Abstract

Nanoparticulate double-heterojunction photocatalysts comprising TiO2(Anatase)/WO3/TiO2(Rutile) were produced by a sol–gel method. The resulting photocatalysts exhibit clear synergistic effects when tested toward the degradation of methyl orange under both UV and visible light. Kinetic studies indicate that the degradation rate on the best double-heterojunction photocatalyst (10 wt % WO3-TiO2) depends mainly on the amount of dye concentration, contrary to pure oxides in which the degradation rate is limited by diffusion-controlled processes. The synergistic effects were confirmed through systematic and careful studies including holes and OH radical formation, X-ray diffraction, electron microscopy, elemental analysis, UV–vis diffuse reflectance spectroscopy, and surface area analysis. Our results indicate that the successful formation of a double heterojunction in the TiO2(Anatase)/WO3/TiO2(Rutile) system leads to enhanced photoactivity when compared to individual oxides and commercial TiO2 P25.
1. Introduction
Technological applications of heterogeneous photocatalysis have been successfully reported in water and air treatment,1−5 CO2 reduction,6−8 and hydrogen production.9−12 Commercial TiO2 P25 is one of the most widely used photocatalyst. It is recognized for its good catalytic activity, high stability, insolubility in water, and low cost.9,13−15 Part of the success of TiO2 P25 under UV irradiation lies on the natural heterojunction formed between rutile and anatase; the presence of this junction enables the separation of photogenerated electron–hole (e––h+) pairs, decreasing their recombination. However, TiO2 P25 can only work efficiently under UV illumination given its large band gap16−19 and suffers from high recombination of the photogenerated e––h+ pairs.20−23
As a result, several efforts have been made to tune the absorption range of TiO2 toward the visible region. A common approach is the addition of dopants, such as noble metals5,19,24,25 or some light elements (e.g., C, N, or S).3,26−29 Another relevant strategy is the formation of heterostructures comprising TiO2 P25 and narrow band gap semiconductors (e.g., WO3) with a more negative (positive) conduction (valence) band level.15,21,30−35 The latter allows an efficient transfer of photogenerated electrons/holes from the guest semiconductor to TiO2, significantly reducing the e––h+ recombination.13 For this particular reason, TiO2-WO3 composites are widely used as photocatalysts,36−38 where the 2.8 eV band gap of WO3, in addition to the positions of its valence and conduction bands, allows an efficient transfer of photogenerated holes from WO3 to TiO2 and electrons from TiO2 to WO3. The latter is possible due to the small standard reduction potential (−0.03 eV) between W(VI) and W(V).39 Subsequently, WO3 can transfer electrons to adsorbed oxygen molecules on TiO2, resulting in enhanced photocatalytic degradation of organic molecules.13
Therefore, here, we report the creation of a double-heterojunction photocatalyst comprising TiO2 rutile, TiO2 anatase, and monoclinic WO3. The larger affinity of WO3 toward anatase is used to minimize anatase transformation to rutile, and in addition, the coupling of WO3 with the anatase/rutile system results in enhanced charge transfer of photogenerated electron and holes, reducing recombination rates and enhancing the production of hydroxyl radicals under both visible and UV light illumination. The TiO2(Anatase)/WO3/TiO2(Rutile) double heterojunction was produced by using a simple sol–gel method. The nanocomposite containing 10% of WO3 in a mixture of anatase and rutile exhibited clear synergistic effects during the photocatalytic degradation of methyl orange (MO) in both UV and visible light. The morphology, elemental composition, kinetic parameters, and degradation mechanisms are investigated.
2. Experimental Section
2.1. Synthesis of WO3
The monoclinic phase of tungsten trioxide was obtained by dissolving 2 g of ammonium tungstate hydrate ((NH4)10(H2W12O42)·4H2O) in deionized (DI) water (85 mL) at 80 °C. Afterward, concentrated HNO3 (15 mL) was added dropwise, and the suspension was kept under constant reflux and agitation for 30 min. The suspension was then transferred to an ultrasonic bath (30 min). The resulting solids were filtered, washed with DI water, and dried (70 °C, 24 h). The obtained powder was ground and calcined (500 °C, 4 h) in air.
2.2. Synthesis of Anatase and Rutile TiO2
Anatase TiO2 was synthetized by the sol–gel method using titanium isopropoxide (4.5 mL) dissolved in 2-propanol (50 mL) and acetic acid (5 mL). The hydrolysis was initiated by adding DI water (5 mL) dropwise. The solution was then kept at 70 °C for 60 min under constant agitation and reflux. Afterward, the sample was dried at 100 °C for 24 h, ground, and finally calcined at 400 °C (4 h) in air. This material was labeled TiO2(Anatase). TiO2 rutile was prepared by direct calcination of 6 g of TiO2 Evonik P25 at 800 °C for 4 h in air, labeled as TiO2 P25(Rutile).
2.3. Synthesis of Double-Heterojunction Photocatalysts
Three double-heterojunction photocatalysts with varying contents of WO3 (10, 20, and 30 wt %) and TiO2 were prepared, denoted as 10%WO3-TiO2, 20%WO3-TiO2, and 30%WO3-TiO2, respectively. Initially, a solution containing titanium isopropoxide (4.5 mL), 2-propanol (50 mL), and acetic acid (5 mL) was prepared. In a separate container, WO3 and TiO2 P25 (1 g) were mixed with 2-propanol (40 mL) for 30 min using an ultrasonic probe (VXC 130, Sonics & Materials, Inc.). This suspension was later combined with the initial acid solution. The hydrolysis was initiated by adding DI water (5 mL) dropwise; the mixture was kept at 70 °C for 60 min under constant agitation and reflux. The samples were dried at 100 °C for 24 h, then ground, and calcined at 800 °C for 4 h under air with a heating rate of 5 °C min–1.
2.4. Materials Characterization
X-ray diffraction was performed using a Bruker D8 Advance diffractometer (Cu Kα, λ = 1.5406 Å; 40 kV and 40 mA), and a step size of 0.05°·s–1 was used. The JCPDS (Joint Committee on Powder Diffraction Standards) database was used to identify the crystalline phases. The proportion of the crystalline phases was evaluated using the software Materials Analysis Using Diffraction (MAUD). The Scherrer equation was used to evaluate the crystallite sizes, L = κλ/β cos(θ), where κ (0.89) is the Scherrer constant, λ is the wavelength of the X-ray radiation, and β is the full width at half maximum of the diffraction peak at 2θ.40 The materials’ morphology was analyzed using a scanning electronic microscope (Carl Zeiss Merlin) equipped with an energy dispersive X-ray spectroscopy (EDX) analyzer, and a thin gold coating was used to improve the conductivity of the samples. Transmission electron microscopy (TEM) images were obtained on an FEI Talos L 120C. X-ray photoelectron spectroscopy (XPS) was performed with a Kratos Axis Ultra DLD electron spectrometer (Al Kα line of 1486.6 eV); the XPS spectra were calibrated with C 1s = 284.4 eV (C–C sp2).41,42 The experimental core-level spectra were fitted by using Gaussian curves, and a Shirley background subtraction was applied in the fitting process. The band gap (Eg) was determined with UV–vis diffuse reflectance spectroscopy (Thermo Scientific Evolution 600) and the Kubelka–Munk method.43 All samples were examined in the 200–800 nm range, and then, by plotting [h(c/λ)f(R)]0.5 vs hc/λ, the Eg can be found when extrapolating the slope to intercept the x axis. Here, h = 6.62607004 × 10–34 m2 kg s–1, c is the speed of light in m s–1, λ is the wavelength (m), and f(R) is the information provided by the UV–vis spectrophotometer. The specific surface area was evaluated by using the Brunauer–Emmett–Teller (BET) method using nitrogen physisorption with the Nova 2000e Quantachrome Instruments. All samples were degassed at 300 °C for 1 h before the analysis.
2.5. Determination of the Photocatalytic Activity
The degradation was carried out in a reacting system comprising an annular stainless steel cylinder with an internal mirror and four symmetrically distributed near-UV light lamps T-15 L (15 W, λmax = 365 nm; emission spectra shown in Figure S1). A Pyrex glass cylindrical cell is located at the center of the photoreactor, and it has three access ports for sampling, feeding, and gas evacuation.1,2 The photocatalytic activity under visible light was investigated using four FL15AQ lamps (15 W) adapted with a polycarbonate filter to ensure that only visible light reached the suspension (Figure S2 depicts the FL15AQ lamp emission spectra with and without a UV filter). A reaction volume of 250 mL, 0.5 g of photocatalyst, and 0.06 mM initial MO concentration were used during the degradation reactions. The total reaction time of 6 h was used, with a constant oxygen flow (100 mL min–1) and agitation. The reaction was monitored by taking a sample every hour, centrifuged, and analyzed using UV–vis spectroscopy.
2.6. Determination of Holes in the Photocatalysts’ Valence Band
The hole formation was investigated using the
iodine ion (I–) reaction as shown in eq 1, where the I–/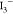 pair has a potential
equal to +0.536 V,
which is smaller than the valence band potentials for WO3 (+2.9 V) and TiO2 (+2.7 V).
pair has a potential
equal to +0.536 V,
which is smaller than the valence band potentials for WO3 (+2.9 V) and TiO2 (+2.7 V).
| 1 |
The 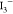 ion was
monitored using UV–vis spectroscopy
utilizing its two absorption peaks at 286 and 345 nm. The experiments
were carried out by using an aqueous solution of potassium iodine
(0.01 M, 250 mL) containing 0.5 g of photocatalyst. The suspension
was kept in the dark for 20 min to reach the adsorption–desorption
equilibrium. Afterward, all near-UV lamps were switched on, and samples
were collected every 30 min. The analysis was performed using a UV–vis
spectrophotometer (2401PC Shimadzu).
ion was
monitored using UV–vis spectroscopy
utilizing its two absorption peaks at 286 and 345 nm. The experiments
were carried out by using an aqueous solution of potassium iodine
(0.01 M, 250 mL) containing 0.5 g of photocatalyst. The suspension
was kept in the dark for 20 min to reach the adsorption–desorption
equilibrium. Afterward, all near-UV lamps were switched on, and samples
were collected every 30 min. The analysis was performed using a UV–vis
spectrophotometer (2401PC Shimadzu).
2.7. Determination of Hydroxyl Radicals
The formation of ·OH radicals was monitored by their oxidation with terephthalic acid where the produced 2-hydroxy-terephthalic acid can be quantified by using its fluorescence signal at 426 nm.30 In these experiments, a solution containing NaOH (0.01 M), terephthalic acid (20 ppm), and a photocatalyst (0.5 g) was prepared. The mixture was kept under dark conditions for 20 min to achieve an adsorption–desorption equilibrium. Afterward, all four near-UV lamps were switched on, and samples were extracted every 15 min in the first hour and then at 90 and 120 min. A Cary Eclipse fluorescence spectrophotometer was used to analyze the samples using an excitation wavelength of 315 nm, an emission range of 350–600 nm, a slit of 2.5, low voltage, and low scan.
3. Results and Discussion
3.1. Photocatalyst Characteristics: Crystal Phase, Band Gap, and Morphology
The crystal structure, phase composition, and average crystallite size of the selected photocatalysts were investigated using powder X-ray diffraction. First, commercial TiO2 Evonik P25 (labeled as TiO2 P25) comprises both anatase and rutile phases (see Figure 1), with an estimated composition of 84.2% anatase and 15.8% rutile and average crystallite sizes of 28 and 167 nm for anatase and rutile, respectively. The subsequent calcination of TiO2 P25 at 800 °C in air results in the transformation of the anatase phase into rutile (JCPDS 00-021-1272) with an average crystallite size of 290 nm; this material was labeled as TiO2 P25(Rutile). The TiO2 synthetized by the sol–gel method and calcined at 400 °C exhibited a pure anatase phase (JCPDS 00-021-1272) with a crystallite size of 12 nm. Last, the produced WO3 had a monoclinic phase type (JCPDS 00-043-1035) with a crystallite size of 54 nm. For the case of the double-heterojunction TiO2(Anatase)/WO3/TiO2(Rutile) photocatalysts, three different WO3-TiO2 mixtures were considered, namely, 10, 20, and 30 wt % WO3 (labeled as 10%WO3-TiO2, 20%WO3-TiO2, and 30%WO3-TiO2, respectively). All heterojunctions consisted of both TiO2 anatase and TiO2 rutile, as well as monoclinic WO3. The proportion of crystalline phases is shown in Table 1. The results indicate that increasing the amount of tungsten trioxide inhibits the transformation of anatase into rutile, typically at 600 °C. This observation is consistent with previous reports,44,45 where tungsten atoms strongly interact with the edge of TiO2 crystals, limiting the crystal growth and anatase-to-rutile transition. The latter indicates an intimate contact between the individual components of our WO3-TiO2 heterojunction.
Figure 1.

X-ray diffractograms of individual oxides and the three double-heterojunction photocatalysts. The reference JCPDS data are plotted for comparison.
Table 1. Composition of Crystal Phases in Double-Heterojunction Photocatalystsa.
| crystal phase | 10% WO3 | 20% WO3 | 30% WO3 |
|---|---|---|---|
| TiO2 rutile | 45.6 (223 nm) | 10.1 | 8.1 |
| TiO2 anatase | 48.6 (45 nm) | 81.7 (39 nm) | 76.7 (38 nm) |
| WO3 monoclinic | 5.8 | 8.2 | 15.2 |
The numbers in parentheses indicate the crystallite size.
The band gap (Eg) of all photocatalysts was evaluated using UV–vis diffuse reflectance spectroscopy. The resulting absorption spectra and Kubelka–Munk (Tauc) plots are shown in Figure 2. The Eg for pure oxides agrees with previous reports, as seen in Table 2. The commercial TiO2 P25 has an Eg of 3.2 eV, while the calcinated TiO2 P25(Rutile) has a slight reduction to 3.0 eV; the reduced Eg is expected due to anatase phase transformation into rutile. The absorption spectra of all three WO3-TiO2 heterojunctions highlight their capacity to additionally absorb parts of the visible light, a desirable characteristic for photocatalysts since the solar spectrum contains predominantly visible light (45%).40 Both 10%WO3-TiO2 and 20%WO3-TiO2 photocatalysts have equal energy band gaps of 2.9 eV, which are smaller than 3 eV for pure TiO2 rutile. On the other hand, 30%WO3-TiO2 exhibits an even smaller Eg of 2.6 eV, an intermediate value between WO3 and rutile, highlighting the proper intermix of all three components. The latter indicates the suitability to modulate Eg by the addition of WO3.
Figure 2.

(a) Absorption spectra and (b) Kubelka–Munk plot of selected photocatalysts.
Table 2. Band Gap of Heterojunctions and Individual Photocatalysts.
We now focus on the 10%WO3-TiO2 nanocomposite to evaluate in detail the morphology and elemental composition. This double heterojunction showed the best photocatalytic activity, as we will discuss later. SEM studies (Figure S3a,b and Figure 3a,b) reveal a homogeneous nanoparticulated material with an average particle size of ∼50 nm. A closer inspection by TEM (Figure S3c–e and Figure 3c–e) shows that WO3 and TiO2 nanoparticles exhibit a thin layer of amorphous material. Although we could not identify the nature of such layer, similar amorphous layers have been previously observed in other WO3-TiO2 systems where it has been identified as tungsten oxides.44 In particular, Figure 3d,e shows two nanoparticles with clear crystalline planes corresponding to WO3 and TiO2 anatase, respectively. We further investigated the oxidation state of Ti and W by XPS studies. The survey spectrum of 10%WO3-TiO2 is displayed in Figure 4a, indicating only the existence of C, O, Ti, and W. The high-resolution spectra of O, Ti, and W are shown in Figure 4b–d, respectively. In Figure 4b, the deconvoluted XPS spectrum of O 1s includes three individual peaks. The main peak at 530.1 eV is assigned to the synthesized TiO2 and WO3, while the peak at 531.0 eV can be assigned to either substoichiometric WOx49,50 or hydroxyl groups adsorbed on the oxides’ surface.51 Another small feature at 532.1 eV can be attributed to the presence of oxygen-containing hydrocarbons,52 probably due to remnants from the synthesis process. Figure 4c shows only one doublet of Ti 2p at 458.8/464.6 eV with no other peaks, indicating that all Ti atoms have the same oxide state (Ti4+).53,54 On the other hand, the deconvolution of W 4f spectra yields two doublets, as shown in Figure 4d. The first one at 35.7/37.9 eV is attributed to W6+ in WO3,55,56 while the second one at 34.2/36.4 eV might be caused by the photoemission of W5+ present in substoichiometric WOx (2 < x < 3);57,58 this feature is consistent with the O 1s peak seen at 531.0 eV.
Figure 3.

(a, b) SEM and (c–e) TEM micrographs of the double heterojunction 10%WO3-TiO2.
Figure 4.
(a) Overall XPS spectra for 10%WO3-TiO2. High-resolution XPS of (b) O, (c) Ti, and (d) W.
3.2. Photocatalytic Activity under Near-UV and Visible Light
The degradation of methyl orange under near-UV and visible light irradiation was used to evaluate the photocatalytic activity; the results are shown in Figure 5a,b. All heterojunctions exhibited better MO degradation when compared to individual oxides, but in particular, both 10%WO3-TiO2 and 20%WO3-TiO2 photocatalysts exhibited the best photocatalytic activity with ∼99% degradation of MO after 300 min under near-UV irradiation, while 30%WO3-TiO2 only achieved ∼90% degradation during the same time. We also evaluated the MO degradation under similar conditions of a simple mixture of TiO2 P25 and WO3 (10 wt %) prepared by grinding the materials in a mortar, labeled as 10%WO3-TiO2(mixture) in Figure 5a. This mixture only achieved ∼50% MO degradation after 300 min, clearly highlighting the importance of an intimate contact between the oxides achieved during the synthesis process. Figure 5b shows the results of MO degradation under visible light (436 nm). In this occasion, the 10%WO3-TiO2 photocatalyst clearly exhibited the best performance, achieving an MO degradation of ∼25% after 300 min, while the other heterojunctions only achieved 15–17%, and pure oxides showed negligible MO degradation. Although the 10%WO3-TiO2 photocatalyst exhibits a relatively large Eg, large particle size, and low surface area (Table S1) when compared to other heterojunctions, the excellent photocatalytic activity indicates that the adequate distribution of the material and the crystal phase (rutile, 45.6 and 5.8% WO3) have a major role in achieving high catalytic activity. These results show the feasibility for a double-heterojunction system to reduce charge recombination, achieving a higher density of positive holes to produce hydroxyl radicals.
Figure 5.

Degradation of methyl orange in aqueous solutions with (a) near-UV and (b) visible light illumination.
3.3. Kinetic Modeling
The kinetics of the photocatalytic degradation of MO when using near-UV illumination was examined with the first-order and modified Freundlich models.59 Here, the apparent reaction rate provides quantitative information regarding the MO degradation and mechanistic details. For the first-order model (see details in the Supporting Information), we assumed the Langmuir–Hinshelwood mechanism,60 in which the reaction rate, r, is given by
| 2 |
Here, k1 is the rate constant that includes parameters such as the maximum amount of compound adsorbed on the photocatalyst surface (see the Supporting Information for more details), k2 is the absorption constant, and C is the dye concentration. At a very low dye concentration, the term k2C ≪ 1, making the reaction rate apparently of first degree. We can now define the apparent reaction rate rapp and its apparent rate constant kapp (eq 3), which, after integration, results in eq 4.
| 3 |
| 4 |
where w is the photocatalyst mass, v is the volume of the reaction, and C0 and C are the initial dye concentration and the dye concentration after the photocatalytic degradation, respectively. On the other hand, the modified Freundlich kinetic model is characterized by eq 5,61
| 5 |
The constant in eq 5 has a similar meaning to that in eq 4. The kinetic parameters were estimated by doing a linear regression of the MO concentration in time (see Figures S4 and S5) and the experimental data in Figure 5a. The coefficient of determination R2 was then used to evaluate the quality of the fittings. The apparent reaction rates and R2 values are shown in Table 3. The obtained R2 values indicate that 10%WO3-TiO2 and 20%WO3-TiO2 heterojunctions follow the first-order model, which means that the degradation rate depends mainly on the amount of dye molecules in the solution. In contrast, other photocatalysts were better described by the modified Freundlich model, which describes a degradation mechanism controlled by ion-exchange and diffusion-controlled processes.62 The value of the apparent rate constant can be used to compare the photocatalytic activity, and as expected, the highest value of kapp was obtained for 10%WO3-TiO2 when compared to other photocatalysts (fitted by the same model). Therefore, independent of the model used, 10%WO3-TiO2 exhibits the best performance in agreement with the experimental observations.
Table 3. Kinetic Model and Apparent Reaction Rate.
| first
order |
modified
Freundlich |
|||
|---|---|---|---|---|
| photocatalyst | kapp × 10–3 (min–1) | R2 | kapp × 10–3 (L g–1 min–1) | R2 |
| 10%WO3-TiO2 | 14.8 | 0.9958 | 96.8 | 0.9219 |
| 20%WO3-TiO2 | 14.1 | 0.9735 | 53.0 | 0.9717 |
| 30%WO3-TiO2 | 7.3 | 0.9576 | 13.0 | 0.9967 |
| TiO2 P25 | 9.3 | 0.9533 | 11.5 | 0.9953 |
| TiO2 P25(Rutile) | 4.9 | 0.9871 | 6.6 | 0.9977 |
| TiO2(Anatase) | 6.0 | 0.9728 | 15.8 | 0.9973 |
| WO3 | 0.6 | 0.8852 | 9.7 | 0.9594 |
3.4. Hole Formation and Determination of ·OH Radicals
Figure 6a depicts the fluorescence spectra of heterojunctions and
pure oxides under near-UV illumination. All photocatalysts showed
the characteristic tri-iodine ion (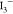 ) peaks, indicating that
holes (h+) are formed at the valence band of both WO3 and TiO2. The spectrum of both 10%WO3-TiO2 and
20%WO3-TiO2 shows the highest intensity, which
is proportional to the h+ density. Additionally, the abundance
of ·OH radicals was evaluated via the production of
2-hydroxy-terephthalic acid using fluorescence spectroscopy (Figure 6b).30 The 10%WO3-TiO2 photocatalyst showed
the highest fluorescence signal, indicating that it not only exhibits
a high h+ density but also yields the
highest production of ·OH radicals. These results
are consistent with MO degradation under near-UV and visible light,
confirming a degradation pathway mainly due to ·OH
radicals.
) peaks, indicating that
holes (h+) are formed at the valence band of both WO3 and TiO2. The spectrum of both 10%WO3-TiO2 and
20%WO3-TiO2 shows the highest intensity, which
is proportional to the h+ density. Additionally, the abundance
of ·OH radicals was evaluated via the production of
2-hydroxy-terephthalic acid using fluorescence spectroscopy (Figure 6b).30 The 10%WO3-TiO2 photocatalyst showed
the highest fluorescence signal, indicating that it not only exhibits
a high h+ density but also yields the
highest production of ·OH radicals. These results
are consistent with MO degradation under near-UV and visible light,
confirming a degradation pathway mainly due to ·OH
radicals.
Figure 6.

(a) Fluorescence spectra of KI solutions with various photocatalysts under near-UV illumination. (b) Fluorescence spectra of 2-hydroxy-terephthalic acid in the presence of various photocatalysts (315 nm excitation).
3.5. Activation Mechanism under Near-UV and Visible Light
We are now in the position to propose an activation mechanism for the TiO2(Anatase)/WO3/TiO2(Rutile) heterojunctions under UV light where the improved performance is attributed to the coexistence of all three materials. Under UV irradiation, all three phases contribute to e––h+ pair production; however, generated electrons in the conduction band (CB) of anatase can be easily transferred to the CB of both rutile and WO3 because of its less negative redox potential in comparison with anatase. Similarly, the generated h+ in the valence band (VB) of WO3 can be transferred to the VB of anatase, resulting in reduced charge recombination and improved production of hydroxyl radicals. In addition, the generated h+ in all phases have suitable redox potentials to produce hydroxyl radicals that can oxidize adsorbed dye molecules, as specified in eqs 6–10 in the reaction mechanism, producing, through several intermediates, CO2 and H2O (eq 10).
Superoxide anions are produced by the photogenerated electrons and oxygen molecules that later form peroxide radicals when interacting with protons. These peroxyl radicals also interact with organic compounds to form intermediates, CO2, and H2O (eqs 11–13). Additionally, peroxyl radicals can form hydrogen peroxide and oxygen molecules (eq 14) by interacting with protons and superoxide anions. The produced hydrogen peroxide can be later broken down into ·OH radicals by UV light (eq 15).
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
| 15 |
It should be emphasized that all three WO3-TiO2 heterojunctions also displayed photocatalytic activity under visible light. In this case, the activation mechanism (Figure 7b) considers that TiO2 rutile and monoclinic WO3 are activated upon visible light irradiation, generating e––h+ pairs. The h+ generated in the VB of WO3 and rutile produce hydroxyl radicals that can be transferred to the anatase VB. As a result, a reduced charge recombination is obtained with a subsequent increase in ·OH radical formation, as shown in eq 8. In this situation, the generated electrons in the conduction bands of WO3 and rutile are not transferred to anatase because of their more positive redox potential than those of anatase.
Figure 7.

Proposed activation mechanism for TiO2(Anatase)/WO3/TiO2(Rutile) double-heterojunction photocatalysts under (a) near-UV and (b) visible light irradiation.
4. Conclusions
Double-heterojunction TiO2(Anatase)/WO3/TiO2(Rutile) photocatalysts were synthetized by a simple sol–gel method. The 10%WO3-TiO2 and 20%WO3-TiO2 composites showed the largest synergistic effect observed during the degradation of methyl orange under UV and visible light. The best MO degradation was achieved with 10 wt % WO3-TiO2, and this photocatalyst exhibited a relatively large Eg, large particle size, and low surface area; however, it exhibited the highest hole density and ·OH production, resulting in enhanced photocatalytic activity. The synergistic effect was explained by considering the formation of a double heterojunction between anatase, rutile, and WO3. Our study shows that tuning the materials’ content and crystal phase during the catalyst production can result in significant changes in the photocatalytic activity.
Acknowledgments
J.A.P.-E. acknowledges experimental support from the laboratory of Photocatalysis at Universidad Autónoma de San Luís Potosí, the laboratory of Ecomaterials at Universidad Autónoma de Nuevo León, and the laboratory of Nanomaterials at Universidad Autónoma de Zacatecas. E.G.-E. acknowledges support from the Swedish Research Council (2018-03937) and the Olle Engkvist Foundation (186-0637). The samples were partially characterized at the Umeå Core Facility for Electron Microscopy (UCEM) and the Vibrational Spectroscopy Core Facility (ViSp) at Umeå University. Andrey Shchukarev (Umeå University, KBC) is also acknowledged for his assistance with XPS measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06054.
Derivation of the first-order model, emission spectra of the light sources, specific surface areas of all photocatalysts, electron microscopy images, and data fitting of kinetic models (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pinedo A.; López M.; Leyva E.; Zermeño B.; Serrano B.; Moctezuma E. Photocatalytic Decomposition of Metoprolol and Its Intermediate Organic Reaction Products: Kinetics and Degradation Pathway. Int. J. Chem. React. Eng. 2016, 14, 809–820. 10.1515/ijcre-2015-0132. [DOI] [Google Scholar]
- Moctezuma E.; Leyva E.; López M.; Pinedo A.; Zermeño B.; Serrano B. Photocatalytic Degradation of Metoprolol Tartrate. Top. Catal. 2013, 56, 1875–1882. 10.1007/s11244-013-0119-x. [DOI] [Google Scholar]
- Likodimos V.; Han C.; Pelaez M.; Kontos A. G.; Liu G.; Zhu D.; Liao S.; de la Cruz A. A.; O’Shea K.; Dunlop P. S. M.; Byrne J. A.; Dionysiou D. D.; Falaras P. Anion-Doped TiO2 Nanocatalysts for Water Purification under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 13957–13964. 10.1021/ie3034575. [DOI] [Google Scholar]
- Shaham-Waldmann N.; Paz Y. Away from TiO2: A Critical Minireview on the Developing of New Photocatalysts for Degradation of Contaminants in Water. Mater. Sci. Semicond. Process. 2016, 42, 72–80. 10.1016/j.mssp.2015.06.068. [DOI] [Google Scholar]
- Pelaez M.; Nolan N. T.; Pillai S. C.; Seery M. K.; Falaras P.; Kontos A. G.; Dunlop P. S. M.; Hamilton J. W. J.; Byrne J. A.; O’Shea K.; Entezari M. H.; Dionysiou D. D. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. 10.1016/j.apcatb.2012.05.036. [DOI] [Google Scholar]
- Truong Q. D.; Liu J.-Y.; Chung C.-C.; Ling Y.-C. Photocatalytic Reduction of CO2 on FeTiO3/TiO2 Photocatalyst. Catal. Commun. 2012, 19, 85–89. 10.1016/j.catcom.2011.12.025. [DOI] [Google Scholar]
- Liu X.; Ye L.; Liu S.; Li Y.; Ji X. Photocatalytic Reduction of CO2 by ZnO Micro/Nanomaterials with Different Morphologies and Ratios of {0001} Facets. Sci. Rep. 2016, 6, 38474. 10.1038/srep38474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A.; Gracia-Espino E.; Wågberg T.; Shchukarev A.; Mohl M.; Rautio A. R.; Pitkänen O.; Sharifi T.; Kordas K.; Mikkola J. P. Photocatalytic Reduction of CO2 with H2O over Modified TiO2 Nanofibers: Understanding the Reduction Pathway. Nano Res. 2016, 9, 1956–1968. 10.1007/s12274-016-1087-9. [DOI] [Google Scholar]
- Rusinque B.; Escobedo S.; de Lasa H. Photocatalytic Hydrogen Production Under Near-UV Using Pd-Doped Mesoporous TiO2 and Ethanol as Organic Scavenger. Catalysts 2019, 9, 33. 10.3390/catal9010033. [DOI] [Google Scholar]
- Yousaf A. B.; Imran M.; Zaidi S. J.; Kasak P. Highly Efficient Photocatalytic Z-Scheme Hydrogen Production over Oxygen-Deficient WO3-x Nanorods Supported Zn0.3Cd0.7S Heterostructure. Sci. Rep. 2017, 7, 6574. 10.1038/s41598-017-06808-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Larios A.; Hernández-Gordillo A.; Morales-Mendoza G.; Lartundo-Rojas L.; Mantilla Á.; Gómez R. Enhancing the H2 Evolution from Water-Methanol Solution Using Mn2+-Mn3+-Mn4+ Redox Species of Mn-Doped TiO2 Sol-Gel Photocatalysts. Catal. Today 2016, 266, 9–16. 10.1016/j.cattod.2015.12.029. [DOI] [Google Scholar]
- Zhong X.; Jin M.; Dong H.; Liu L.; Wang L.; Yu H.; Leng S.; Zhuang G.; Li X.; Wang J. G. TiO2 Nanobelts with a Uniform Coating of G-C3N4 as a Highly Effective Heterostructure for Enhanced Photocatalytic Activities. J. Solid State Chem. 2014, 220, 54–59. 10.1016/j.jssc.2014.08.016. [DOI] [Google Scholar]
- Daghrir R.; Drogui P.; Robert D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. 10.1021/ie303468t. [DOI] [Google Scholar]
- Fagan R.; McCormack D. E.; Dionysiou D. D.; Pillai S. C. A Review of Solar and Visible Light Active TiO2 Photocatalysis for Treating Bacteria, Cyanotoxins and Contaminants of Emerging Concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. 10.1016/j.mssp.2015.07.052. [DOI] [Google Scholar]
- Schneider J.; Matsuoka M.; Takeuchi M.; Zhang J.; Horiuchi Y.; Anpo M.; Bahnemann D. W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- Zhang S. T.; Ruan Y. R.; Liu C.; Wang P.; Ma Y. Q. The Evolution of Structure, Chemical State and Photocatalytic Performance of α-Fe/FeTiO3/TiO2 with the Nitridation at Different Temperatures. Mater. Res. Bull. 2017, 95, 503–508. 10.1016/j.materresbull.2017.08.042. [DOI] [Google Scholar]
- Ctibor P.; Pala Z.; Stengl V.; Musalek R. Photocatalytic Activity of Visible-Light-Active Iron-Doped Coatings Prepared by Plasma Spraying. Ceram. Int. 2014, 40, 2365–2372. 10.1016/j.ceramint.2013.08.007. [DOI] [Google Scholar]
- Hu L.; Wang J.; Zhang J.; Zhang Q.; Liu Z. An N-Doped Anatase/Rutile TiO2 Hybrid from Low-Temperature Direct Nitridization: Enhanced Photoactivity under UV-/Visible-Light. RSC Adv. 2014, 4, 420. 10.1039/c3ra44421j. [DOI] [Google Scholar]
- Xie W.; Li R.; Xu Q. Enhanced Photocatalytic Activity of Se-Doped TiO2 under Visible Light Irradiation. Sci. Rep. 2018, 8, 8752. 10.1038/s41598-018-27135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozzi M. V.; Selli E. Doping TiO2 with P-Block Elements: Effects on Photocatalytic Activity. J. Photochem. Photobiol. C 2013, 14, 13–28. 10.1016/j.jphotochemrev.2012.09.002. [DOI] [Google Scholar]
- Yu C.; Zhou W.; Yu J. C.; Liu H.; Wei L. Design and Fabrication of Heterojunction Photocatalysts for Energy Conversion and Pollutant Degradation. Chin. J. Catal. 2014, 35, 1609–1618. 10.1016/S1872-2067(14)60170-4. [DOI] [Google Scholar]
- Huo Y.; Chen X.; Zhang J.; Pan G.; Jia J.; Li H. Ordered Macroporous Bi2O3/TiO2 Film Coated on a Rotating Disk with Enhanced Photocatalytic Activity under Visible Irradiation. Appl. Catal. B Environ. 2014, 148-149, 550–556. 10.1016/j.apcatb.2013.11.040. [DOI] [Google Scholar]
- Zhao Z. J.; Hwang S. H.; Jeon S.; Hwang B.; Jung J. Y.; Lee J.; Park S. H.; Jeong J. H. Three-Dimensional Plasmonic Ag/TiO2 Nanocomposite Architectures on Flexible Substrates for Visible-Light Photocatalytic Activity. Sci. Rep. 2017, 7, 8915. 10.1038/s41598-017-09401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.; Park Y.; Kim W.; Choi W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C 2013, 15, 1–20. 10.1016/j.jphotochemrev.2012.10.001. [DOI] [Google Scholar]
- Sheikh F. A.; Appiah-Ntiamoah R.; Zargar A. M.; Chandradass J.; Chung W.-J.; Kim H. Photocatalytic Properties of Fe2O3-Modified Rutile TiO2 Nanofibers Formed by Electrospinning Technique. Mater. Chem. Phys. 2015, 172, 62–68. 10.1016/j.matchemphys.2015.12.060. [DOI] [Google Scholar]
- Liu C.; Zhang L.; Liu R.; Gao Z.; Yang X.; Tu Z.; Yang F.; Ye Z.; Cui L.; Xu C.; Li Y. Hydrothermal Synthesis of N-Doped TiO2 Nanowires and N-Doped Graphene Heterostructures with Enhanced Photocatalytic Properties. J. Alloys Compd. 2016, 656, 24–32. 10.1016/j.jallcom.2015.09.211. [DOI] [Google Scholar]
- Saien J.; Mesgari Z. Highly Efficient Visible-Light Photocatalyst of Nitrogen-Doped TiO2 Nanoparticles Sensitized by Hematoporphyrin. J. Mol. Catal. A: Chem. 2016, 414, 108–115. 10.1016/j.molcata.2015.12.027. [DOI] [Google Scholar]
- Znad H.; Kawase Y. Synthesis and Characterization of S-Doped Degussa P25 with Application in Decolorization of Orange II Dye as a Model Substrate. J. Mol. Catal. A: Chem. 2009, 314, 55–62. 10.1016/j.molcata.2009.08.017. [DOI] [Google Scholar]
- Sui Y.; Su C.; Yang X.; Hu J.; Lin X. Ag-AgBr Nanoparticles Loaded on TiO2 Nanofibers as an Efficient Heterostructured Photocatalyst Driven by Visible Light. J. Mol. Catal. A: Chem. 2015, 410, 226–234. 10.1016/j.molcata.2015.09.018. [DOI] [Google Scholar]
- Rawal S. B.; Bera S.; Lee D.; Jang D.-J.; Lee W. I. Design of Visible-Light Photocatalysts by Coupling of Narrow Bandgap Semiconductors and TiO2: Effect of Their Relative Energy Band Positions on the Photocatalytic Efficiency. Catal. Sci. Technol. 2013, 3, 1822. 10.1039/c3cy00004d. [DOI] [Google Scholar]
- Sood S.; Mehta S. K.; Sinha A. S. K.; Kansal S. K. Bi 2 O 3 /TiO 2 Heterostructures: Synthesis, Characterization and Their Application in Solar Light Mediated Photocatalyzed Degradation of an Antibiotic, Ofloxacin. Chem. Eng. J. 2016, 290, 45–52. 10.1016/j.cej.2016.01.017. [DOI] [Google Scholar]
- Huang H.; Li D.; Lin Q.; Zhang W.; Shao Y.; Chen Y.; Sun M.; Fu X. Efficient Degradation of Benzene over LaVO4/TiO2 Nanocrystalline Heterojunction Photocatalyst under Visible Light Irradiation. Environ. Sci. Technol. 2009, 43, 4164–4168. 10.1021/es900393h. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Zeng L.; Song W.; Qin Z.; Zeng D.; Xie C. In Situ Synthesis of C-TiO2/g-C3N4 Heterojunction Nanocomposite as Highly Visible Light Active Photocatalyst Originated from Effective Interfacial Charge Transfer. Appl. Catal. B Environ. 2017, 202, 489–499. 10.1016/j.apcatb.2016.09.052. [DOI] [Google Scholar]
- Yao Y.; Qin J.; Chen H.; Wei F.; Liu X.; Wang J.; Wang S. One-Pot Approach for Synthesis of N-Doped TiO2/ZnFe2O4 Hybrid as an Efficient Photocatalyst for Degradation of Aqueous Organic Pollutants. J. Hazard. Mater. 2015, 291, 28–37. 10.1016/j.jhazmat.2015.02.042. [DOI] [PubMed] [Google Scholar]
- Lu B.; Ma N.; Wang Y.; Qiu Y.; Hu H.; Zhao J.; Liang D.; Xu S.; Li X.; Zhu Z.; Cui C. Visible-Light-Driven TiO2/Ag3PO4/GO Heterostructure Photocatalyst with Dual-Channel for Photo-Generated Charges Separation. J. Alloys Compd. 2015, 630, 163–171. 10.1016/j.jallcom.2015.01.008. [DOI] [Google Scholar]
- Ke D.; Liu H.; Peng T.; Liu X.; Dai K. Preparation and Photocatalytic Activity of WO3/TiO2 Nanocomposite Particles. Mater. Lett. 2008, 62, 447–450. 10.1016/j.matlet.2007.05.060. [DOI] [Google Scholar]
- Ismail M.; Bousselmi L.; Zahraa O. Photocatalytic Behavior of WO3-Loaded TiO2 Systems in the Oxidation of Salicylic Acid. J. Photochem. Photobiol., A 2011, 222, 314–322. 10.1016/j.jphotochem.2011.07.001. [DOI] [Google Scholar]
- Gao L.; Gan W.; Qiu Z.; Zhan X.; Qiang T.; Li J. Preparation of Heterostructured WO3/TiO2 Catalysts from Wood Fibers and Its Versatile Photodegradation Abilities. Sci. Rep. 2017, 7, 1102. 10.1038/s41598-017-01244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves De Castro I.; Ariane De Oliveira J.; Cristina Paris E.; Regina Giraldi T.; Ribeiro C. Production of Heterostructured TiO2/WO3 Nanoparticulated Photocatalysts through a Simple One Pot Method. Ceram. Int. 2015, 41, 3502–3510. 10.1016/j.ceramint.2014.11.001. [DOI] [Google Scholar]
- Huerta-Flores A. M.; Torres-Martínez L. M.; Moctezuma E.; Singh A. P.; Wickman B. Green Synthesis of Earth-Abundant Metal Sulfides (FeS2, CuS, and NiS2) and Their Use as Visible-Light Active Photocatalysts for H2 Generation and Dye Removal. J. Mater. Sci.: Mater. Electron. 2018, 29, 11613–11626. 10.1007/s10854-018-9259-x. [DOI] [Google Scholar]
- Wu X.; Yin S.; Dong Q.; Guo C.; Li H.; Kimura T.; Sato T. Synthesis of High Visible Light Active Carbon Doped TiO2 Photocatalyst by a Facile Calcination Assisted Solvothermal Method. Appl. Catal. B Environ. 2013, 450. 10.1016/j.apcatb.2013.05.052. [DOI] [Google Scholar]
- Greczynski G.; Hultman L. X-Ray Photoelectron Spectroscopy: Towards Reliable Binding Energy Referencing. Prog. Mater. Sci. 2020, 100591. 10.1016/j.pmatsci.2019.100591. [DOI] [Google Scholar]
- Huerta-Flores A. M.; Mora-Hernández J. M.; Torres-Martínez L. M.; Moctezuma E.; Sánchez-Martínez D.; Zarazúa-Morín M. E.; Wickman B. Extended Visible Light Harvesting and Boosted Charge Carrier Dynamics in Heterostructured Zirconate–FeS2 Photocatalysts for Efficient Solar Water Splitting. J. Mater. Sci.: Mater. Electron. 2018, 29, 18957–18970. 10.1007/s10854-018-0019-8. [DOI] [Google Scholar]
- Riboni F.; Bettini L. G.; Bahnemann D. W.; Selli E. WO3-TiO2 vs. TiO2 Photocatalysts: Effect of the W Precursor and Amount on the Photocatalytic Activity of Mixed Oxides. Catal. Today 2013, 209, 28–34. 10.1016/j.cattod.2013.01.008. [DOI] [Google Scholar]
- Couselo N.; García Einschlag F. S.; Candal R. J.; Jobbágy M. Tungsten-Doped TiO2 vs Pure TiO2 Photocatalysts: Effects on Photobleaching Kinetics and Mechanism. J. Phys. Chem. C 2008, 1094. 10.1021/jp0769781. [DOI] [Google Scholar]
- Wang G.; Xu L.; Zhang J.; Yin T.; Han D. Enhanced Photocatalytic Activity of Powders (P25) via Calcination Treatment. Int. J. Photoenergy 2012, 2012, 1–9. 10.1155/2012/265760. [DOI] [Google Scholar]
- Li Y.; Lv K.; Ho W.; Dong F.; Wu X.; Xia Y. Hybridization of Rutile TiO2 (RTiO2) with g-C3N4 Quantum Dots (CN QDs): An Efficient Visible-Light-Driven Z-Scheme Hybridized Photocatalyst. Appl. Catal. B Environ. 2017, 611. 10.1016/j.apcatb.2016.09.055. [DOI] [Google Scholar]
- Sánchez-Martínez D.; Gomez-Solis C.; Torres-Martinez L. M. CTAB-Assisted Ultrasonic Synthesis, Characterization and Photocatalytic Properties of WO3. Mater. Res. Bull. 2015, 165. 10.1016/j.materresbull.2014.10.034. [DOI] [Google Scholar]
- Ghasempour F.; Azimirad R.; Amini A.; Akhavan O. Visible Light Photoinactivation of Bacteria by Tungsten Oxide Nanostructures Formed on a Tungsten Foil. Appl. Surf. Sci. 2015, 55. 10.1016/j.apsusc.2015.01.217. [DOI] [Google Scholar]
- Kunyapat T.; Xu F.; Neate N.; Wang N.; De Sanctis A.; Russo S.; Zhang S.; Xia Y.; Zhu Y. Ce-Doped Bundled Ultrafine Diameter Tungsten Oxide Nanowires with Enhanced Electrochromic Performance. Nanoscale 2018, 4718. 10.1039/c7nr08385h. [DOI] [PubMed] [Google Scholar]
- Song H. Y.; Jiang H. F.; Liu X. Q.; Jiang Y. Z.; Meng G. Y. Preparation of WOx-TiO2 and the Photocatalytic Activity under Visible Irradiation. Key Eng. Mater. 2007, 336-338, 1979–1982. 10.4028/www.scientific.net/kem.336-338.1979. [DOI] [Google Scholar]
- Baklanova I. V.; Krasil’nikov V. N.; Zhukov V. P.; Gyrdasova O. I.; Kuznetsov M. V.; Buldakova L. Y.; Yanchenko M. Y. Synthesis, Spectral, Optical and Photocatalytic Properties of Vanadium- and Carbon-Doped Titanium Dioxide with Three-Dimensional Architecture of Aggregates. J. Photochem. Photobiol., A 2016, 6. 10.1016/j.jphotochem.2015.08.002. [DOI] [Google Scholar]
- Lange F.; Schmelz H.; Knözinger H. An X-Ray Photoelectron Spectroscopy Study of Oxides of Arsenic Supported on TiO2. J. Electron Spectros. Relat. Phenomena 1991, 57, 307–315. 10.1016/0368-2048(91)80017-O. [DOI] [Google Scholar]
- Liu X. W.; Yang Z. Z.; Pan F. S.; Gu L.; Yu Y. Anchoring Nitrogen-Doped TiO2Nanocrystals on Nitrogen-Doped 3D Graphene Frameworks for Enhanced Lithium Storage. Chem. - A Eur. J. 2017, 1757. 10.1002/chem.201604603. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Wang L.; Su J.; Guo L. Branched Tungsten Oxide Nanorod Arrays Synthesized by Controlled Phase Transformation for Solar Water Oxidation. Chem CatChem 2016, 8, 2119–2127. 10.1002/cctc.201600267. [DOI] [Google Scholar]
- Bertus L. M.; Faure C.; Danine A.; Labrugere C.; Campet G.; Rougier A.; Duta A. Synthesis and Characterization of WO3 Thin Films by Surfactant Assisted Spray Pyrolysis for Electrochromic Applications. Mater. Chem. Phys. 2013, 140, 49–59. 10.1016/j.matchemphys.2013.02.047. [DOI] [Google Scholar]
- Yu H.; Guo J.; Wang C.; Zhang J.; Liu J.; Zhong X.; Dong G.; Diao X. High Performance in Electrochromic Amorphous WOx Film with Long-Term Stability and Tunable Switching Times via Al/Li-Ions Intercalation/Deintercalation. Electrochim. Acta 2019, 644. 10.1016/j.electacta.2019.06.129. [DOI] [Google Scholar]
- Alov N. V. XPS Study of MoO3and WO3Oxide Surface Modification by Low-Energy Ar+ Ion Bombardment. Phys. Status Solidi C 2015, 263. 10.1002/pssc.201400108. [DOI] [Google Scholar]
- Vaez Z.; Javanbakht V. Synthesis, Characterization and Photocatalytic Activity of ZSM-5/ZnO Nanocomposite Modified by Ag Nanoparticles for Methyl Orange Degradation. J. Photochem. Photobiol., A 2020, 388, 112064. 10.1016/j.jphotochem.2019.112064. [DOI] [Google Scholar]
- Herrmann J.-M. Photocatalysis Fundamentals Revisited to Avoid Several Misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. 10.1016/j.apcatb.2010.05.012. [DOI] [Google Scholar]
- Uddin M. J.; Islam M. A.; Haque S. A.; Hasan S.; Amin M. S. A.; Rahman M. M. Preparation of Nanostructured TiO2-Based Photocatalyst by Controlling the Calcining Temperature and PH. Int. Nano Lett. 2012, 2, 19. 10.1186/2228-5326-2-19. [DOI] [Google Scholar]
- Talebi S.; Chaibakhsh N.; Moradi-Shoeili Z. Application of Nanoscale ZnS/TiO2 Composite for Optimized Photocatalytic Decolorization of a Textile Dye. J. Appl. Res. Technol. 2017, 15, 378–385. 10.1016/j.jart.2017.03.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



