Abstract

Crystalline biofilm formation in indwelling urinary catheters is a serious health problem as it creates a barrier for antibacterial coatings. This emphasizes the failure of antibacterial coatings that do not have a mechanism to reduce crystal deposition on catheter surfaces. In this study, trifluoropropyl spray-coated polydimethylsiloxane (TFP–PDMS) has been employed as an antibiofilm forming surface without any antibacterial agent. Here, TFP was coated on half-cured PDMS using the spray coating technique to obtain a durable superhydrophobic coating for a minimum five cycles of different sterilization methods. The crystalline biofilm-forming ability of Proteus mirabilis in artificial urine, under static and flow conditions, was assessed on a TFP-PDMS surface. In comparison to the commercially available silver-coated latex and silicone catheter surfaces, TFP–PDMS displayed reduced bacterial attachment over 14 days. Moreover, the elemental analysis determined by atomic absorption spectroscopy and energy-dispersive X-ray analysis revealed that the enhanced antibiofilm forming ability of TFP-PDMS was due to the self-cleaning activity of the surface. We believe that this modified surface will significantly reduce biofilm formation in indwelling urinary catheters and further warrant future clinical studies.
Introduction
Formation of bacterial biofilms in medical devices causes threatening issues in healthcare applications as they spread and cause infections in the internal organs. Catheter-associated urinary tract infections (CAUTI) is one of the most common healthcare device-associated infections that may develop until secondary bloodstream infections and many other complications.1 The persistence of CAUTI for a prolonged period eventually tends to mortality.2−4 The most common uropathogens causing urinary catheter biofilms are Enterococcus faecalis,Pseudomonas aeruginosa,Escherichia coli, and Proteus mirabilis.5
Urease-producing bacteria, mainly Proteus mirabilis, are critical because they can encrust urinary catheter surfaces and thereby form biofilms, although they have already been treated with antibacterial agents.6,7 Uropathogens use different mechanisms to form biofilms.8 As the first step in urinary catheter biofilm formation, bacteria form a conditioning film which consists of proteins, electrolytes, and other organics adhering to the catheter surface.9,10 Then, free-floating bacteria attach to the conditioning layer through hydrophobic interactions, electrostatic interactions, and flagella. The attached cells secrete exopolysaccharides forming gel-like matrix to secure their attachment.8 Bacteria multiply and spread over the surface and form a loosely packed three-dimensional (3D) structure. Finally, individual organisms detach from the biofilm in order to complete the life cycle.11 It is reported that P. aeruginosa, K. pneumonia and P. mirabilis are responsible for catheter blockages because they can produce urease in urine.12 These organisms can hydrolyze urea into ammonium and carbonate ions by increasing the pH of urine. Magnesium and calcium phosphate crystals are precipitated in this alkaline medium and aggregated with microorganisms to form an encrusted biofilm which is known as the crystalline biofilm. P. mirabilis was found to be the most common uropathogen causing crystalline biofilms.13
The treatments over CAUTI are limited as uropathogens develop resistance over most antibiotic drugs undergoing mechanisms that encode virulence factors.14−16 For example, P. mirabilis is classified to a multidrug-resistant bacteria as there are many recent cases reported around the world.17,18 Although alternatives to antibiotic drugs are emerging,19−21 it is still worth preventing CAUTI than treating them.
In recent times, scientists have focused on the modifications of the catheter wall to prevent biofilm formation and encrustation. A single or combination of different antibiotics and antiseptics such as nitrofurazone have been coated or impregnated. However, because of the high resistance of biofilm bacteria, these organic coatings failed.22 As inorganic coatings, silver, titanium, and magnesium species (e.g., oxides, alloys, and nanoparticles) have been commonly used for inhibiting or controlling biofilm formation on catheters. Moreover, prolonged activity has been maintained by applying controlled releasing mechanisms such as hydrogels, liposome encapsulation, structural polymers, and biodegradable coatings. However, the above strategies may consist of disadvantages such as swelling. Also, inflammatory responses of the urinary tract may be due to catheter friction producing fibrinogen, which is essential for biofilm formation.23
Thus, scientists have focused on combating bacteria in multiple directions. Together with the bactericidal activity, antifouling surfaces for urinary catheters have been introduced to reduce biofilm formation and inhibit host–protein deposition. Wang et al. immobilized silver nanoparticles on polydopamine pretreated silicone catheter to gain controlled release behavior and grafted poly(sulfobetaine methacrylate-co-acrylamide) to introduce antifouling properties.24 However, several bacteria including P. mirabilis have shown resistance against the well-performed silver nanoparticles recently.25,26 Therefore, high-performance antifouling coatings without any antibiotics or bactericidal elements have become an advantage to address the above issues.
Polydimethylsiloxane (PDMS) is currently used to produce many medical devices because of its multiple advantages such as nontoxicity, flexibility, biocompatibility, and autoclavability.27−30 PDMS can also be developed as an antiadhesion surface through enhancing hydrophobicity. Once the contact angle of PDMS with water (CAwater) increases beyond 150°, the surface becomes superhydrophobic and may exhibit self-cleaning ability.31,32 Superhydrophobicity of PDMS medical devices can be achieved through several techniques such as micropatterning of surfaces and coating fabrication.33,34 Spray coating is one of the simple methods as a number of chemicals can be deposited once or in a repeated way on PDMS.28,35 However, maintaining the durability of the coatings on PDMS is important for prolonged usage of catheters.
Scientists have been focusing on the modifications of urinary catheter wall for the prevention or reduction of biofilm adherence as well as to reduce encrustation on the catheter wall while maintaining its durability. However, currently available antimicrobial catheters are less successful for prolonged catheterization, and some novel designs are still in preclinical stages.36,37 Therefore, proposing new practical methods for the implementation of infection-free catheters is continuously researched. This study aimed at applying a superhydrophobic and superoleophilic surface prepared using a simple spray-coating fabrication method to reduce the adhesion of P. mirabilis on PDMS surfaces. The antibiofilm-forming efficacy was evaluated using both static and dynamic models and compared to latex and all-silicone catheters.
Results and Discussion
Characterization
The higher surface roughness together with extremely low surface free energy results in super antiwetting ability of TFP–PDMS. TFP–PDMS surface is superhydrophobic and superoleophilic as the CAWater and CAHexadecane were observed as 167° ± 2° and 0°. This hydrophobicity is higher than that of hydrophobic 100% silicone and hydrophilic siliconized latex catheter surfaces.38,39 The sliding angle of TFP–PDMS was 2° ± 0°. Atomic force microscopy (AFM) images revealed that the surface of TFP–PDMS has nano-/micrometer-level distributed surface roughness with Rq of 273 nm (Figure 1). Siliconized latex catheters also have nano/micrometer-level roughness with less surface roughness (Rq = 144 nm). The 100% silicone catheter has the least surface roughness that is of 35 nm. Furthermore, the total surface free energy was 1.9 mJ m–2, which is an extremely low value caused for enhancing wetting resistance. These results represent the property of a super antiwetting surface (sliding angle <10°) and thus show the self-cleaning ability.
Figure 1.

AFM images of catheter materials: (a) 100% silicone; (b) TFP–PDMS; (c) siliconised latex.
Durability and Self-Healing Study
The sterilization of urinary catheters can result in the corrosion of the catheter coating. On the other hand, the durability of many fluorinated nanoparticle coatings is limited as they are difficult to be attached to the substrates because of low surface energy.40 Such coatings can be detached with urine flow over a period. Because TFP–PDMS is a nanoparticle coating (Figure 1), we conducted durability studies as a function of the sliding angle to use TFP–PDMS as a self-cleaning material in biomedical applications. The self-healing of TFP–PDMS was determined using two techniques: heat treatment and THF treatment followed by heat treatment (Figure 2). The latter method was more effective in terms of reconstructing the material with less sliding angle. Interestingly, the coating was highly durable under UV exposure, making UV treatment a promising sterilization method without healing the material, and the coating could be UV-sterilized for at least five 20 min cycles. TFP–PDMS exhibited better stability to acidity than basicity and exhibited considerable resistance under flow conditions. TFP–PDMS can be exposed to at least five cycles of 6 min ultrasonication to remove any nonadhered substances. These results indicate that TFP–PDMS can be used as a durable self-cleaning material over sterilization methods, and the functionality can be regained by THF and heat treatment, promising its reusability.
Figure 2.
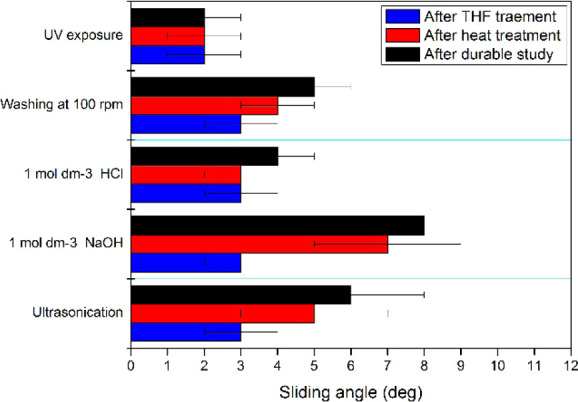
Durability and self-healing ability of TFP–PDMS.
Determination of the Biofilm-Forming Ability of P. mirabilis on the Prepared Superhydrophobic Material
Both 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) and crystal violet (CV) assays provided a better understanding of the formation of biofilms quantitatively. The MTT assay serves as a respiratory indicator of live cells, while the CV assay serves as an indicator of adhesion representing both live and dead cells.41
The mean CV activities for TFP–PDMS and silicone were slowly increased while the mean CV activity of siliconized latex gradually increased up to 8th day under static conditions (Figure 3(a)). The biofilm formation on all the three materials increased from 8th day to 11th day at different rates under static conditions followed by constant biofilm formation after the 11th day. However, 14th day biofilms under flow conditions were observed in fewer amounts than 14th day biofilms under static conditions. The different rates of biofilm formation are due to the different adhesion capabilities between the catheter materials and the biofilm components. For the adhesion of P. mirabilis onto the catheter surface, Armbruster and Mobley have suggested that P. mirabilis expresses mannose-resistant fimbriae which are similar to that of Klebsiella sp.(42)P. mirabilis does not require swarming for adherence and subsequent development of the biofilm on the silicone surface but determines the dispersion on the surface.43 Furthermore, there is no experimental evidence for the contribution of fimbriae to catheter adherence. Therefore, we suggest that there is no direct interaction between the surfaces and P. mirabilis.42 However, P. mirabilis cells can embed in an organic adhesive matrix which may also be trapped with amorphous apatite in a medium above pH 7.5 and deposited on the catheter surface.44 Hence, the biofilm on TFP–PDMS is loosely bound to the substrate than the other two catheter materials, and the delayed biofilm formation may occur as a result of the flushing action of such depositions.
Figure 3.

P. mirabilis biofilm formation on catheter materials determined by colorimetric assays (s, static condition; f, flow condition): (a) CV assay; (b) MTT assay.
When considering the MTT function, a similar increasing trend was observed for TFP–PDMS and silicone surfaces (Figure 3(b)). In contrast, siliconized latex material resulted for a considerable deviation, which is a high MTT value throughout the experiment from the beginning. This observation may not be due to the amount of biofilm but resulted from the interferences by other releasing additives from the siliconized latex material such as antioxidants.45,46 However, further studies are required to identify the exact releasing agent.
Under the flow conditions, TFP–PDMS exhibited the least amount of biofilm according to both the CV and MTT assays. It might be a result of the flushing of the substances caused for forming the biofilm as TFP–PDMS has self-cleaning ability itself. Therefore, TFP–PDMS can be considered as a better catheter material than commercially available silicone and siliconized latex. Among the commercially available silicone and siliconized catheter materials, silicone showed better resistance for biofilm formation as a similar trend was stated by Jones et al.47 Overall, TFP–PDMS showed the most resistant activity, although it failed to achieve a perfect antibiofilm forming surface. However, the newly prepared material in this study was more efficient over 14 days under flow conditions than other commercially available indwelling urinary catheters including silver-coated latex (17.7 h) and all silicone (47 h), as reported in previous studies using a catheterized bladder model.48
Quantitative Analysis of Calcium and Magnesium in the Biofilms
P. mirabilis, the main urease positive microorganism in the urinary system, increases the pH of urine by producing ammonia and carbonate. In urine above pH 8, the macroscopic aggregates of cells and crystals form, deposit on the catheter surface, and initiate the formation of crystalline biofilms. Especially, magnesium and calcium ions may precipitate as calcium oxalate or CaOx type, calcium phosphate, and magnesium ammonium phosphate.49,50Figure 4 presents the concentrations of magnesium and calcium in the biofilms formed on the catheter materials quantified by atomic absorption spectroscopy. Under static conditions, calcium concentration (2–20 mg L- 1) was considerably higher than magnesium concentration (<5 mg L–1), indicating the higher possibility to deposit calcium crystals in the urinary catheter environment. At flow conditions, the content of both magnesium and calcium reduced from siliconized latex surface to TFP–PDMS that has a similar trend of biofilm amount observed in the biofilm assays.
Figure 4.

Element concentrations in the biofilms formed on catheter materials (s, static condition; f, flow condition): (a) magnesium concentration; (b) calcium concentration.
The initial stage of crystalline biofilm formation is the development of a conditioning layer which is generated by the deposition of host urinary components such as electrolytes, proteins, and other organic molecules.5 The similar trend of the amount of biofilm with the amount of calcium and magnesium indicates that the conditioning layer initiates with the deposition of electrolytes (calcium and magnesium ions) other than the deposition of proteins and organic molecules provided by artificial urine. Therefore, the superhydrophobicity of TFP–PDMS with the collaborative property of self-cleaning ability has resulted in washing out the deposited particles, minimizing the growth rate of P. mirabilis than other two catheter materials that were moderately hydrophobic (silicone) and hydrophilic (siliconized latex).51
However, this phenomenon was valid only for the flow system. In the static system, the calcium and magnesium particles were free to be deposited on the catheter surface, providing sufficient time for bacterial growth. Static artificial urine medium provided enough time to attach proteins and organics to the catheter surface. Previous studies have revealed that catheters have exhibited both hydrophobic and hydrophilic regions, allowing biofilm growth for P. mirabilis, although P. mirabilis has a negatively charged hydrophilic cell surface.10,52−55 Hence, the static system facilitated more time for the TFP–PDMS surface to be attached to more oleophilic compounds as a binder of calcium and magnesium particles. Because silicone was moderately hydrophobic and moderately oleophobic, silicone exhibited resistance to both types of compounds that resulted in poor deposition of calcium and magnesium.
Total Elements Present in the Biofilms
Energy-dispersive X-ray analysis (EDX) was employed to determine the relative amounts of the elements present on the surfaces of the materials. There should be new elements present or increase of the existing elements after the biofilm formation. As shown in Table 1, TFP–PDMS has no new element even after the microbial treatment. Importantly, the absence of calcium proved the suitability of using TFP–PDMS as a better catheter material. The durability of TFP–PDMS over usage can be further confirmed because the weight percentage of fluorine has not reduced, indicating minimal damage for the TFP coating.
Table 1. Elements Present on the Surface of Catheter Materials before and after the Biofilm Formation.
| element | TFP–PDMS | silicone | siliconized latex | |||
|---|---|---|---|---|---|---|
| before | after | before | after | before | after | |
| C | 46.5 | 46.6 | 26.6 | 41.0 | 83.5 | 42.5 |
| O | 28.0 | 37.9 | 37.1 | 40.3 | 9.9 | 19.6 |
| Si | 19.2 | 9.4 | 36.3 | 18.4 | 0.4 | 16.7 |
| F | 6.4 | 6.0 | ||||
| Ca | 0.3 | 0.1 | 0.6 | |||
| Mg | ||||||
| S | 3.2 | |||||
| Cl | 2.9 | 0.5 |
When considering silicone, calcium has been introduced after the microbial treatment with an even distribution. When considering siliconized latex, although calcium was not a guest element for siliconized latex, the increment of the calcium content from 0.1 to 0.6 (w/w %) may be due to crystalline biofilms. The deposited calcium might be due to calcium oxalate or CaOx or calcium phosphate. However, any of EDX spectra did not show a value for phosphorous governed for a controversial EDX observation compared to the literature which has stated that the main constituent of crystalline biofilms in urinary catheters is calcium phosphate in the presence of the high amount of P. mirabilis (1 × 109).56 In addition to calcium and phosphorous, a considerable increment of carbon and oxygen was observed. This result might be due to the presence of P. mirabilis, organic substances in the conditioning layer, and oxalates. Hence, we suggest that calcium existed preferably as calcium oxalates than calcium phosphates. Moreover, a considerable increment of carbon and oxygen was observed coming from P. mirabilis, organic substances in the conditioning layer, and oxalates. A similar pattern of the distribution of calcium and the dense distribution of carbon and oxygen is shown in Figure 5.
Figure 5.

Elemental distribution detected by EDX over the surfaces of catheter materials after treating with P. mirabilis.
In EDX results, magnesium was not observed in any of the materials, although magnesium was detected in atomic absorption spectroscopy. Such results can be obtained because magnesium was under the detection limit of the instrument (<0.01 wt %).57 Therefore, even if calcium phosphate and magnesium ammonium phosphate were present in the biofilms, they might not be detected.
Ultrastructure of Catheter Materials
Figure 6 shows the scanning electron microscopy (SEM) images of the catheter material surfaces before and after exposure to P. mirabilis for 48 h under flow conditions. The siliconized latex catheter consisted of a rough surface. After exposure to P. mirabilis, the defects disappeared. However, microscale crystals were observed. According to the mechanism of crystalline biofilm formation suggested by Wilks et al., adheredP. mirabilis were covered by a microcrystalline sheet, then by diffused crystalline materials, and finally by defined struvites.58 Therefore, bacterial cells were embedded on crystalline biofilms formed on the surface of the siliconized latex catheter. The difference in the structure of biofilms on silicone and siliconized latex further indicates their stage of biofilm formation after 48 h.
Figure 6.
SEM images of catheter material surfaces. (a), (b) Silicone before and after exposure to P. mirabilis; (c), (d) siliconized latex before and after exposure to P. mirabilis; (e), (f) TFP–PDMS before and after exposure to P. mirabilis.
The silicone catheter surface was uniform prior to the set up in the flow cell system (Figure 6(a)). After 48 h, the smoothness of the surface had depleted because of the deposition of ions in the artificial urine media (Figure 6(b)). The uniform distribution of those deposits was previously confirmed by the EDX studies, as shown in Figure 5. Furthermore, the silicone surface facilitated the adhesion of P. mirabilis, followed by microcolony formation.28 Jones et al. have observed that P. mirabilis in artificial urine forms a flat layer of cells, entirely free from nutrient channels on glass slides at 24 h, suggesting citrate as the sole carbon source.59 Conversely, in the present study, artificial urine produced nutrient channels on the silicone surface which consists of not only citrate but also nutritious tryptone soy broth including glucose.
On the TFP–PDMS surface, significant change was not observed (Figure 6(e,f)). There was no change observed in either microorganisms or crystals in the SEM images taken before and after exposure to P. mirabilis. It shows that biofilm formation on the TFP–PDMS surface has not initiated after 48 h.
Previous studies performed using both hydrophobic and hydrophilic catheter surfaces have speculated that free-energy polymer surfaces would not be employed for the prevention of crystalline biofilm formation.54 In contrast, the present study provides one step toward designing a potential antibiofilm-forming surface using low surface free-energy polymer surfaces, especially with superhydrophobic coatings such as TFP. However, we will focus on AFM studies for the determination of interactions between P. mirabilis and the TFP catheter surface with and without the deposition of the conditioning layer. Furthermore, the expansion of this study over a series of uropathogens and their cocultures is required to strengthen the finding.
Conclusions
P. mirabilis is the most common uropathogen which forms crystalline biofilms in urinary catheters and ultimately results in CAUTI. Multidrug resistance of P. mirabilis intensifies the importance of antiadhesion catheter walls other than treating CAUTI or modifying catheter walls with antimicrobials. Biofilm formation on urinary catheters can be delayed by applying with low surface-energy coatings such as TFP. Spraying TFP on partially-cured PDMS was identified as a potential method for improving the superhydrophobic and self-cleaning properties of PDMS. Under the flow conditions, TFP–PDMS exhibited the least amount of biofilm compared with commercially available catheter materials of siliconized latex and silicone because of the self-cleaning ability of TFP–PDMS. There is a relationship between the deposition of magnesium and calcium and biofilm formation on catheter materials used in the study. Surface structures and hydrophobicity might result in differences in P. mirabilis adsorption on the catheter materials. Therefore, TFP–PDMS can be applied as an antibiofilm-forming catheter material rather than commercially available silicone and siliconized latex catheter materials, as confirmed by in vitro studies with P. mirabilis.
Methodology
Materials
PDMS Sylgard 184 Kit with the components of base and curing agent was purchased from Dow Corning. Trifluoropropyl polyhedral oligomeric silsesquioxane (TFP, FL0583) was purchased from Hybrid Plastics, USA. All other chemicals used throughout the experiments were procured from Sigma Aldrich, USA. Tetrahydrofuran (THF, 99%) was used without any further purification.
Cultures and Broths
Proteus mirabilis (ATCC 12453) was used to study biofilm formation on the prepared silicone materials. Stock cultures were maintained on nutrient agar (NA, Sigma-Aldrich, USA) slant. Congo red indicator (Oxoid, UK) and bacteriological agar no. 1 (Oxoid, UK) were used to prepare congo red agar.
Fabrication of the TFP–PDMS Catheter Material
TFP-PDMS was prepared by the spray coating of TFP on partially cured PDMS.60 For the preparation of partially-cured PDMS sheets, the PDMS prepolymer was mixed in a 10:1 (w/w) base/catalyst ratio, stirred at 400 rpm for 5 min, and degassed for 5 min. The mixture was heated at 80 °C until the gel point was reached. A concentration of 30 mg mL–1 of TFP was prepared in THF and sprayed for 100 s on the half-cured PDMS sheet using a spray gun (over 10 cm from the sample with an approximate N2 pressure of 0.35 MPa) with continuous heating at 105 °C until the gel point of PDMS. The spray-coated samples were further cured at 80 °C for 1 h, followed by curing at 100 °C for 2 h.
To verify superhydrophobicity and superoleophilicity, the contact angles of TFP–PDMS against water (CAwater) and hexadecane (CAhexadecane) were tested using the sessile drop method. The sliding angle of TFP–PDMS for water was measured by rotating the stage together with the camera and the lamp as a whole unit. The total surface energy of the prepared surfaces (γs) were calculated using the contact angle hysteresis method using water (eqs 1 and 2).60 All the readings were taken after averaging five independent measurements of 5 μL liquid drops at room temperature in atmospheric pressure.
| 1 |
| 2 |
where θa is the advancing contact angle of water, θr is the receding contact angle of water, and θ is the static contact angle of the liquid. γs and γl are the total surface energy of the solid surface and the total surface energy of the liquid surface, respectively. (water γl = 72.8 mJ m–2; hexadecane γl = 27 mJ m–2).
The surface roughness was imaged using an atomic force microscope (AFM, PARK System×100).
Durability and Self-Healing Study
To determine the effect of UV exposure, a TFP–PDMS piece (2 cm × 2 cm) was placed under a UV lamp at a wavelength of 254 nm. The contact angles were measured after 3 h of continuous UV exposure. The effect of washing was tested at 100 rpm of shaking speed using deionized water for 1 h. Acid and base corrosion tests were carried out by immersing the samples in 1 mol dm–3 H2SO4 (pH = 0.0) and 1 mol dm–3 NaOH (pH = 14.0), respectively, for 1 h. The acid- and base-treated samples were washed with deionized water followed by 15 min heating at 100 °C. The sliding angles were measured after the temperature reached room temperature.
For self-healing studies, heat treatment was carried out by curing the corroded surfaces at 150 °C for 1 h. THF treatment was done by the spray-coating of THF over 4 s on the corroded surface followed by heating at 150 °C for 1 h.
Preparation of Artificial Urine
Artificial urine was prepared, as described by Torzewska and Rozalski.61 The solution was prepared by adding CaCl2.2H2O (0.651 g L–1), MgCl2·6H2O (0.651 g L–1), NaCl (4.6 g L–1), Na2SO4 (2.3 g L–1), sodium citrate (0.65 g L–1), sodium oxalate (0.02 g L–1), KH2PO4 (2.8 g L–1), KCl (1.6 g L–1), NH4Cl (1.0 g L–1), urea (25.0 g L–1), and creatine (1.1 g L–1). Tryptic soy broth (10.0 g L–1) was added as the organic medium. The pH of the solution was adjusted to 5.8 and sterilized by passing through a 0.20 μm pore-size filter. Artificial urine was stored at 4 °C.
Study Design for the Biofilm-Forming System
Square-shaped (0.5 cm × 0.5 cm) TFP–PDMS samples, commercially available silicone catheter segments (16 Fr, two-way silicone Foley catheter, Zhanjiang Star Enterprise Co. Ltd., Europe), and commercially available siliconized latex catheter segments (16 Fr, two-way siliconised foley catheter, Shree Umiya Surgical Pvt. Ltd., India) were used for the following experiment. The noncoated surface of TFP-PDMS and the outer surface of the commercial catheter segments were glued to a syringe using PDMS. For that, the PDMS base and curing agent were mixed with a mixing ratio of 10:1 and cured at 80 °C. The syringes were sterilized under UV radiation for 30 min.
A simple bladder model was designed, as described by Maierl et al. with modifications.62 The catheter materials were fixed to the flow cells using the PDMS base/curing agent mixture with a mixing ratio of 10:1 and cured at 80 °C. The bladder model consists of a peristaltic pump (BT 100SV2-W, Golander, USA) to allow a continuous urine flow, a glass bottle as the reservoir of urine, three bladder models prepared using syringes and silicone tubing to connect all the parts (Figure 7). All the parts were sterilized by UV irradiation before and after setting up the apparatus. After connecting the tubes, the system was sealed using silicone glue and set up in an incubator with a temperature of 37 °C. A volume of 150 mL of 5 × 105P. mirabilis cell suspension was supplied to the bladder model via a peristaltic pump. In this way, a constant volume of urine (30 mL) was collected in each syringe.
Figure 7.

Study design for the biofilm-forming system.
To mimic the flow conditions, the system was incubated for 14 days at a continuous flow rate of 5 mL min–1 with only one intervention that was the addition of 50 mL of urine every fortnight. After 14 days, urine was drained, and the catheter materials were carefully taken out under sterile conditions to use for further analysis.
For static conditions, the system was incubated at 37 °C for 14 days.63,64 The test samples were carefully taken out (three samples per day) under sterile conditions for further analysis.
3-(4, 5-Dimethylthiazolyl-2)-2, 5-Diphenyltetrazolium Bromide (MTT) Assay
Principle: The most viable cells’ mitochondrial activity is constant, and consequently, the number of viable cells is linearly related to the mitochondrial activity.65 The mitochondrial activity of the cells is reflected by the conversion of yellow tetrazolium MTT into purple formazan crystals, which can be solubilized for homogenous measurements. Thus, any alternation in the viable cell number can be detected by measuring the formazan concentration reflected in optical density (OD) that can be quantified by spectrophotometric means.
A volume of 50 μL of MTT working solution was added to the wells of a previously washed microtiter plate and incubated at 37 °C in a dark environment. After 4 h, remaining MTT was carefully aspirated using a micropipette. Purple-colored insoluble formazan product was solubilized in 100 μL of dimethyl sulfoxide (DMSO), and the absorbance was measured using a microtiter plate reader (SPECTRAmaxPLUS384 Molecular Devices, Inc., USA) by setting the detecting and reference wavelengths at 595 and 620 nm, respectively.
Crystal Violet Assay
Crystal violet assay is a measure of the DNA mass of living cells by staining DNA. The samples were washed with sterile PBS to remove substances excluded from the biofilm. The biofilms were stained with 200 μL of 0.1% crystal violet for 15 min, followed by washing with distilled water three times to remove any excess dye. For destaining, 200 μL of 30% acetic acid was introduced into wells and shaken for 15 min. The solubilized CV was then collected, and absorbance was measured at 595 nm using the microtiter plate reader. The mean CV activity for microorganism-treated materials was interpreted by deducing the absorbance of the control (eq 3).
| 3 |
Quantification of Calcium and Magnesium in the Biofilm
To determine the calcium and magnesium concentration, three catheter material segments processed under flow conditions were obtained after 14 days and moved to 94-well plates. The segments were washed twice with phosphate buffer (pH = 7.4). A volume of 200 μL of 10% nitric acid was added into each well. After 48 h, the plate was shaken at 200 rpm. The solutions in each well were aspirated into 25 mL volumetric flasks and diluted. The calcium and magnesium concentrations were determined by Atomic Adsorption Spectroscopy (AAS, Thermo Scientific iCE 3000).
Determination of the Ultrastructure of Biofilms on Catheter Materials
Biofilms were grown according to the procedure reported in the previous section. However, in the flow system, sterile flow cells purchased from Sigma Aldrich were used instead of syringes. The catheter materials with biofilms were washed three times with PBS. Then, they were transferred to an additional 24-well plate containing 2.5% glutaraldehyde at 4 °C. After 48 h, samples were subsequently washed with distilled water, dehydrated in a series of ethanol solutions to the ascending order (30, 50, 70, 80, 90, 95, and 100%), and air-dried overnight in a desiccator. Then, the biofilms were visualized using a scanning electron microscope following gold plating. Elemental analysis was conducted using a SEM–EDX detector.
Statistical Analysis
The statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS) version 16. Multiple means of more than three data sets were compared using one-way analysis of variance (ANOVA) and two-way ANOVA. The level of significance was taken at 5% (p < 0.05).
* Manjula Manoji Weerasekera - Department of Microbiology, Faculty of Medical Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka; Centre for Advanced Material Research, Faculty of Applied Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka.
Acknowledgments
We acknowledge the Department of Zoology, Faculty of Applied Sciences, USJ and Instrument center, Faculty of Applied Sciences for offering facilities for the research. For technical assistance in microbiology we thank Chamila Vithana and Dharmasiri, Department of Microbiology, Faculty of Medical Sciences, USJ.
Glossary
Abbreviations
- TFP-PDMS
trifluoropropyl spray-coated polydimethylsiloxane
- CAUTI
catheter-associated urinary tract infections
- CA
contact angle
- AFM
atomic force microscopy
- OD
optical density
- CV
crystal violet
- MTT
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
- EDX
energy-dispersive X-ray spectroscopy
- SEM
scanning electron microscopy
- AAS
atomic absorption spectroscopy
Author Present Address
# Discovery Labs, MAS Holdings, Sri Lanka(D.R.R.)
Author Present Address
⊥ Department of Basic Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka(A.D.)
Author Present Address
∥ Intelligent Polymer Research Institute/AIIM Faculty, Innovation Campus, University of Wollongong, NSW, Australia(B.G.)
Author Contributions
All authors have given approval to the final version of the manuscript.
This work was financially supported by the Centre for Advanced Materials Research, University of Sri Jayewardenepura, Sri Lanka, under the grant AMRC/RE/2016/MPhil-05.
The authors declare no competing financial interest.
References
- Stickler D. J. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J. Intern. Med. 2014, 276, 120–129. 10.1111/joim.12220. [DOI] [PubMed] [Google Scholar]
- Yamamichi F.; Shigemura K.; Kitagawa K.; Fujisawa M. Comparison between non-septic and septic cases in stone-related obstructive acute pyelonephritis and risk factors for septic shock: A multi-center retrospective study. J. Infect. Chemother. 2018, 24, 902–906. 10.1016/j.jiac.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Patel P. K.; Greene M. T.; Rogers M. A. M.; Ratz D.; Kuhn L.; Davis J.; Saint S. The epidemiology of hospital-acquired urinary tract-related bloodstream infection in veterans. Am. J. Infect. Control 2018, 46, 747–750. 10.1016/j.ajic.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B. G.; Ferguson J. K.; Anderson M.; Sear J.; Barnett A. Length of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state model. J. Hosp. Infect. 2016, 93, 92–99. 10.1016/j.jhin.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Trautner B. W.; Darouiche R. O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. 10.1016/j.ajic.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomfield R. J.; Morgan S. D.; Khan A.; Stickler D. J. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J. Med. Microbiol. 2009, 58, 1367–1375. 10.1099/jmm.0.012419-0. [DOI] [PubMed] [Google Scholar]
- Sabir N.; Ikram A.; Zaman G.; Satti L.; Gardezi A.; Ahmed A.; Ahmed P. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am. J. Infect. Control 2017, 45, 1101–1105. 10.1016/j.ajic.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Flores-Mireles A. L.; Walker J. N.; Caparon M.; Hultgren S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denstedt J. D.; Wollin T. A.; Reid G. Biomaterials Used in Urology: Current Issues of Biocompatibility, Infection, and Encrustation*. J. Endourol. 1998, 12, 493–500. 10.1089/end.1998.12.493. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod S. M.; Stickler D. J. Species interactions in mixed-community crystalline biofilms on urinary catheters. J. Med. Microbiol. 2007, 56, 1549–1557. 10.1099/jmm.0.47395-0. [DOI] [PubMed] [Google Scholar]
- Kunin C. M. Blockage of urinary catheters: Role of microorganisms and constituents of the urine on formation of encrustations. J. Clin. Epidemiol. 1989, 42, 835–842. 10.1016/0895-4356(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Stickler D. J. Bacterial biofilms in patients with indwelling urinary catheters. Nat. Clin. Pract. Urol. 2008, 5, 598–608. 10.1038/ncpuro1231. [DOI] [PubMed] [Google Scholar]
- Bardoloi V.; Yogeesha Babu K. V. Comparative study of isolates from community-acquired and catheter-associated urinary tract infections with reference to biofilm-producing property, antibiotic sensitivity and multi-drug resistance. J. Med. Microbiol. 2017, 66, 927–936. 10.1099/jmm.0.000525. [DOI] [PubMed] [Google Scholar]
- Vipin C.; Mujeeburahiman M.; Arun A. B.; Ashwini P.; Mangesh S. V.; Rekha P. D. Adaptation and diversification in virulence factors among urinary catheter-associated Pseudomonas aeruginosa isolates. J. Appl. Microbiol. 2019, 126, 641–650. 10.1111/jam.14143. [DOI] [PubMed] [Google Scholar]
- Pawar S.; Ashraf M. I.; Mujawar S.; Mishra R.; Lahiri C. In silico Identification of the Indispensable Quorum Sensing Proteins of Multidrug Resistant Proteus mirabilis. Front. Cell. Infect. Microbiol. 2018, 8, 269. 10.3389/fcimb.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korytny A.; Riesenberg K.; Saidel-Odes L.; Schlaeffer F.; Borer A. Bloodstream infections caused by multi-drug resistant Proteus mirabilis: Epidemiology, risk factors and impact of multi-drug resistance. Infect. Dis. 2016, 48, 428–431. 10.3109/23744235.2015.1129551. [DOI] [PubMed] [Google Scholar]
- Shelenkov A.; Petrova L.; Fomina V.; Zamyatin M.; Mikhaylova Y.; Akimkin V. Multidrug-Resistant Proteus mirabilis Strain with Cointegrate Plasmid. Microorganisms 2020, 8, 8111775 10.3390/microorganisms8111775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Deng Y.; Huang J.; Fan X.; Cheng C.; Nie C.; Ma L.; Zhao W.; Zhao C. Size-Transformable Metal–Organic Framework–Derived Nanocarbons for Localized Chemo-Photothermal Bacterial Ablation and Wound Disinfection. Adv. Funct. Mater. 2019, 29, 1900143 10.1002/adfm.201900143. [DOI] [Google Scholar]
- Yang Y.; Wu X.; Ma L.; He C.; Cao S.; Long Y.; Huang J.; Rodriguez R. D.; Cheng C.; Zhao C.; Qiu L. Bioinspired Spiky Peroxidase-Mimics for Localized Bacterial Capture and Synergistic Catalytic Sterilization. Adv. Mater. 2021, 33, 2005477 10.1002/adma.202005477. [DOI] [PubMed] [Google Scholar]
- Fan X.; Yang F.; Huang J.; Yang Y.; Nie C.; Zhao W.; Ma L.; Cheng C.; Zhao C.; Haag R. Metal–Organic-Framework-Derived 2D Carbon Nanosheets for Localized Multiple Bacterial Eradication and Augmented Anti-infective Therapy. Nano Lett. 2019, 19, 5885–5896. 10.1021/acs.nanolett.9b01400. [DOI] [PubMed] [Google Scholar]
- Singha P.; Locklin J.; Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. 10.1016/j.actbio.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mireles A. L.; Walker J. N.; Bauman T. M.; Potretzke A. M.; Schreiber H. L.; Park A. M.; Pinkner J. S.; Caparon M. G.; Hultgren S. J.; Desai A. Fibrinogen Release and Deposition on Urinary Catheters Placed during Urological Procedures. J. Urol. 2016, 196, 416–421. 10.1016/j.juro.2016.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Neoh K. G.; Kang E.-T.; Tambyah P. A.; Chiong E. Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J. Biomed. Mater. Res. Part B 2015, 103, 519–528. 10.1002/jbm.b.33230. [DOI] [PubMed] [Google Scholar]
- Neoh K. G.; Li M.; Kang E.-T.; Chiong E.; Tambyah P. A. Surface modification strategies for combating catheter-related complications: recent advances and challenges. J. Mater. Chem. B 2017, 5, 2045–2067. 10.1039/C6TB03280J. [DOI] [PubMed] [Google Scholar]
- Saeb A. T. M.; Al-Rubeaan K. A.; Abouelhoda M.; Selvaraju M.; Tayeb H. T. Genome sequencing and analysis of the first spontaneous Nanosilver resistant bacterium Proteus mirabilis strain SCDR1. Antimicrob. Resist. Infect. Control 2017, 6, 119. 10.1186/s13756-017-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H.; Moon J.-H.; Kim J. H.; Jeong S. M.; Lee S.-H. Flexible, stretchable and implantable PDMS encapsulated cable for implantable medical device. Biomed. Engineer. Lett. 2011, 1, 199. 10.1007/s13534-011-0033-8. [DOI] [Google Scholar]
- Martin S.; Bhushan B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. 10.1016/j.jcis.2016.10.094. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wu Y.; Zhang D.; Li L.; Wang T.; Duan S. Preparation of superhydrophobic flexible tubes with water and blood repellency based on template method. Colloids Surf., A 2020, 587, 124331 10.1016/j.colsurfa.2019.124331. [DOI] [Google Scholar]
- Li Z.; Nguyen B. L.; Cheng Y. C.; Xue J.; MacLaren G.; Yap C. H. Durable, flexible, superhydrophobic and blood-repelling surfaces for use in medical blood pumps. J. Mater. Chem. B 2018, 6, 6225–6233. 10.1039/C8TB01547C. [DOI] [PubMed] [Google Scholar]
- Lafuma A.; Quéré D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. 10.1038/nmat924. [DOI] [PubMed] [Google Scholar]
- Zimmermann J.; Seeger S.; Reifler F. A. Water Shedding Angle: A New Technique to Evaluate the Water-Repellent Properties of Superhydrophobic Surfaces. Text. Res. J. 2009, 79, 1565–1570. 10.1177/0040517509105074. [DOI] [Google Scholar]
- Han Z.; Feng X.; Jiao Z.; Wang Z.; Zhang J.; Zhao J.; Niu S.; Ren L. Bio-inspired antifogging PDMS coupled micro-pillared superhydrophobic arrays and SiO2 coatings. RSC Adv. 2018, 8, 26497–26505. 10.1039/C8RA04699A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyaei H.; Makki H.; Mohseni M.; Chapter 14 - Superhydrophobic coatings for medical applications. In Superhydrophobic Polym. Coat., Samal S. K.; Mohanty S.; Nayak S. K. (eds.) Elsevier, 2019, 321–338. [Google Scholar]
- Liu H.; Huang J.; Chen Z.; Chen G.; Zhang K.-Q.; Al-Deyab S. S.; Lai Y. Robust translucent superhydrophobic PDMS/PMMA film by facile one-step spray for self-cleaning and efficient emulsion separation. Chem. Eng. J. 2017, 330, 26–35. 10.1016/j.cej.2017.07.114. [DOI] [Google Scholar]
- Yu Q.; Wu Z.; Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. 10.1016/j.actbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Anjum S.; Singh S.; Benedicte L.; Roger P.; Panigrahi M.; Gupta B. Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances. Global Challenges 2018, 2, 1700068 10.1002/gch2.201700068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey V. K.; Srivastava K. R.; Ajmal G.; Thakur V. K.; Gupta V. K.; Upadhyay S. N.; Mishra P. K. Differential Susceptibility of Catheter Biomaterials to Biofilm-Associated Infections and Their Remedy by Drug-Encapsulated Eudragit RL100 Nanoparticles. Int. J. Mol. Sci. 2019, 20, 205110 10.3390/ijms20205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Wang L.; Liang X.; Vorstius J.; Keatch R.; Corner G.; Nabi G.; Davidson F.; Gadd G. M.; Zhao Q. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater Sci. Eng. 2019, 5, 2804–2814. 10.1021/acsbiomaterials.9b00071. [DOI] [PubMed] [Google Scholar]
- Nosonovsky M.; Bhushan B. Why re-entrant surface topography is needed for robust oleophobicity. Phil. Trans. R. Soc., A 2016, 374, 20160185 10.1098/rsta.2016.0185. [DOI] [PubMed] [Google Scholar]
- Jadhav S.; Shah R.; Bhave M.; Palombo E. A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. 10.1016/j.foodcont.2012.05.071. [DOI] [Google Scholar]
- Armbruster C. E.; Mobley H. L. T. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat. Rev. Microbiol. 2012, 10, 743–754. 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. V.; Mahenthiralingam E.; Sabbuba N. A.; Stickler D. J. Role of swarming in the formation of crystalline Proteus mirabilis biofilms on urinary catheters. J. Med. Microbiol. 2005, 54, 807–813. 10.1099/jmm.0.46123-0. [DOI] [PubMed] [Google Scholar]
- Stickler D. J.; Lear J. C.; Morris N. S.; Macleod S. M.; Downer A.; Cadd D. H.; Feast W. J. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J. Appl. Microbiol. 2006, 100, 1028–1033. 10.1111/j.1365-2672.2006.02840.x. [DOI] [PubMed] [Google Scholar]
- Wang P.; Henning S. M.; Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One 2010, 5, e10202 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tonder A.; Joubert A. M.; Cromarty A. D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC. Res. Notes 2015, 8, 47. 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. L.; Russell A. D.; Caliskan Z.; Stickler D. J. A Strategy for the Control of Catheter Blockage by Crystalline Proteus mirabilis Biofilm Using the Antibacterial Agent Triclosan. Eur. Urol. 2005, 48, 838–845. 10.1016/j.eururo.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sabbuba N.; Hughes G.; Stickler D. J. The migration of Proteus mirabilis and other urinary tract pathogens over Foley catheters. BJU Int. 2002, 89, 55–60. 10.1046/j.1464-410X.2002.02560.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen S. M.; Stickler D. J.; Mobley H. L. T.; Shirtliff M. E. Complicated Catheter-Associated Urinary Tract Infections Due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26. 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutipongtanate S.; Sutthimethakorn S.; Chiangjong W.; Thongboonkerd V. Bacteria can promote calcium oxalate crystal growth and aggregation. J. Biol. Inorg. Chem. 2013, 18, 299–308. 10.1007/s00775-012-0974-0. [DOI] [PubMed] [Google Scholar]
- Wei F.; Yu H.; Zeng Z.; Liu H.; Wang Q.; Wang J.; Li S. Preparation and Structural Characterization of Hydroxylethyl Methacrylate Grafted Natural Rubber Latex. Polímeros 2014, 24, 283–290. 10.4322/polimeros.2014.068. [DOI] [Google Scholar]
- Silva S.; Araújo L.; Nascimento Junior J. A.; Silva T.; Lopes A. C.; Correia M. T.; Silva M.; Oliveira M. B. Effects of Cefazolin and Meropenem in Eradication Biofilms of Clinical and Environmental Isolates of Proteus mirabilis. Curr. Microbiol. 2020, 77, 1681–1688. 10.1007/s00284-020-01984-7. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer A.; Morris N.; Feast W. J.; Stickler D. Polymer surface properties and their effect on the adhesion of Proteus mirabilis. Proc. Inst. Mech. Eng. Part H 2003, 217, 279–289. 10.1243/095441103322060730. [DOI] [PubMed] [Google Scholar]
- Czerwonka G.; Guzy A.; Kal̷uża K.; Grosicka M.; Dańczuk M.; Lechowicz L̷.; Gmiter D.; Kowalczyk P.; Kaca W. The role of Proteus mirabilis cell wall features in biofilm formation. Arch. Microbiol. 2016, 198, 877–884. 10.1007/s00203-016-1249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holling N.; Dedi C.; Jones C. E.; Hawthorne J. A.; Hanlon G. W.; Salvage J. P.; Patel B. A.; Barnes L. M.; Jones B. V. Evaluation of environmental scanning electron microscopy for analysis of Proteus mirabilis crystalline biofilms in situ on urinary catheters. FEMS Microbiol. Lett. 2014, 355, 20–27. 10.1111/1574-6968.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf A. S. H.; Herrera V.; Muñoz E.; Chapter 6 - Corrosion and protection of the metallic structures in the petroleum industry due to corrosion and the techniques for protection. In Handbook of Materials Failure Analysis, Makhlouf A. S. H.; Aliofkhazraei M. (eds.) Butterworth-Heinemann, 2018, 107–122. [Google Scholar]
- Wilks S. A.; Fader M. J.; Keevil C. W. Novel Insights into the Proteus mirabilis Crystalline Biofilm Using Real-Time Imaging. PLoS One 2015, 10, e0141711–e0141711. 10.1371/journal.pone.0141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen S. M.; Shirtliff M. E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2011, 2, 460–465. 10.4161/viru.2.5.17783. [DOI] [PubMed] [Google Scholar]
- Gayani B.; Senarathna D.; Weerasekera M. M.; Kottegoda N.; Ratnaweera D. R. Improving superhydrophobicity of polydimethylsiloxanes using embedding fluorinated polyhedral oligomeric silsesquioxanes cages. SN Appl. Sci. 2020, 2, 1944. 10.1007/s42452-020-03721-y. [DOI] [Google Scholar]
- Torzewska A.; Różalski A. Various intensity of Proteus mirabilis-induced crystallization resulting from the changes in the mineral composition of urine. Acta Biochim. Pol. 2015, 62, 127–132. 10.18388/abp.2014_882. [DOI] [PubMed] [Google Scholar]
- Maierl M.; Jörger M.; Rosker P.; Reisner A. In vitro Dynamic Model of a Catheterized Bladder and Biofilm Assay. Bio-protoc. 2015, 5, e1381 10.21769/BioProtoc.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgadevi R.; Kaleeshwari R.; Swetha T. K.; Alexpandi R.; Karutha Pandian S.; Veera Ravi A. Attenuation of Proteus mirabilis colonization and swarming motility on indwelling urinary catheter by antibiofilm impregnation: An in vitro study. Colloids Surf. B. Biointerfaces 2020, 194, 111207 10.1016/j.colsurfb.2020.111207. [DOI] [PubMed] [Google Scholar]
- Frant M.; Dayyoub E.; Bakowsky U.; Liefeith K. Evaluation of a ureteral catheter coating by means of a BioEncrustation in vitro model. Int. J. Pharm. 2018, 546, 86–96. 10.1016/j.ijpharm.2018.04.023. [DOI] [PubMed] [Google Scholar]
- van Meerloo J.; Kaspers G. J.; Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 2011, 731, 237–245. 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]



