Abstract
Biobased materials such as cellulose, chitin, silk, soy, and keratin are attractive alternatives to conventional synthetic materials for filtration applications. They are cheap, naturally abundant, and easily fabricated with tunable surface chemistry and functionality. With the planet’s increasing crisis due to pollution, the need for proper filtration of air and water is undeniably urgent. Additionally, fibers that are antibacterial and antiviral are critical for public health and in medical environments. The current COVID-19 pandemic has highlighted the necessity for cheap, easily mass-produced antiviral fiber materials. Biopolymers can fill these roles very well by utilizing their intrinsic material properties, surface chemistry, and hierarchical fiber morphologies for efficient and eco-friendly filtration of physical, chemical, and biological pollutants. Further, they are biodegradable, making them attractive as sustainable, biocompatible green filters. This review presents various biopolymeric materials generated from proteins and polysaccharides, their synthesis and fabrication methods, and notable uses in filtration applications.
1. Introduction
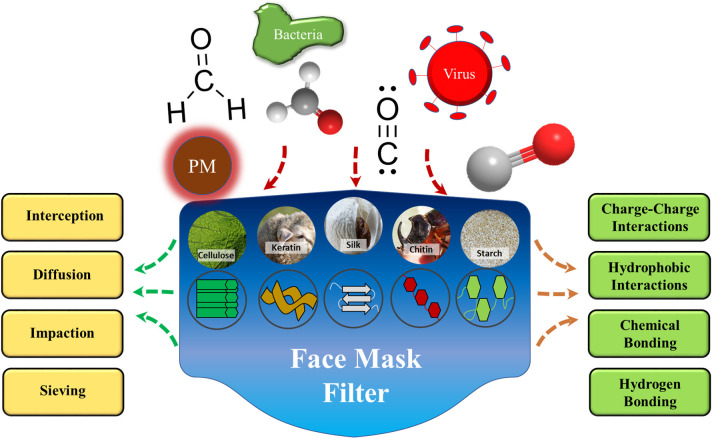
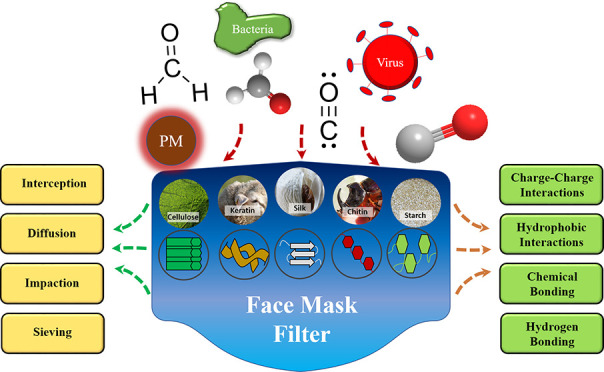
Creating efficient filters is critical to environmental health, manufacturing, and healthcare. While many conventional filters already exist, they can be expensive, inefficient, or contribute to polluting the environment when disposed of improperly or if they are not recyclable or biodegradable. Biopolymers offer an attractive alternative for filter materials that can potentially address the aforementioned problems. Biopolymers are readily available and often cheap and easy to process. They mainly encompass proteins and polysaccharides from both animal and plant sources, including silkworm silks, animal wools, corn and soy proteins, and cotton cellulose (Figure 1). Biopolymer-based materials are already widely used in biomedical applications such as drug delivery and tissue engineering due to their biocompatibility and bioactivity.1 In addition, their applications in filtering have received increasing attention in the past decade.2 For biopolymers to be useful in filter applications, they should exhibit certain desirable physical and chemical properties that are important for absorption and the elimination of specific chemical contaminants or provide bactericidal or viricidal functionality. The wide range of functional groups available in proteins and polysaccharides allows for highly selective filtration for pollutants and other contaminants. Functionalization of biopolymers through postprocessing or combining different biopolymers together can improve their overall filtration abilities, leading to comprehensive filtration of physical, chemical, and biological molecules. This review surveys several biopolymers and their applications in filtration. Major filtration methods are discussed in order to better illustrate the roles of biopolymers (Figure 1).
Figure 1.
Biopolymer-based filtration materials fabricated from a variety of protein and polysaccharide sources (inserted cellulose, keratin, silk, chitin, and starch photo credits: pixabay.com). These unique surface chemistries and diverse molecular interactions aid filtration of various contaminants, including particulate matter (PM), bacteria, viruses, and smoke pollutants (O–H, HCHO, and C≡O).
2. Biopolymer Materials
Proteins and their plethora of active functional groups make them great candidates for air filtration. Silk is a biomaterial produced naturally from insects such as silkworms, moths, and spiders. Silk fibroin (SF), extracted from silk, has been widely used as a textile for both daily wear and medical applications. For example, silk-based air filters have been used in hospitals, offices, and living spaces.1 The most common components of silk are alanine, glycine, and serine, which give it an overall charge that can interact with contaminants.
Soy proteins (SPs), in particular, have a wide variety of functional groups that are polar, nonpolar, hydrophobic, or hydrophilic.3 The ionizable groups are of particular interest for capturing charged pollutants and invoking antibacterial properties.3 This versatility has made SPs attractive for a wide range of applications, including food packaging, adhesives, drug delivery, tissue engineering, and antibacterial agents.4 Native soy protein isolate (SPI) is a large, bulky particle comprising a vast variety of intermolecular reactions from its side chains.3 When native SPI is denatured, the soy protein chains are unfolded to expose the interactive functional groups that are responsible for capturing pollutants.
Keratin is the major component of wool and hair fibers, a protein found in virtually all mammals. The primary structure of keratin is a series of monomers that are chemically cross-linked via disulfide bonds through the side chains. Thus, wool is not readily soluble and must be reduced or oxidized to enhance its solubility for processing into a useful material. Meanwhile, the disulfide bonds give wool keratin the unique ability to adsorb metal ions once oxidized. The oxidation process cleaves sulfur–sulfur bonds, leaving behind cysteic acid and carboxylic acid to bond with metal ions.5 The oxidized form of wool keratin, called wool keratose (WK), has been found to have very useful properties in filtration. A WK/SF blend can filter heavy metal ions such as Cu2+ with over 90% efficiency.5 This is largely due to the ion-binding cysteic acid groups. It is also highly reusable. Even after being recycled six times, the filter maintains a greater than 90% adsorption efficiency of Cu2+.5 Such a filter would be very useful in metal ion heavy environments, such as air filters in mines. The recycling ability of WK and SF appeals to both industrial and ecological parties by preventing the buildup of waste and the costs of fabricating new filters.
Cellulose is a biomaterial lignan made from natural plant fibers.6 It is very rigid, largely attributed to hydrogen bonding between cellulose molecules. Cellulose molecules arrange themselves in parallel to form microfibrils via hydrogen bonding. Cellulose-based filters in this arrangement have crystalline structures, leading to a high degree of stability and resistance to chemical degradation.6 However, this rigid framework makes it difficult to process. Chemical processes such as oxidation are often necessary to break it down for processing.6 The right balance of rigidity and processability is important when using cellulose-based materials.
Chitin is widely found in the shells of shrimp and crabs, mollusk shells, and endoskeleton, the exoskeleton of arthropods, fungi, yeast, and microbial cell walls. While γ-chitin typically forms microfibers, the others form nanofibers. Chitin forms chains of 1000–3000 residues through p1,4 glycoside linkages. Chitin is treated by concentrated alkali solution to remove acetyl groups via deacetylation to form chitosan. The higher the degree of deacetylation, the more positively charged groups on the chitosan backbone are exposed, which is important in filtration applications.7
3. Filtration Principles of Biopolymers
Air filtering can be categorized into two general approaches. First is physical adsorption, where the toxic contaminants bind themselves to either the filter surface or an embedded particle on the filter. The other is chemisorption, where either the filter membrane surface or an embedded particle reacts with the pollutant, rendering it inert. In both cases, the limiting factor is the availability of the active sites within the filter. That is, once there are no active sites available for which the toxin to bind, the remaining toxins may continue to flow through the porous filter.8 Previous air filters have relied on synthetic polymers to remove particles based on impaction, interception, diffusion, and electrostatic interactions, as illustrated in Figure 2.8 These same interactions apply to biopolymer-based filters. The primary filtration interaction varies with the size and nature of the pollutant (Figure 3); whereas larger pollutants (>1000 nm dust and spores) may be physically blocked with ease, smaller pollutants (100–1000 nm) are primarily filtered using electrostatics.8Figure 3b also shows the fractional collection efficiency for different mechanical filters with respect to the diameter of the contaminant.
Figure 2.
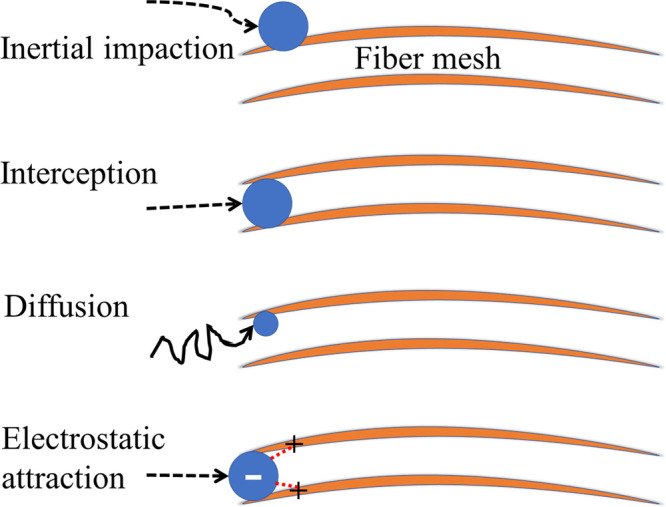
Typical mechanisms behind biopolymer (orange lines) filtration include impaction, interception, diffusion, and electrostatic interaction. Black lines indicate movement path of pollutant (VOC, bacteria, virus, etc.), shown as a blue sphere; red dotted lines indicate electrostatic interactions between biopolymer and pollutant.8
Figure 3.
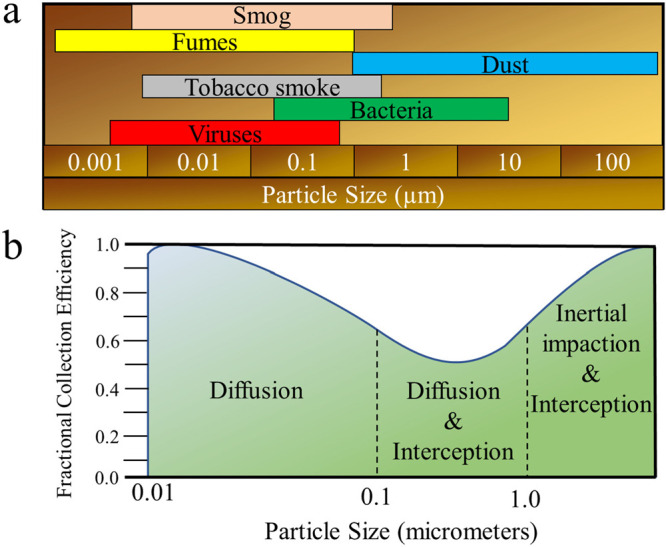
(a) Relative size of common air contaminants and (b) fractional collection efficiency for different mechanical filters with respect to the diameter of the contaminant.8
Electrostatic attraction results from either functional groups on biopolymer-based fibers or particles implanted in the fibers, which provide an electrostatic charge (Figure 2). This charge interacts with charged pollutants, trapping them, such as in the ability of ionizable functional groups on silk and wool keratin to trap metal ions discussed previously.5 In this example, cystic and carboxylic acid residues were especially praised for their adsorption ability. Electrostatic filtration is most effective for particles between 100 and 1000 nm in size. The path of the particle through the filter can also be altered, allowing for easier physical filtering. Applications of electrostatically enhanced filters can be anywhere that has ion-rich environments, such as construction zones.
Furthermore, the choice of material itself is often important in the fabrication of filters. In the past decade, cellulose, soy protein, chitosan, and corn zein have all been commonly used to create fibrous filtering materials. These materials are all easy to work with and form fibers from, plus contain, exposed functional groups along their backbones that can interact with pollutants to assist in capturing them. Often, filters are created from layered materials in order to improve their efficacy.2 Maintaining a low pressure drop through the filter is a critical property of face mask filters that the aforementioned materials are able to accomplish. In short, the pressure drop, ΔP, is the change in pressure before (Px) and after (Py) filtration through the material. A low pressure drop ensures good fluid flow through the filter and, in the case of wearable face masks, also ensures good breathability.
Discussing pressure drop and layered filters brings attention to more details—how the lamination and geometry of stacking fibers will affect filtration efficacy and wearer comfort. One study comparing the comfort of various face masks and their efficiency against SARS-CoV-2 showed how increasing the amount of layers will decrease the air permeability of the mask, which prevents moisture and viruses from escaping the wearer’s mask, but at the trade-off of breathability. Even among samples with the same amount of layers, different structures can be formed between the two layers through cut piles that affect the air permeability, filtration efficiency, and pressure drop.9
The geometry and layering of fibers will also affect the porosity of the filter, which not only further influences the filtration efficiency but also affects the mechanical properties of the fiber. This is an important property for user compliance, reusability, and the ability to wash and clean the filter. Biopolymers are an attractive option for tunable porosity and mechanical properties, as they are easily modified from their natural-derived forms into more useful forms. Silk, for example, is one of the toughest mechanical materials found in nature, but its molecular weight can be decreased during processing while remaining a highly viable material for fiber formation. Various fabrication processes, which will be discussed next, can also influence the porosity and mechanical property of the fibers produced and ultimately the filtration properties of the product.
4. Fabrication Methods
4.1. Processing
Some biopolymers are strong enough to sustain the pressure and strain experienced during the filtration process, while others may not be, depending on how they are prepared. For example, silk needs to be treated by transforming it from a cocoon form into a usable fibrous material. Likewise, cellulose, which exists in crystalline and amorphous forms, will need to be degraded or hydrolyzed chemically. As a byproduct of plants, cellulose typically needs to undergo hydrolysis of β-1,4-linkages by adding enzymes into the raw extracted cellulose pulp in order to modify it into a more useful form.1 This broken down form of cellulose is what is used to prepare micro- and nanofibers.
4.2. Nanofibers
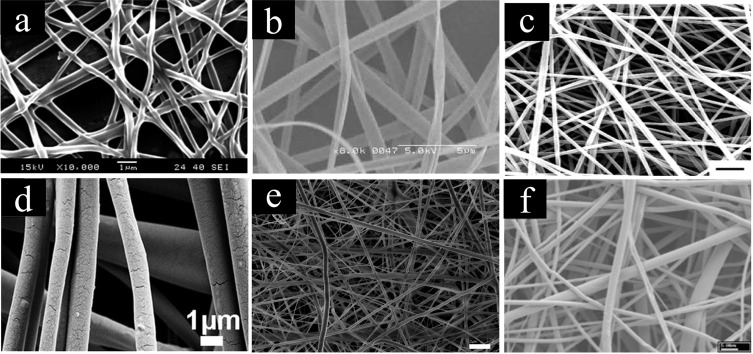
Nanofibers refer to fibers with diameters smaller than 1 μm. To use nanofibers in filtration, proper fabrication is important to increase the fiber efficiency, pollutant capacity, and filter life. Smaller fiber sizes can increase efficiency by improving the single collection efficiency while accommodating a smaller pressure drop.10 Importantly, smaller fibers also supply heightened capture and inertial impaction efficiencies to improve filtration. Nanofibers allow the same filter efficiency as microfibers at a smaller pressure drop or a better efficiency at the same pressure drop.11 The size of the fibers also affects the local flow conditions of filtrates through the fibers. Compared to microfibers, nanofibers have larger surface areas and highly versatile permeability, porosity, and stability.2 Nanofibers can be fabricated from several different kinds of biopolymers in order to create nanofibers with properties for specific applications while maintaining the benefits of nanofibers. As examples, Figure 4 shows scanning electron microscopy (SEM) images of nanofibers created for filtering applications fabricated from (a) silk, (b) corn zein, (c) soy protein, (d) starch, (e) chitosan, and (f) cellulose.
Figure 4.
SEM images of nanofibers fabricated from various biopolymers that can be employed for filtering applications. (a–c) Proteins and (d–f) polysaccharides, specifically, (a) silk, (b) corn zein, (outer scale bar = 5 μm), (c) soy (scale bar = 1 μm), (d) starch, (e) chitosan, and (f) cellulose. (a) Reproduced with permission from ref (12). Copyright 2015 Elsevier. (b) Reproduced with permission from ref (13). Copyright 2005 Wiley. (c) Reproduced from ref (3). Copyright 2016 American Chemical Society. (d) Reproduced with permission from ref (14). Copyright 2018 Wiley. (e) Reproduced from ref (15). Copyright 2007 American Chemical Society. (f) Reproduced with permission from ref (16). Copyright 2002 Wiley.
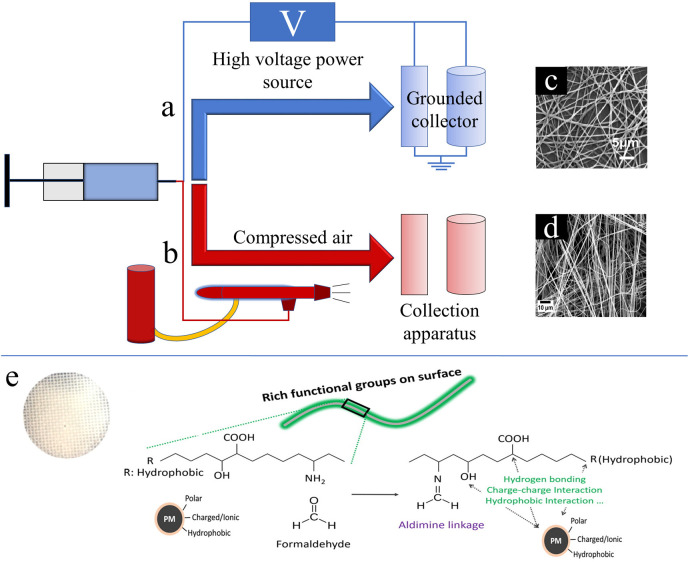
There are various approaches to create nanofibers. Among them, electrospinning has become one of the most dominant fabrication methods. In part, this is due to its simple setup: a syringe pump controls the flow rate of a polymer solution from a syringe needle connected to a high voltage generator, which shears the solution into fibers through electrostatic forces (Figure 5a). Once the polymer solution exits the syringe needle, solvent is evaporated, and the polymer fibers are jetted and collected on a substrate attached to a grounded metal plate at a certain distance. The polymer solution is typically prepared by dissolving biopolymers in organic solvents or ionic liquids.17 The size of the fibers, porosity, permeability, and stability of the resulting membranes will be tuned by the choice of polymers, solvent, applied voltage (typically in the kilovolts), distance, ambient humidity, solution viscosity, and postprocessing.8 Metal ions, including copper, chromium, and arsenic, can be introduced in the polymer solution to enhance the surface chemistry of the nanofibers to better interact with pollutants via electrostatic attraction.8
Figure 5.
Schematics of (a) electrospinning and (b) air-spraying of biopolymer solutions to form fibers for filtration devices, including SEM images of (c) electrospun silk-based nanofibers and (d) air-spun silk-based nanofibers. Filter paper made from (e) soy proteins is included as examples to show how biopolymer-based nanofibers interact with pollutants. (c) Reproduced with permission from ref (19). Copyright 2017 Elsevier. (d) Reproduced from ref (18). Copyright 2018 American Chemical Society. (e) Reproduced from ref (3). Copyright 2016 American Chemical Society.
Similar to electrospinning, solution-spraying or air-spraying of biopolymer solutions also allows for the fabrication of nanofibers (Figure 5b). Air-spraying, however, is much more cost-effective as it does not require the use of a high-voltage power source.18 Regardless, both methods are versatile fabrication techniques for creating biopolymer-based nanofibers with tunable characteristics for filtration applications. Typically, air-spraying results in fibers with a larger diameter and less control over their orientation, but postprocessing and current research are working to improve this. The differences in fiber morphology can be seen in the SEM images of silk nanofibers in Figure 5c,d. Within the same figure, the unique properties of biopolymers that make them attractive for filtering are highlighted with a soy protein filter (Figure 5e).
5. Novel Applications
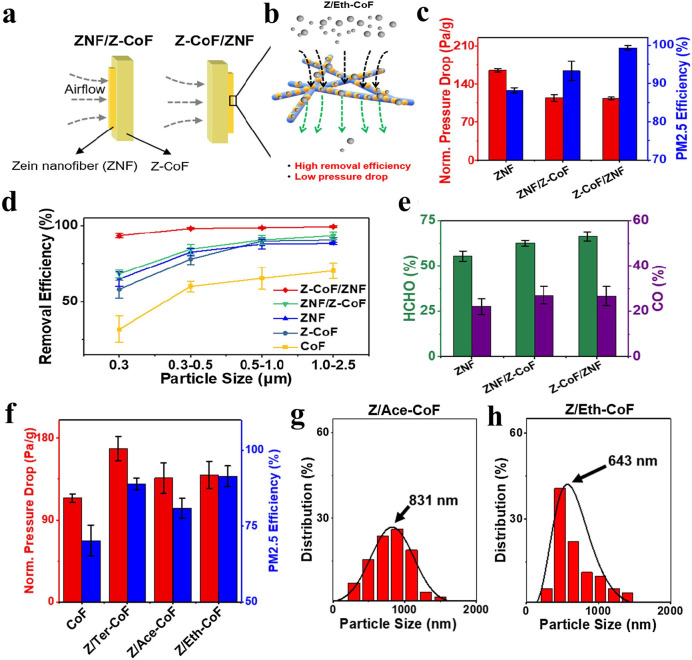
Traditionally, biopolymer fibers such as cellulose and silk are both good filters with the ability to be modified for specialized tasks to make them even more versatile. Novel biopolymer filters with added functional groups are able to react with chemical pollutants as they come in contact or close proximity, cleansing air and water of dangerous chemicals. Often, research will combine fiber materials from different biopolymers in order to combine the benefits of both individual polymer or overcome each other’s weaknesses. In one recent example, Liu et al. combined cotton and zein to create an air filter with the porosity, breathability, and efficiency of common cotton filters with the additional filtration ability of functionalized zein to catch fine PM pollutants.2 By combining a dense layer of thick zein nanofibers (ZNF) with a breathable layer of zein nanofiber cotton fibers (Z-CoF), a low pressure drop of 112.5 Pa/g was maintained. This was much lower than the individual pressure drops of ZNF (165.0 Pa/g) or Z-CoF (139.4 Pa/g) alone. The Z-CoF were cotton fibers soaked in zein solutions in order to coat them in zein nanoparticles. Through this process, the fibers gain the useful surface chemistry of zein to improve their filtration abilities. These bilayer filters showed excellent removal efficiency over a wide range of PM sizes from 0.3 to 2.5 μm. Specifically, the filtration efficiencies were 93.3, 98.2, 98.7, and 99.0% for PM0.3, PM0.5, PM1.0, and PM2.5, respectively. An interesting note, seen in Figure 6a–e, is that the order of the layers appears to make a difference in the removal efficiency of the filter (99.0% vs 93.2% for PM2.5). The filters were also effective against common pollutants, with a 66.2% removal efficiency toward HCHO and a 26.6% removal efficiency toward CO. Ethanol was found to be the best solvent for filtration efficiency due to its effects on zein’s surface properties and the homogeneous distribution of zein nanoparticles along the cotton fiber surface. The team also studied how 1-butanol (ternary solvent) and acetone affected the surface properties. Changing the solvent affected the pressure drop and PM2.5 filtration efficiency (Figure 6f) as well as the size of the zein nanoparticles on the surface of the cotton fibers (Figure 6g,h).
Figure 6.
(a) Zein nanofiber cotton fibers (Z-CoF) with a thin layer of zein nanofibers (ZNF) before (ZNF/Z-CoF) or after (Z-CoF/ZNF) the layer of Z-CoF. (b) Z-CoF formed from soaking in ethanol showed the highest removal efficiency and lowest pressure drop of all three solvents tested. (c) Normalized pressure drop and PM2.5 removal. (d) Removal efficiency of regular cotton fibers and functionalized fibers for a range of PM sizes. (e) Efficiency of functionalized fibers against HCO and CO. (f) Normalized pressure drop and PM2.5 removal efficiency for CoF and Z-CoF prepared from 1-butanol (Ter-), acetone (Ace-), or ethanol (Eth-). Particle size distribution of zein nanoparticles on the CoF surface is also shown for (g) acetone and (h) ethanol. Reproduced with permission from ref (2). Copyright 2019 Elsevier.
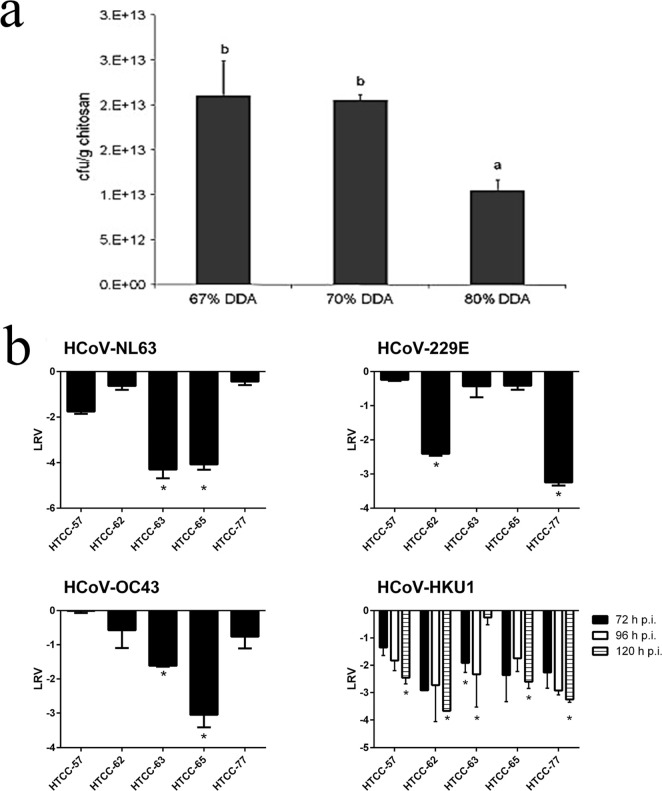
Settings such as hospitals and nursing homes can host many types of biological pollutants. Therefore, filters with antibacterial and antiviral properties are of particular interest.1 Biopolymer-based filters have been widely used to exploit their antibacterial and antiviral properties. Cellulose has been used for hundreds of years to make cotton surgical masks that are used in hospitals to protect patients from bacterial contamination.1 The antibacterial and antiviral properties of biopolymers are believed to be due to various surface interactions including charge–charge, hydrophobic interactions, hydrogen bonding, and chemical bonding.1 In proteins, the series of amino acids dictate the functional groups and surface properties that are able to interact with and capture pollutants, while polysaccharides will have several functional groups along their monomers for these interactions. For example, chitosan fibers showed antimicrobial properties due to charged ammonium ions on their surface. These ions bind to negatively charged components of the bacterial cell wall, thereby inhibiting cell growth and leading to microbial death.20 The extent of antimicrobial activity can be tailored by varying the chitosan content, the molecular weight of chitosan, and the deacetylation procedure. Chitosan filters made from nonwoven nanofibers have been tested for antimicrobial properties, which show that higher degrees of deacetylation also contribute to higher antimicrobial capacity.20 Tests are performed under both static, and dynamic conditions measured the survival of Escherichia coli (E. coli) using the pour plate method. The results, seen in Figure 7a, show a direct correlation with the chitosan content to stronger antimicrobial capacity, with higher degrees of deacetylation also contributing to higher antimicrobial capacity.
Figure 7.
(a) Antibacterial property of 1.33 wt % HMW, 90% chitosan, 10% poly(ethylene oxide) (PEO) blended fibers is shown, as well as how the degree of deacetylation (DDA) plays an effect. Increasing the DDA% increases the amount of available protonated amine sites for antibacterial activity, but 80% DDA fibers here had a larger diameter, which leads to a smaller number of available sites. (b) Log reduction values of several common human coronaviruses by substituted chitosan derivatives (57–77% substituted). Error bars represent the standard error, and asterisks signify statistically significant differences (P < 0.05); hpi = hours post-infection. (a) Reproduced from ref (7). Copyright 2009 Elsevier. (b) Reproduced from ref (21) with open access CC-BY-4.0 license, 2016 PLoS.
Catatonically modified chitosan with different degrees of substitution (57–77%) have also been shown to inhibit infection by human coronaviruses by blocking the virus’ interaction with cellular receptors in vivo.21Figure 7b shows the log reduction value of several common coronaviruses (HCoV-NL63, HCoV-OC43, HCoV-229E, and HCoV-HKU1) for trimethylammonium chitosan chloride (HTCC) ranging from 55 to 77% degrees of substitution (DS). Currently, there are no marketed drugs or vaccines for the treatment of coronaviruses, including the deadly SARS-CoV, MERS-CoV, and SARS-CoV-2 coronaviruses. The inhibitory ability of these substituted chitosan derivatives to prevent coronavirus infection by inhibiting the virus from binding to the ACE2 receptor is therefore a promising field for potential coronavirus treatment.
Like deacetylation, other surface modifications can improve antiviral and antibacterial capabilities including doping with various molecules or chemical modifications. Table 1 summarizes filter uses, pressure drops, and overall filtering effectiveness of various biopolymers toward different pollutants. Where multiple references are given, maximum filtration efficiencies and minimum pressure drops are given; PMn represents particulate matter (PM) aerosol particles of n diameter in micrometers; VOC represents volatile organic compound; CFU represents colony-forming unit.
Table 1. Summary of Biopolymer-Based Filter Materials.
| filtering
efficiency |
|||||
|---|---|---|---|---|---|
| biopolymer | pollutants | virus | bacteria | pressure drop | ref |
| silk | VOC 99.4% | nucleopolyhedrovirus | E. coli | 98 Pa | (5,12,22) |
| PM2.5: 98.8% | M. luteus | ||||
| PM0.3: 96.2% | |||||
| Cu2+: 1.65 μg/mg | |||||
| keratin | HCHO: 70% | N/A | E. coli: 99.9% | N/A | (5,23) |
| Cu2+: 2.88 μg/mg | S. aureus: 99.9% | ||||
| Cr3+ | |||||
| soy | HCHO, CO: 90% | N/A | E. coli: 80% | 136 Pa | (3,4) |
| PM2.5: 99.8% | B. subtilis: 80% | ||||
| PM10–2.5: 99.99% | |||||
| zein | PM0.1–10: >99.5% | N/A | N/A | 175–180 Pa | (24) |
| HCHO, CO: >70% | |||||
| cellulose | PM2.5: 99.0% | influenza A | S. aureus | 112.5 Pa/g | (1,2) |
| PM0.3: 93.3% | caliciviruses | E. coli: 3 log reduction of CFU | |||
| hepatitis A | C. freundii | ||||
| hepatitis C | K. pneumoniae | ||||
| herpes simplex | |||||
| enterovirus | |||||
| astrovirus | |||||
| norovirus | |||||
| West Nile | |||||
| chitin, chitosan | NaCl aerosols: 92% | HIV-1 | E. coli: 99.4% | 147.6 Pa | (7,20,25) |
| PM2.5: 100% removal from 999 μg m–3 in 33 min | S. aureus: 99.5% | ||||
| Cr(VI) | P. aeruginosa | ||||
| B. subtilis | |||||
| S. choleraesuis | |||||
| P. mirabilis | |||||
| S. enteritidis | |||||
| E. aerogenes | |||||
| Corynebacterium | |||||
| S. epidermidis | |||||
| E. faecalis | |||||
| P. gingivalis | |||||
| A. actinomycetemcomitans | |||||
| S. mutans | |||||
| starch | N/A | adenovirus 41 | E. coli: 100% | 1619 Pa | (1) |
| MS2 enterobacteria phage | S. aureus: 100% | ||||
6. Conclusion
Biopolymer-based materials have been used in a wide range of filtration applications, including air filters for homes, cars, and hospitals, filters for removing heavy metals, and antibacterial or antiviral filters for medical applications. Additionally, they are reusable and biodegradable. Biopolymers are cheap and widely available, but their tunable structures and ease of processing make them attractive as filtering materials. The functional groups provided by biopolymers provide useful surface chemistry, which can be used as-is or further optimized for specific, desired applications to improve filtration. Recent applications have already shown the potential of biopolymers in filtering but also highlight areas for improvement in future applications.
Biography

Dr. Xiao Hu is an Associate Professor in the Department of Physics and Astronomy at Rowan University, USA. He also holds faculty appointments in the Department of Molecular and Cellular Biosciences, Department of Biomedical Engineering, Graduate Program in Pharmaceutical Sciences, and Graduate Program in Materials Science and Engineering at Rowan. He received his Ph.D. degree in Physics and M.S. degree in Biomedical Engineering at Tufts University in 2009. Prior to joining Rowan, he was a Research Associate in the NIH P41 Tissue Engineering Resource Center at Tufts University. His research focuses on the interdisciplinary area between biophysics, polymer science, and biomedical engineering.
Author Contributions
C.G., K.C., E.S., K.L., G.J., and X.H. wrote the manuscript. C.G., Y.S., and X.H. edited the manuscript.
This study was supported by the Rowan University Seed Grants. C.G. and X.H. are supported by the NSF Biomaterials Program (DMR-1809541). X.H. and S.Y. are supported by NSF Future Eco Manufacturing Research program (CMMI-2037097).
The authors declare no competing financial interest.
References
- Junter G.-A.; Lebrun L. Cellulose-based virus-retentive filters: a review. Rev. Environ. Sci. Bio/Technol. 2017, 16 (3), 455–489. 10.1007/s11157-017-9434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Dunne F. O.; Fan X.; Fu X.; Zhong W.-H. A protein-functionalized microfiber/protein nanofiber Bi-layered air filter with synergistically enhanced filtration performance by a viable method. Sep. Purif. Technol. 2019, 229, 115837. 10.1016/j.seppur.2019.115837. [DOI] [Google Scholar]
- Souzandeh H.; Johnson K. S.; Wang Y.; Bhamidipaty K.; Zhong W.-H. Soy-Protein-Based Nanofabrics for Highly Efficient and Multifunctional Air Filtration. ACS Appl. Mater. Interfaces 2016, 8 (31), 20023–20031. 10.1021/acsami.6b05339. [DOI] [PubMed] [Google Scholar]
- Lubasova D.; Netravali A.; Parker J.; Ingel B. Bacterial filtration efficiency of green soy protein based nanofiber air filter. J. Nanosci. Nanotechnol. 2014, 14 (8), 4891–4898. 10.1166/jnn.2014.8729. [DOI] [PubMed] [Google Scholar]
- Ki C. S.; Gang E. H.; Um I. C.; Park Y. H. Nanofibrous membrane of wool keratose/silk fibroin blend for heavy metal ion adsorption. J. Membr. Sci. 2007, 302 (1), 20–26. 10.1016/j.memsci.2007.06.003. [DOI] [Google Scholar]
- Delmer D. P. CELLULOSE BIOSYNTHESIS: Exciting Times for A Difficult Field of Study. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50 (1), 245–276. 10.1146/annurev.arplant.50.1.245. [DOI] [PubMed] [Google Scholar]
- Desai K.; Kit K.; Li J.; Michael Davidson P.; Zivanovic S.; Meyer H. Nanofibrous chitosan non-wovens for filtration applications. Polymer 2009, 50 (15), 3661–3669. 10.1016/j.polymer.2009.05.058. [DOI] [Google Scholar]
- Centers for Disease Control Department of Health and Human Services . Filtration And Air-Cleaning Systems To Protect Building Environments; National Institute for Occupational Safety and Health, 2003.
- Park S.; Jayaraman S. From containment to harm reduction from SARS-CoV-2: a fabric mask for enhanced effectiveness, comfort, and compliance. J. Text. Inst. 2020, 1–15. 10.1080/00405000.2020.1805971. [DOI] [Google Scholar]
- Bao L.; Seki K.; Niinuma H.; Otani Y.; Balgis R.; Ogi T.; Gradon L.; Okuyama K. Verification of slip flow in nanofiber filter media through pressure drop measurement at low-pressure conditions. Sep. Purif. Technol. 2016, 159, 100–107. 10.1016/j.seppur.2015.12.045. [DOI] [Google Scholar]
- Graham K.; Ouyang M.; Raether T.; Grafe T.; McDonald B.; Knauf P.. Polymeric Nanofibers in Air Filtration Applications. Presented at the Fifteenth Annual Technical Conference & Expo of the American Filtration & Separations Society, Galveston, Texas, April 9–12, 2002.
- Zhou W.; Huang H.; Du S.; Huo Y.; He J.; Cui S. Removal of copper ions from aqueous solution by adsorption onto novel polyelectrolyte film-coated nanofibrous silk fibroin non-wovens. Appl. Surf. Sci. 2015, 345, 169–174. 10.1016/j.apsusc.2015.03.036. [DOI] [Google Scholar]
- Miyoshi T.; Toyohara K.; Minematsu H. Preparation of ultrafine fibrous zein membranes via electrospinning. Polym. Int. 2005, 54 (8), 1187–1190. 10.1002/pi.1829. [DOI] [Google Scholar]
- Li X.; Hou T.; Lu Y.; Yang B. A method for controlling the surface morphology of centrifugally spun starch-based fibers. J. Appl. Polym. Sci. 2018, 135 (6), 45810. 10.1002/app.45810. [DOI] [Google Scholar]
- Schiffman J. D.; Schauer C. L. One-Step Electrospinning of Cross-Linked Chitosan Fibers. Biomacromolecules 2007, 8 (9), 2665–2667. 10.1021/bm7006983. [DOI] [PubMed] [Google Scholar]
- Liu H.; Hsieh Y.-L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci., Part B: Polym. Phys. 2002, 40 (18), 2119–2129. 10.1002/polb.10261. [DOI] [Google Scholar]
- Liu H.; Gough C. R.; Deng Q.; Gu Z.; Wang F.; Hu X. Recent Advances in Electrospun Sustainable Composites for Biomedical, Environmental, Energy, and Packaging Applications. Int. J. Mol. Sci. 2020, 21 (11), 4019. 10.3390/ijms21114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaz A. n.; Roberts A. D.; Faraji S.; Nascimento T. R. L.; Medeiros E. S.; Zhang W.; Greenhalgh R. D.; Mautner A.; Li X.; Blaker J. J. Porous, Aligned, and Biomimetic Fibers of Regenerated Silk Fibroin Produced by Solution Blow Spinning. Biomacromolecules 2018, 19 (12), 4542–4553. 10.1021/acs.biomac.8b01233. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y.; Morikawa H.; Yamanaka S.; Tamada Y. Electrospinning of silk fibroin from all aqueous solution at low concentration. Mater. Sci. Eng., C 2017, 73, 498–506. 10.1016/j.msec.2016.12.113. [DOI] [PubMed] [Google Scholar]
- Kong M.; Chen X. G.; Xing K.; Park H. J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144 (1), 51–63. 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Milewska A.; Kaminski K.; Ciejka J.; Kosowicz K.; Zeglen S.; Wojarski J.; Nowakowska M.; Szczubiałka K.; Pyrc K. HTCC: Broad Range Inhibitor of Coronavirus Entry. PLoS One 2016, 11 (6), e0156552 10.1371/journal.pone.0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Wu S.; Jian M.; Xie J.; Xu L.; Yang X.; Zheng Q.; Zhang Y. Silk nanofibers as high efficient and lightweight air filter. Nano Res. 2016, 9 (9), 2590–2597. 10.1007/s12274-016-1145-3. [DOI] [Google Scholar]
- Hu Y.; Wang W.; Yu D. Preparation of antibacterial keratin fabrics via UV curing and click chemistry. RSC Adv. 2016, 6 (85), 81731–81735. 10.1039/C6RA15657F. [DOI] [Google Scholar]
- Tian H.; Fu X.; Zheng M.; Wang Y.; Li Y.; Xiang A.; Zhong W.-H. Natural polypeptides treat pollution complex: Moisture-resistant multi-functional protein nanofabrics for sustainable air filtration. Nano Res. 2018, 11 (8), 4265–4277. 10.1007/s12274-018-2013-0. [DOI] [Google Scholar]
- Karagozlu M. Z.; Karadeniz F.; Kim S.-K. Anti-HIV activities of novel synthetic peptide conjugated chitosan oligomers. Int. J. Biol. Macromol. 2014, 66, 260–266. 10.1016/j.ijbiomac.2014.02.020. [DOI] [PubMed] [Google Scholar]