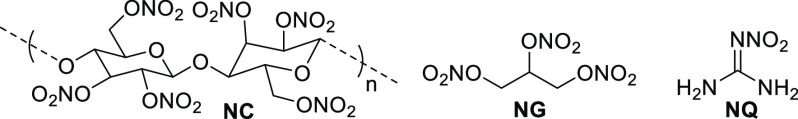Abstract

A review of energetic materials based on the nitric ester functionality is presented. Examined are materials that are classified as primary explosives, pressable secondary explosives, melt-castable secondary explosives, and rocket- and gun-propellant materials. Disclosed are the molecular structures, physical properties, performances, and sensitivities of the most important legacy nitric esters, as well as the relevant new materials developed within the past several years. Where necessary, discussions of the synthetic protocols to synthesize these materials are also presented.
1. Introduction
The field of energetic materials encompasses the areas of explosives, propellants, and pyrotechnics. In designing suitable materials, the incorporation of nitro groups represents the classic way to increase density and energetic power of a given energetic material. Nitro groups can be added to nitrogen atoms to form nitramines, aromatic carbons to form nitroaromatics, aliphatic carbons to form nitroalkanes and polynitroalkanes, and alcohols to form nitric esters.1a−1d The nitric ester moiety is not only of high medicinal importance,2 but also one of the most useful explosophores in designing rational energetic materials.1a Conversion of hydroxyl functionalities to the nitric ester can be achieved by several means and is an appealing way to significantly increase the density, oxygen balance, and energetic power of a molecule. Nitric esters are commonly found in materials that can be classified as primary explosive, secondary explosive, and propellant ingredients. While there are hundreds of nitric ester-based energetic materials that have been synthesized to date, this review will focus on materials that are of interest from an application standpoint.
Nitric Ester Explosives
2.1. Primary Explosives
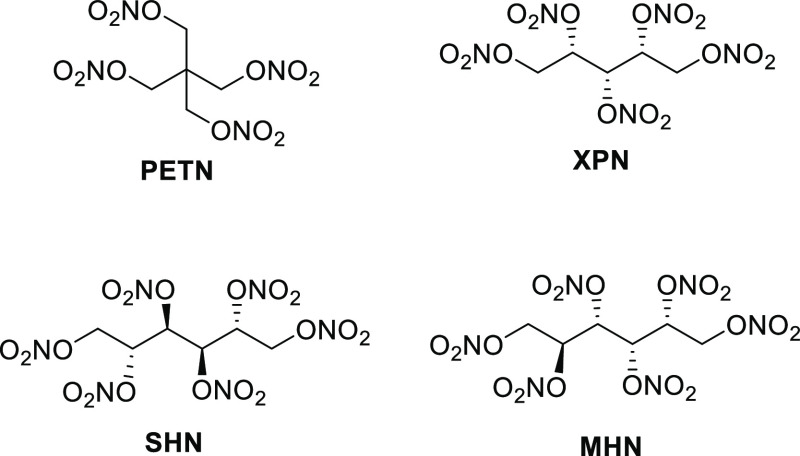
A primary explosive material is characterized as a material that has a high sensitivity to impact, friction, and electrostatic discharge. It is typically present in small amounts and is used as part of an ignition train to initiate a more powerful secondary explosive in a weapons system. Legacy primary explosives include the highly effective but also toxic mercury fulminate, lead azide, and lead styphnate, to name a few.3 However, there are nitric ester materials that also fall into the primary explosives category and are thus “greener” due to their heavy-metal-free nature. Officially classified as a “borderline” primary explosive due to its respective impact and friction sensitivity of 3 J and 60 N, pentaerythritol tetranitrate4 (PETN) is a common ingredient in many pyrotechnic percussion primer mixtures, blasting caps, and detonation cord due to its high performance, coupled with its sensitivity values. Highly nitrated sugars have also been prepared and, because of their high sensitivities to impact, friction, and shock, are firmly classified as primary explosive materials (Figure 1). This includes xylitol pentanitrate (XPN), sorbitol hexanitrate (SHN), and mannitol hexanitrate (MHN).5a−5d The performance paramenters of these materials and PETN are given in Table 1.
Figure 1.
Molecular structure of PETN and some primary explosive sugar nitrates.
Table 1. Performance, Physical Properties, nd Sensitivity Values of PETN, XPN, SHN, and MHN.
| data category | PETN | XPN | SHN | MHN |
|---|---|---|---|---|
| Tma [°C] | 141.3 | 45.5 | 53.0 | 112.0 |
| Tdecb [°C] | 172.9 | 169 | 163.3 | 181.4 |
| ΩCO2c [%] | –10.1 | +6.4 | +7.1 | +7.1 |
| ΩCOd [%] | +15.2 | +27.6 | +28.3 | +28.3 |
| ρe [g cm–3] | 1.76 | 1.852 | 1.868 | 1.73 |
| Pcjf [GPa] | 31.5 | 32.6 | 32.0 | 30.4 |
| Vdetg [ms–1] | 8400 | 8780 | 8700 | 8260 |
| ΔfH°ih[kJ mol–1] | –538.6 | –500.5 | –547 | –547.2 |
| ISi [J] | 3 | 1.9 | 0.62 | 2.2 |
| FSj [N] | 60 | 18 | 37 | 37 |
| ESDk [J] | 0.0625 | 4.5 | - | - |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
ESD = electrostatic discharge sensitivity.
XPN has sometimes been characterized as a viscous liquid material, but this is due to impurities that arise during nitration. Pure XPN is a white, crystalline solid. XPN possesses an explosive power that is similar to RDX. However, unlike the secondary explosive nature of RDX, XPN is classified as a primary explosive material, because it possesses a significantly higher impact and friction sensitivity as compared to PETN. A reason for this increased sensitivity can be attributed to the presence of electron withdrawing nitric ester moieities on five consecutive atoms. Since each of these carbon atoms has a partial positive charge, and like charges have a tendency to repel, a molecule such as XPN becomes highly unstable toward ignition and mechanical stimuli.
Like XPN, SHN is also a highly sensitive material. According to Hahma,6 nitration of sorbitol is not a trivial process. Even the most optimal nitration conditions—in which the HNO3 employed is free of nitrites—mixtures of SHN and its pentanitrate derivatives are typically obtained. This mixture is reported to be quite unstable, and gives off red fumes upon storage. Therefore, practical applications of SHN are very limited. SHN has been determined to be comparable to nitroglycerin in its high sensitivity to impact, shock, and friction.
Interestingly, MHN, which is a diastereomer of SHN, is reported to be obtained easily and in high purity after nitration of mannitol and recrystallization from acetone. Due to its highly pure nature, MHN has a higher stability as compared to SHN. However, because of the presence of contiguous nitric ester functionalities and partial positively charged carbons, a stabilizer such as akardite or ethyl centralite is typically added in order to prolong stability.7
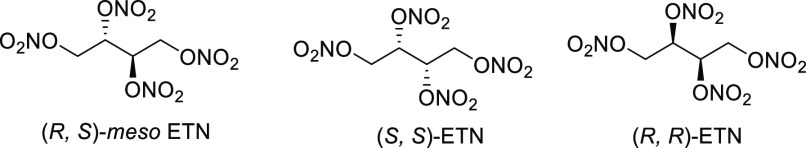
A rather distinct class of nitrated sugars that is worthy of discussion is the erythritol tetranitrate (ETN) series of nitric esters. Although the (R,S)-meso diastereomer of ETN was first synthesized in 1849, its potential use as an explosive was limited due to the lack of availability of its erythritol precursor for many years. Today, however, meso-erythritol is widely available from corn using enzymatic chemistry.8
Manner and co-workers recently prepared the three diastereomers of ETN (Figure 2).9a,9b As shown in Table 2, (R,S)-meso ETN is a solid and is well-known to possess a primary explosive-like sensitivity to friction, and borderline primary explosive impact sensitivity. However, the (S,S)- and (R,R)-diastereomers were found to be liquids, with evidence of a glass transition temperature at −50 °C. Interestingly, the latter two diastereomers were found to exhibit very insensitive friction sensitivities, yet very high impact sensitivity values. While it is expected that the ETN materials will exhibit similar calculated performance values, the physical properties of energetic materials are demonstrated to change based on the orientations of the explosophores in space.
Figure 2.
Synthesized isomers of erythritol tetranitrate.
Table 2. Performance, Physical Properties, and Sensitivity Values of ETN Isomers.
| data category | (R,S)-meso-ETN | (S,S)-ETN | (R,R)-ETN |
|---|---|---|---|
| Tma [°C] | 62.5 | - | - |
| Tdecb [°C] | 163.4 | 154.3 | 154.8 |
| ΩCO2c [%] | +5.3 | +6.4 | +7.1 |
| ΩCOd [%] | +26.5 | +27.6 | +28.3 |
| ρe [g cm–3] | 1.68 | - | - |
| Pcjf [GPa] | 27.2 | - | - |
| Vdetg [ms–1] | 8015 | - | - |
| ΔfH°h [kJ mol–1] | –474.8 | - | - |
| ISi [J] | 3.6 | 0.74 | 0.74 |
| FSj [N] | 38 | >360 | >360 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
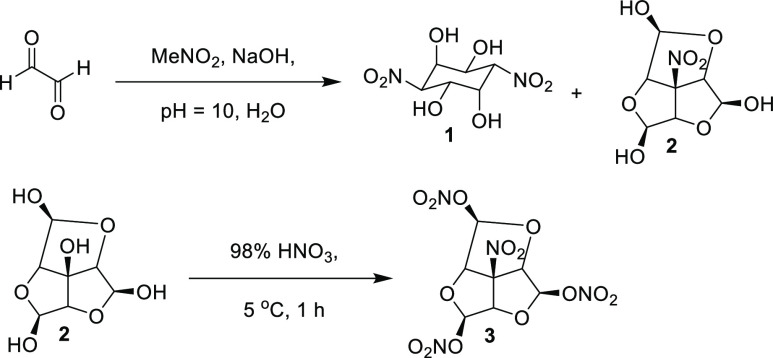
As depicted in Scheme 1, Polish researchers condensed glyoxal and nitromethane under basic conditions.10 Contrary to the literature, cyclohexane 1 was not the major product. Instead, the fused tricycle 2 was the major product, and was isolated in high purity by recrystallization. Nitration of 2 yielded trinitrate 3.
Scheme 1. Synthesis of Trinitric Ester 3.
The physical properties, sensitivities, and performance of 3 is given in Table 3. The trinitrate is characterized as having a high sensitivity to impact and friction, but also a high density and detonation velocity. 3 was able to be pressed into pellets for detonation velocity measurements once their sensitivity was reduced through the addition of 5 wt.% Viton to the material.
Table 3. Performance, Physical Properties, and Sensitivities of Trinitrate 3.
| data category | 3 |
|---|---|
| Tma [°C] | 115 |
| Tdecb [°C] | 155 |
| ΩCO2c [%] | –13 |
| ΩCOd [%] | +17.3 |
| ρe [g cm–3] | 1.73 |
| Vdetf [ms–1] | 8000 |
| ΔfH°g [kJ mol–1] | –649.2 |
| ISh [J] | 2 |
| FSi [N] | 50 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
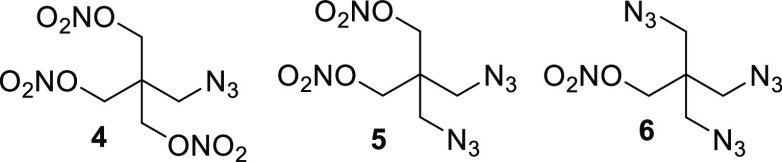
A group of highly sensitive azido materials derived from PETN, provided in Figure 3, were recently synthesized and characterized by Klapötke.11 As the nitrate moiety was replaced with the azide explosophore, the density, detonation pressure, and detonation velocity declined, while the heat of formation, as expected, rose significantly (Table 4). Additionally, the melting point was observed to decrease significantly as the number of azide groups increased, culminating with triazide 6 exhibiting liquidous behavior. As expected, impact sensitivities were in the primary explosive range for all materials, with sensitivities to impact increasing with higher azide content. Although 4–6 exhibited high friction sensitivities, triazide 6 possessed a lower friction sensitivity than the monoazide and diazide. While this may seem counterintuitive, it is to be expected, given that 4 and 5 are solids, and 6 is a liquid.
Figure 3.
Molecular structure of azido nitrate derivatives 4–6.
Table 4. Performance, Physical Properties, and Sensitivities of Azidonitrates 4–6.
| data category | 4 | 5 | 6 |
|---|---|---|---|
| Tma [°C] | 82 | 32 | –24 |
| Tdecb [°C] | 180 | 172 | 175 |
| ΩCO2c [%] | –27 | –46.4 | –68.7 |
| ΩCOd [%] | 0 | –17.4 | –37.5 |
| ρe [g cm–3] | 1.67 | 1.59 | 1.53 |
| Pcjf [GPa] | 27.8 | 24.2 | 21.2 |
| Vdetg [ms–1] | 8092 | 7842 | 7740 |
| ΔfH°ih [kJ mol–1] | –59 | +362 | +795 |
| ISi [J] | 2 | 3 | 1 |
| FSj [N] | 36 | 15 | 80 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
2.2. Secondary Explosives
A secondary explosive, unlike a primary explosive, has a lower sensitivity to impact, friction, and electrostatic discharge, but yields higher detonation velocities and detonation pressures.6 Due to their relatively insensitive nature, secondary explosive materials are usually present in large quantities in munitions such as mortar and artillery rounds and are thus referred to as “main fill” materials. Some of the most common secondary explosives include the pressable materials RDX and HMX.1 CL-20 also fits within the pressable secondary explosive category, but finds little practical application due to its expensive cost. Interestingly, despite the large number of nitric ester molecules that have been synthesized to date, it is difficult to find such a standalone material that fits into the secondary explosive profile. However, several secondary explosive formulations containing PETN have been developed, in which these formulations feature a high performance but a reduced sensitivity.
One of the most common PETN-based formulation classes is Semtex. The Semtex series of explosives is currently produced in the Czech Republic and has many variants, including 1A, 1H, 10, 10-SE, and S 30.12a They are all classified as plastic explosives with the capability to be shaped into charges. Semtex mixtures contain large amounts of PETN and/or RDX as the main fill, inert binders/plasticizers to reduce sensitivity, and a small amount of stabilizer to prolong shelf life. Semtex 1A, 1H, and 10 are used mainly for demolition and underwater blasting, and can also be used as an initiator. SEMTEX 10-SE is used for the explosive hardening of metal materials, while the S 30 variant is used for explosive cladding and welding of metals. Some physical and performance properties of the aforementioned Semtex formulations are given in Table 5.
Table 5. Physical and Performance Properties of Semtex Formulations.
| data category | 1A | 1H | 10 | 10-SE | S 30 |
|---|---|---|---|---|---|
| ΩCO2c [%] | –63.4 | –64 | –44 | –59 | –3 |
| ρb [g cm–3] | 1.4 | 1.46 | 1.51 | 1.47 | 1.05 |
| Vdetc [ms–1] | 7300 | 7500 | 7600 | 7100 | 2200 |
| Pcjd [GPa] | 17.5 | - | 20.9 | - | - |
| % PETN/RDX | 83 | 85 | 86 | 78 | 30 |
| Shelf Life [years] | 2 | 5 | 10 | 2 | 1 |
| ISe [J] | 21.1 | - | 15.7 | - | - |
| FSf [N] | 187 | - | 204 | - | - |
ΩCO2 = CO2 oxygen balance.
ρ = density.
Vdet = detonation velocity.
Pcj = detonation pressure.
IS = impact sensitivity.
FS = friction sensitivity.
Other PETN-based secondary explosive formulations used for blasting and demolition include the French plastic explosive Formex P1, the Swedish plastic explosive Sprängdeg m/46, and the Egyptian plastic explosive EPX-1.12b As is summarized in Table 6, these three formulations have similar detonation velocities and pressures as compared to many of the Semtex formulations listed in Table 5.
Table 6. Physical and Performance Properties of Formex P1, Sprängdeg m/46, and EPX-1 Plastic Explosives.
| data category | Formex P1 | Sprängdeg m/46 | EPX-1 |
|---|---|---|---|
| ρa [g cm–3] | 1.53 | 1.52 | 1.55 |
| Vdetb [ms–1] | 7544 | 7520 | 7636 |
| Pcjc [GPa] | 20.0 | 19.3 | 21.1 |
| % PETN | 89 | 86 | 86 |
| ISd [J] | 13.5 | 14.2 | 13.9 |
| FSe [N] | 194 | 183 | 176 |
ρ = density.
Vdet = detonation velocity.
Pcj = detonation pressure.
IS = impact sensitivity.
FS = friction sensitivity.
In addition to pressable materials, the area of melt-castable explosives also fits into the realm of secondary explosives. Like pressable secondary explosives, melt-castable materials should have high density, high detonation velocities, high detonation pressures, and should be reasonably insensitive. For a material to be melt-castable, however, the material should also possess a melting point of 70–125 °C, with a decomposition temperature occurring significantly after the melting point.13 TNT, the state-of-the-art melt-castable material, melts at 80 °C and decomposes at 295 °C. Historically, nitric ester materials were not believed to exhibit melt-castable properties and were determined to be too sensitive and too unstable to be classified as such. This assumption has been overturned in recent years, however.
In 2008, Chavez synthesized 2,3-hydroxymethyl-2,3-dinitro-1,4-butanediol tetranitrate (SMX, Figure 4). SMX is unique in that it is completely oxygen balanced. SMX possesses a melting point in the melt-castable range, was found to have twice the detonation pressure of TNT, and has a detonation pressure and detonation velocity equivalent to the pressable secondary explosive HMX (Table 7).14 Unfortunately, SMX was found to exhibit a fairly high sensitivity to impact and friction, and was also found to decompose at a fairly low temperature. Especially because of the latter, the prospect of SMX being a melt-castable material is likely very limited.
Figure 4.
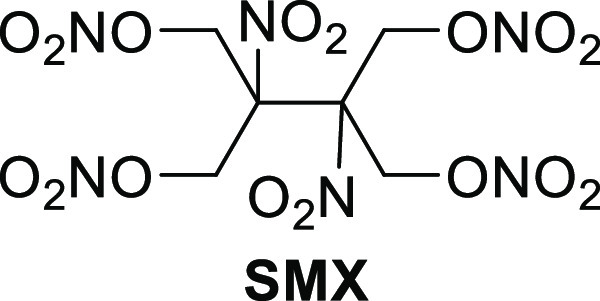
Structure of oxygen balanced SMX.
Table 7. Performance, Physical Properties, and Sensitivities of Tetranitrate SMX.
| data category | SMX |
|---|---|
| Tma [°C] | 85–86 |
| Tdecb [°C] | 141 |
| ΩCO2 [c%] | 0 |
| ΩCOd [%] | +38.1 |
| ρe [g cm–3] | 1.912 |
| Vdetf [ms–1] | 9100 |
| Pcjg [GPa] | 40.0 |
| ΔfH°h [kJ mol–1] | –371 |
| ISi [J] | 3 |
| FSj [N] | 60 |
| ESDk [N] | 0.625 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Vdet = detonation velocity.
detonation pressure.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
electrostatic discharge.
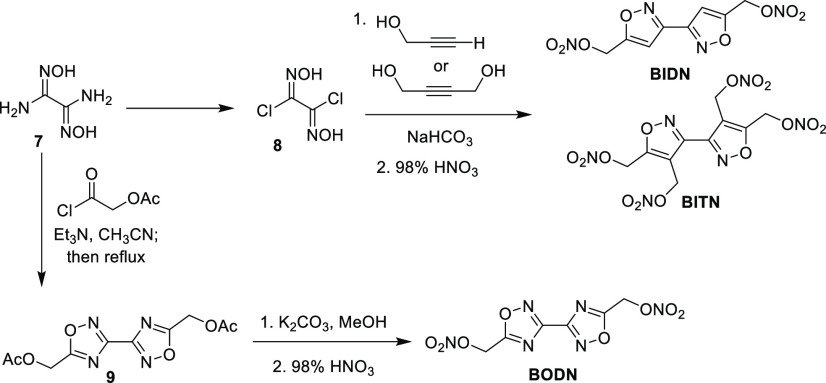
More recent work has demonstrated that nitric ester materials can exhibit low sensitivities, high performance, and optimal melting points and decomposition temperatures to be classified as melt-castable. Three such materials, summarized in Scheme 2, were derived from the common intermediate diaminoglyoxime (7).15a−15c 3,3′-Biisoxazole-5,5′-bis(methylene) dinitrate (BIDN) was found to have performance properties (i.e., detonation pressure and detonation velocity) similar to TNT, while also fitting into the standalone melt-castable range (Table 8). A standalone melt-castable material is one that is able to be melted with steam at ambient pressure. 3,3′-Biisoxazole-4,4′,5,5′-tetra(methylene) tetranitrate (BITN) was higher performing than BIDN due to its higher density, and more favorable oxygen balance. However, BITN also exhibited a PETN-like sensitivity to impact, friction, and electrostatic discharge because of these reasons, coupled with the presence of more nitric ester functionalities. The higher melting point of BITN also eliminates it as a standalone melt-castable material. However, BITN can serve as a potential melt-castable eutectic compound. Melt-castable eutectics are materials that exhibit melting points slightly above 100 °C, but whose melting point can be lowered into the standalone melt-castable range when formulated with other materials.
Scheme 2. Synthesis of Melt-Castable Nitric Esters BIDN, BITN, and BODN.
Table 8. Physical and Performance Properties of Melt-Castable Nitric Esters BIDN, BITN, BODN, and TNT.
| data category | TNT | BIDN | BITN | BODN |
|---|---|---|---|---|
| Tma [°C] | 80.4 | 92 | 121.9 | 84.5 |
| Tdecb [°C] | 295 | 189.2 | 193.7 | 183.4 |
| ΩCO2c [%] | –74 | –62 | –37 | –33.3 |
| ΩCOd [%] | –24.7 | –16.8 | 0 | 0 |
| ρe [g cm–3] | 1.65 | 1.605 | 1.76 | 1.832 |
| Pcjf [GPa] | 20.5 | 19.3 | 27.1 | 29.4 |
| Vdetg [m s–1] | 6950 | 7060 | 7837 | 8180 |
| ΔfH°h,i [kJ mol–1] | –59.3 | –139 | –395 | –79.4 |
| ISi [J] | 15 | 11.2 | 30 | 8.7 |
| FSj [N] | 240 | >360 | 60 | 282 |
| ESDk [J] | 0.25 | 0.25 | 0.0625 | 0.125 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
ESD = electrostatic discharge sensitivity.
Bis(1,2,4-oxadiazole)-bis(methylene) dinitrate (BODN) exhibits very promising properties as a standalone melt-castable material, with a detonation pressure ca. 50% higher than TNT, and a low sensitivity. The high performance is owed to the presence of a higher nitrogen content as compared to BIDN, its higher density, and favorable oxygen balance. The low sensitivity of BODN likely stems from its crystal structure, in which the nitric ester moieties engaged in intramolecular hydrogen bonding with the methylene hydrogen atoms.
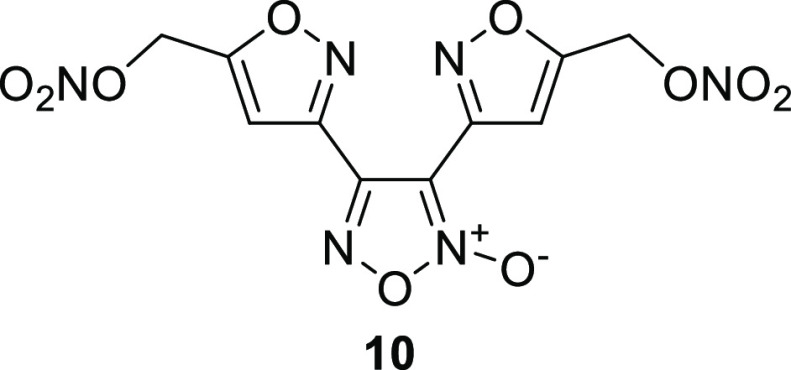
Standalone melt-castable material 10 was recently synthesized, in which the well-known furoxan ring16 was inserted between the 5-nitroxymethyl isoxazole rings (Figure 5). When comparing 10 to BIDN, introduction of the furoxan ring was observed to significantly increase the density, heat of formation, detonation velocity, and detonation pressure (Table 9).17 Interestingly, the melting and decomposition temperatures remained largely unchanged, meaning that 10 also exhibits standalone melt-castable behavior.
Figure 5.
Molecular structure of standalone melt-castable nitric ester 10.
Table 9. Physical and Performance Properties of Standalone Melt-Castable Nitric Ester 10.
| data category | TNT | 10 |
|---|---|---|
| Tma [°C] | 80.4 | 91 |
| Tdecb [°C] | 295 | 194 |
| ΩCO2c [%] | –74 | –56.2 |
| ΩCOd [%] | –24.7 | –13 |
| ρe [g cm–3] | 1.65 | 1.72 |
| Pcjf [GPa] | 20.5 | 25.1 |
| Vdetg [ms–1] | 6950 | 7374 |
| ΔfH°h,i [kJ mol–1] | –59.3 | +49.5 |
| ISi [J] | 15 | 7.8 |
| FSj [N] | 240 | 240 |
| ESDk [J] | 0.25 | 0.125 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
ESD = electrostatic discharge sensitivity.
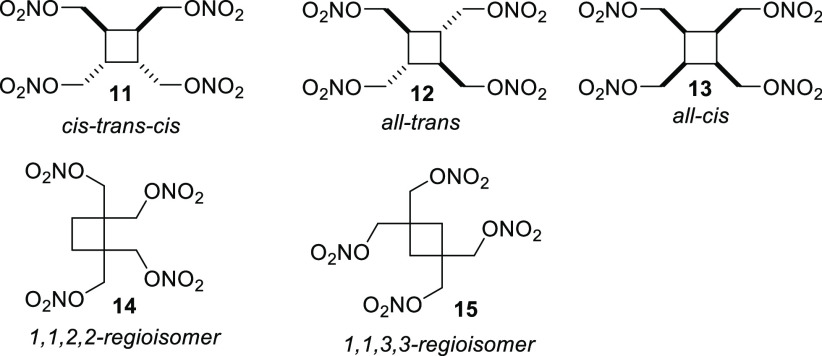
Recently, several stereo- and regioisomeric cyclobutane tetranitratic esters were synthesized (Figure 6).18 Although the energetic performances were found to be similar, the physical properties of the compounds were found to be significantly different. Of the six compounds synthesized, 11 and 13 were classified as melt-castable eutectic materials, as evidenced by the melting and decomposition temperatures of these materials (Table 10). 14 can be classified as a standalone melt-castable explosive due to its 85.9 °C melting point. Unsurprisingly, the impact sensitivity of 14 is higher, which is likely due to the nitric ester moieties being in closer proximity to one another. However, the impact sensitivity is still lower than PETN. 12 and 15 are classified as explosives, but their undesirable melting points make them unappealing for practical applications.
Figure 6.
Structure of stereo- and regioisomeric cyclobutane tetranitrates 11–15.
Table 10. Physical and Performance Properties Cyclobutane Tetranitrates 11–15.
| data category | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|
| TMa [°C] | 106 | 47.5 | 100.8 | 85.9 | 146.9 |
| Tdecb [°C] | 198.5 | 199.7 | 194.3 | 192.8 | 196.2 |
| Ρc [g cm–3] | 1.64 | 1.605 | 1.656 | 1.668 | 1.597 |
| Pcjd [GPa] | 24.5 | 22.9 | 24.5 | 24.6 | 24.4 |
| Vdete [m s–1] | 7438 | 7544 | 7504 | 7604 | 7472 |
| ΔfH°f [kJ mol–1] | –510.1 | –535.9 | –480.8 | –465.5 | –509.2 |
| ISg [J] | 6.2 | 6.2 | 6.2 | 4.7 | 6.2 |
| FSh [N] | 240 | 240 | 240 | >360 | >360 |
| ESDi [J] | 0.125 | 0.125 | 0.125 | 0.125 | 0.25 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
ESD = electrostatic discharge sensitivity.
2. Nitric Ester Propellants
As the name implies, the purpose of a propellant is to provide the energy for a projectile, vehicle, or object to fly a specified distance. Propellants that contain nitric esters include double-base (nitrocellulose and nitroglycerin) and triple-base (nitrocellulose, nitroglycerin, and nitroguanidine) mixtures (Figure 7). NG is typically synthesized using mixed acid at cold temperatures. When producing NG in mass, ensuring that the material is free from acid is critical to ensuring its long-term stability. Acid buildup of nitroglycerin mixtures is well-known to exothermic autocatalytic decomposition, which can lead to severe explosions.1a
Figure 7.
Molecular structure of nitrocellulose (NC), nitroglycerin (NG), and nitroguanidine (NQ).
NC is prepared by nitrating high-quality cellulose (derived from wood pulp or cotton linters) with mixed acid. By controlling the concentration and ratios of mixed acid used, various degrees of nitrated NC can be obtained. The degree of nitration is measured by determining the nitrogen content of the product. The percent nitrogen content for NC is typically 10.3–13.5%. NC that possesses a nitrogen content between 10.3% and 12.3% typically finds applications as lacquers, coatings, and inks, and as an ingredient in fingernail polishes. NC with a nitrogen content exceeding 12.6% is classified as an explosive material. A nitrogen content between 12.6% and 12.7% is classified as pyrocellulose, which is an ingredient in the manufacture of smokeless powders, while a nitrogen content between 13.35% and 13.45% is classified as guncotton. Guncotton also finds applications in smokeless powder formulations, as well as in blasting and mining applications.
NQ is ubiquitous in triple-base gun propellants. It is not solubilized in NC/NG mixtures but is imbedded within these mixtures as a fine powder. The main role of NQ is twofold: to moderate the temperature of the propellant, thus serving to reduce gun barrel erosion, and to suppress the fireball flash of the muzzle. NG is a very impact sensitive material, about an order of magnitude more sensitive than NC. NQ, however, is a very insensitive material, which has been observed to tolerate maximum settings on impact sensitivity machines. The performance and sensitivity values of NC, NG, and NQ is provided in Table 11.
Table 11. Performance and Sensitivity Values of Some Commonly Encountered Ingredients in Double- and Triple-Base Propellant Formulations.
| data category | NC | NG | NQ | EGDN | DEGDN | TEGDN | BTTN | TMETN | BuNENA | DINA |
|---|---|---|---|---|---|---|---|---|---|---|
| Tma [°C] | 14 | –22 | –10.9 | –19 | –27 | –3 | –9 | 51.3 | ||
| Tdecb [°C] | 192–209 | 150 | 248.9 | 175 | 160 | 170 | 182 | 180 | 177.5 | |
| ΩCO2c [%] | –28.7 | +3.5 | –30.7 | 0 | –40.8 | –66.7 | –16.6 | –34.5 | –104.3 | –26.6 |
| ΩCOd [%] | +24.7 | –15.7 | +21 | –8.2 | –26.7 | –10.5 | –3.14 | –57.9 | 0 | |
| ρe [g cm–3] | 1.67 | 1.591 | 1.71 | 1.48 | 1.38 | 1.335 | 1.52 | 1.46 | 1.22 | 1.488 |
| Vdete [ms–1] | 7300 | 7600 | 8200 | 7300 | 6600 | 2000 | 7050 | 7580 | ||
| ΔfH°f [kJ mol–1] | –1435.1 | –370.1 | –93 | –244.8 | –436.8 | –629.9 | –406 | –425.2 | –192.5 | –448.2 |
| ISh [J] | 3 | 0.2 | >49 | 0.2 | 0.2 | 12.7 | 1 | 9.2 | 6 | 6 |
| FSi [N] | >353 | >353 | >353 | >353 | >353 | >353 | >353 | >353 | >353 | >353 |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
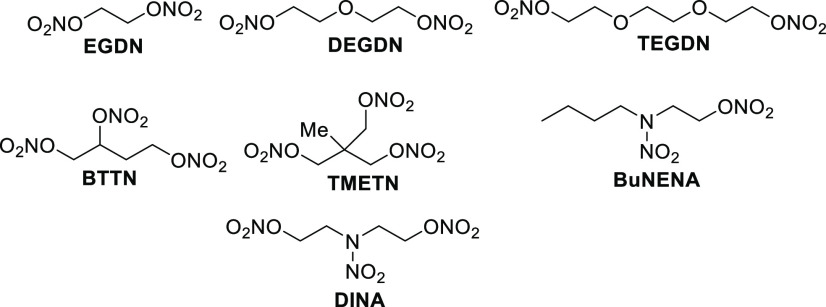
While nitric ester materials comprise the foundation of double- and triple-base propellant materials, there are many other ingredients that make up a propellant formulation. These include oxidizer and fuel additives, stabilizers (added to prolong shelf life and reduce/eliminate autocatalytic decomposition of many propellant nitric esters), burn rate modifiers, anticaking agents, and inert as well as energetic plasticizers. Of these additional ingredients, energetic plasticizers are commonly nitric ester-based when added into double-base rocket or triple-base gun propellant formulations. Some of the most commonly encountered energetic propellant plasticizers are summarized in Figure 8. Ethylene glycol dinitrate (EGDN), diethylene glycol dinitrate (DEGDN), and triethylene glycol dinitrate (TEGDN) all gelatinize with NC better than NG does.19 The ability to gelatinize—thus forming a solid material from liquids—is important from the processing standpoint. The ability to gelatinize helps to reduce the overall sensitivity of propellant mixtures. Although EGDN, DEGDN, and TEGDN are not considered NG replacements due to their lower performances and oxygen balances, they do possess the ability to depress the freezing temperature of NG.19
Figure 8.
Molecular structures of commonly encountered nitric ester plasticizers in double- and triple-base rocket propellant formulations.
Butanetriol trinitrate (BTTN) not only gelatinizes well with NC, but has also been explored as a potential replacement to NG.21 At low temperatures, NG has been found to crystallize in double-base formulations, causing the propellant grain to crack, thus rendering a formulation unusable. Partial replacement of NG with BTTN has been found to solve the cracking problem. BTTN has been found to possess a higher thermal stability, a lower shock sensitivity, and a lower freezing point, and it is six times less volatile than NG.20a Despite this, NG remains the plasticizer of choice on the basis of cost. Currently, on a pound basis, BTTN costs $60 to produce, while NG costs $2. There are recent efforts, however, to employ directed evolution/microbe technologies to manufacture butanetriol (the precursor to BTTN). If successful, this could significantly reduce the cost.20b
Due to the presence of only primary nitric ester functionality, its low volatility, and because of its lower oxygen balance, trimethyloltrinitrate (TMETN) is one of the more stable and less sensitive nitric esters commonly encountered in propellant mixtures.21 Unlike the aforementioned plasticizers, TMETN gelatinizes NC only to a moderate extent, and gelatinization only occurs at elevated temperature. Thus, TMETN finds only limited applications as a plasticizer in propellant mixtures. It has, however, been found to be a favorable plasticizer for glycidyl azide polymer (GAP).
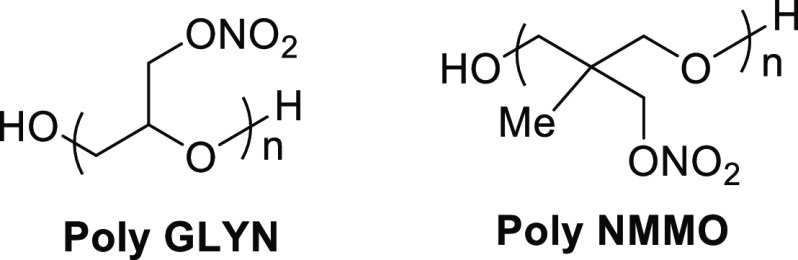
Nitroxyethyl nitramines (NENAs) are of high interest to the rocket and especially gun propulsion community. Two of the most popular NENAs are n-butyl nitroxyethylnitramine (BuNENA) and dioxyethylnitramine dinitrate (DINA). NENAs are typically prepared with a mixture of Ac2O and HNO3 in the presence of ZnCl2 as a catalyst.22 ZnCl2, which forms the transient chloramine intermediate, is critical in obtaining high yields of product. Like many in the NENA class, BuNENA and DINA have been shown to plasticize cellulose-based polymers. This includes NC, GAP, polyglycidyl nitrate (poly GLYN), and poly(3-nitratomethyl-3-methyl) oxetane (poly NMMO) (Figure 9).23a BuNENA and DINA are characterized as having a low impact sensitivity and low glass transition temperatures. They are capable of forming eutectic mixtures, exhibiting lower melting points than conventional nitric esters, and thus preserving optimal low-temperature mechanical properties. NENAs have been found to impart high burning rates in formulation, with reduced flame temperature. BuNENA and DINA has been used as a replacement for NG in the development of insensitive, low vulnerability (LOVA) double-base rocket and triple-base gun propellants.23b The Navy has produced DINA on large scales for flashless gun propellant, and more recent work has demonstrated that DINA can be employed successfully in CL-20- and HMX-based double-base rocket propellants.24
Figure 9.
Molecular structures of energetic nitric ester polymers poly GLYN and poly NMMO.
Despite the positive characteristics of NENA plasticizers, they suffer from long-term aging issues, and formulations containing these plasticizers have difficulty maintaining a 10 year service life. Although NENAs possess excellent plasticizing effects initially, their low molecular weights and high volatility allow these materials to move readily from polymeric binder systems in formulation.23a
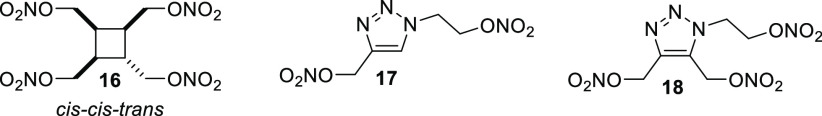
Although their utility has been well demonstrated, the aforementioned nitric ester liquid propellant materials are relatively historical materials, and there have been very few new materials synthesized in this area. However, some recently synthesized liquid nitric esters were found to have potential energetic plasticizer properties. (Figure 10). Cyclobutane tetranitrate 16 was one of the family of materials synthesized in Figure 6, but was the only one in that family that exhibited liquid behavior, with a freezing point of <−40 °C, a high decomposition temperature, a good specific impulse (240 s), and a low sensitivity to impact, friction, and electrostatic discharge (Table 12).18 Another class of materials recently disclosed were energetic 1,2,3-triazole nitric ester materials, synthesized using click chemistry between azidoethanol and propargyl alcohol or 1,4-butynediol, followed by nitration to give 17 and 18.25 With a low decomposition temperature of 120 °C, 17 is likely not to find application as a propellant plasticizer material. The fully substituted nature of 18 evidently increased the decomposition temperature significantly, and this material therefore has the potential to find use in propellant formulations.
Figure 10.
Molecular structure of potential nitric ester liquid plasticizers 16–18.
Table 12. Performance and Sensitivity Values of Energetic Liquid Plasticizers 16–18.
| data category | 16 | 17 | 18 |
|---|---|---|---|
| Tma [°C] | <−40 | - | - |
| Tdecb [°C] | 186.8 | 120 | 155 |
| ΩCO2c [%] | –44.9 | –51.5 | –36.4 |
| ΩCOd [%] | –9 | –17.2 | –5.2 |
| ρe [g cm–3] | 1.543 | 1.44 | 1.73 |
| Pcjf [GPa] | 24.5 | 19.9 | 26.3 |
| Vdetg [ms–1] | 7577 | 7170 | 7950 |
| ΔfH°h,i [kJ mol–1] | –512 | –70.9 | 36 |
| ISi [J] | 9 | 5.7 | 9 |
| FSj [N] | >360 | - | - |
| ESDk [J] | 0.125 | - | - |
Tm = onset temperature of melting.
Tdec = onset temperature of decomposition.
ΩCO2 = CO2 oxygen balance.
ΩCO = CO oxygen balance.
ρ = derived density from X-ray data.
Pcj = detonation pressure.
Vdet = detonation velocity.
ΔfH° = molar enthalpy of formation.
IS = impact sensitivity.
FS = friction sensitivity.
ESD = electrostatic discharge.
Conclusion
In summary, not only does the nitric ester functionality contribute significantly to the overall energy of energetic molecules, but its presence also spans the many classes of energetic materials. Disclosed in this review are nitric ester materials that have the potential to serve as ingredients in primary explosives, pressable secondary explosives, melt-castable secondary explosives, double-base rocket propellants, and triple-base gun propellants. Although believed to be linked to a high degree of sensitivity, with unpredictable chemical and thermal stability, many of the newer nitric ester materials disclosed over the past 5 years were found to exhibit a significant degree of insensitivity and chemical/thermal stability. The nitric ester moiety—one of the earliest discovered and known explosophores—still plays an important role in the discovery of new energetic materials, across many potential energetic applications and platforms.
Acknowledgments
The US Army is acknowledged for funding of this work. The authors are grateful to ACS Omega for the invitation to write this review article.
Glossary
Abbreviations
- PETN
pentaerythritol tetranitrate
- XPN
xylitol pentanitrate
- MHN
mannitol hexanitrate
- SHN
sorbitol hexanitrate
- RDX
royal demolition explosive
- HNO3
nitric acid
- ETN
erythritol tetranitrate
- HMX
her majesty explosive
- CL-20
hexanitrohexaazaisowurtzitane
- TNT
2,4,6-trinitrotoluene
- SMX
2,3-hydroxymethyl-2,3-dinitro-1,4-butanediol tetranitrate
- BIDN
3,3′-biisoxazole-5,5′-bis(methylene) dinitrate
- BITN
3,3′-biisoxazole-4,4′,5,5′-tetra(methylene) tetranitrate
- BODN
bis(1,2,4-oxadiazole)-bis(methylene) dinitrate
- NC
nitrocellulose
- NG
nitroglycerin
- NQ
nitroguanidine
- EGDN
ethylene glycol dinitrate
- DEGDN
diethylene glycol dinitrate
- TEGDN
triethylene glycol dinitrate
- BTTN
butanetriol trinitrate
- TMETN
trimethylol trinitrate
- BuNENA
n-butyl nitroxyethylnitramine
- DINA
dioxyethylnitramine dinitrate
- GAP
glycidyl azide polymer
- Poly GLYN
polyglycidyl nitrate
- poly NMMO
poly(3-nitratomethyl-3-methyl) oxetane
Biographies
Dr. Jesse J. Sabatini received his BS in chemistry from Binghamton University in 2004, and a PhD in organic synthesis from the University of Virginia in 2007. After postdoctoral studies at the University of Pittsburgh, he began his independent career with the US Army at Picatinny Arsenal, NJ in 2009 as a research chemist, specializing in pyrotechnic and primary explosive formulations. In 2014, Dr. Sabatini moved to the US Army Research Laboratory at the Aberdeen Proving Ground, MD, and assumed Team Leader duties. In this capacity, he leads the synthesis programs relating to the development of new explosive, propellant, and pyrotechnic ingredients for applications in energetics formulation. Dr. Sabatini is currently the President of the International Pyrotechnics Society and is a member of the Editorial Advisory Boards of the international peer-reviewed journals of ChemPlusChem and Propellants, Explosives, Pyrotechnics.
Mr. Eric C. Johnson earned a double major in chemical engineering and chemistry from the University of Delaware. After graduation, Mr. Johnson worked at DuPont on real-time analysis applications that improved process cycle time and safety. He also worked at TA Instruments and at Mettler-Toledo Autochem before joining the US Army Research Laboratory (ARL) in 2010. During his 11 years at ARL, Mr. Johnson has worked on numerous formulation, synthesis, and reaction scale-up projects involving propellant, explosive, and pyrotechnic materials.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- a Klapötke T. M.Chemistry of High-Energy Materials, 5th ed.; De Gruyter: Berlin, 2019. [Google Scholar]; b Szala M.; Sałaciński T. New energetic materials derived from common explosives. Materiały Wysokoenergetyczne High Energy Materials 2020, 12, 90–110. 10.22211/matwys/0114E. [DOI] [Google Scholar]; c Agrawal J. P.; Hodgson R. D.. Organic Chemistry of Explosives; Wiley: Hoboken, NJ, 2007. [Google Scholar]; d Chin C.-L.; Liu S.-H.; Chen W.-C.; Cheng K.-L.. Method For Preparing Nitrate Ester. US Patent 10703707 B2, 2020.
- Litchfield M. H. J. Pharm. Sci. 1971, 60, 1599–1607. 10.1002/jps.2600601102. [DOI] [PubMed] [Google Scholar]
- Sabatini J. J.; Oyler K. D. Recent Advances in the Synthesis of High Explosive Materials. Crystals 2016, 6, 5–22. 10.3390/cryst6010005. [DOI] [Google Scholar]
- Meyer R.; Köhler J.; Homburg A.. Explosives, 6th ed.; 2007; p 250. [Google Scholar]
- a Stark K. -A. S; Alvino J. F.; Kirkbride P.; Sumby C. J.; Metha G. F.; Lenehan C. E.; Fitzgerald M.; Wall C.; Mitchell M.; Prior C. Crystal Structure, Sensitiveness and Theoretical Explosive Performance of Xylitol Pentanitrate (XPN). Propellants, Explos., Pyrotech. 2019, 44, 541–549. 10.1002/prep.201800337. [DOI] [Google Scholar]; b Gribanov P. S.; Topchiy M. A.; Fedyanin I. V.; Asachenko A. S.; Nechaev M. S.; Pleshakov D. V. Reexamination of an Energetic Nitrate Ester SHN. Propellants, Explos., Pyrotech. 2017, 42, 1014–1019. 10.1002/prep.201700117. [DOI] [Google Scholar]; c Meyer R.; Köhler J.; Homburg A.. Explosives, 6th ed.; 2007; p 207. [Google Scholar]; d Wang G.-X.; Gong X.-D.; Du H.-C.; Liu Y.; Xiao H.-M. Theoretical Prediction of Properties of Aliphatic Polynitrates. J. Phys. Chem. A 2011, 115, 795–804. 10.1021/jp1054155. [DOI] [PubMed] [Google Scholar]
- E-mail communication, https://ipfs.io/ipfs/QmdA5WkDNALetBn4iFeSepHjdLGJdxPBwZyY47ir1bZGAK/explosives/mannitol_hexanitrate.html, Accessed 29 March, 2021.
- Oxley J. C.; Smith J. L.; Brady J. E.; Brown A. C. Characterization and Analysis of Tetranitrate Esters. Propellants, Explos., Pyrotech. 2012, 37, 24–39. 10.1002/prep.201100059. [DOI] [Google Scholar]
- Rzechonek D. A.; Dobrowolski A.; Rymowicz W.; Mironczuk A. M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. 10.1080/07388551.2017.1380598. [DOI] [PubMed] [Google Scholar]
- a Lease N.; Kay L. M.; Brown G. W.; Chavez D. E.; Robbins D.; Byrd E. F. C.; Imler G. H.; Parrish D. A.; Manner V. W. Synthesis of Erythritol Tetranitrate Derivatives: Functional Group Tuning of Explosive Sensitivity. J. Org. Chem. 2020, 85, 4619–4626. 10.1021/acs.joc.9b03344. [DOI] [PubMed] [Google Scholar]; b Lease N.; Kay L. M.; Brown G. W.; Chavez D. E.; Leonard P. W.; Robbins D.; Manner V. W. Modifying Nitrate Ester Sensitivity Properties Using Explosive Isomers. Cryst. Growth Des. 2019, 19, 6708–6714. 10.1021/acs.cgd.9b01062. [DOI] [Google Scholar]
- Cudziło S.; Nita M.; Chołuj A.; Szala M.; Danikiewicz W.; Spólnik G.; Krompiec S.; Michalik S.; Krompiec M.; Świtlicka A. Synthesis, Structure and Explosive Properties of a New Trinitrate Derivative of an Unexpected Condensation Product of Nitromethane with Glyoxal. Propellants, Explos., Pyrotech. 2012, 37, 261–266. 10.1002/prep.201000133. [DOI] [Google Scholar]
- Lenz T.; Klapotke T. M.; Muhlemann M.; Stierstorfer J. About the Azido Derivatives of Petaerythritol Tetranitrate. Propellants, Explos., Pyrotech. 2021, 1. 10.1002/prep.202000265. [DOI] [Google Scholar]
- a https://www.finexplo.fi/?page_id=439, Accessed 29 March 2021.; b Elbeih A. Characteristics of a New Plastic Explosive Named EPX-1. J. Chem. 2015, 2015, 1–6. 10.1155/2015/861756. [DOI] [Google Scholar]
- Ravi P.; Badgujar D. M.; Gore G. M.; Tewari S. P.; Sikder A. K. Review on Melt Cast Explosives. Propellants, Explos., Pyrotech. 2011, 36, 393–403. 10.1002/prep.201100047. [DOI] [Google Scholar]
- Chavez D. E.; Hiskey M. A.; Naud D. L.; Parrish D. Synthesis of an Energetic Nitrate Ester. Angew. Chem., Int. Ed. 2008, 47, 8307–8309. 10.1002/anie.200803648. [DOI] [PubMed] [Google Scholar]
- a Wingard L. A.; Guzman P. E.; Johnson E. C.; Sabatini J. J.; Drake G. W.; Byrd E. F. C. Synthesis of bis-Isoxazole-bis-Methylene Dinitrate: A Potential Nitrate Plasticizer and Melt-Castable Energetic Material. ChemPlusChem 2017, 82, 195–198. 10.1002/cplu.201600470. [DOI] [PubMed] [Google Scholar]; b Wingard L. A.; Johnson E. C.; Guzman P. E.; Sabatini J. J.; Drake G. W.; Byrd E. F. C.; Sausa R. C. Synthesis of biisoxazoletetrakis(methyl nitrate): A Potential Nitrate Plasticizer and Highly Explosive Material. Eur. J. Org. Chem. 2017, 2017, 1765–1768. 10.1002/ejoc.201700280. [DOI] [Google Scholar]; c Johnson E. C.; Sabatini J. J.; Chavez D. E.; Sausa R. C.; Byrd E. F. C.; Wingard L. A.; Guzman P. E. Bis(1,2,4-oxadiazole)bis(methylene) Dinitrate: A High-Energy Melt-Castable Explosive and Energetic Propellant Plasticizing Ingredient. Org. Process Res. Dev. 2018, 22, 736–740. 10.1021/acs.oprd.8b00076. [DOI] [Google Scholar]
- Larin A. A.; Bystrov D. M.; Fershtat L. L.; Konnov A. A.; Makhova N. N.; Monogarov K. A.; Meerov D. B.; Melnikov I. N.; Pivkina A. N.; Kiselev V. G.; Muravyev N. V. Nitro-, Cyano-, and Methylfuroxans, and Their Bis-Derivatives: From Green Primary to Melt-Cast Explosives. Molecules 2020, 25, 5836. 10.3390/molecules25245836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. C.; Sabatini J. J.; Chavez D. E.; Wells D. E.; Banning J. E.; Sausa R. C.; Byrd E. F. C.; Orlicki J. A. Bis(Nitroxymethylisoxazolylyl) Furoxan: A Promising Standalone Melt-Castable Explosive. ChemPlusChem 2020, 85, 237–239. 10.1002/cplu.201900710. [DOI] [PubMed] [Google Scholar]
- Barton L. M.; Edwards J. T.; Johnson E. C.; Bukowski E. J.; Sausa R. C.; Byrd E. F. C.; Orlicki J. A.; Sabatini J. J.; Baran P. S. Impact of Stereo- and Regiochemistry on Energetic Materials. J. Am. Chem. Soc. 2019, 141, 12531–12535. 10.1021/jacs.9b06961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal J. P.High Energy Materials: Propellants, Explosives, Pyrotechnics; Wiley VCH: Weinheim, 2010; p 275. [Google Scholar]
- a Pisacane F. J.1,2,4-Butanetriol: Analysis and Synthesis; NSWC TR 82–380; Defense Technical Information Center, 1982.; b Cramer R. J.Biosynthesis of Energetic Ingredients; JSEM Annual Conference, March 20–23, 2006.
- Xuebao H. Synthesis of Insensitive Nitrate Plasticizers TMETN and PGDN by Micro Reaction Technology. Chinese Journal of Explosives and Propellants 2018, 41, 359–362. [Google Scholar]
- Frankel M. B.; Vanneman C. R. Preparation of Aliphatic Secondary Nitramines. J. Org. Chem. 1958, 23, 1810. 10.1021/jo01105a625. [DOI] [Google Scholar]
- a Provatas A.Energetic Polymers and Plasticizers for Explosive Formulations-A Review of Recent Advances; DSTO-TR-0966; Weapons Systems Division; Aeronautical and Maritime Research Laboratory, 2000.; b Urenovitch J. V.Low vulnerability propellant plasticizers. US Patent 5,482,481, 1996.
- Wu Z.; Liu N.; Zheng W.; Chen J.; Song X.; Wang J.; Cui C.; Zhang D.; Zhao F. Application and Properties of CL-20/HMX Cocrystal in Composite Modified Double Base Propellants. Propellants, Explos., Pyrotech. 2020, 45, 92–100. 10.1002/prep.201900245. [DOI] [Google Scholar]
- Gaur P.; Dev S.; Kumar S.; Kumar M.; Vargeese A. A.; Soni P.; Siril P. M.; Ghosh S. Dendritic Polynitrato Energetic Motifs: Development and Exploration of Physiochemical Behavior through Theoretical and Experimental Approach. ACS Omega 2017, 2, 8227–8233. 10.1021/acsomega.7b00880. [DOI] [PMC free article] [PubMed] [Google Scholar]