Abstract
The excellent conductivity and versatile surface chemistry of MXenes render these nanomaterials attractive for sensor applications. This mini-review puts recent advances in MXene-based sensors into perspective and provides prospects for the area. It describes the attractive properties and the working principles of MXene-based sensors fabricated from a MXene/polymer nanocomposite or a pristine MXene. The importance of surface modification of MXenes to improve their affinity for polymers and to develop self-healing and durable sensors is delineated. Several novel sensor fabrication methods and their challenges are discussed. Emerging applications of MXene-based sensors including moisture, motion, gas, and humidity detection as well as pressure distribution mapping are critically reviewed. Potential applications of MXene-based sensors in the food industry to monitor food materials and production plants are highlighted.
1. Introduction
Electroconductivity, rich surface chemistry, and high aspect ratio of MXenes are attractive properties for sensor fabrication. An ideal sensor has high sensitivity, a low limit of detection, low fabrication costs, low hysteresis, a quick and preferably linear response, and fast recovery for repetitive use. For pressure and strain sensors, a linear response in a wide pressure range as well as high durability over thousands of deformation cycles is required. Sensors made from MXenes, MXene/polymer nanocomposites, and mixed-dimensional two-dimensional (2D) MXene-based heterostructures have shown these properties. The latter ones include a heterostructure of a MXene and a 0D, 1D, or 2D nanomaterial. As an example, silver nanoparticles, carbon nanotubes, and graphene oxide nanosheets are 0D, 1D, and 2D nanoparticles, respectively, which have been used together with a MXene to fabricate mixed-dimensional 2D MXene-based heterostructures.1 These heterostructures along with pristine MXene and MXene/polymer nanocomposites have been used in sensor applications to detect toxic compounds in water and food materials,2 monitor human body movements and human health, measure gas and humidity levels, and recognize voices, etc.3Table 1 lists many MXene-based sensors and their corresponding applications.
Table 1. MXene-Based Sensors and Their Corresponding Applications.
| nanocomposite components | application | ref |
|---|---|---|
| Ti3C2 and reduced graphene oxide | pressure sensor | (1) |
| Ti3C2–Ag nanowire and polydopamine/Ni2+ | strain sensor | (1) |
| Ti3C2 and chitosan | biosensor for detecting pesticides | (2) |
| Ti3C2 and Nafion | detecting nitrile ions | (3) |
| Ti3C2 and PANI | ethanol, methanol, ammonia, and acetone detection | (5) |
| Ti3C2 and polyurethane | stretchable strain sensing fabric | (6) |
| Ti3C2 and poly(vinylidene fluoride-trifluoroethylene) | capacitive pressure sensor | (7) |
| Ti3C2 and natural microcapsules | epidermal flexible pressure sensors | (8) |
| surface-modified Ti3C2 and epoxidized natural rubber | self-healable intelligent sensors | (9) |
| hollow MXene spheres and reduced graphene | piezoresistive pressure sensor | (10) |
| Ti3C2 and ink | strain sensor for health monitoring | (11) |
| modified Ti3C2 and amino poly(dimethylsiloxane) | elastomeric wearable strain sensors | (12) |
| Ti3C2 and poly(vinyl alcohol) | wearable electronic sensors for robotic applications | (13) |
| Ti3C2 and poly(diallyldimethylammonium chloride) | humidity sensor | (14) |
| Ti3C2 and polyacrylamide and poly(vinyl alcohol) and ethylene glycol | subzero temperature strain sensor | (15) |
| Ti3C2 and poly(dimethylsiloxane) | skin conformal sensors for health monitoring | (16) |
| Ti3C2 and modified sodium alginate and polyacrylamide | self-healing capability, self-adhesiveness, moisture retention, human motion biomonitoring sensor | (17) |
| Ti3C2 | NH3 detection | (18) |
| Ti3C2 and gold nanoparticles | glucose detection biosensor | (19) |
| Ti3C2 and TiO2 | H2O2 detection | (20) |
The performance of a MXene in sensor applications depends on the type and the concentration of its surface functional groups (hydroxyl, oxygen, fluorine, chlorine). For example, simulation results have shown that an oxygen-terminated MXene has excellent performance for ammonia sensing, while a hydroxyl-terminated MXene has a better performance for ethanol detection.4,5 For real-world applications, MXene-based sensors should be produced in large quantities, and sensor materials should have high mechanical endurance for machine processability. In other words, conductivity and stretchability are needed simultaneously, which can be obtained through the addition of a MXene to a polymer, mostly an elastomer. However, adjusting mechanical and electrical properties of the resulting MXene/polymer nanocomposites is still challenging.6
This mini-review puts recent advances in MXene-based sensors into perspective and provides prospects for the area. It first describes novel techniques for manufacturing MXene-based sensors and discusses challenges in this area. Many of these challenges can be addressed by surface modification of MXene. Thus, techniques to modify the surface of MXenes are discussed in section 3. Next, some emerging applications of MXene-based sensors are critically reviewed.
2. Sensor Manufacturing Techniques and Challenges
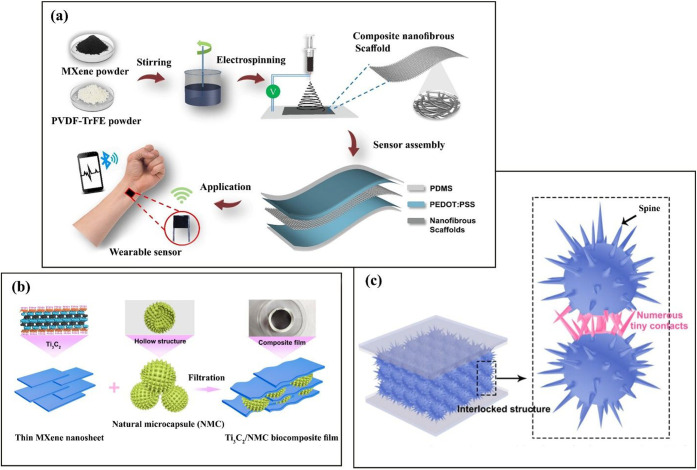
There is an ongoing need for developing more sensitive and more flexible pressure sensors. Materials or structures that efficiently convert an external pressure into an electrical signal are favorable for pressure sensor applications. A common approach for fabricating MXene/polymer nanocomposite sensors is depicted in Figure 1a. As this figure shows, MXene and a polymer are dissolved in a solvent. The sensing layer of the sensor is then created from the MXene/polymer mixture using a processing technique such as electrospinning. Next, the sensing layer is sandwiched between two conductive electrodes. The sensor is then placed or mounted on a plate to transfer its output signal to an analyzer via a wireless or wired connection. Research in the area of sensors has mostly concentrated on developing more efficient sensing layers, which play a central role in sensors.
Figure 1.
(a) Fabrication steps of a capacitive pressure sensor with a dielectric material made from PVDF-TrFE and electrodes made from poly(3,4-ethylenedioxythiophene) polystyrenesulfonate/polydimethylsiloxane. Reprinted in part with permission from ref (7). Copyright 2020 American Chemical Society. (b) Fabrication steps of a Ti3C2/natural microcapsules biocomposite sensing film. (c) Schematic showing the contact between MXene sheets and NMC particles. Reprinted in part with permission from ref (8). Copyright 2019 American Chemical Society.
One way to develop state-of-the-art pressure sensors is to mimic the human body. Human skin possesses a micro/nanostructure with a hierarchy interlocking pattern, providing excellent pressure sensitivity. By mimicking human skin, researchers have developed MXene/natural microcapsules (NMC) biocomposite films that show 9.4 times greater sensitivity compared to that of planar MXene-based sensors.8Figure 1b,c shows the fabrication steps and the structure of this sensor, respectively. In a hierarchal interlocking MXene/NMC sensor (Figure 1c), there are many spine–tip contacts in the sensing layer. Compared with planar MXene sheets, these NMCs show higher deformability, causing MXene/NMC nanocomposites to show higher sensibility compared with pristine MXene sensors. In fact, the presence of NMC between MXene sheets creates a porous structure which provides higher deformability and consequently greater ability to convert applied pressure into an electrical signal. In sensor technology, the response time is defined as the time it takes for the response of a sensor (sensor reading) to reach 90% of the ultimate change in the sensor reading when the sensor is subjected to a step change in the actual value of the quantity that the sensor measures. It can be decreased by fabricating a sensor from a hierarchically interlocked structure, like MXene/NMC. Based on previous studies, two recommendations to fabricate state-of-the-art MXene-based sensors can be made. First, improve the deformability of the sensing layer to fully exploit the conductivity of MXene and to increase the contact area between MXene sheets with other components inside the sensing layer.8 Second, form networks of a MXene in the polymeric matrix. Well-organized networks of MXenes are very sensitive to external mechanical forces and consequently are able to detect very small movements.9
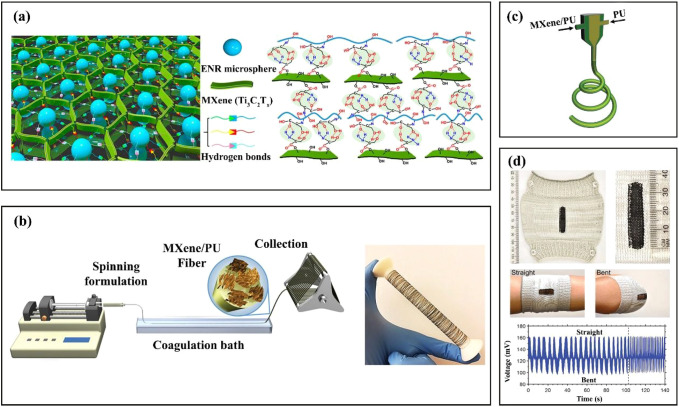
MXenes have excellent dispersibility in water. Thus, any polymer which is soluble or dispersible in water can be easily mixed with a MXene via a simple aqueous solution mixing process to fabricate a MXene/polymer nanocomposite sensor. In addition to improvement in mechanical properties, polymers can protect a MXene from oxidation if they show a great affinity for a MXene and finely mix with it.9 Elastomeric polymer particles are usually spherical with a size higher than sheets of a MXene. Thus, when these materials are mixed with each other, the polymer particles push the MXene sheets to the interstitial space between the particles, and this causes the formation of a 3D continuous network of conductive nanosheets (Figure 2a).9 The presence of this continuous network of a MXene is essential to fabricate a state-of-the-art sensor.
Figure 2.
(a) Schematic representing the formation of a 3D network of a MXene in the presence of polymer particles. Reprinted in part with permission from ref (9). Copyright 2020 American Chemical Society. (b) Wet spinning setup for the production of MXene/polymer fibrillar nanocomposite sensors. (c) Apparatus used for the fabrication of a coaxial nanocomposite sensing fiber. (d) Sensing fabrics made by knitting sensing fibers. Reprinted in part with permission from ref (6). Copyright 2020 Wiley.
Wet spinning is a commercially viable approach to produce MXene/polymer nanocomposite sensors. Figure 2b shows a schematic of a wet spinning setup for the production of fibrillar nanocomposite sensors. Using an elastomeric polyurethane (PU) and a suspension of MXene in an organic solvent (dimethylsulfoxide or dimethylformamide), Seyedin et al.6 successfully fabricated MXene/PU sensors with a gauge factor of 12900, which is the highest ever reported in the literature. They were also able to produce continuous fibers with a length higher than 1 km, which is promising for the large-scale production of strain sensors. However, a wet spinning process has several variables which must be adjusted carefully. A process variable is the type of nonsolvent used in the coagulation bath. The nonsolvent affects the coagulation rate of the fiber and also influences the conductivity percolation threshold. For example, under constant MXene and polymer concentrations, changing only the nonsolvent type of the coagulation bath from isopropyl alcohol to acetic acid altered the MXene percolation threshold from 1 to 2 wt %.6 Many other properties including the morphology of the nanocomposite sensing fibers, their geometry, conductivity, and maximum filler loading capability are dependent on the type of the nonsolvent.6
A challenge in the fabrication of MXene/polymer nanocomposite sensors especially for wearable sensor applications is the decrease of flexibility and stretchability with increased MXene concentration beyond a threshold. Numerous mechanical characterization tests should be carried out to determine the MXene concentration threshold for each polymer.6 On the other hand, the addition of a small amount of a MXene to a polymer may not lead to the formation of a MXene network needed for good electroconductivity. These facts point to the existence of trade-offs among the composite properties and the importance of MXene loading level in nanocomposite sensors.6
Another challenge in the fabrication of MXene/polymer strain sensors is the occurrence of a permanent hysteresis after several cycling deformations. In other words, during a period of cyclic strain, the resistivity of a MXene/polymer nanocomposite sensor increases with each cycle. Each stretch disrupts some interconnections of MXene flakes in the conductive network. The interconnections are then re-formed once the strain force is not applied anymore. However, over time with repeated cyclic strains, some MXene flakes break, leading to some unrecoverable degradation of the connectivity of the MXene conductive network and thus to an increase in the resistivity of the sensor. This is called drift in the resistivity response or hysteresis.6 To overcome this challenge, a coaxial wet spinning approach can be used to fabricate a coaxial fiber with a core made from a pure elastomeric polymer like PU and a sheath made from MXene/PU. Figure 2c shows a schematic of the apparatus used for the fabrication of this coaxial fiber.6 MXene/polymer nanocomposite sensing fibers with a coaxial morphology have a higher strain range of sensing and show a lower drift in the resistivity response over repeated cyclic strains. These improvements originate first from the ability of the pure elastomeric core of the coaxial fiber for complete recovery after the removal of each force and second from increased recovery of the MXene conductive network of the elastomeric PU sheath during unloading.6
It is essential for a MXene/polymer nanocomposite sensing fiber to have enough mechanical strength to fabricate textile sensors using a knitting machine. In fact, a fiber needs to undergo several intermeshing loops to be a part of a fabric. When these sensing fabrics are produced (Figure 2d), they show a completely different resistivity response compared with individual fibers. As a load is applied to a sensing fabric, the resistivity decreases. This completely opposite resistivity response behavior can be attributed to less physical contact resistance upon stretching. In fact, the individual MXene-based sensing fibers give a sensing fabric a greater chance, under a tensile force, of coming into contact with each other which ultimately reduces the resistivity of the whole of the fabric. So, this difference in the resistivity responses of individual sensing fibers and sensing fabrics should be accounted for.6
Zhu et al.10 proposed a novel fabrication method of MXene-based sensors. They used positively charged spherical polymer particles and then mixed the particles with a MXene, which inherently has a negative surface charge. Due to electrostatic attractions, MXene flakes attached onto the surface of the polymer particles. This mixture was then mixed with a graphene oxide suspension using an aqueous solution mixing technique. Through a freeze-drying process, an aerogel was formed while thermal annealing of the obtained aerogel at 450 °C removed the polymeric spheres, converted graphene oxide to reduced graphene oxide (rGO), and generated a 3D porous network. In fact, the removal of the polymeric spheres on which the MXene flakes were attached causes the production of a hollow MXene sphere. The thermal expansion of a polymer in a sensor may cause signal instability of the sensor; this is why some researchers have tried to remove polymers from their sensor structure specially when the sensor is supposed to work at high temperatures where thermal expansion is more likely.10 To improve electrical contact and enhance conduction paths, Zhu et al.10 mixed MXene hollow spheres with rGO. They were able to fabricate sensors with improved sensitivity in a broad linear range. Their pressure sensors showed excellent durability over 6000 cycles, a low detection limit of 6 Pa, and a low response time of 230 ms.10
Finding the optimal amount of a MXene that should be added to a polymer to fabricate a sensor is very challenging. An adequate amount of a MXene is needed to form a conductive network in the polymer matrix, while this network should break up in a specific strain range due to increased distance between MXene flakes. As the strain increases, the MXene flakes which form a conductive network in the sensor move away from each other gradually. Further strain ultimately causes the complete breakage of the network. These changes in conductive network configuration are manifested by a gradual reduction in the electrical current passing through the sensor and are correlated to the extent of the strain that the sensor experiences. When the concentration of MXene in a polymer matrix is high, the formed conductive network of MXene flakes does not break in a desired strain range. In other words, MXene flakes always interact with each other, and no detectable change in resistivity/conductivity occurs even at high strains.11 Thus, the amount of MXene loaded to a polymer matrix highly affects the sensitivity and the gauge factor of the sensor.
3. MXene Surface Modification
Some challenges that were discussed in the previous section can be overcome by the surface modification of MXene. In many MXene/polymer nanocomposite sensors, surface modification is necessary to improve the affinity between the MXene and the polymeric continuous phase. Hydroxyl groups on the surface of MXenes are reactive and can undergo many different surface modification reactions. Surface modification is also needed when a self-healing MXene/polymer sensor is of interest. For every sensor in real-world applications, long lifespan and workability after being damaged are desired. These appealing properties can be achieved in a MXene/polymer nanocomposite sensor by modifying the surface of MXene and using a reactive polymer (mostly elastomers) as the hosting matrix. Guo et al.9 conducted an esterification reaction between hydroxyl surface groups of Ti3C2 MXene and carboxylic acid groups of serin. The surface-modified MXene was then mixed with a reactive elastomeric polymer to simultaneously improve the self-healing properties and prolong their lifespan. These nice properties are due to the formation of hydrogen bonds between the serin-modified MXene and the hydroxyl or carboxylic groups of an epoxidized natural rubber elastomer. The hydrogen bonding also decreases the percolation threshold of a MXene in a nanocomposite sensor due to the formation of a well-oriented organized MXene network. In a research study, a randomly distributed pristine MXene in a nonreactive polymer showed a percolation threshold around 40 wt % to form a conductive network. However, this value reduced to 6 wt % when the surface of a MXene was modified and then was mixed with a reactive polymer with the capability of establishing hydrogen bonds.9 These hydrogen bonds also improve the mechanical properties of the sensor and endow it with flexibility, self-healing ability, and twistability as they work as bridges for stress transfer between a polymer matrix and MXene nanosheets.9 In a similar research by Zhang et al.,21 MXene/(polydimethylsiloxane) self-healing nanocomposite sensors were developed. This MXene/polymer nanocomposite sensor showed almost the same detection accuracy after being cut into two pieces and then were self-healed for 24 h. Both of the pristine and healed sensors were sensitive enough to detect and distinguish the movements of the throat of a human when he pronounced different words.21 Guo et al.9 reported that a completely cut MXene/elastomer sensor self-healed and recovered about 100% of its initial tensile strength just after 90 min. It was also possible to twist, bend, and stretch the healed nanocomposite sensor without any problem.9 All of these self-healing sensors benefited from hydrogen bond formation due to either surface modification of a MXene or the use of a reactive polymer.
Nanoparticles of a different type, here called secondary nanoparticles, can also be bonded to the surface of MXene flakes, resulting in the formation of a mixed-dimensional heterostructure. The size of the secondary nanoparticle can be less than 100 nm, which technically is called a nanoparticle with 0 dimension. The secondary nanoparticle can have a tubular or rod shape structure (1D nanoparticle) like a carbon nanotube. It can also have a sheet-like structure (2D nanoparticle) like a MXene. The best example of a 2D/2D heterostructure is the hybrid of a MXene and a modified graphene oxide. Usually, the bonding of 0D nanoparticles into the surface of a MXene starts by using a salt such as AgNO3 or SbCl3. Due to electrostatic attraction in an aqueous environment, the cation of each of these salts can be absorbed on the surface of MXene, and the 0D nanoparticle, such as a silver nanoparticle, is then formed by a reduction reaction. The same electrostatic attractions can be utilized for the modification of the MXene surface by secondary 1D and 2D nanoparticles to form different heterostructures. Usually, secondary nanoparticles are functionalized using a modifier like a silane coupling agent to endow them with a positive surface charge. The latter positively charged constituent interacts with the negative charges on the surface of MXene easily. These mixed-dimensional MXene-based structures have found widespread application in sensor technology.1
4. Applications
4.1. Pressure and Strain Sensors
Generally, there are three types of pressure/strain sensors, that is, capacitive, piezoresistive, and piezoelectric. The piezo prefix has its root in Greek which means pressure. An applied pressure causes a change in the electrical capacitance of a capacitive pressure material, a change in the resistivity of a piezoresistive material, and the generation of charges in a piezoelectric material. In the case of capacitive pressure sensors, the working mechanism can be explained using the following equation for the capacitance of parallel-plate capacitors:
| 1 |
where ε is the dielectric constant, A is the surface area of the electrodes, and d is the distance between the two electrodes. For a constant A, an applied force causes a change in ε or d and thus in C. To achieve a high level of sensitivity, a measurable change should occur in ε or d upon the exertion of a subtle force. To satisfy this requirement, the dielectric material should have a low compressive modulus. Elastomeric materials and aerogels are good examples of materials with a low compressive modulus. In fact, a dielectric layer made from such materials shows a big deformation upon receiving a weak force. Under these conditions, a MXene can effectively improve the sensitivity of a polymeric dielectric material like poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) by increasing its dielectric constant and reducing its compression modulus. For example, MXene/PVDF-TrFE sandwiched between two flexible polymeric electrodes can act as a wearable, mechanically stable, pressure sensor,7 leading to the fabrication of capacitive MXene/polymer pressure sensors. The fabrication steps of such MXene/polymer sensors are depicted in Figure 1a.
In MXene-based piezoresistive strain sensors, when pressure/force is applied, a change in resistivity occurs. The resistivity change is usually expressed in the form of ΔR/R0, where R0 is the resistivity of the sensor’s material at rest, no stretch, and ΔR is the difference between resistivity after and before the strain. A measure of the sensitivity of a strain sensor is its gauge factor (GF):
| 2 |
where ΔL is the absolute change in length and L0 is the original length of the sensor before strain. A higher GF means a greater change in the resistivity upon a strain. Recently MXene/poly(dimethylsiloxane) strain sensors with a GF 3.6 have been successfully fabricated.21 MXene/elastomer sensors with a GF 43–107 in the strain range of 0–10% were also reported.9 On top of these, MXene-based sensors with a GF on the order of several thousands were discussed in the previous section; these are impressive achievements as other state-of-the-art sensors have a GF around 2. The sensitivity of a pressure sensor is described using
| 3 |
where Δl and ΔP are, respectively, the changes in electrical current and pressure readings before and after applying the pressure, and l0 is the electrical current when no pressure is exerted.10
As mentioned before, in most MXene/polymer sensors, a conductive network of MXene flakes is formed beyond a MXene concentration threshold, called the percolation concentration. When the sensors are subjected to a pressure force, the MXene flakes of the network move closer to each other, while when they are subject to a strain force, they separate from each other. The larger the distances are between MXene flakes in a MXene network, the harder the transport is of electrical charges in the network. In fact, separated flakes or weakly connected flakes create extra resistivity for charge transport. This correlation between the extent of the electrical charge transport and the amount of the applied force is the working principle behind strain and pressure sensors that contain electroconductive nanoparticles including MXene.13
4.2. Moisture and Humidity Detection
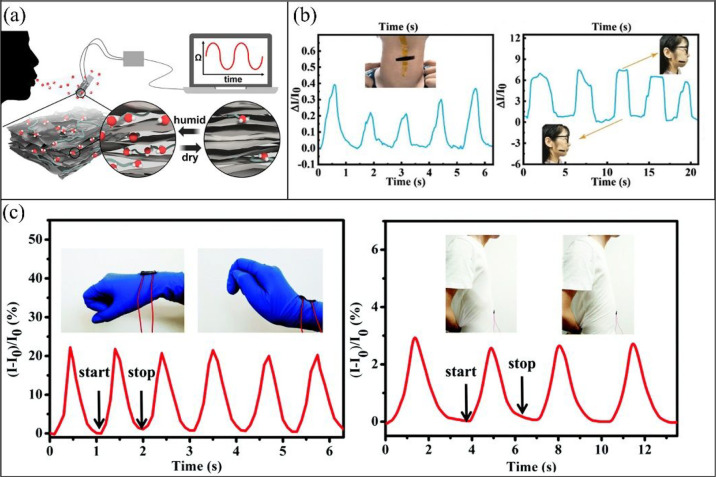
As MXene nanosheets are hydrophilic, they can be used to detect humidity. This means MXene-based sensors can be used in humidity detection and monitoring. An understanding is that the electrical conductivity of a MXene-based sensor increases due to the penetration of water in the MXene network.9 In a direct contrast, An et al.14 reported that the intercalation of water molecules between MXene flakes increases the interlayer spacing and thus tunneling resistance between the sheets. They mentioned that this process is reversible, and the deintercalation of water molecules reduces the interlayer distance and consequently reduces the resistivity. The humidity sensor introduced by An et al.14 was sensitive enough to detect and distinguish inhalation/exhalation rates of a human during running and walking (Figure 3a). The rate of inhalation/exhalation is higher during running than during walking, and this difference can be detected by the humidity-detecting sensor due to the higher rate of water intercalation between MXene layers during running. Regardless of the mechanism, it is important to know that the conductivity of a MXene changes upon the penetration of water molecules between its flakes, and this behavior renders MXenes attractive materials for use in moisture/humidity-detecting sensors.
Figure 3.
(a) MXene-based humidity sensor responds by water intercalation/deintercalation between MXene layers. Reprinted in part with permission from ref (14). Copyright 2019 American Chemical Society. (b) Skin-mountable MXene-based sensors for voice detection. Reprinted in part with permission from ref (10). Copyright 2020 Wiley. (c) MXene-based sensors are attached to different parts of body to detect tiny movements. Reprinted in part with permission from ref (17). Copyright 2020 Royal Society of Chemistry.
4.3. Human Motion Detection
Stretchable nanocomposite sensors made from elastomeric polymers are called wearable sensors. MXene/polymer sensors have been reported to be accurate enough to detect a tiny movement of a human body.9 When a person changes his/her facial expression or pronounces different words, her/his facial muscles stretch and contract.9 For example, when a person talks, his/her throat muscles stretch or relax in a unique way. When a motion-detection sensor is attached to the front of the neck or face skin (Figure 3b), the movements of throat muscles upon the pronunciation of a specific word create a unique pattern which can be detected by these sensors. Thus, MXene-based sensors can be used in voice detection.7 The analyzed voice signals then can be converted to commands to operate a vehicle. This represents opportunities in driverless transportation, artificial intelligence, and robotics.9
There are numerous examples in the literature that wearable MXene/polymer nanocomposite sensors are worn to detect the movement of an eyebrow, finger, hand, stomach, and so on (Figure 3c13,15). MXene-based motion sensors not only detect a movement, but also quantify the intensity of the movement by correlating it to ΔR/R0. One interesting application of MXene-based motion sensors is for signature authentication. Every person puts a unique amount of pressure on a sheet over a unique period of time to sign. Such a motion sensor can be produced in the form of a thin sheet and can be placed under the paper on which to be signed. The sensor then generates a unique electric signal, which is processed by artificial intelligence with access to a signature database to indicate the authenticity of the signature. So, the MXene-based motion sensors can find widespread applications in security.13 A wearable motion sensor can also be used as a pulse meter to measure heartbeat rate. The recorded signal can then be analyzed to evaluate the heart performance.16
4.4. Gas Detection
Although this paper mostly deals with MXene/polymer nanocomposite sensors, a gas sensor can be fabricated from a pristine MXene. Different gases show different affinities to the surface of MXene. Thus, they have different chances of adsorption on the MXene surface. When molecules of a gas with good affinity to the MXene surface are adsorbed to the surface, electrons transfer from gas molecules to the MXene surface. This causes a change in the electrical conductivity of the MXene, as the conductivity depends on surface functional groups and electronic configuration of MXenes. Gases which do not show affinity to a MXene or the ones that interact weakly with its surface do not cause significant change in MXene conductivity.18 The response of a MXene-based gas sensor is described in terms of
| 4 |
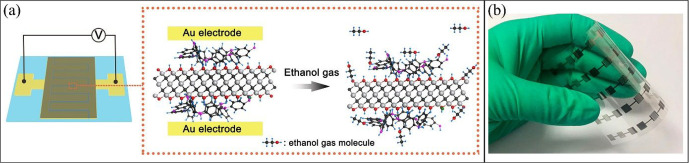
where Rg and Ra are the sensor resistance in a gas and the sensor resistance in air, respectively.18 Similarly, the gas sensitivity can be expressed as ΔI/I0, where ΔI is the change in electrical current upon gas contact and I0 is the current before gas adsorption. As gas detection is based on the interaction of a gas molecule with the surface of a MXene, a more available MXene surface facilitates gas detection. As a result, a single-layer MXene with a higher surface area is a better candidate for the sensor fabrication compared with a multilayer or stacked MXene. To prevent MXene flakes from stacking and thus keeping the surface available for the adsorption of gaseous molecules, Zhao et al.5 grew polyaniline (PANI) particles on the surface of Ti3C2 by in situ polymerization. This provided an open structure between MXene layers (i.e., wider gas diffusion pathways) and assured good access to the MXene sensing layer.5 This modification helped MXene/PANI sensor show a sensitivity to ethanol 2.3 times higher than that of the pristine MXene. It also endowed the MXene-based sensor with high flexibility (Figure 4).5
Figure 4.
Structure of a MXene-based gas-detection sensor containing a sensing layer customized for (a) ethanol detection and (b) flexibility of the sensor made from PANI-modified-MXene. Reprinted in part with permission from ref (5). Copyright 2019 Wiley.
The cleanness of the MXene surface used for sensor fabrication is also important. It depends on the reactions conducted during the MXene synthesis, especially etching reactions. MXene etching is usually performed by the direct addition of HF or by the in situ formation of HF using a mixture of LiF/HCl. When the latter mixture is used, some lithium atoms remain on the surface of MXene and occupy potential sites for gas adsorption. This means lower chance for a gas molecule to be adsorbed on the surface of MXene. A MXene etched with NaF/HCl has a cleaner surface than a MXene etched with LiF/HCl, as the former does not have metallic atoms of the etching mixture on its surface. It is easier to remove Na than Li from the surface of MXene when the etched MAX phase is washed. So, the degree of MXene surface cleanness strongly affects the performance of MXene-based gas sensors.18 The recovery of a gas sensor, that is, the desorption of the previously adsorbed gases, is essential for repetitive use and ensures sensor long service life. Easy and quick desorption of gas molecules adsorbed to the MXene surface permits the recovery of the sensor for successive use. Molecules of a gas with a high adsorption energy do not detach easily from the sensor surface when the sensor is exposed to air.5
Wu et al.,18 who used a pristine MXene as a gas sensor, showed that the sensor can accurately detect NH3 even at a concentration as low as 10 ppm. When they used the sensor to measure the concentration of a 25 ppm of NH3 mixture, the sensor had a response time of 45 s and a recovery time of 94 s. NH3 selectivity of their pristine MXene sensor was 4 times higher than the ethanol selectivity of the sensor. Thus, their sensor is suitable for ammonia detection. In contrast, the MXene/PANI gas sensor of Zhao et al.5 showed a better performance in detecting ethanol than in detecting ammonia. These differences originate from the strength of the interactions of a gas molecule with the surface of a sensor. Compared to the pristine MXene sensor of Wu et al.,18 the presence of PANI in the MXene/PANI sensor facilitated electron transfer from ethanol to the MXene surface and also improved the likelihood of the functional groups to interact with ethanol molecules. Thus, customizing the surface chemistry of MXenes via polymer grafting enhances the selectivity of MXene-based sensors for a specific gas detection.
In addition to Ti3C2, other MXenes can also be used for sensor fabrication. For example, Ti2C MXene was theoretically and experimentally investigated for gas sensor applications.22 Simulation studies confirmed that single-layer Ti2C shows higher sensibility for NH3 than for CO2, O2, H2, and CH4 when its surface is enriched with oxygen functional groups. In addition, fabricated flexible Ti2C/polyimide sensors showed excellent NH3 detection. V2C, a MXene, was incorporated into polyimide to fabricate a sensor for the detection of nonpolar gases. The grafting of poly(2-(dimethylamino)ethyl methacrylate) on the V2C surface allowed for the fabrication of sensors that are sensitive to CO2 and temperature.22
4.5. Pressure Distribution Mapping
MXene-based pressure sensors are sensitive enough to show the gradient of pressure when their surface is subjected to spatially varying pressure.23 For example, when six chest pieces with different masses were placed on a MXene sphere/rGO aerosol sensor, the sensor was able to map the pressure distribution and the locations of these pieces accurately.10 This application can be useful for paraplegic patients who sit for a long time without any movement. Due to the inactivity of their body and the permanent pressure on their hips, the skin of their hips degrades over time, which causes bedsores. This problem can be prevented if stress concentration points on the hips of these patients are recognized at early stages of the diseases. Preventive actions can then be taken to avoid skin degradation in those highly pressurized hip regions.
4.6. Food Engineering Applications
Quick and reliable sensors to warn about meat spoilage are needed in the food industry. Ammonia is one of the gases released by spoiled meat due to protein metabolism.24 As a result, the presence of this gas can be an indication of meat spoilage. Detecting any change in the quality and the smell of meats has always been of importance. Smart packages that monitor and record any deterioration in the packaged foods are used widely in the food industry. The introduction of MXene/polymer nanocomposite sensors capable of detecting ammonia can further advance the smart packaging industry for monitoring of meat spoilage.25
Glucose exists in many food products. Its removal from food raw materials is often needed, as it causes color change and browning in processed foods when stored for a long time or become dehydrated. This change in color occurs due to the Maillard reaction.26 A good example is the production of a dehydrated egg powder from egg white. More than 80% of the dry matter of egg white are proteins, and thereby this product is widely used in bakery products, meringues, and meat products due to its foaming and gel-formation abilities. Compared with its natural liquid (nondried) counterpart, egg white powder can be stored and transported for a longer period under milder storage conditions. However, there are some challenges in egg white powder production. The 4 g·dm–3 glucose concentration in egg white causes browning during the heating process of spray drying, hindering the production of egg white powder. As a result, it is important to monitor the level of glucose during the production of egg white powder.27 Rakhi et al.19 fabricated an amperometric glucose biosensor. They immobilized glucose oxidase on a Nafion-solubilized Au/MXene substrate and showed that their sensor has a linear response with a high accuracy for glucose detection. These glucose-detecting MXene-based sensors can be used for monitoring the glucose concentration before the production of egg white powder to inhibit browning reactions during the process.
The wine industry can also benefit from MXene-based glucose-detecting sensors. The conversion of grape to wine happens through a primary glucose alcoholic fermentation by yeast. Next, there is a malolactic fermentation process which is based on lactic acid bacteria. Quantifying the amount of glucose during the winemaking process is important, as it affects the alcohol level as well as the residual sugar in the wine. Consequently, the sugar level influences the sweetness and the quality of the wine.28 The control and optimization of the fermentation processes can be advanced by a MXene-based glucose-detecting sensor.
Oxygen and oxidation reactions are also of importance in wine manufacturing. A tiny amount of oxygen in wine is beneficial, as it improves the wine quality. However, the exposure of wine to a high concentration of oxygen for a long period causes wine spoilage, excessive oxidative reactions, and flatness. In wine terminology, flatness means the lack of bouquet and freshness. In conventional wine production methods, experienced wine experts are asked to taste a wine to determine its quality. However, a more scientific way is needed to correlate the wine quality to the level of H2O2 in wine, which is related to the extent of oxidation reactions occurring during wine fermentation. Unfortunately, H2O2 is a reactive intermediate chemical, and it stays in wine solution for a short period of time. This has motivated the development of several MXene-based sensors for quick H2O2 detection. Wang et al. immobilized hemoglobin on a MXene/TiO2 surface and reported that their sensor has a H2O2 limit of detection as low as 14 nM.20 As another example, a MXene/chitosan nanocomposite H2O2 detection sensor with a wide linear detection range of 5–1650 μmol·L–1 and a detection limit of 0.74 μmol·L–1 was introduced.29 These highly sensitive MXene-based sensors can allow the wine industry to monitor the extent of oxidation reactions and to improve the quality and the taste of wines.
The detection of H2O2 is also important in the dairy industry. In hot rural areas where cooling facilities are typically scarce, the shelf life of raw milk can be increased by adding a tiny amount of H2O2 after milking. After milking, lactoperoxidase, an enzyme, exists for a short period of time in milk and can react with H2O2 to increase the shelf life of the milk. A H2O2 concentration of around 1.4 × 10–4 g H2O2/mL milk is usually adequate for lactoperoxidase to catalyze the oxidation of thiocyanate, while greater amounts of H2O2 are detrimental.30 As a replacement for conventional detergents, H2O2 is also used in the dairy industry for cleaning of instruments and machines. Thus, it is possible that H2O2 leftovers on instruments’ surfaces mix with milk and increase the amount of H2O2 in the milk product.31 This challenge can be addressed using MXene-based H2O2 sensors.
5. Conclusion, Challenges, and Future Outlook
The unique 2D structure, metallic-like conductivity, and rich surface chemistry of MXenes are expanding their applications in sensor technology, specifically wearable sensors. Pristine MXenes or their nanocomposites with other nanoparticles or polymers have been used to develop sensors for gas detection, strain, pressure, and humidity. The principal working mechanism behind all of these MXene-based sensors is a change in conductivity upon an external stimulus. It is desirable to fabricate sensors, external stimulus to conductivity relationships of which are linear.
There are several challenges in developing MXene-based sensors. The first challenge relates to the preparation of the MXene precursor, i.e., the MAX phase. A MAX phase is usually formed by high-temperature processing of titanium, aluminum, etc. in powder form, which all are combustible materials. Several milling processes are required to get a fine MAX powder. If HF is used as an etchant, proper HF handling and waste treatment are needed. MXenes can be synthesized using LiF/HCl instead of HF. The second challenge is large-scale production of MXene-based sensors where the inadequate mechanical stability of MXene prevents it from being processed by industrial machines. To address this challenge, researchers have focused on mixing of MXenes with other materials especially polymers to fabricate nanocomposites. The type of the polymer (glassy vs rubbery) and the kind of interactions between MXene and the polymer are two important parameters which should be taken into consideration to maximize synergistic interactions between the sensor’s constituents. One effective approach to expedite research in this direction is the use of first-principles and machine learning models. These models can be used to accelerate the research and development. The third challenge is the optimal amount of MXene that should be incorporated into a polymer matrix to develop a high-performance sensor. On one hand, the MXene concentration should be high enough to form a conductive network. On the other hand, the MXene concentration should not be too high to form a conductive network that does not show any change in conductivity under strain. The fourth challenge relates to real world applications where durability and workability even after sensor damage are desired. This can be realized with improved mechanical properties of the components of a sensor, which is usually achieved through surface modification. Generally, an appropriate selection of a polymer, a modifier, and a processing method is needed to ensure the development of durable and accurate MXene/polymers sensors. The fifth challenge is the need for clean and perfect MXene surfaces, which improves the sensitivity of MXene-based sensors. Etchants better than HF and HCl/LiF are needed.
Addressing the challenges will further accelerate the development of novel MXene-based sensors. These sensors are expected to be used in human health monitoring, voice recognition, and robotics. Less explored areas like food engineering can also benefit from MXene-based sensors. MXene-based sensors have the potential to impact many industries.
Acknowledgments
H.R. was partially supported by the U.S. National Science Foundation under Grant No. CBET-1804285. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Biographies
Hossein Riazi received his B.S. (2010) and M.S. (2012) degrees in polymer engineering from Amirkabir University of Technology, Tehran, Iran, and Ph.D. degree in chemical engineering from Drexel University (2020), Philadelphia, U.S.A. He conducted his Ph.D. project under the supervision of Professor Masoud Soroush. His research interests are polymer membranes, polymerization science, surface chemistry, and polymerization modeling.
Golnoush Taghizadeh received her B.Sc. (2012) and M.Sc. (2015) degrees in food engineering from the department of food science and engineering, Islamic Azad University, Isfahan, Iran. Since 2015, she has been working in the dairy and medicinal herb processing industries as an R&D researcher. Her research interests are chemical analysis of foods and dairies.
Masoud Soroush is a professor of Chemical and Biological Engineering at Drexel University, U.S.A. He received his B.S. (Chemical Engineering) from Abadan Institute of Technology, Iran, and M.S.E. (Chemical Engineering), M.S.E. (Electrical Engineering: Systems), and Ph.D. (Chemical Engineering) all from the University of Michigan, Ann Arbor, U.S.A. His research interests include polymer membranes, polymer reaction engineering, model-predictive safety, multiscale modeling, probabilistic modeling and inference, and process systems engineering. He has edited/coedited 6 books and authored/coauthored more than 330 publications including over 200 refereed papers. He is an elected fellow of AIChE and a senior member of IEEE.
The authors declare no competing financial interest.
References
- Huang W.; Hu L.; Tang Y.; Xie Z.; Zhang H. Recent Advances in Functional 2D MXene-Based Nanostructures for Next-Generation Devices. Adv. Funct. Mater. 2020, 30 (49), 2005223. 10.1002/adfm.202005223. [DOI] [Google Scholar]
- Zhou L.; Zhang X.; Ma L.; Gao J.; Jiang Y. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243–249. 10.1016/j.bej.2017.10.008. [DOI] [Google Scholar]
- Michael J.; Qifeng Z.; Danling W. Titanium carbide MXene: Synthesis, electrical and optical properties and their applications in sensors and energy storage devices. Nanomater. Nanotechnol. 2019, 9, 184798041882447. 10.1177/1847980418824470. [DOI] [Google Scholar]
- Xiao B.; Li Y.; Yu X.; Cheng J. MXenes: Reusable materials for NH3 sensor or capturer by controlling the charge injection. Sens. Actuators, B 2016, 235, 103–109. 10.1016/j.snb.2016.05.062. [DOI] [Google Scholar]
- Zhao L.; Wang K.; Wei W.; Wang L.; Han W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. Info Mat 2019, 1 (3), 407–416. 10.1002/inf2.12032. [DOI] [Google Scholar]
- Seyedin S.; Uzun S.; Levitt A.; Anasori B.; Dion J.; Gogotsi Y.; Razal J. MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles. Adv. Funct. Mater. 2020, 30 (12), 1910504. 10.1002/adfm.201910504. [DOI] [Google Scholar]
- Sharma S.; Chhetry A.; Sharifuzzaman M.; Yoon H.; Park J. Y. Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition. ACS Appl. Mater. Interfaces 2020, 12 (19), 22212–22224. 10.1021/acsami.0c05819. [DOI] [PubMed] [Google Scholar]
- Wang K.; Lou Z.; Wang L.; Zhao L.; Zhao S.; Wang D.; Han W.; Jiang K.; Shen G. Bioinspired interlocked structure-induced high deformability for two-dimensional titanium carbide (MXene)/natural microcapsule-based flexible pressure sensors. ACS Nano 2019, 13 (8), 9139–9147. 10.1021/acsnano.9b03454. [DOI] [PubMed] [Google Scholar]
- Guo Q.; Zhang X.; Zhao F.; Song Q.; Su G.; Tan Y.; Tao Q.; Zhou T.; Yu Y.; Zhou Z.; Lu C. Protein-Inspired Self-Healable Ti3C2MXenes/Rubber-Based Supramolecular Elastomer for Intelligent Sensing. ACS Nano 2020, 14 (3), 2788–2797. 10.1021/acsnano.9b09802. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Yue Y.; Cheng Y.; Zhang Y.; Su J.; Long F.; Jiang X.; Ma Y.; Gao Y. Hollow MXene Sphere/Reduced Graphene Aerogel Composites for Piezoresistive Sensor with Ultra-High Sensitivity. Adv. Electron. Mater. 2020, 6 (2), 1901064. 10.1002/aelm.201901064. [DOI] [Google Scholar]
- Li B.; Ma K.; Lu S.; Liu X.; Ma Z.; Zhang L.; Wang X.; Wang S. Structural health monitoring for polymer composites with surface printed MXene/ink sensitive sensors. Appl. Phys. A: Mater. Sci. Process. 2020, 126 (10), 791. 10.1007/s00339-020-03979-4. [DOI] [Google Scholar]
- Zhang K.; Sun J.; Song J.; Gao C.; Wang Z.; Song C.; Wu Y.; Liu Y. Self-Healing Ti3C2MXene/PDMS Supramolecular Elastomers Based on Small Biomolecules Modification for Wearable Sensors. ACS Appl. Mater. Interfaces 2020, 12 (40), 45306–45314. 10.1021/acsami.0c13653. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Z.; Lee K.; Anjum D.; Sougrat R.; Jiang Q.; Kim H.; Alshareef H. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4 (6), eaat0098 10.1126/sciadv.aat0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H.; Habib T.; Shah S.; Gao H.; Patel A.; Echols I.; Zhao X.; Radovic M.; Green M.; Lutkenhaus J. Water sorption in MXene/polyelectrolyte multilayers for ultrafast humidity sensing. ACS Appl. Nano Mater. 2019, 2 (2), 948–955. 10.1021/acsanm.8b02265. [DOI] [Google Scholar]
- Liao H.; Guo X.; Wan P.; Yu G. Conductive MXene Nanocomposite Organohydrogel for Flexible, Healable, Low-Temperature Tolerant Strain Sensors. Adv. Funct. Mater. 2019, 29 (39), 1904507. 10.1002/adfm.201904507. [DOI] [Google Scholar]
- Kedambaimoole V.; Kumar N.; Shirhatti V.; Nuthalapati S.; Sen P.; Nayak M.; Rajanna K.; Kumar S. Laser-Induced Direct Patterning of Free-standing Ti3C2-MXene Films for Skin Conformal Tattoo Sensors. ACS sensors 2020, 5 (7), 2086–2095. 10.1021/acssensors.0c00647. [DOI] [PubMed] [Google Scholar]
- Wu X.; Liao H.; Ma D.; Chao M.; Wang Y.; Jia X.; Wan P.; Zhang L. A wearable, self-adhesive, long-lastingly moist and healable epidermal sensor assembled from conductive MXene nanocomposites. J. Mater. Chem. C 2020, 8 (5), 1788–1795. 10.1039/C9TC05575D. [DOI] [Google Scholar]
- Wu M.; He M.; Hu Q.; Wu Q.; Sun G.; Xie L.; Zhang Z.; Zhu Z.; Zhou A. Ti3C2MXene-based sensors with high selectivity for NH3 detection at room temperature. ACS sensors 2019, 4 (10), 2763–2770. 10.1021/acssensors.9b01308. [DOI] [PubMed] [Google Scholar]
- Rakhi R.; Nayak P.; Xia C.; Alshareef H. Erratum: Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 38465. 10.1038/srep38465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Yang C.; Duan M.; Tang Y.; Zhu J. TiO2 nanoparticle modified organ-like Ti3C2MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. 10.1016/j.bios.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Sun J.; Song J.; Gao C.; Wang Z.; Song C.; Wu Y.; Liu Y. Self-Healing Ti3C2MXenes/PDMS Supramolecular Elastomer Based on Small Biomolecules Modification for Wearable Sensor. ACS Appl. Mater. Interfaces 2020, 12, 45306. 10.1021/acsami.0c13653. [DOI] [PubMed] [Google Scholar]
- Xin M.; Li J.; Ma Z.; Pan L.; Shi Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297. 10.3389/fchem.2020.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Liu X.; Graham N.; Yu W.; Sun K. Two-dimensional MXene incorporated graphene oxide composite membrane with enhanced water purification performance. J. Membr. Sci. 2020, 593, 117431. 10.1016/j.memsci.2019.117431. [DOI] [Google Scholar]
- Kartika V., Rivai M., Purwanto D.. Spoiled meat classification using semiconductor gas sensors, image processing and neural network. 2018 International Conference on Information and Communications Technology (ICOIACT); IEEE, 2018; pp 418–423.
- Yu X.; Li Y.; Cheng J.; Liu Z.; Li Q.; Li W.; Yang X.; Xiao B. Monolayer Ti2CO2: a promising candidate for NH3 sensor or capturer with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 2015, 7 (24), 13707–13713. 10.1021/acsami.5b03737. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Ames J.; Smith R.; Baynes J.; Metz T. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8 (2), 754–769. 10.1021/pr800858h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M.; Batista K.; Batista G.; Fernandes K. Biosensor for determination of glucose in real samples of beverages. Cienc. Tecnol. Aliment. 2012, 32 (1), 65–69. 10.1590/S0101-20612012005000003. [DOI] [Google Scholar]
- Goriushkina T.; Soldatkin A.; Dzyadevych S. Application of amperometric biosensors for analysis of ethanol, glucose, and lactate in wine. J. Agric. Food Chem. 2009, 57 (15), 6528–6535. 10.1021/jf9009087. [DOI] [PubMed] [Google Scholar]
- Khan R.; Andreescu S. MXenes-Based Bioanalytical Sensors: Design, Characterization, and Applications. Sensors 2020, 20 (18), 5434. 10.3390/s20185434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefin S.; Sarker M.; Islam M.; Harun-Ur-Rashid M.; Islam M. Use of Hydrogen Peroxide (H2O2) in raw cow’s milk preservation. J. Adv. Vet. Anim. Res. 2017, 4 (4), 371–377. 10.5455/javar.2017.d236. [DOI] [Google Scholar]
- Gleeson D.; O'Brien B.; jordan k. The effect of using nonchlorine products for cleaning and sanitising milking equipment on bacterial numbers and residues in milk. Int. J. Dairy Technol. 2013, 66 (2), 182–188. 10.1111/1471-0307.12037. [DOI] [Google Scholar]