Abstract
The aggregation of tau protein is one of the hallmarks for Alzheimer’s disease, resulting in neurodegeneration. The peptidomimetics strategy to prevent tau aggregation is more specific over other small molecules. In the present study, we analyzed the effect of amyloid-β-derived peptidomimetics for inhibiting heparin-induced tau aggregation in vitro. These peptides and their derivatives were known to prevent aggregation of amyloid-β. KLVFF is a hydrophobic sequence of the pentapeptide that prevented tau aggregation as observed by thioflavin S fluorescence, transmission electron microscopy, and circular dichroism spectroscopy. P4 and P5 also prevented assembly of tau into aggregates and formed short fibrils. The β-sheet breaker LPFFD was however ineffective in preventing tau aggregation. The peptides further demonstrated reversal of tau-induced cytotoxicity in a dose-dependent manner. Our results suggested that these peptides can also be used to inhibit tau aggregation and also, toxicity induced by tau could be considered as potential molecules that have an effect on tau as well as amyloid-β.
Introduction
Protein misfolding and aggregation are common feature of neurodegenerative disease that occur because of a characteristic protein–protein interaction. Alzheimer’s diseases (AD) is one of the neurodegenerative diseases caused because of misfolding and aggregation of tau and amyloid-β. Failure in developing successful therapeutics for inhibiting amyloid-β pathology has now turned the focus toward tau protein.1 Tau is a natively unfolded, microtubule-associated protein that interacts and helps in stabilizing microtubules.2,3 The AD triggers pathological modifications of tau, leading to the loss of interaction with microtubules and their disassembly.4 This causes loss of axonal integrity and neuronal degeneration. Although protein–protein interactions are indispensable for various cellular functions, the altered protein interactions are evidenced in neurodegenerative disorders.5 Hence, modulating these pathological interactions has been proposed as therapeutics in neurodegenerative diseases.6 Several small molecules from plants, fungal origin molecules, and synthetic molecules were employed to prevent protein aggregation.7−9 In this scenario, designing a molecule that can specifically interact and dissociate these amyloid tangles would be the ideal approach.10,11 Peptides and peptidomimetics are one of the strategies that can break pathological interactions and prevent protein aggregation.12 The amino acid sequences carry information for both, structured organization as well as disorder of the protein. Understanding this nature of amino acids aids in designing peptides that can be used to prevent protein–protein interactions. The two hexapeptides, VQIINK and VQIVYK, play a major role in tau aggregation.13,14 The rationally designed peptides with each amino acids substituted with proline in the hexapeptide sequence prevented their aggregation.15 The macrocyclic arrangement of hexapeptides inhibited tau aggregation by capping the growth of aggregates.16 Similarly, tau-derived peptides were designed to understand the interface formed during tau aggregation.17 These peptides inhibited tau aggregation by capping and preventing the electrostatic interactions in the interface. In the present work, we have studied the effect of amyloid-β-derived peptides in preventing tau aggregation. These peptides were previously evaluated for their role in inhibiting amyloid-β aggregation and preventing metal-induced cytotoxicity.18,19 Initially, the amyloid-β paradigm was extensively studied in AD and several small molecule inhibitors were screened, which also includes amyloid-β-derived peptides.20,21 The hydrophobic peptide sequence KLVFF was studied by different groups; KLVFF and its modified forms inhibited aggregation of amyloid-β and also reduced their toxicity.22 In the present study, we have analyzed the efficacy of LPFFD and KLVFF as control peptides in preventing tau aggregation, along with peptides P3, P4, P5, and P6. In addition, LPFFD and its modified forms were the β-sheet breakers known to prevent aggregation of amyloid-β.23−25 The peptidomimic modulators were deigned based on the amyloid-β recognition unit (KLVFF). In general, amyloid-β aggregation is predominantly driven by hydrophobic interactions, which is later stabilized by multiple hydrogen bonding. We envision blocking the hydrogen bonds by introducing thymine, with multiple hydrogen bond forming capacity (P3) and further introducing sarcosine units to prevent lateral association during aggregation (P4 and P5). These synthetic modifications have successfully led to the development of effective amyloid-β aggregation modulators.18 The knowledge of these peptides is limited and the role of these peptides are not well known in tau hypothesis.26 This study would address the viewpoint of screening peptides with dual properties of inhibiting aggregates of tau and amyloid-β. The peptidomimetics KLVFF, P4, and P5 prevented tau aggregation, as analyzed by thioflavin S (ThS), transmission electron microscopy (TEM), and circular dichroism (CD) analysis; furthermore, the nontoxic nature of these peptides in neuro2a cells suggest them to be a possible lead toward AD therapeutics.
Results and Discussion
Amyloid-β-Derived Peptides KLVFF, P4, and P5 Prevented Tau Aggregation
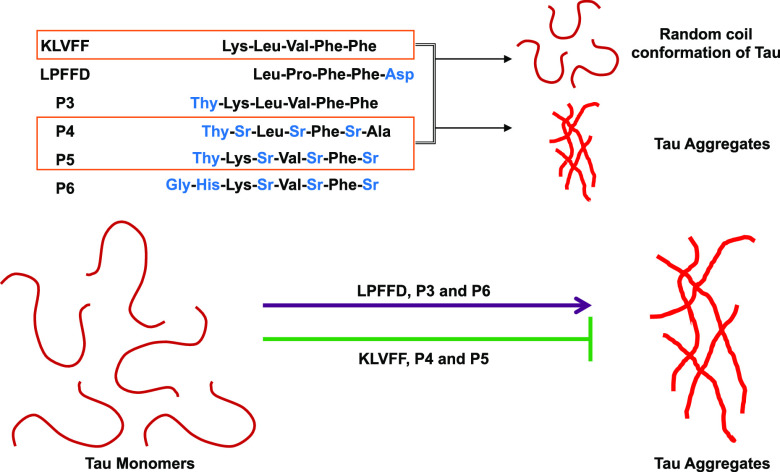
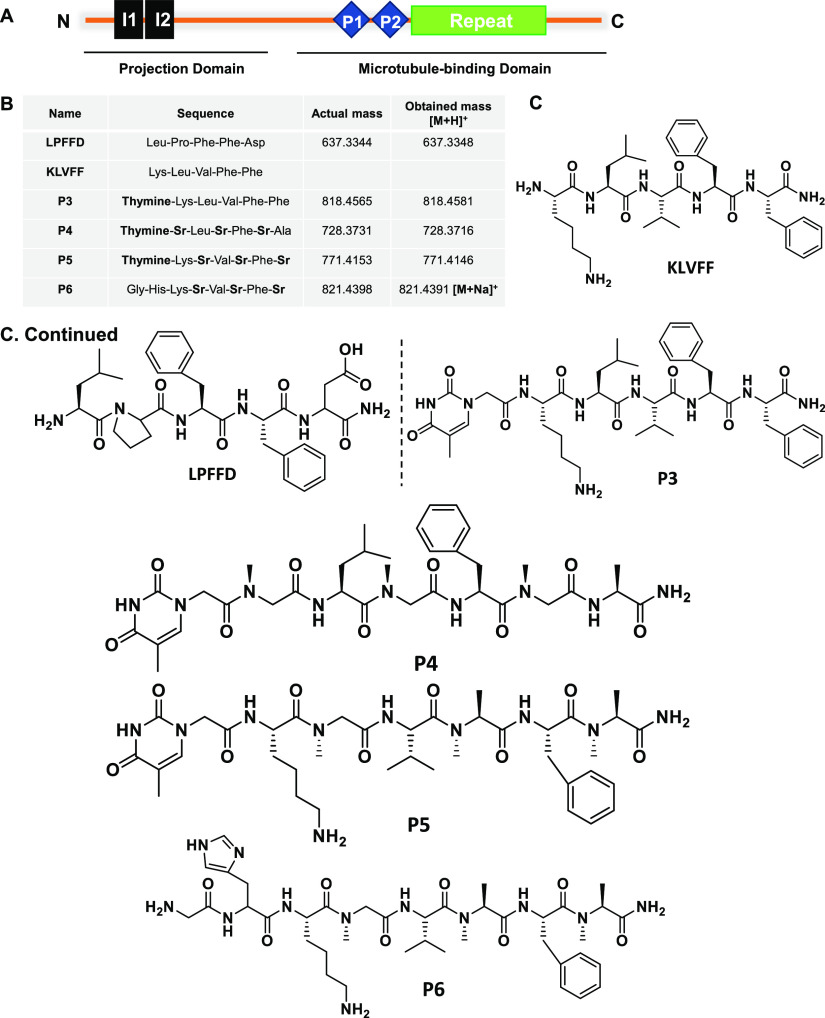
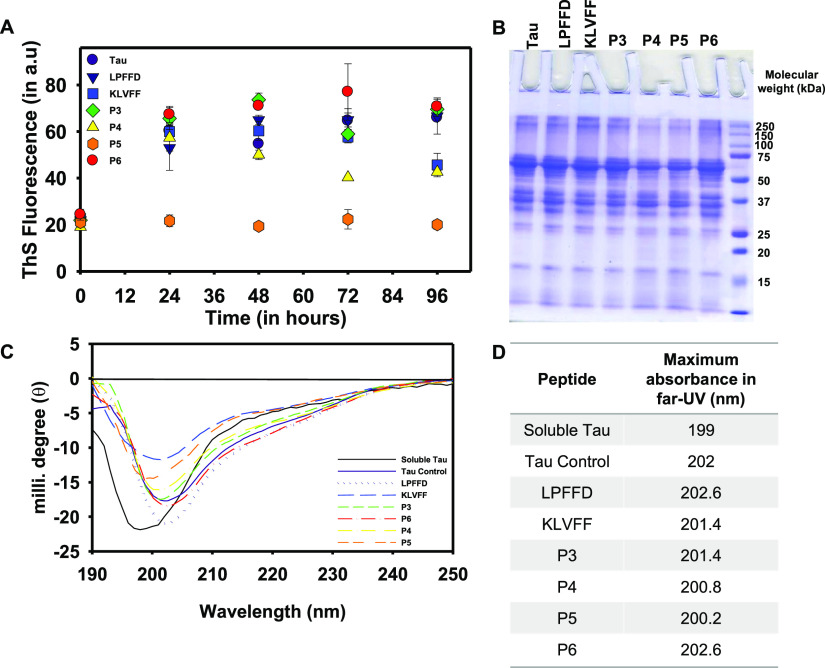
The accumulations of amyloid proteins, amyloid-β, and tau are the hallmarks of AD.2,27 The increasing number of AD incidents every year indicates an alarming situation to develop therapeutics against AD.28 Several approaches have been tried and failed in preventing these pathological accumulations. Peptidomimetics are one of the strategies widely studied in the aspect of amyloid-β aggregation. However, its role in tau aggregation is not known; in this present study, we have screened amyloid-β peptides LPFFD, KLVFF, and their derivatives P3–P6 in tau aggregation (Table S1 and Figures S1–S5). These peptides were studied to inhibit amyloid-β aggregation and their metal chelation property was elucidated to prevent metal-induced toxicity in AD.18,19 These peptides were now employed to understand their potency of inhibiting tau aggregation provided which, it would exhibit the dual role in preventing amyloid-β as well as tau aggregation. The longest isoform of tau has 441 amino acids with an N-terminal projection domain that aids in spacing microtubules and a proline-rich domain that harbors kinase-binding sites; followed by C-terminal repeats R1–R4 (Figure 1A).29 These repeats play a key role in microtubule assembly and during aggregation the hexapeptide motifs VQIINK and VQIVYK in R2 and R3 serve as nucleating sites for aggregation.13,30 In the present study, the effect of amyloid-β-derived peptides (Figure 1B) on heparin-induced tau aggregates have been analyzed by ThS fluorescence. LPFFD and KLVFF were the control peptides and P3–P6 were their derivatives (Figure 1C). It was observed that P5 was most effective in inhibiting tau aggregation, followed by P4 and KLVFF, whereas LPFFD, P3, and P6 showed no inhibition (Figure 2A). Similarly, amyloid-β aggregation inhibition was studied by LPFFD derivatives,31,32 where these peptides reduced fluorescence, indicating inhibition of aggregation. LPFFD is a β-breaker that has no role in inhibiting tau aggregation in our studies, whereas KLVFF prevents tau aggregation. This might indicate that tau forms hydrophobic interactions initially, which is prevented by KLVFF. The higher-order aggregates formed in the presence and absence of peptides were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Tau protein in the absence of peptides showed distinct higher-order aggregates above 150 kDa; the intensity of these higher-order aggregates was reduced in the presence of P4 and P5 (Figure 2B). This observation clearly indicates the inhibitory effect of P4 and P5 on tau aggregation.
Figure 1.
Domain organization of full-length tau and amyloid-β-derived peptides (A). Full-length tau orchestrates assembly and stability of microtubules. It consists of the N-terminal projection domain with two inserts, I1 and I2 and proline-rich region followed by four repeats that acts as the microtubule-binding domain. The microtubule domain functions to interact with tubulin and aids in its assembly to the microtubules. During AD pathology, the aberrant PTMs and cellular insults modulate aggregation of tau, viz., microtubule-binding domain. (B) Synthetic peptides LPFFD and its derivatives P3 to P6 composed of thymine and N-methyl glycine, i.e., sarcosine (Sr). (C) Structure of amyloid-β-derived peptidomimetics used in the present study to analyze their effect in preventing tau aggregation.
Figure 2.
Tau aggregation inhibition by peptides. (A) In vitro heparin-induced hTau40Wt aggregation resulted in increased fluorescence. A decrease in ThS fluorescence was observed in LPFFD-treated tau, indicating aggregation inhibition; similarly, peptide P5 also showed a decrease in fluorescence. P3, P4, and P6 did not affect tau aggregation and showed ThS fluorescence similar to hTau40Wt. (B) SDS-PAGE resolved the higher-order aggregates of tau above 150 kDa. These aggregates were evident in tau control and in the presence of peptides; however, their intensity was reduced by P4 and P5. This indicates the inhibitory effect of P4 and P5 on tau aggregation. (C) Heparin induced β-sheet conformation of tau (indicated in red) as observed by CD spectroscopy. The peptides P4, P5, and KLVFF prevented conformational changes and exhibited random coil conformation of tau. However, in the presence of other peptides, LPFFD, P3, and P6 tau exhibited β-sheet conformation. (D) Maximum absorbance of tau in the far-UV region in the presence of peptides indicates that P5 showed a minor shift in the spectra when compared to other peptides.
KLVFF, P4, and P5 Transit Tau toward Random Coil Conformation
Tau exhibits random coil conformation of native conditions and undergoes transition to β-sheet on aggregation.33−35 In AD, the change of tau conformation leads to the pathological assembly of tau into aggregates.36 CD spectroscopy was performed to analyze the change in tau conformation because of peptides. The soluble tau (indicated in black) shows minimum ellipticity at 198 nm and additional arm at 214 nm, indicating random coil conformation. The transition of the wavelength is from 198 to 213 nm. Moreover, the intensity at 214 nm also increases, indicating aggregation of tau control (Figure 2C).37 Amongst the peptides, P5 followed by KLVFF and P4 drive tau conformation to random coil conformation and show reduction in intensity at 214 nm (Figure 2D). In the presence of LPFFD, P3, and P6, tau conformation was altered to β-sheet conformation where LPFFD shows a high intensity at 214 nm when compared to other peptides and tau control.24 This observation was in accordance with ThS fluorescence and SDS-PAGE analysis.
Peptides Impede Fibrillization of Tau
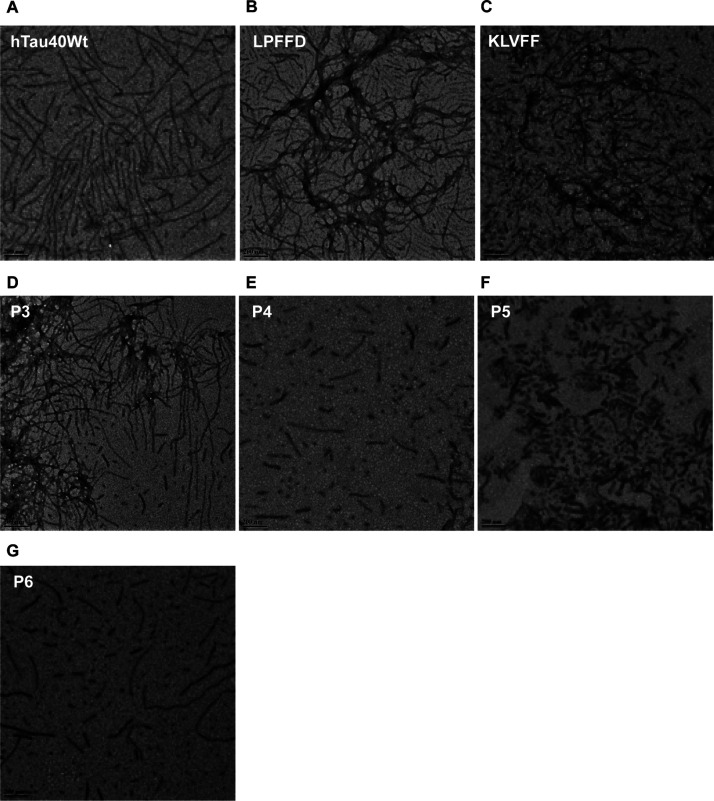
An electron microscopy analysis showed the change in tau morphology upon incubating with peptides. The heparin-induced aggregates of tau exhibit long fibrillar morphology with a darkly stained appearance because of uranyl acetate (Figure 3A). However, tau formed short, broken filaments like morphology upon incubating with KLVFF, P4, and P5 (Figure 3C,E,F), whereas LPFFD, P3, and P6 showed no inhibition (Figure 3B,D,G). This observation suggests the effective role of peptides in inhibiting tau aggregation. These observations were in accordance with our ThS fluorescence data. Previous studies with these peptides also showed inhibition of amyloid-β aggregation, indicating the effective role of KLVFF, P4, and P5 in preventing aggregation of both, tau and amyloid-β.18,38,39 Thus, our study indicates the inhibitory role of KLVFF, P4, and P5 in preventing tau aggregation.
Figure 3.
LPFFD and its derivatives prevent tau fibrillization. (A) The tendency of tau assembly into fibrils was mapped by TEM where their morphology is visualized as long and fibrillar aggregates. (B) Control peptide LPFFD prevents fibrillization of tau and forms short-length aggregates. (C–G) Similar to the control peptide, treatment with KLVFF, P4, and P5 resulted in inhibition of tau assembly into aggregates and P3 and P6 showed no inhibition.
Peptides Attenuates Tau-Induced Toxicity in SH-SY5Y Cells
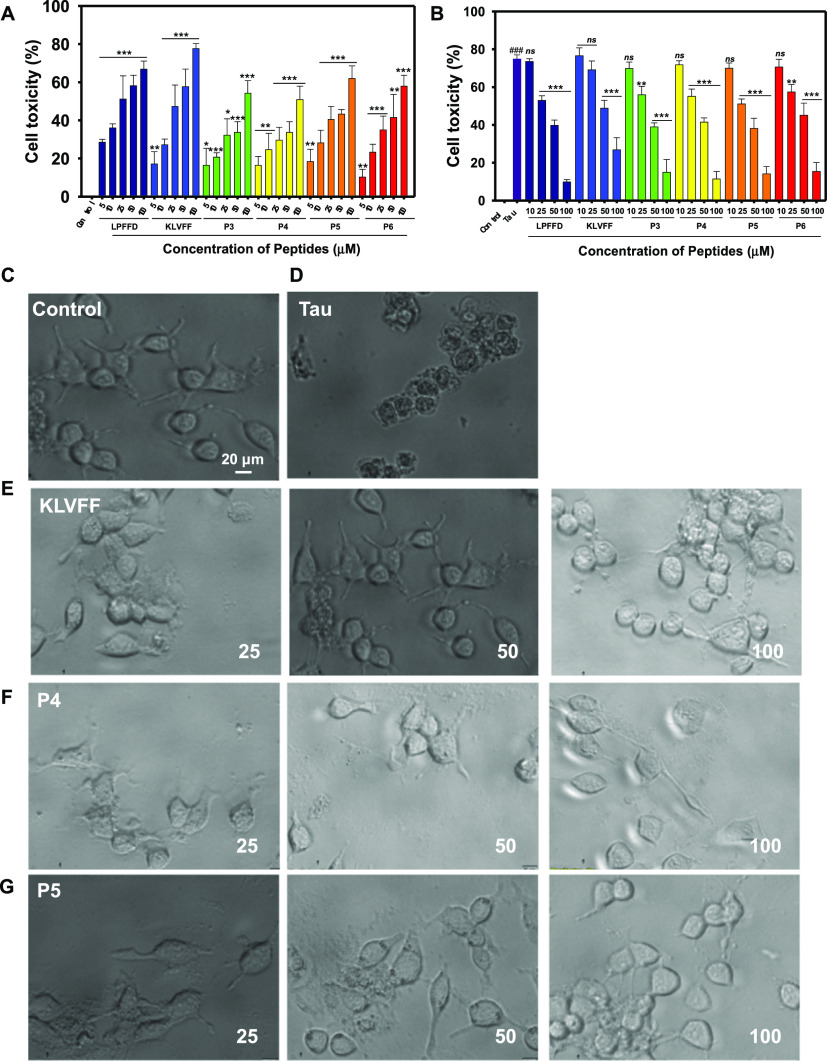
From the in vitro assays so far, it is evident that the peptides show promising tau disaggregation properties. Further, to evaluate the effect of the peptides on tau aggregate toxicity toward cells, we first examined if the peptides themselves exhibited any cytotoxicity on SH-SY5Y neuroblastoma cells. The cells were incubated with the various peptides at different concentrations for 12 h and the viability of cells and the cell morphology were analyzed by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay and microscopy, respectively (Figure 3). The peptides showed approximately 60–70% cell toxicity at the highest concentration of 100 μM.
Studies showed that the tau aggregates exert cytotoxicity probably because of hyperphosphorylation40 or microtubule dysfunction inside the cell.41−43 Hence, to evaluate whether the tested peptides can reduce tau aggregate-mediated toxicity in neural cells, we incubated the cells with both tau aggregates and peptides and toxicity was measured by MTT. As shown in Figure 4A,B, tau aggregates (hTau40wt) when incubated alone showed 70% cell toxicity. However, in the presence of peptides, the cytotoxic effect was significantly attenuated in a dose-dependent manner, which was evident from increased cell viability. At lower concentrations of peptides (10 μM), none of the inhibitors could increase the cell viability. Nevertheless, with increased concentrations of peptides (50 & 100 μM), there was a significant decrease in cell toxicity. Similar effects were observed in microscopic images.
Figure 4.
Evaluation of tau-induced cytotoxicity in the presence of different concentrations of peptides. (A) Cell viability of SH-SY5Y cells in the presence of peptides. Cells were incubated with different concentrations of peptides; LPFFD, KLVFF, P3, P4, P5, P6 and (10–100 μM) for 12 h and the viability was measured by MTT assay. DMEM media was used to dissolve the indicated peptides, used as untreated control and set to 100% viability. (B) Full-length tau aggregates (hTau40wt) at 5 μM concentration in the absence or presence of indicated concentrations of peptides (LPFFD, KLVFF, and P3–P6) was added to the SH-SY5Y cells and incubated for 12 h at 37 °C. The cell toxicity was measured by MTT assay. (C–G) Cell imaging. Images of the cells post incubation for 12 h with peptides at various concentrations in the absence and presence of 5 μM tau. The cells were observed under a bright field microscope with a magnification of 20×.
Conclusions
The amyloid-β-derived peptides were studied for their aggregation inhibition effect on amyloid-β. In our present study, we have analyzed the effect of these peptides on tau aggregation and observed that KLVFF, P4, and P5 prevented in vitro tau aggregation. These peptides decreased ThS fluorescence in heparin-induced tau aggregation and P4 and P5 reduced the higher-order aggregates as evidenced by SDS-PAGE. The random coil conformation of tau was maintained by P5, followed by KLVFF and P4. The TEM micrographs also suggested the inhibition of tau assembly into fibrillar aggregates. In conclusion, the inhibitory role of these peptides on tau and their nontoxic nature suggest them to be of therapeutic importance in overcoming AD by targeting tau aggregation.
Materials and Methods
Chemical and Reagents
Luria broth was purchased from HiMedia; ampicillin, ethylene glycol tetraacetic acid (EGTA), phenylmethylsulfonyl fluoride (PMSF), MgCl2, NaCl, disodium phosphate, and monopotassium phosphate were purchased from MP. Isopropyl β-d-1-thiogalactopyranoside (IPTG), dithiothreitol (DTT), protease inhibitor cocktail, heparin, and sodium azide were purchased from Calbiochem. 2-(N-Morpholino)ethanesulfonic acid (MES), bicinchoninic acid (BCA), bovine serum albumin, BES, MTT, and ThS were purchased from Sigma. Copper grids with carbon type-B, with 400 mesh (01814-F) for TEM were purchased form Ted Pella, Inc. SH-SY5Y cells were purchased from ATCC (CRL-2266), and Dulbecco’s modified Eagle medium (DMEM)-F12, penicillin–streptomycin mixture, trypsin–ethylenediaminetetraacetic acid, and fetal bovine serum (FBS) were purchased from Thermo Scientific Pvt. Ltd.
Synthesis of Peptidomimetics and Purification
The control peptide (LPFFD) and N-methyl glycine (sarcosine: Sr) substituted peptidomimetics; P3 (thymine-Lys-Leu-Val-Phe-Phe), P4 (thymine-Sr-Leu-Sr-Phe-Sr-Ala), P5 (thymine-Lys-Sr-Val-Sr-Phe-Sr) and P6 (Gly-His-Lys-Sr-Val-Sr-Phe-Sr) were synthesized following standard 9-fluorenylmethoxycarbonyl (Fmoc) chemistry on an automated peptide synthesizer from Syro II (MultiSynTech).18 Rink amide resin (Novabiochem) was used as a solid support for the peptidomimetic synthesis. Fmoc-protected sarcosine (Sr) was prepared and directly used for the synthesis of P4 and P5 in the peptide synthesizer. Hexafluorophosphate benzotriazole tetramethyl uronium was used as a coupling reagent for amino acids in the presence of N,N-diisopropylethylamine with dimethylformamide (DMF) as solvent. For deprotection of Fmoc, 40% piperidine in DMF was used. P3 and LPFFD were synthesized with a coupling time of 1 h per amino acid, whereas for P4, P5, and P6 the coupling time was increased to 2 h to obtain higher coupling yields. All the peptides and peptidomimetics were purified using a reverse-phase preparative high-performance liquid chromatography (HPLC) on the C18 column at 40 °C. Product purity was greater than 98% as ascertained by analytical HPLC. The molecular masses of the peptides and their mimetics were verified with HRMS (Q-TOF).
Preparation of Tau Protein
Expression of tau protein was induced by 0.5 mM IPTG in BL21* at 37 °C. Postinduction cells were incubated for 4 h before harvesting by centrifugation at 4000 rpm for 10 min. The cells were homogenized at 15,000 PSI using a constant cell disruption system and purified by cation-exchange chromatography followed by size-exclusion chromatography as described previously.44 The lysate was extracted in 50 mM MES pH 6.8, 1 mM EGTA, 1 mM PMSF, 2 mM MgCl2, 5 mM DTT, and protease inhibitor cocktail. NaCl and DTT were added to a final concentration of 0.5 M and 5 mM, respectively, and heated for 20 min at 90 °C. The resultant lysate was cooled and subjected to centrifugation at 40,000 rpm at 4 °C for 45 min. The supernatant was dialyzed overnight in the above buffer with 20 mM MES buffer and 50 mM NaCl (buffer A). The dialyzed protein was centrifuged and loaded onto preequilibrated Sepharose fast-flow column (GE17-0729-01). The bound protein was eluted by linearly increasing the NaCl gradient to 1 M. The fractions obtained from ion exchange chromatography were pooled, concentrated, and loaded onto a HiLoad 16/600 Superdex 75 pg column (GE28-9893-33). The protein concentration was measured by a BCA assay.
Aggregation Inhibition Assay
Tau aggregation was induced by heparin at 4:1 ratios (tau/heparin) in an assembly buffer composed of 20 mM BES pH 7.4, 25 mM NaCl, 1 mM DTT, 0.01% NaN3, and protease inhibitor cocktail.8,45 The effect of peptides on tau aggregation was studied in the presence of 200 μM peptides with 20 μM tau. This mixture was incubated at 37 °C and ThS fluorescence was measured at the time interval of 24 h, till 96 h.
ThS Fluorescence Assay
The extent of tau aggregation was studied by ThS assay as described previously.8 Tau aggregation results in the formation of β-sheet structures, which was probed by ThS. ThS binds to the β-sheet structure and fluorescence, indicating aggregation, whereas incorporation of peptides that may result in decrease in ThS fluorescence shows inhibition of tau aggregation. The fluorophore was prepared in 50 mM ammonium acetate at pH 7.0. Tau was incubated with ThS at a 1:4 ratio (tau/ThS), that is, 2 μM of tau was added along with 8 μM of ThS to 384 black well plate. ThS fluorescence was measured at 521 nm upon exciting the fluorophore at 441 nm. The measurements were recorded in triplicate in a Tecan Infinite 200 PRO multimode microplate reader and the kinetics was plotted using SigmaPlot 10.0.
SDS-PAGE Assay
Tau aggregate formation was analyzed by SDS-PAGE. At 96 h, 20 μL of tau protein was aliquoted from the reaction mixture and resolved on 10% SDS-PAGE gel. SDS-PAGE was run by using a Mini-PROTEAN System from Bio-Rad.8,45
CD Spectroscopy
The change in tau conformation from a random coil to a β-sheet was analyzed by CD spectroscopy as described previously.44 Tau was diluted to 3 μM in 50 mM phosphate buffer, pH 6.8, and the spectra were recorded in the far-UV region at 25 °C in Jasco J-815 by using a 1 mm path length cuvette. The measurement parameters were as follows; the wavelength range was 250–190 nm, data pitch of 1.0, and 100 nm/min scanning speed. The spectra were plotted by using SigmaPlot 10.0.
TEM Analysis
In vitro tau aggregation results in deeply stained, long fibrillar aggregates, as observed by TEM. Tau (1 μM) was collected at the end of 96 h and spotted onto 400 mesh carbon-coated copper grids. The excess protein was removed by incubating the grid in water for 30 s, which was followed by staining using 2% uranyl acetate for 2 min. The grid was dried before imaging by Tecnai T20, at 120 kV.7,8
Cytotoxicity Assay
The SH-SY5Y cells were cultured in DMEM-F12 media (Gibco) supplemented with 20% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin. Subconfluent cells were trypsinized and 25,000 cells/well were seeded in a 96-well plate (100 μL/well) and allowed to adhere overnight at 37 °C. Tau (5 μM) and the peptides at different concentrations (10, 25, 50, and 100 μM) were dissolved in DMEM. The negative control, represented by control, was prepared as a medium without any molecules and treated in the same manner. The medium (100 μL) with or without the tau or peptides was added to each well. After 12 h of incubation at 37 °C, cell viability was evaluated by thiazolyl-blue-tetrazolium-bromide (MTT) assay as per the manufacturer’s instructions. Briefly, 10 μL of 0.5 mg/mL MTT was added into each well and further incubated for 4 h at 37 °C. Later, 100 μL of dimethyl sulfoxide was added into each well and the color intensity was measured using a microplate reader at 570 nm. Each treatment was performed in triplicate. Further, to monitor the cell viability in the presence of peptides, the cells were treated with 100 μL of DMEM containing 5 μM full-length tau aggregates (hTau40wt), followed by the indicated amounts of peptides. The full-length tau aggregates alone was used as control. The cell viability was evaluated as described earlier using the MTT assay and the results are represented as percentages.
Microscopy
SH-SY5Y cells subjected to cytotoxicity studies were also scanned for bright field-time-series images using a fluorescent microscope (Olympus microscope-TH4 200 Shinjuku, Tokyo Japan) at 20× magnification.
Statistical Analysis
The data was analyzed by Student’s t-test, two-tailed and unpaired at 95% confidence interval; and represented in terms of mean ± sem. The statistical significance was represented as ***, **, and *, which indicated a p value < 0.001, <0.01, and <0.05 respectively; and the p value > 0.05 was represented as ns.
Acknowledgments
Nalini V Gorantla acknowledges the University Grant Commission (UGC) for the fellowship. We acknowledge Prof. Roland Brandt from the University of Osnabruck, Germany, and Prof. Jeff Kuret from the Ohio State University College of Medicine, USA, for being generous in providing tau constructs. The authors greatly acknowledge Chinnathambi’s lab members for their critical reading and fruitful discussion.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03497.
Synthesis of peptidomimetics and purification by analytical HPLC and molecular masses analyzed by HRMS (Q-TOF) (PDF)
Author Contributions
N.V.G. and S.C. designed the experiments. N.V.G. and L.P.S. executed the experiments. P.G.N. and P.P.C. contributed to the toxicity assays. Peptides and peptidomimetics were synthesized and characterized by K.R. under the guidance of T.G. N.V.G. and S.C. wrote the paper. S.C. conceived the idea of the project, supervised the project, provided resources, and wrote the paper. All the authors reviewed the results and checked the final paper.
This work was supported by an in-house CSIR-National Chemical Laboratory grant MLP029526.
The authors declare no competing financial interest.
Supplementary Material
References
- Congdon E. E.; Sigurdsson E. M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399. 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla N. V.; Chinnathambi S. Tau Protein Squired by Molecular Chaperones During Alzheimer’s Disease. J. Mol. Neurosci. 2018, 66, 356–368. 10.1007/s12031-018-1174-3. [DOI] [PubMed] [Google Scholar]
- Sonawane S. K.; Chinnathambi S. Prion-Like Propagation of Post-Translationally Modified Tau in Alzheimer’s Disease: A Hypothesis. J. Mol. Neurosci. 2018, 65, 480–490. 10.1007/s12031-018-1111-5. [DOI] [PubMed] [Google Scholar]
- Fontaine S. N.; Sabbagh J. J.; Baker J.; Martinez-Licha C. R.; Darling A.; Dickey C. A. Cellular factors modulating the mechanism of tau protein aggregation. Cell. Mol. Life Sci. 2015, 72, 1863–1879. 10.1007/s00018-015-1839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T. L.; Attems J.; Thal D. R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. 10.1007/s00401-017-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C.; Brunden K. R.; Trojanowski J. Q.; Lee V. M.-Y.; Smith A. B.; Huryn D. M. Modulation of protein-protein interactions as a therapeutic strategy for the treatment of neurodegenerative tauopathies. Curr. Top. Med. Chem. 2011, 11, 317–330. 10.2174/156802611794072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane S. K.; Balmik A. A.; Boral D.; Ramasamy S.; Chinnathambi S., Baicalein suppresses Tau fibrillization by sequestering oligomers. 2019, bioRxiv:704536. [DOI] [PubMed] [Google Scholar]
- Gorantla N. V.; Das R.; Mulani F. A.; Thulasiram H. V.; Chinnathambi S. Neem Derivatives Inhibits Tau Aggregation. J. Alzheimers Dis. Rep. 2019, 3, 169–178. 10.3233/adr-190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla N. V.; Landge V. G.; Nagaraju P. G.; Priyadarshini CG P.; Balaraman E.; Chinnathambi S. Molecular Cobalt (II) Complexes for Tau Polymerization in Alzheimer’s Disease. ACS Omega 2019, 4, 16702. 10.1021/acsomega.9b00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Nisha C. M.; Silakari C.; Sharma I.; Anusha K.; Gupta N.; Nair P.; Tripathi T.; Kumar A. Current and novel therapeutic molecules and targets in Alzheimer’s disease. J. Formos. Med. Assoc. 2016, 115, 3–10. 10.1016/j.jfma.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Cao J.; Hou J.; Ping J.; Cai D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 64. 10.1186/s13024-018-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P.; Patel B.; Makwana V.; Jadhav H. R.; Kiefel M.; Davey A.; Reekie T. A.; Rudrawar S.; Kassiou M. Peptides, Peptidomimetics, and Carbohydrate-Peptide Conjugates as Amyloidogenic Aggregation Inhibitors for Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 1530–1551. 10.1021/acschemneuro.8b00185. [DOI] [PubMed] [Google Scholar]
- von Bergen M.; Barghorn S.; Li L.; Marx A.; Biernat J.; Mandelkow E.-M.; Mandelkow E. Mutations of Tau Protein in Frontotemporal Dementia Promote Aggregation of Paired Helical Filaments by Enhancing Local β-Structure. J. Biol. Chem. 2001, 276, 48165–48174. 10.1074/jbc.m105196200. [DOI] [PubMed] [Google Scholar]
- Von Bergen M.; Friedhoff P.; Biernat J.; Heberle J.; Mandelkow E.-M.; Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming beta structure. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 5129–5134. 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemerovski-Glikman M.; Frenkel-Pinter M.; Mdah R.; Abu-Mokh A.; Gazit E.; Segal D. Inhibition of the Aggregation and Toxicity of the Minimal Amyloidogenic Fragment of Tau by Its Pro-Substituted Analogues. Chem.—Eur J. 2017, 23, 9618–9624. 10.1002/chem.201701218. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Liu C.; Sawaya M. R.; Vadla B.; Khan S.; Woods R. J.; Eisenberg D.; Goux W. J.; Nowick J. S. Macrocyclic β-Sheet Peptides That Inhibit the Aggregation of a Tau-Protein-Derived Hexapeptide. J. Am. Chem. Soc. 2011, 133, 3144–3157. 10.1021/ja110545h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler P. M.; Boyer D. R.; Murray K. A.; Yang T. P.; Bentzel M.; Sawaya M. R.; Rosenberg G.; Cascio D.; Williams C. K.; Newell K. L. Structure-based inhibitors halt prion-like seeding by Alzheimer’s disease-and tauopathy-derived brain tissue samples. J. Biol. Chem. 2019, 294, 16451. 10.1074/jbc.ra119.009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar K.; Suresh S. N.; Manjithaya R.; Govindaraju T. Rationally designed peptidomimetic modulators of Aβ toxicity in Alzheimer’s disease. Sci. Rep. 2015, 5, 8139. 10.1038/srep08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar K.; Madhu C.; Govindaraju T. Natural Tripeptide-Based Inhibitor of Multifaceted Amyloid β Toxicity. ACS Chem. Neurosci. 2016, 7, 1300–1310. 10.1021/acschemneuro.6b00175. [DOI] [PubMed] [Google Scholar]
- Estrada L.; Soto C. Disrupting β-amyloid aggregation for Alzheimer disease treatment. Curr. Top. Med. Chem. 2007, 7, 115–126. 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- Ribarič S. Peptides as potential therapeutics for Alzheimer’s disease. Molecules 2018, 23, 283. 10.3390/molecules23020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen B. M.; Paleologou K. E.; Ali S. A. E.; Qureshi M. M.; Allsop D.; El-Agnaf O. M. A. Designing Peptide Inhibitors for Oligomerization and Toxicity of Alzheimer’s β-Amyloid Peptide. Biochemistry 2008, 47, 1984–1992. 10.1021/bi701415b. [DOI] [PubMed] [Google Scholar]
- Liu F.; Du W.; Sun Y.; Zheng J.; Dong X. Atomistic characterization of binding modes and affinity of peptide inhibitors to amyloid-β protein. Front. Chem. Sci. Eng. 2014, 8, 433–444. 10.1007/s11705-014-1454-6. [DOI] [Google Scholar]
- Minicozzi V.; Chiaraluce R.; Consalvi V.; Giordano C.; Narcisi C.; Punzi P.; Rossi G. C.; Morante S. Computational and Experimental Studies on β-Sheet Breakers Targeting Aβ1-40Fibrils. J. Biol. Chem. 2014, 289, 11242–11252. 10.1074/jbc.m113.537472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adessi C.; Frossard M.-J.; Boissard C.; Fraga S.; Bieler S.; Ruckle T.; Vilbois F.; Robinson S. M.; Mutter M.; Banks W. A. Pharmacological profiles of peptide drug candidates for the treatment of Alzheimer’s disease. J. Biol. Chem. 2003, 278, 13905–13911. 10.1074/jbc.m211976200. [DOI] [PubMed] [Google Scholar]
- Lu J.; Cao Q.; Wang C.; Zheng J.; Luo F.; Xie J.; Li Y.; Ma X.; He L.; Eisenberg D. Structure-based peptide inhibitor design of amyloid-β aggregation. Front. Mol. Neurosci. 2019, 12, 54. 10.3389/fnmol.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R.; Chinnathambi S. Microglial priming of antigen presentation and adaptive stimulation in Alzheimer’s disease. Cell. Mol. Life Sci. 2019, 76, 3681. 10.1007/s00018-019-03132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler J.; James B.; Johnson T.; Marin A.; Weuve J. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- Goedert M.; Spillantini M. G.; Jakes R.; Rutherford D.; Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3, 519–526. 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Ganguly P.; Do T. D.; Larini L.; LaPointe N. E.; Sercel A. J.; Shade M. F.; Feinstein S. C.; Bowers M. T.; Shea J.-E. Tau assembly: the dominant role of PHF6 (VQIVYK) in microtubule binding region repeat R3. J. Phys. Chem. B 2015, 119, 4582–4593. 10.1021/acs.jpcb.5b00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viet M. H.; Ngo S. T.; Lam N. S.; Li M. S. Inhibition of aggregation of amyloid peptides by beta-sheet breaker peptides and their binding affinity. J. Phys. Chem. B 2011, 115, 7433–7446. 10.1021/jp1116728. [DOI] [PubMed] [Google Scholar]
- Di Natale G.; Zimbone S.; Bellia F.; Tomasello M. F.; Giuffrida M. L.; Pappalardo G.; Rizzarelli E. Potential therapeutics of Alzheimer’s diseases: New insights into the neuroprotective role of trehalose-conjugated beta sheet breaker peptides. Pept. Sci. 2018, 110, e24083 10.1002/pep2.24083. [DOI] [Google Scholar]
- Schweers O.; Schönbrunn-Hanebeck E.; Marx A.; Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J. Biol. Chem. 1994, 269, 24290–24297. [PubMed] [Google Scholar]
- Jeganathan S.; von Bergen M.; Mandelkow E.-M.; Mandelkow E. The natively unfolded character of tau and its aggregation to Alzheimer-like paired helical filaments. Biochemistry 2008, 47, 10526–10539. 10.1021/bi800783d. [DOI] [PubMed] [Google Scholar]
- Von Bergen M.; Barghorn S.; Biernat J.; Mandelkow E.-M.; Mandelkow E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim. Biophys. Acta, Mol. Basis Dis. 2005, 1739, 158–166. 10.1016/j.bbadis.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Battisti A.; Ciasca G.; Grottesi A.; Bianconi A.; Tenenbaum A. Temporary secondary structures in tau, an intrinsically disordered protein. Mol. Simul. 2012, 38, 525–533. 10.1080/08927022.2011.633347. [DOI] [Google Scholar]
- Maeda S.; Sahara N.; Saito Y.; Murayama M.; Yoshiike Y.; Kim H.; Miyasaka T.; Murayama S.; Ikai A.; Takashima A. Granular tau oligomers as intermediates of tau filaments. Biochemistry 2007, 46, 3856–3861. 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- Chalifour R. J.; McLaughlin R. W.; Lavoie L.; Morissette C.; Tremblay N.; Boulé M.; Sarazin P.; Stéa D.; Lacombe D.; Tremblay P. Stereoselective interactions of peptide inhibitors with the β-amyloid peptide. J. Biol. Chem. 2003, 278, 34874–34881. 10.1074/jbc.m212694200. [DOI] [PubMed] [Google Scholar]
- Wei C.-W.; Peng Y.; Zhang L.; Huang Q.; Cheng M.; Liu Y.-N.; Li J. Synthesis and evaluation of ferrocenoyl pentapeptide (Fc-KLVFF) as an inhibitor of Alzheimer’s Aβ1-42 fibril formation in vitro. Bioorg. Med. Chem. Lett. 2011, 21, 5818–5821. 10.1016/j.bmcl.2011.07.111. [DOI] [PubMed] [Google Scholar]
- Cowan C. M.; Mudher A. Are tau aggregates toxic or protective in tauopathies?. Front. Neurol. 2013, 4, 114. 10.3389/fneur.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay B.; Li G.; Yin H.; Kuret J. Tau Aggregation and Toxicity in a Cell Culture Model of Tauopathy. J. Biol. Chem. 2007, 282, 16454–16464. 10.1074/jbc.m700192200. [DOI] [PubMed] [Google Scholar]
- Avila J.; Santa-María I.; Pérez M.; Hernández F.; Moreno F. Tau phosphorylation, aggregation, and cell toxicity. J. Biomed. Biotechnol. 2006, 2006, 74539. 10.1155/jbb/2006/74539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt M.; Biernat J.; Hübschmann S.; Dennissen F. J. A.; Timm T.; Aho A.; Mandelkow E.-M.; Mandelkow E. Time course of Tau toxicity and pharmacologic prevention in a cell model of Tauopathy. Neurobiol. Aging 2017, 57, 47–63. 10.1016/j.neurobiolaging.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Gorantla N. V.; Shkumatov A. V.; Chinnathambi S.. Conformational Dynamics of Intracellular Tau Protein Revealed by CD and SAXS. Tau Protein; Springer, 2017; pp 3–20. [DOI] [PubMed] [Google Scholar]
- Sonawane S. K.; Ahmad A.; Chinnathambi S. Protein-Capped Metal Nanoparticles Inhibit Tau Aggregation in Alzheimer’s Disease. ACS Omega 2019, 4, 12833–12840. 10.1021/acsomega.9b01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.