 |
where mtar is the content of tar in the coal gas, mg/Nm3; Vgas is the volume of the coal gas, Nm3/h; Mtar is the mass of tar injected into the gasifier, mg/h; η is the tar conversion rate.
The gas production volume Vgas was calculated by the nitrogen balance method.
The tar samples collected from the coal gas were analyzed by GC–MS (GCMS QP2010 Ulltra, Shimadzu, Japan) to determine the chemical compounds and relative content.
The char sample was collected from the sample point at the bottom of the gasifier after each working condition.
The pore structure of char was analyzed by a specific surface area analyzer (BET, ASAP 2010, Micromeritics, America). The adsorption medium is 77 K low-temperature liquid nitrogen. Scanning electron microscope (SEM, JSM-7800, JEOL, Japan) was used to analyze the surface morphology of char. The metal content of the char surface was analyzed by energy-dispersive X-ray spectroscopy (EDX, JSM-7800, JEOL, Japan).The metal content of the char was analyzed by X-ray fluorescence spectroscopy (XRF, AXIOS, PANalytical B.V, Netherlands).
Acknowledgments
Financial support of this work by National Key Research and Development Program of China (no. 2018YFB0605405) is gratefully acknowledged.
The authors declare no competing financial interest.
This paper was published on March 18, 2021. It was reposted on April 19, 2021, with the name of an author removed.
References
- Stiegel G. J.; Maxwell R. C. Gasification technologies: the path to clean, affordable energy in the 21st century. Fuel Process. Technol. 2001, 71, 79–97. 10.1016/s0378-3820(01)00138-2. [DOI] [Google Scholar]
- Emami Taba L.; Irfan M. F.; Wan Daud W. A. M.; Chakrabarti M. H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renewable Sustainable Energy Rev. 2012, 16, 5584–5596. 10.1016/j.rser.2012.06.015. [DOI] [Google Scholar]
- Giuliano A.; Poletto M.; Barletta D. Pure hydrogen co-production by membrane technology in an IGCC power plant with carbon capture. Int. J. Hydrogen Energy 2018, 43, 19279–19292. 10.1016/j.ijhydene.2018.08.112. [DOI] [Google Scholar]
- Wan W.; Dai Z.; Li C.; Yu G.; Wang F. Innovative concept for gasification for hydrogen based on the heat integration between water gas shift unit and coal–water–slurry gasification unit. Int. J. Hydrogen Energy 2014, 39, 7811–7818. 10.1016/j.ijhydene.2014.03.032. [DOI] [Google Scholar]
- Font Palma C. Modelling of tar formation and evolution for biomass gasification: A review. Appl. Energy 2013, 111, 129–141. 10.1016/j.apenergy.2013.04.082. [DOI] [Google Scholar]
- Ji Q.; Tabassum S.; Hena S.; Silva C. G.; Yu G.; Zhang Z. A review on the coal gasification wastewater treatment technologies: past, present and future outlook. J. Cleaner Prod. 2016, 126, 38–55. 10.1016/j.jclepro.2016.02.147. [DOI] [Google Scholar]
- Anis S.; Zainal Z. A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renewable Sustainable Energy Rev. 2011, 15, 2355–2377. 10.1016/j.rser.2011.02.018. [DOI] [Google Scholar]
- Juan W. U.; Haijun C.; Yuezhao Z.; Chuanhua L.; Li Y. Biomass producer gas tar removal technology based on recovery idea. Chem. Ind. Eng. Prog. 2013, 32, 2099. [Google Scholar]
- Brandt P.Decomposition of tar in gas from updraft gasifier by thermal cracking. 1st World Conference and Exhibition on Biomass for Energy and Industry, Seville, 2000.
- Tao K.; Ohta N.; Liu G.; Yoneyama Y.; Wang T.; Tsubaki N. Plasma enhanced catalytic reforming of biomass tar model compound to syngas. Fuel 2013, 104, 53–57. 10.1016/j.fuel.2010.05.044. [DOI] [Google Scholar]
- Ahrenfeldt J.; Egsgaard H.; Stelte W.; Thomsen T.; Henriksen U. B. The influence of partial oxidation mechanisms on tar destruction in TwoStage biomass gasification. Fuel 2013, 112, 662–680. 10.1016/j.fuel.2012.09.048. [DOI] [Google Scholar]
- Zhang S.; Chen Z.; Zhang H.; Wang Y.; Xu X.; Cheng L.; Zhang Y. The catalytic reforming of tar from pyrolysis and gasification of brown coal: Effects of parental carbon materials on the performance of char catalysts. Fuel Process. Technol. 2018, 174, 142–148. 10.1016/j.fuproc.2018.02.022. [DOI] [Google Scholar]
- Zeng X.; Wang F.; Han Z.; Han J.; Zhang J.; Wu R.; Xu G. Assessment of char property on tar catalytic reforming in a fluidized bed reactor for adopting a two-stage gasification process. Appl. Energy 2019, 248, 115–125. 10.1016/j.apenergy.2019.04.122. [DOI] [Google Scholar]
- Zeng X.; Wang F.; Sun Y.; Zhang J.; Tang S.; Xu G. Characteristics of tar abatement by thermal cracking and char catalytic reforming in a fluidized bed two-stage reactor. Fuel 2018, 231, 18–25. 10.1016/j.fuel.2018.05.043. [DOI] [Google Scholar]
- Serrano D.; Kwapinska M.; Horvat A.; Sánchez-Delgado S.; Leahy J. J. Cynara cardunculus L. gasification in a bubbling fluidized bed: The effect of magnesite and olivine on product gas, tar and gasification performance. Fuel 2016, 173, 247–259. 10.1016/j.fuel.2016.01.051. [DOI] [Google Scholar]
- Quitete C. P. B.; Souza M. M. V. M. Application of Brazilian dolomites and mixed oxides as catalysts in tar removal system. Appl. Catal., A 2017, 536, 1–8. 10.1016/j.apcata.2017.02.014. [DOI] [Google Scholar]
- Shen Y.; Fu Y. Advances in in situ and ex situ tar reforming with biochar catalysts for clean energy production. Sustainable Energy Fuels 2018, 2, 326–344. 10.1039/c7se00553a. [DOI] [Google Scholar]
- Liu F.; Yang L.; Song C. Chemical looping hydrogen production using activated carbon and carbon black as multi-function carriers. Int. J. Hydrogen Energy 2018, 43, 5501–5511. 10.1016/j.ijhydene.2018.01.098. [DOI] [Google Scholar]
- Wang X.; Chen H.; Yang H.; Ju F.; Zhang S. Influence of pyrolysis condition and coal type on char gasification reactivity. Asia-Pac. J. Chem. Eng. 2012, 7, S171–S176. 10.1002/apj.632. [DOI] [Google Scholar]
- Min Z.; Yimsiri P.; Asadullah M.; Zhang S.; Li C.-Z. Catalytic reforming of tar during gasification. Part II. Char as a catalyst or as a catalyst support for tar reforming. Fuel 2011, 90, 2545–2552. 10.1016/j.fuel.2011.03.027. [DOI] [Google Scholar]
- Guan G.; Kaewpanha M.; Hao X.; Abudula A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renewable Sustainable Energy Rev. 2016, 58, 450–461. 10.1016/j.rser.2015.12.316. [DOI] [Google Scholar]
- Li D.; Ishikawa C.; Koike M.; Wang L.; Nakagawa Y.; Tomishige K. Production of renewable hydrogen by steam reforming of tar from biomass pyrolysis over supported Co catalysts. Int. J. Hydrogen Energy 2013, 38, 3572–3581. 10.1016/j.ijhydene.2013.01.057. [DOI] [Google Scholar]
- Kim Y.-K.; Park J.-I.; Jung D.; Miyawaki J.; Yoon S.-H.; Mochida I. Low-temperature catalytic conversion of lignite: 3. Tar reforming using the supported potassium carbonate. J. Ind. Eng. Chem. 2014, 20, 9–12. 10.1016/j.jiec.2013.04.003. [DOI] [Google Scholar]
- Miyazawa T.; Kimura T.; Nishikawa J.; Kado S.; Kunimori K.; Tomishige K. Catalytic performance of supported Ni catalysts in partial oxidation and steam reforming of tar derived from the pyrolysis of wood biomass. Catal. Today 2006, 115, 254–262. 10.1016/j.cattod.2006.02.055. [DOI] [Google Scholar]
- Min Z.; Zhang S.; Yimsiri P.; Wang Y.; Asadullah M.; Li C.-Z. Catalytic reforming of tar during gasification. Part IV. Changes in the structure of char in the char-supported iron catalyst during reforming. Fuel 2013, 106, 858–863. 10.1016/j.fuel.2012.11.063. [DOI] [Google Scholar]
- Striu̅gas N.; Zakarauskas K.; Stravinskas G.; Grigaitienė V. Comparison of steam reforming and partial oxidation of biomass pyrolysis tars over activated carbon derived from waste tire. Catal. Today 2012, 196, 67–74. 10.1016/j.cattod.2012.04.065. [DOI] [Google Scholar]
- Abu El-Rub Z.; Bramer E. A.; Brem G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. 10.1016/j.fuel.2008.01.004. [DOI] [Google Scholar]
- Morin M.; Nitsch X.; Hémati M. Interactions between char and tar during the steam gasification in a fluidized bed reactor. Fuel 2018, 224, 600–609. 10.1016/j.fuel.2018.03.050. [DOI] [Google Scholar]
- Feng D.; Zhao Y.; Zhang Y.; Sun S. Effects of H2O and CO2 on the homogeneous conversion and heterogeneous reforming of biomass tar over biochar. Int. J. Hydrogen Energy 2017, 42, 13070–13084. 10.1016/j.ijhydene.2017.04.018. [DOI] [Google Scholar]
- Klinghoffer N. B.; Castaldi M. J.; Nzihou A. Catalyst Properties and Catalytic Performance of Char from Biomass Gasification. Ind. Eng. Chem. Res. 2012, 51, 13113–13122. 10.1021/ie3014082. [DOI] [Google Scholar]
- Frackowiak E.; Béguin F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. 10.1016/s0008-6223(00)00183-4. [DOI] [Google Scholar]
- Asadullah M.; Zhang S.; Min Z.; Yimsiri P.; Li C.-Z. Effects of biomass char structure on its gasification reactivity. Bioresour. Technol. 2010, 101, 7935–7943. 10.1016/j.biortech.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Deng C.; Song W.; Chai Z.; Guo S.; Zhu Z. Characteristics of Tar Thermal Cracking and Catalytic Conversion during Circulating Fluidized Bed Char Gasification. Energy Fuels 2020, 34, 142–149. 10.1021/acs.energyfuels.9b03346. [DOI] [Google Scholar]