Abstract

Endometrial cancer (EC) is one of the three most common gynecological cancers in female groups. Gambogic acid (GA), a natural caged xanthone, exerts significantly antitumor effects on many cancers. However, its efficacy on EC and pharmacological mechanism of action remain marginal up to now. This study suggested that GA had significant inhibitory effects on EC in vitro and in vivo, and no toxicity to normal cells or mice. In detail, GA suppressed cell proliferation, induced cell apoptosis, and cell cycle arrest at G0/G1 stage, complied with the network pharmacology analysis, showed that the PI3K/Akt pathways were the most important signaling, and their protein and mRNA expression levels were confirmed by qRT-PCR and Western blot experiments. In all, our study first proved that GA could inhibit cell proliferation, induce cell apoptosis, and cell cycle arrest at G0/G1 stage via the PI3K/Akt pathways, so GA would be a good therapy for EC.
1. Introduction
Endometrial cancer (EC) is one of the three most common malignant cancers in the female reproductive tract, accounting for 8% of the total malignant cancers in the female body.1 Recently, people’s living habits and diet have changed dramatically with the development of society and economy. Also, the incidence rate of EC is increasing every year, causing a serious social threat. Now the main treatments of EC included surgery, radiotherapy, and chemotherapy. However, they have related side effects and toxicities.2,3 Therefore, it is urgent to find potently effective and relatively safe drugs for the treatment of EC.
Natural xanthones from Garcinia exhibited antitumor effects.4 Our group had discovered several novel antitumor xanthones and explored their pharmacological mechanisms of action.5−9 During our research for screening anti-EC xanthones from the genus Garcinia, gambogic acid (GA) (Figure 1) was isolated from the orange gamboge resin secreted by Garcinia hanburyi, showing significant antitumor effects on many human cancers, such as liver, lung, gastric, ovarian, pancreatic, and prostate cancer.10 However, the effects of GA on EC remained margin now.
Figure 1.
GA effectively suppresses cell viability in endometrial cells. (A) Chemical structure of gambogic acid. (B, C) Inhibitory action of GA on Ishikawa cells. (D) Inhibitory action of GA on HEC-1B cells. Data are presented as mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, vs the control group.
Network pharmacology is a novel subject derived from systembiology, featured by multicomponents acting on multitargets via multipathways, exerted synergistic effects on complicate diseases, widely used for exploring the pharmacological mechanisms of action of drugs against miscellaneous diseases.11,12
In this paper, we performed experimental pharmacology to validate the potential efficiency of GA against EC in vitro and in vivo and explored its pharmacological mechanisms of action, also guided by the analysis of network pharmacology.
2. Results
2.1. GA Suppressed the Proliferation of EC Cells
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay results suggested that GA could inhibit the proliferation of Ishikawa and ECC-1 cell lines in a dose- and time-dependent manner (Figure 1B,C). The IC50 values of GA against Ishikawa and ECC-1 cell lines were 0.35 ± 0.02 and 0.26 ± 0.03 μM at 24 h, respectively. At 48 h, they were 0.29 ± 0.01 and 0.21 ± 0.01 μM, respectively. Also, GA exhibited no cytotoxicity to a normal human bronchial epithelial cell line 16HBE (Figure 1D). In the following study, we tried to explore the mechanisms of action of GA against EC by the network pharmacology prediction and experimental pharmacology validation.
2.2. GA Changed the Morphology of EC Cells
In the control of EC cells, Ishikawa and ECC-1 cell lines were mostly spindle-shaped, fully extended, and firmly attached to the plate. After the treatment with 0.2 and 0.4 μM of GA for 24 h, respectively, we could see the cell shrinkage, cell size reduction, and loose arrangement, and some free cells suspended in the medium (Figure 2). Furthermore, there were a lot of death cells suspended in the medium when added with GA at the concentration of 0.4 μM. The results indicated that GA could suppress proliferation and might induce apoptosis of the Ishikawa and ECC-1 cell lines in a dose- and time-dependent manner.
Figure 2.
GA induced the morphological changes of human EC cells Ishikawa and ECC-1 (100×, scale bar = 50 μm).
2.3. EC-Related Targets of GA Predicted by Network Pharmacology
To clarify the mechanism of action of inhibition of EC by GA, we employed PharmMapper, CTD, Similarity Ensemble Approach, Pubmed, and SwissTargetPrediction to collect the potential targets of GA (Table S1). On the other hand, the targets of EC were collected from GeneCards and DrugBank. Finally, the common targets (in total 68) from those two sources were considered as EC-related targets of GA (Figure 3A). The compound-target network is exhibited in Figure 3B. Based on the analysis of the network, considering the degree value, the key targets included STAT3, VEGFA, TP53, AKT1, MAPK1, SRC, EP300, PTPN11, EGFR, ESR1, IGF1, EGF, MAPK8, MMP9, IL6, CTNNA1, STAT1, TIMP1, TGFB1, IGF1R, TNF, and MDM2. Furthermore, the potential targets were evaluated by the GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis (Figure S1A–C). The GO analysis results suggested that most of the targets existed in the cytoplasmic part with protein homodimerization or heterodimerization. These targets were involved in these biological processes, such as cellular response to chemical stimulus, regulation of cell population proliferation, regulation of cell death, and regulation of programmed cell death. Besides, 68 targets participated in 284 pathways by KEGG analysis. The top 20 pathways with the lowest p-value are exhibited in Figure 4. Among them, the PI3K-Akt signaling pathway was the most important signaling pathway, the compound-target network showed Akt was one of the most important targets with the highest degree. Therefore, we deduced that GA inhibited EC via the PI3K-Akt signaling pathway.
Figure 3.
Network pharmacology of the inhibition of EC by GA. (A) Venn map of endometrial cancer-related genes and GA-target genes. (B) “Endometrial cancer targets–GA” network.
Figure 4.
KEGG enrichment analysis of potential targets of GA. The top 20 with lower p-value are shown.
2.4. GA Caused Apoptosis of EC Cells and the Related Genes Were Analyzed by qRT-PCR
From the above results, GA could inhibit the growth of EC cells, to confirm the underlying mechanism involved in apoptosis, ECC-1 cells were treated with 0.2 and 0.4 μM of GA for 24 h, respectively, following added with annexin V-FITC and PI, and then analyzed on a flow cytometer, the results are shown in Figure 5A,B. As compared with the control, GA could induce cell apoptosis at the concentration of 0.2 μM; furthermore, the higher concentration of GA at 0.4 μM remarkably induced cell death. Thus, GA caused ECC-1 cells apoptosis in a dose-dependent manner. On the other hand, the network pharmacology analysis suggested that the internal factor genes (BCL-2, BAD, BAX, caspase-3, and caspase-9) participated in cell apoptosis. The relative expression levels of those genes were analyzed using qRT-PCR; the results suggest that the mRNA expression levels of BAD, BAK, BAX, and Cyto-c were increased, while those of Apf-1, Bcl-2, and caspase-3, -8, -9, and -10 were decreased in a dose-dependent manner (Figure 5C).
Figure 5.
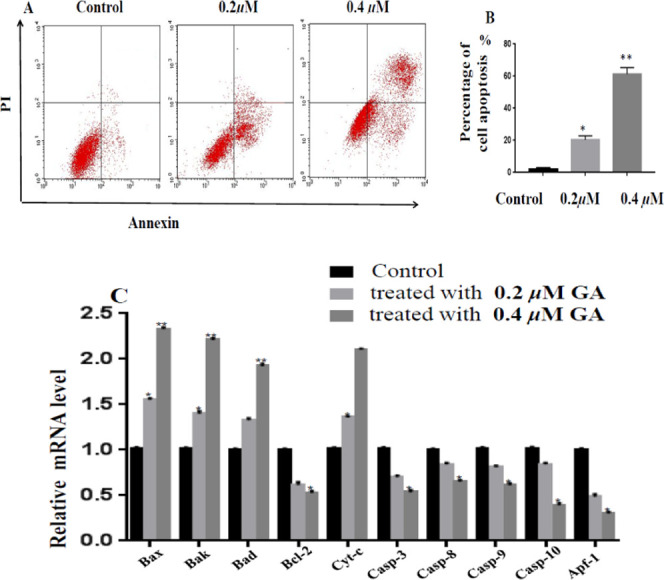
GA induced apoptosis in ECC-1 cells. (A) Apoptosis of ECC-1 cells treated with GA for 24 h, which was examined by Annexin V-FITC/PI staining. (B) Percentage of cell apoptosis. (C) Relative mRNA level of apoptosis-related genes treated with GA for 24 h, which was examined by qRT-PCR. Error bars represent mean ± SD. *p < 0.05, **p < 0.01, vs control (no GA).
2.5. GA Caused Cell Cycle Arrest of EC Cells and the Related Genes Were Analyzed by qRT-PCR
The cell cycle disorder also might play an important role in the anti-EC process. To confirm this hypothesis, 1 × 106 per well of ECC-1 cells were cultivated in six-well plates overnight; subsequently, they were mixed with 0.2 and 0.4 μM of GA, respectively. Then, they were stained with PI and FxCycle PI/RNase. Finally, the treated cells were detected on a flow cytometer. The cell cycle assay demonstrated that the proportion of G0/G1 phase in ECC-1 cells increased gradually, following the supplement with the higher concentration of GA. On the other hand, the proportions of the phase of the ECC-1 cells at S and G2/M stages exhibited an opposite trend (Figure 6A,B). Furthermore, the mRNA expression levels of genes related to the cell cycle were measured by qRT-PCR (Figure 6C). Compared with the control, ECC-1 cells were treated with 0.2 and 0.4 μM of GA for 24 h, respectively; the mRNA expression levels of p27, p21, p16, and FoxO1 were remarkably upregulated, whereas those of CDK6, 4, and 2 and Cyclin A2, D1, and E1 were downregulated.
Figure 6.

GA mediated the cell cycle arrest in G0/G1 of ECC-1 cells. (A) Results showed the percent of cell population in G0/G1, S, and G2/M phases of the cell cycle analyzed using FACScan. (B) Rates of the cell cycle in different stages. (C) mRNA expression levels of the cell cycle of the involved genes were measured by RT-PCR. Error bars represent mean ± SD. *p < 0.05, **p < 0.01, vs control (no GA).
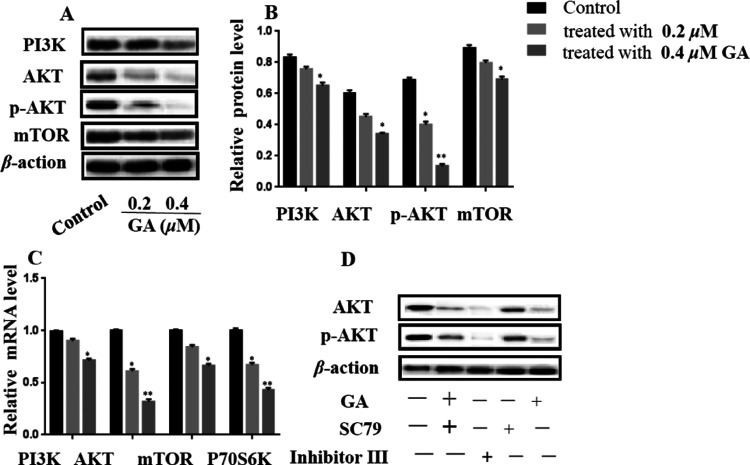
2.6. GA Exhibited Inhibitory Effects on EC via PI3K/AKT Pathway
Our network pharmacology study showed that PI3K/AKT played an important role in the process of inhibition of EC by GA. To validate the role of the PI3K/AKT pathway in the process of inhibition of EC by GA, the gene expression levels of PI3K, AKT, and mTOR were measured by qRT-PCR and WB. ECC-1 cells treated with GA decreased the mRNA expression levels of PI3K, AKT, P70S6K, and mTOR in a dose-dependent manner (Figure 7C). On the other hand, the Western blot experiments were performed; the protein expression levels of PI3K, AKT, p-AKT, and mTOR also showed similar trends (Figure 7A,B). Moreover, the AKT activator ethyl 2-amino-6-chloro-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (SC79) and inhibitor (inhibitor III) were used for evaluating their effects on EC. First, ECC-1 cells were exposed to SC79 and/or inhibitor III before treatment with 0.2 μM of GA independently. The MTT assay showed that the cell viability was sharply reduced following the treatment of inhibitor III, while the cell viability almost did not change when exposed to SC79. Moreover, the Western blot results are exhibited in Figure 7D; GA and inhibitor III had similar effects on reducing the expression levels of AKT and p-AKT. On the contrary, SC79 increased the expression levels of AKT and p-AKT.
Figure 7.
Relative expression level of PI3K/AKT pathway-related genes in GA-treated ECC-1 cells. (A) Protein expression profile and (B) expression levels of related proteins. (C) mRNA expression profile. (D) Effects of AKT inhibitor (inhibitor III) and activator (SC79) on GA-induced apoptosis in ECC-1 cells. ± SD is the mean value for the data (n = 3). *p < 0.05, **p < 0.01, vs control (no GA).
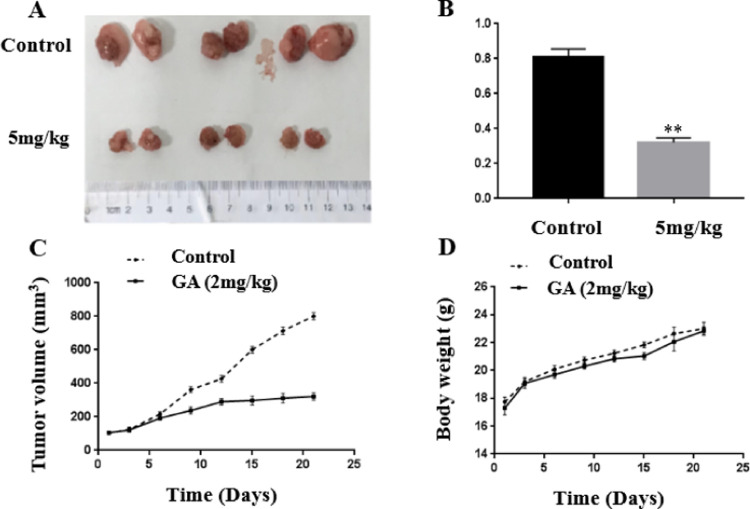
2.7. GA Suppressed EC In Vivo
To examine the anti-EC effects of GA in vivo, the bodyweight of mice increased gradually; however, those in the treatment groups and control groups showed no significant difference (Figure 8D). The results showed that GA had no toxicity on mice, which was consistent with the fact that GA had no toxicity to the normal cells in the MTT assay.
Figure 8.
GA inhibited the growth of ECC-1 cell xenografts in nude mice. (A) Images of tumors at the end of experiments. (B) Average tumor weight in mice. (C) Average tumor volume in mice. (D) Bodyweight changes of mice. Error bars represent mean ± SD. * p < 0.05, **p < 0.01 vs control. n.s., no significant.
GA remarkably suppressed the growth of EC (Figure 8A–C). The mean tumor volume of the mice treated with GA obviously decreased compared to those of the control group. Meanwhile, the mean tumor weight of the mice treated with GA showed a similar trend.
3. Discussion
Gambogic acid, a natural caged xanthone, isolated from the orange gamboge resin secreted by Garcinia hanburyi grown in Southeast Asia. In China, the orange gamboge resin was a Chinese medicine used for the treatment of tumors, ulcers, stubborn dermatitis, and empyrosis.14 It had a lot of biological activities, included anticancer,15 antimicrobial, anti-inflammatory, antiliver fibrosis, and antipulmonary fibrosis effects. Among them, many studies focused on the anticancer effects and showed remarkably inhibitory effects on many kinds of cancers. The underlying mechanisms of the anticancer action were quite diverse,15 such as suppression of proliferation, induction of apoptosis, induction of autophagy, induction of cell cycle arrest, inhibition of migration and metastasis, and antiangiogenesis. However, its efficiency on EC has not been reported so far. To the best of our knowledge, this is the first study on the effects of GA on EC and the mechanisms of action.
The anti-EC activity was evaluated by the MTT assay using two human EC cell lines included Ishikawa and ECC-1. The results indicated that GA could inhibit the proliferation of Ishikawa and ECC-1 cell lines in a time- and dose-dependent manner. Furthermore, GA had no cytotoxicity to an immortalized normal human bronchial epithelial cell lines. The result suggested that GA had selective toxicity to EC, which was consistent with the results from the experiments in vivo. GA suppressed the growth of EC in mice, had no toxicity, and showed stronger inhibitory effects on ECC-1 than Ishikawa cells. Thus, our subsequent pharmacological mechanisms of action focused on ECC-1 cells. The IC50 values of GA against Ishikawa and ECC-1 cell lines were 0.35 ± 0.02 and 0.26 ± 0.03 μM at 24 h, which suggested it had stronger cytotoxic than the positive control used in the previous study.16
Natural products play a significant role in drug discovery and development.17 They have relatively complex structures and exert their effects on multiple targets through many signaling pathways in the body. Which is different from the classical medical theory highlighting one drug, one target.18 Thus, studying the mechanisms of action of natural products is costly, time-consuming, and challenging.19 The omics data hold promise for their use for identifying potential targets for the treatment of EC.20,21
Network pharmacology, derived from system biology, is a novel perspective that combines the complex network relationship among a lot of compounds, genes, targets, signal pathways, and diseases. It has been successfully used for exploring the pharmacological mechanisms of multiple components acting on multiple targets, such as traditional Chinese medicine, herbal medicine, and natural products. In this study, the network pharmacology analysis of GA suppressed against EC via the PI3K-Akt signaling pathway.
To explore the underlying mechanism of GA suppressing the growth of EC, our previous study found that 0.4 μM of GA could kill the EC cells, so we speculated that GA could induce apoptosis in EC cells. Apoptosis was a kind of programmed cellular suicide, which played a key role in several human diseases including EC. Thus, apoptosis was a requisite target for the treatment of EC. The ECC-1 cells stained with Annexin V-FITC and PI were tested on a flow cytometer, the results showed that GA induced cell apoptosis in a dose-dependent manner. Also, the molecular mechanism of this phenomenon aroused our attention. In combination with network pharmacology, it showed that the key targets related to apoptosis were Bcl-2, Bad, Bax, Casp-3, and Casp-9. Also, their mRNA expression levels were measured by qRT-PCR. In addition, their up- and downstream signal genes were also measured. In detail, following the treatment of GA, the antiapoptotic gene (Bcl-2, caspase-3, 8, 9, and 10), and apoptotic protease activator gene (Apf-1) were downregulated, while proapoptotic genes (Bad, Bak, and Bax) and Cyt-c were upregulated. Antiapoptotic and proapoptotic genes (Bcl family protein) could regulate the mitochondrial pathway. Thus, the process was speculated as follows: GA damaged mitochondria to trigger the release of Cyto-c. At the same time, Apaf-1 participated in activating caspase-9, advancing caspase-3 divergence, and leading to apoptosis. On the other hand, Bcl-2 family genes activated caspase-3, increased the levels of Cyto-c, and accelerated apoptosis.22 The Bcl-2 family dimer ratio was an important indicator for the apoptosis or live cells.
The cell cycle played an important role in the growth, development, and differentiation of many kinds of cancer cells, which is a well-established target for cancers.23 Our study showed that GA induced cell cycle arrest at the G0/G1 stage. GA was reported to cause cell cycle arrest at the phases of G0/G1, S, and G2/M, which suggested that GA exerted inhibitory effects on various cancers through different mechanisms of action. In combination with network pharmacology study, which demonstrated the key target related to cell cycle was CDK2. Thus, we measured their mRNA expression levels by qRT-PCR. CDK and cyclin family proteins regulated cell cycle progress via transporting nutrients and transducing growth factors into cells. CDK4/6 related to Cyclin D1 was essential for regulating G1,24 and they were inhibited by p16. Cyclin E1 combined with CDK2 induced cell cycle in the late G1 stage, and CDK2/Cyclin E1 complex was inhibited by p21 (tumor suppressor gene).25 The data showed that GA induced cell cycle arrest at the G0/G1 stage via activating gene expression levels of p16, p21, and p27, and inhibiting the gene expression levels of CDK2/Cyclin E1 and Cyclin D1/CDK4/6 complex. PI3K/AKT played an important role in the process of proliferation, cell cycle, differentiation, and apoptosis of numerous cancer cells. A larger number of studies have stated that the disturbance of PI3K/AKT signaling pathway led to several gynecological cancers including EC.26 Therefore, the PI3K/AKT signaling pathway was an important target for the treatment of EC. Fortunately, the network pharmacology study showed that GA suppressed EC via PI3K/AKT signaling pathway. Also, the inferences were validated by measuring the molecular expression levels of mRNA and protein. Moreover, we also selected AKT inhibitor and activator prior to exposing ECC-1 cells to GA. GA showed similar behavior with inhibitor III, such as inhibiting the proliferation of EC cells and lowering the phosphorylation of AKT, which was contrary to the activator.
4. Conclusions
Our study first investigated the effects of GA in inhibiting EC in vitro and in vivo and explored the molecular mechanism of action-integrated network pharmacology with experimental pharmacology. In detail, GA inhibited EC by suppressing proliferation, inducing cell cycle arrest at G0/G1 stage, and inducing apoptosis by triggering the mitochondrial pathway and inactivating the PI3K/AKT pathway. Therefore, GA was a potential inhibitor of EC via the PI3K/AKT signal pathway.
5. Methods and Materials
5.1. Reagents and Antibodies
3-(4,5-Dimeth,ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), GA (purity ≥98%, Figure S2B), ethyl 2-amino-6-chloro-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (SC79), and inhibitor III were obtained from Bailingwei Science and Technology Company (Beijing, China). F12 and RPMI 1640 media were purchased from Hyclone (Logan, UT). Fetal bovine serum (FBS) was obtained from Gibco Industries (Invirogen, Australia). Antibodies against PI3K, AKT, p-AKT, mTOR, and β-action were purchased from Cell Signaling Technology (Beverly, MA). The mouse and rabbit IgG horseradish peroxidase-conjugated antibodies were supplied from Beyotime (Shanghai, China).
5.2. Cytotoxicity Bioassay
GA was dissolved in dimethyl sulfoxide (DMSO) to make stock solutions, then diluted in culture medium for experiments. To test the effects of GA on EC cells viability, which was calculated using the MTT test.16
5.3. GA Inhibited the Proliferation of EC Cells
Ishikawa and ECC-1 cell lines were plated in a six-hole plate (1 × 105 cells/well) and fixed for 24 h. Then, every plate was added with 0.2 or 0.4 μM GA for 24 h, and the cell morphology was studied by an optical microscope.
5.4. Apoptosis Assay
The apoptosis experiment was performed as described before.27
5.5. Cell Cycle Assay
ECC-1 cells at a density of 1 × 106/well were cultivated in six-well plates for 24 h and then added with 0.2 and 0.4 μM GA, respectively. Then, the treated cells were stained with propidium iodide (PI, Biolegend) and FxCycle PI/RNase according to the manufacturer’s instruction. The cell cycle analysis was tested on a flow cytometer (FACSCalibur, Becton Dickinson). Each assay was repeated three times.
5.6. qRT-PCR Analysis
Total RNA isolated by TRIzol reagent (Beyotime, Shanghai, China) according to the manufacturer’s method. qRT-PCR was performed using the PrimeScript RT Reagent Kit (TaKaRa, DRR037A). qRT-PCR analysis was performed on a Veriti Thermal Cycler (Applied Biosystems, Life Technologies) using an SYBR green real-time PCR kit (TOYOBO, QPK-201). Data collection was performed using a StepOnePlus Real-Time PCR System Thermal Cycling Block (Applied Biosystems, Life Technologies). Table 1 exhibited the sequences of primers.
Table 1. Primers for qPCR.
| gene | primer | sequence (5′-3′) |
|---|---|---|
| Apaf-1 | forward | CCTTCTCTGTGGACAGTAC |
| reverse | TCCGACCCCTGACTGGAAA | |
| AKT | forward | GTCGCCTGCCCTTCTACAAC |
| reverse | CACACGATACCGGCAAAGAA | |
| Bad | forward | AGAGTTTGAGCCGAGTGAGC |
| reverse | CATCCCTTCGTCGTCCTCC | |
| Bak | forward | ACTTGCTCCCAACCCATTC |
| reverse | CCCACTTAGAACCCTCCAGAT | |
| Bax | forward | ACGGCCTCCTCTCCTACTTT |
| reverse | AAACACAGTCCAAGGCAGCT | |
| Bcl-2 | forward | GAGGATTGTGGCCTTCTTTG |
| reverse | GCCGGTTCAGGTACTCAGTC | |
| Caspase-3 | forward | TGGACTGTGGCATTGAGACA |
| reverse | CAGGTGCTGTGGAGTATGCA | |
| Caspase-8 | forward | TATCCCGGATGGCTGACT |
| reverse | GACATCGCTCTCAGGCTC | |
| Caspase-9 | forward | GCTCTTCCTTTGTTCATCTCC |
| reverse | CATCTGGCTCGGGGTTACTGC | |
| Caspase-10 | forward | CAGGGGCAGGAAGAGAACAG |
| reverse | ACTAGGAAACGCTGCTCCAC | |
| CDK2 | forward | AACACAGAGGGGGCCATCAAGC |
| reverse | CAGGAGCTCGGTACCACAGGGTC | |
| CDK4 | forward | CTGACCGGGAGATCAAGGTA |
| reverse | TCCACCACTTGTCACCAGAA | |
| CDK6 | forward | CGTGGTCAGGTTGTTTGATGT |
| reverse | CGGTGTGAATGAAGAAAGTCC | |
| Cyclin A2 | forward | GACTGGCTGGTTGAGGTGG |
| reverse | GTGGCGGTTTGAGGTAGGT | |
| Cyclin D1 | forward | ATGGAACACCAGCTCCTGTGCTGC |
| reverse | TCAGATGTCCACGTCCCGCACGT | |
| Cyclin E1 | forward | GGATTATTGCACCATCCAGAGGCT |
| reverse | CTTGTGTCGCCATATACCGGTCAA | |
| Cyto-C | forward | CCTCTGGGGCATTATCCATC |
| reverse | ATATTTGCACAGTGAAACATAGGA | |
| mTOR | forward | TTATGGGCAGCAACGGACAT |
| reverse | CTTCTCCCTGTAGTCCCGGA | |
| P70S6K | forward | ACTGGAAGCCTTGGAATGGG |
| reverse | CCTTGCCGACCACAGTATGT | |
| p16 | forward | TGAGAAACCTCGGGAAACTTA |
| reverse | AAAGGCAGAAGCGGTGTT | |
| p21 | forward | CCACAGCGATATCCAGACATTC |
| reverse | GAAGTCAAAGTTCCACCGTTCTC | |
| p27 | forward | TCCCTGGATTAAGGCATTCTT |
| reverse | TTTGGTTTGGGAGGGTCATA | |
| PI3K | forward | GAAAAGTTTGGCCGGTTCCG |
| reverse | GCAGTCAACATCAGCGCAAA | |
| β-actin | forward | CTCGCCTTTGCCGATCC |
| reverse | GAATCCTTCTGACCCATGCC |
5.7. Western Blotting Analysis
The attached cells were treated with 0.2 and 0.4 μM GA, respectively. The total protein of each sample was extracted by RIPA (Beyotime, Shanghai, China) on ice for 30 min, collected in a 1.5 mL centrifugation tube, then centrifuged at 12 000 rpm at 4 °C for 10 min; the supernatant was reserved and quantified using a BCA protein quantitative kit. One volume of liquid protein was added with five volumes of loading buffer and the mixture heated in a metal bath at 99 °C for 10 min; 20 μg of the sample was separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to poly(vinylidene difluoride) (PVDF) membrane. The membrane was blotted with 1/1000 primary antibodies at 4 °C overnight. Subsequently, it was incubated with the corresponding 1/3000 secondary antibody at 37 °C for 2 h. The visualization of protein was performed on an ECL Plus Western blotting detection system. The antibodies used in the study were anti-PI3K, anti-AKT, anti-p-AKT, anti-mTOR, and anti-β-action.
5.8. Prediction of EC-Related Targets of GA
To collect the potential targets of GA, databases, such as PharmMapper (http://lilab-ecust.cn/pharmmapper/), CTD (http://ctdbase.org/), Similarity Ensemble Approach (http://sea16.docking.org/), Pubmed (https://www.ncbi.nlm.nih.gov/pubmed), and SwissTargetPrediction (http://swisstargetprediction.ch/), were employed. On the other hand, the potential targets of EC were collected from GeneCards (https://www.genecards.org/) and DrugBank (https://go.drugbank.com/). Those common targets were computed via http://jvenn.toulouse.inra.fr/app/example.html.
5.9. Construction of Compound-Target Network
The interactions among the EC-related targets of GA were analyzed via the STRING database (https://string-db.org/) and interactions with a combined score higher than 0.9 were selected for the study. The compound-target network was constructed according to the PPI data (protein–protein interaction) by the software Cytoscape-v3.2.1.
5.10. Enrichment Analysis of the Pathways on GA Inhibiting EC
Gene ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using the DAVID program (https://david.ncifcrf.gov/).
5.11. Xenograft Mouse Experiments
The animal experiments were carried out in Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Tongji University (TJKQ), in line with the Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee in TJKQ (TJKQ-DW-19). Six-week-old nude mice (BALB/c, female) were purchased from the laboratory animal center at Tongji University; 1 × 106 of ECC-1 cells diluted with 100 μL of PBS were subcutaneously implanted into the dorsal flanks of the mice. Five days later, the mice were randomly divided into two groups (n = 6 per group), such as the vehicle and GA (2 mg/(kg day) s.c.) groups. Tumor volume (L × H × W mm3) and body weight were recorded every 3 days during the in vivo study. After 21 days, the mice were sacrificed, and the tumors were removed, photographed, and weighed.
5.12. Statistical Analysis
Pictures were captured using GraphPad Prism 7. Statistical analysis was performed by the Statistical Package for the Social Sciences (SPSS) 12.0. All experimental data were determined in line with one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons, and there was a significant difference in the variance homogeneity measurement (p < 0.05).
Acknowledgments
This work was supported by the Special Subject for Scientific Research of Chinese Medicine from Shanghai Municipal Commission of Health and Family Planning (No. 2018JP007).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00696.
The potential targets of GA were evaluated by the GO and KEGG pathway enrichment analysis; the results include biological processes, cellular components, and molecular functions; the purity of GA was analyzed by HPLC in this study; the results showed the targets of GA from PharmMapper, CTD, Similarity Ensemble Approach, Pubmed, and SwissTargetPrediction; and the targets of EC were collected from GeneCards and DrugBank (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Siegel R. L.; Miller K. D.; Jemal A. Cancer statistics, 2020. Ca-Cancer J. Clin. 2020, 70, 7–30. 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Brooks R. A.; Fleming G. F.; Lastra R. R.; Lee N. K.; Moroney J. W.; Son C. H.; Tatebe K.; Veneris J. L. Current recommendations and recent progress in endometrial cancer. Ca-Cancer J. Clin. 2019, 69, 258–279. 10.3322/caac.21561. [DOI] [PubMed] [Google Scholar]
- Morice P.; Leary A.; Creutzberg C.; Abu-Rustum N.; Darai E. Endometrial cancer. Lancet 2016, 387, 1094–1108. 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- Winter D. K.; Sloman D. L.; Porco J. A. Polycyclic xanthone natural products: structure, biological activity and chemical synthesis. Nat. Prod. Rep. 2013, 30, 382–391. 10.1039/c3np20122h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. X.; Zhang D. D.; Liang S.; Lao Y. Z.; Zhang H.; Tan H. S.; Chen S. L.; Wang X. H.; Xu H. X. Bioassay-guided isolation of prenylated xanthones and polycyclic acylphloroglucinols from the leaves of Garcinia nujiangensis. J. Nat. Prod. 2012, 75, 1459–64. 10.1021/np3003639. [DOI] [PubMed] [Google Scholar]
- Tang Z. Y.; Xia Z. X.; Qiao S. P.; Jiang C.; Shen G. R.; Cai M. X.; Tang X. Y. Four new cytotoxic xanthones from Garcinia nujiangensis. Fitoterapia 2015, 102, 109–114. 10.1016/j.fitote.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Xia Z. X.; Zhang H.; Xu D. Q.; Lao Y. Z.; Fu W. W.; Tan H. S.; Cao P.; Yang L.; Xu H. X. Xanthones from the Leaves of Garcinia cowa Induce Cell Cycle Arrest, Apoptosis, and Autophagy in Cancer Cells. Molecules 2015, 20, 11387–11399. 10.3390/molecules200611387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. Y.; Lu L. H.; Zhou X. Y.; Shen J.; Song W. P.; Tang Y. D.; Xia Z. X. A new cytotoxic polycyclic polyprenylated acylphloroglucinol from Garcinia nujiangensis screened by the LC-PDA and LC-MS. Nat. Prod. Res. 2020, 34, 2448–2455. 10.1080/14786419.2018.1539983. [DOI] [PubMed] [Google Scholar]
- Tang Z. Y.; Lu L. H.; Xia Z. X. Anti-Tumor Xanthones from Garcinia nujiangensis Suppress Proliferation, and Induce Apoptosis via PARP, PI3K/AKT/mTOR, and MAPK/ERK Signaling Pathways in Human Ovarian Cancers Cells. Drug Des., Dev. Ther. 2020, 14, 3965–3976. 10.2147/DDDT.S258811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik K.; Harsha C.; Bordoloi D.; Sailo B. L.; Sethi G.; Leong H. C.; Arfuso F.; Mishra S.; Wang L. Z.; Kumar A. P.; Kunnumakkara A. B. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. 10.1016/j.canlet.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Zhang C. N.; Liu C. X.; Qu Y. X.; Cao Y. J.; Liu R. H.; Sun Y.; Nyima T.; Zhang S. F.; Sun Y. K. LC–MS-Based Qualitative Analysis and Pharmacokinetic Integration Network Pharmacology Strategy Reveals the Mechanism of Phlomis brevidentata H.W.Li Treatment of Pneumonia. ACS Omega 2021, 6, 4495–4505. 10.1021/acsomega.0c06201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z. Q.; Yu G. H.; Han X.; Liu Y.; Wang G. P.; Li X. Y.; Yang H. Y.; Sun W. Y. Exploring the Active Components of Simotang Oral Liquid and Their Potential Mechanism of Action on Gastrointestinal Disorders by Integrating Ultrahigh-Pressure Liquid Chromatography Coupled with Linear Ion Trap-Orbitrap Analysis and Network Pharmacology. ACS Omega 2021, 6, 2354–2366. 10.1021/acsomega.0c05680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantarasriwong O.; Batova A.; Chavasiri W.; Theodorakis E. A. Chemistry and biology of the caged Garcinia xanthones. Chem. - Eur. J. 2010, 16, 9944–9962. 10.1002/chem.201000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. L.; Chen Y. C.; Lin L. F.; Li H. Gambogic Acid as a Candidate for Cancer Therapy: A Review. Int. J. Nanomed. 2020, 15, 10385–10399. 10.2147/IJN.S277645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Zhang Y. Y.; Sun Y. S.; Ma R. H.; Thakur K.; Jian-Guo Zhang J. G.; Asparanin Z. J. A from Asparagus officinalis L. Induces G0/G1 Cell Cycle Arrest. Asparanin A from Asparagus officinalis L. Induces G0/G1 Cell Cycle Arrest and Apoptosis in Human Endometrial Carcinoma Ishikawa Cells via Mitochondrial and PI3K/AKT Signaling Pathways. J. Agric. Food Chem. 2020, 68, 213–224. 10.1021/acs.jafc.9b07103. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Rodrigues T.; Schneider P.; Schneider G.; et al. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A.; Mantravadi P. K.; Kalesh K. Detectives and helpers: Natural products as resources for chemical probes and compound libraries. Pharmacol. Ther. 2020, 216, 107688 10.1016/j.pharmthera.2020.107688. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Ni Z. J.; Ye L.; Zhang Y. Y.; Thakur K.; Cespedes-Acuña C. L.; Han J. Z.; Zhang J. G.; Wei J. Z. Asparanin A inhibits cell migration and invasion in human endometrial cancer via Ras/ERK/MAPK pathway. Food Chem. Toxicol. 2021, 150, 112036–112050. 10.1016/j.fct.2021.112036. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Zhang Y. Y.; R H; Thakur K.; Han J. Z.; Hu F.; Zhang J. G.; Wei J. Z. Multi-omics reveals the anticancer mechanism of asparagus saponin-asparanin A on endometrial cancer Ishikawa cells. Food Funct. 2021, 12, 614–632. 10.1039/D0FO02265A. [DOI] [PubMed] [Google Scholar]
- Hartman M. L.; Malgorzata C. BCL-w: apoptotic and non-apoptotic role in health and disease. Cell Death Dis. 2020, 11, 260 10.1038/s41419-020-2417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray U.; Raghavan S. C. Modulation of DNA double-strand break repair as a strategy to improve precise genome editing. Oncogene 2020, 39, 6393. 10.1038/s41388-020-01445-2. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Zhou S. F.; Zhang Z. P.; Xu G. P.; Li X. B.; Yan J. L. Gambogic acid inhibits the growth of osteosarcoma cells in vitro by inducing apoptosis and cell cycle arrest. Oncol. Rep. 2011, 25, 1289–1295. 10.3892/or.2011.1189. [DOI] [PubMed] [Google Scholar]
- Yue Q. X.; Feng L. X.; Cao B. Y.; Liu M.; Zhang D. M.; Wu W. Y.; Jiang B. H.; Yang M.; Liu X.; Guo D. A. Proteomic Analysis Revealed the Important Role of Vimentin in Human Cervical Carcinoma HeLa Cells Treated With Gambogic Acid. Mol. Cell. Proteomics 2016, 15, 26–44. 10.1074/mcp.M115.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F.; Yap T. A.; Meric-Bernstamb F. Targeting the PI3K pathway in cancer: are we making headway?. Nat. Rev. Clin. Oncol. 2018, 15, 273–291. 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- Ma R. H.; Ni Z. J.; Zhang F.; Zhang Y.Y.; Liu M. M.; Thakur K.; Zhang J. G.; Wang S.; Wei Z. J. 6-Shogaol mediated ROS production and apoptosis via endoplasmic reticulum and mitochondrial pathways in human endometrial carcinoma Ishikawa cells. J. Funct. Foods 2020, 74, 104178–104193. 10.1016/j.jff.2020.104178. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.