Abstract

Catalyst-free photoinduced processes in aqueous medium represent significant advancement toward development of green and sustainable pathways in organic synthesis. tert-Butyl hydroperoxide (TBHP) is a widely used oxidant in organic reactions, where the decomposition of TBHP into its radicals by metal catalysts or other reagents is a key factor for efficient catalytic outcome. Herein, we report a simple and environmentally friendly visible light-promoted synthetic pathway for the synthesis of N-heterocyclic moieties, such as quinazolinones and quinoxalines, in the presence of TBHP as an oxidizing agent in aqueous medium that requires no catalysts/photocatalysts. The enhanced rate of decomposition to generate free radicals from TBHP upon visible light irradiation is the driving force for the domino reaction.
Introduction
The search for green and sustainable synthetic protocols for organic transformations is an urgent need for obtaining fine chemicals and bioactive compounds. Quinazolinone and quinoxaline cores are privileged nitrogenous heterocycles, owing to their existence in a variety of bioactive natural products (Scheme 1), and play a significant role in medicinal chemistry as anticancer agents, anti-inflammatory agents, antibacterial agents, and anticonvulsant piriqualone.1−5 In addition, some of the quinazolinones are known to have therapeutic values in the treatment of tuberculosis. Consequently, development of sustainable, cost-effective, and more efficient methods for the preparation of these heterocycles is of continuous interest. The classical method of quinazolinone synthesis involves condensation of aldehydes and 2-aminobenzamides, resulting in aminal intermediates followed by their oxidation to quinazolinones. However, the use of chemically unstable aldehydes as starting materials and hazardous oxidants, such as KMnO4, CuCl, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), etc., causes significant limitations to this method.6,7 Among several synthetic strategies developed so far, direct oxidative cyclization leading to N-heterocyclic rings has received significant importance due to the wide availability of the starting materials, anthranilamides, and alcohols.8,9 However, a catalytic approach is necessary for achieving high activity and selectivity as the reaction involves dehydrogenation of both C–H and N–H bonds in a one-pot procedure. Various transition metal complexes, such as Ir, Pd, Cu, etc., show efficient activity for the synthesis of these heterocycles; however, most of the reactions are performed either at elevated temperatures or involve organic solvents.10−15 Considering the great emphasis on green and sustainable chemistry, development of environmentally friendly catalytic processes using water as a nontoxic and abundant solvent is one of the prime challenges. Recently, Kundu et al. reported the synthesis of N-heterocyclic moieties using Ir complexes in water medium under reflux conditions.16 Hu et al. also reported the catalyst-free synthesis of quinazolinones using aldehydes as the starting material in aqueous medium at high temperatures (120–130 °C).17 However, alcohols are the preferred starting materials over aldehydes in organic synthesis due to their wide availability and stability. Several other methodologies, including precious and nonprecious metals with O2 or the transition metal-free KOH system, have also been developed for the synthesis of 2-arylquinazolinone.18−23 Free radical chemistry is a powerful tool in the construction of useful reactive intermediates under mild reaction conditions, and various synthetic strategies have been adopted for the generation of free radicals and the use of these radical intermediates in organic synthesis. TBHP is a cost-effective and widely used oxidant and radical initiator. TBHP is relatively stable as compared to H2O2 toward thermal decomposition and has been certified for truck shipment in many countries.24 Various heterogeneous catalysts, such as ZnI2, α-MnO2, Fe3O4-CND, etc., show efficient catalytic activity for the quinazolinone synthesis when coupled with TBHP as an oxidant.8,25,26 The catalytic efficiencies of TBHP-mediated reactions largely depend on the rate of TBHP decomposition into its radicals induced by supporting metal-based catalytic systems. Hence, the objective is to look for a green catalytic pathway to increase the TBHP decomposition rate, which can subsequently catalyze the desired chemical reaction.
Scheme 1. Biologically Active Quinazolinones.
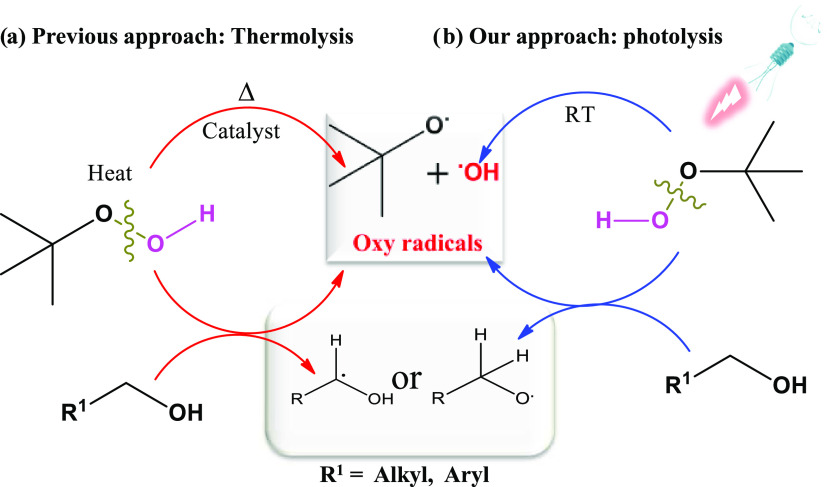
Over the years, visible light irradiation has become an environmentally friendly and economic source of energy for various useful and unique organic reactions. Visible light provides an alternative and more sustainable pathway for organic transformations with excellent functional group tolerance under mild reaction conditions.27,28 Various photocatalysts, such as Ru(II)- and Ir(III) metal-based molecular systems, dyes, and semiconducting nanoparticles, have been developed for visible light-catalyzed organic transformations.29−35 Recently, N-hydroxyphthalimide (NHPI) has been developed as an effective organophotoredox catalyst for cyclization reactions.36 TBHP is well-known to form free radicals at elevated temperatures through the homolysis of the O–O bond, which is potentially used for the oxidation of benzylic alcohol and the C–H bond.37,38 We envisioned that t-BuOO• free radicals can be generated through the homolysis of O–H bonds under visible light without the presence of external photocatalysts. Satisfyingly, our strategy worked well, which could be harnessed for the synthesis of quinazolinones from benzyl alcohol as a starting material through a one-pot cascade reaction. The involvement of α-hydroxyalkyl or alkoxy radical intermediates led to the formation of aldehydes, which undergo cyclization in the presence of benzylamides to yield the final products, thus providing a simple, catalyst-free strategy to obtain these biologically important moieties in water under mild reaction conditions (Scheme 2).
Scheme 2. Reactive Oxy Free Radical Generated from TBHP.
Results and Discussion
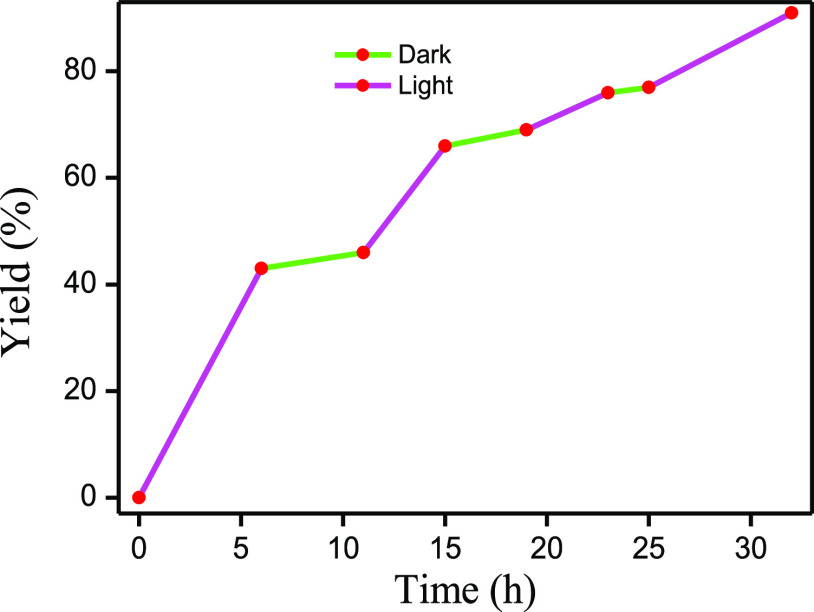
The initial assessment for the synthesis of quinazolinones by visible light irradiation using TBHP without the presence of any other photocatalyst was obtained by performing a model reaction between benzyl alcohol and anthranilamide. In the presence of 1.0 equiv of TBHP, 24% of the desired product 2-phenylquinazolin-4(3H)-one (3aa) was obtained after 12 h of white-light illumination in water at 25 °C (entry 1, Table 1). The product yield was enhanced to 63% when the TBHP concentration was increased to 2.0 equiv and 89% using 3.2 equiv of TBHP, keeping all of the other conditions similar (entries 2 and 3, Table 1). A further increase in the TBHP amount resulted in no appreciable increase of the product yield (entry 4, Table 1). The results were pleasantly surprising as the use of other commercially available oxidants, such as H2O2, di-tert-butyl hydroperoxide (DTBP), or urea peroxide, did not result in a satisfactory yield of the desired product under similar reaction conditions (entries 6–8, Table 1). To compare the photolytic behavior of TBHP with thermolysis, we performed the model reaction at 90 °C in the presence of 3.2 equiv of TBHP in the absence of visible light irradiation, which resulted in only 36% of the desired product (entry 5, Table 1). The results suggest that visible light played a critical role in TBHP activation for the cyclo-oxidative reaction. Among all of the solvents screened, performing the reaction in water as the solvent afforded the best yield of quinazolinone. Performing the reaction under a N2 environment did not have a significant impact on the product yield (entry 9, Table 1). Additionally, to confirm the role of visible light in the reaction, the progress of the reaction was monitored by turning off the visible light source from time to time. Negligible progress in the formation of the C–N coupling product was observed without visible light irradiation (Figure 1).
Table 1. Optimization of Reaction Conditionsa.
| entry | catalyst | oxidant | solvent | yieldb (%) | sel (%) |
|---|---|---|---|---|---|
| 1 | TBHP (1 equiv) | H2O | 24 | 88 | |
| 2 | TBHP (2 equiv) | H2O | 53 | 86 | |
| 3 | TBHP (3.2 equiv) | H2O | 89 | 97 | |
| 4 | TBHP (5.0 equiv) | H2O | 92 | 98 | |
| 5c | TBHP (3.2 equiv) | H2O | 32 | 93 | |
| 6d | TBHP (3.2 equiv) | H2O | 36 | 95 | |
| 7 | H2O2 (3.2 equiv) | H2O | 32 | 53 | |
| 8 | DTBP (3.2 equiv) | H2O | trace | ||
| 9 | urea peroxide (3.2 equiv) | H2O | trace | ||
| 10e | TBHP (3.2 equiv) | H2O | 83 | 91 | |
| 11 | TBHP (3.2 equiv) | toluene | 53 | 93 | |
| 12 | TBHP (3.2 equiv) | MeOH | 26 | 87 | |
| 13 | TBHP (3.2 equiv) | DMSO | 23 | 91 |
Unless otherwise specified, all of the reactions were carried out with benzyl alcohol (2.0 mmol, 208 μL) and 2-aminobenzamide (1.0 mmol, 136 mg) as the model substrates, illuminated under a 40 W white light-emitting diode (LED) lamp for 12 h at 25 °C.
Isolated yield.
Under dark conditions at 25 °C.
Under dark conditions at 90 °C.
Under a N2 environment.
Figure 1.
Progress of the photo-oxidative coupling reaction of benzyl alcohol and 2-aminobenzamide under visible light irradiation (pink) and in dark conditions (green, in the absence of ambient light) under the optimized reaction conditions. The lamp was turned off from time to time, and the formation of the desired product quinazoline was monitored using a gas chromatograph (GC) and the internal standard 1,4-di-tertbutylbenzene (19.4 mg, 0.1 mol). The temperature of the reactor was maintained up to 30–35 °C under both conditions.
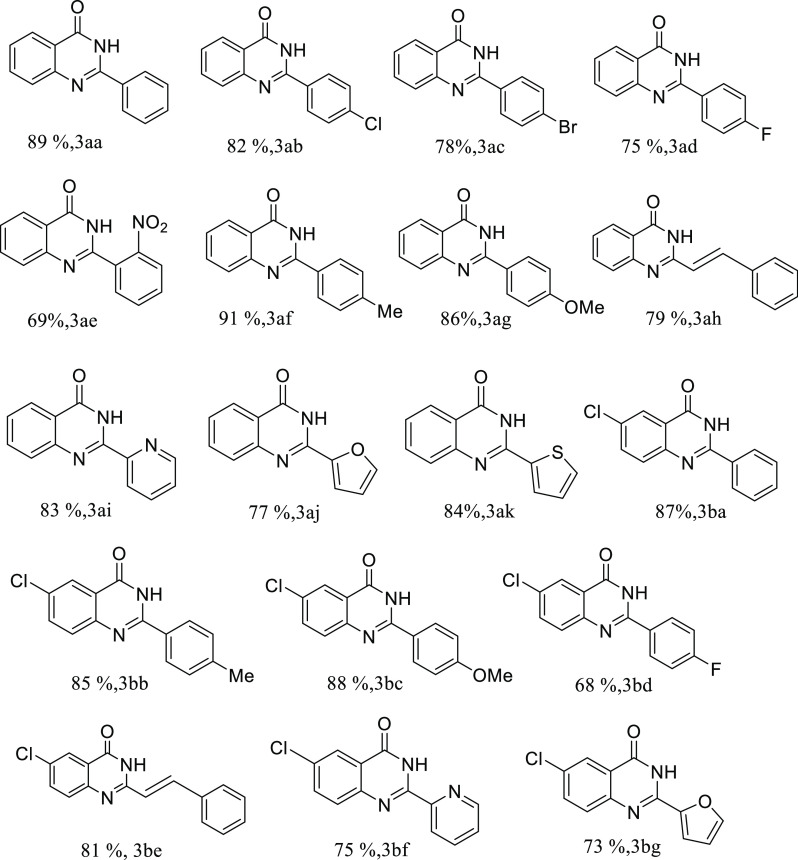
With the optimized reaction conditions in hand, we evaluated the substrate scope for the reaction. A wide range of quinazolinones could be synthesized using various primary alcohols as substrates that react with 2-aminobenzamide to afford good-to-excellent yields in the presence of TBHP under visible light irradiation. Both electron-withdrawing and -donating substituents (−NO2, −CH3, and −OCH3) in the phenyl ring of alcohol could be inserted into the quinazolinone skeleton with significant yield (entries 3ae, 3af, and 3ag; Table 2). Halo-substituted benzyl alcohols could be coupled effectively to form the corresponding quinazolinones with good yield under the optimized reaction conditions (entries 3ab, 3af, and 3ag; Table 2). The olefinic C=C bond of cinnamyl alcohol also persists well under the reaction conditions and results in the corresponding quinazolinone with 79% yield (entry 3ah, Table 2). 5-Chloro-2-aminobenzamide was also found to be compatible under the present reaction conditions and yielded the desired products with excellent yields (entries 3ba, 3bb, 3bc, 3bd, and 3be; Table 2). Heteroatom-containing primary alcohols, such as 2-pyridine methanol, furfuryl alcohol, and 2-thiophene methanol, could also be coupled effectively with both 2-aminobenzamide and 5-chloro-2-aminobenzamide under the optimized reaction conditions with their corresponding products in high yield (entries 3ai, 3aj, 3ak, 3be, 3bf, and 3bg; Table 2). Aliphatic alcohols were not suitable as substrates under the present reaction conditions, as we obtained a negligible yield of the desired coupling products. The present methodology is also suitable for large-scale synthesis as we obtained a high yield of quinazolinone (75% yield) when the model coupling reaction of benzyl alcohol and 2-aminobenzamide was performed on gram scale using TBHP under visible light irradiation (Scheme 3).
Table 2. Visible Light-Mediated Synthesis of Quinazolinone in the Presence of TBHPa.
Unless otherwise specified, all of the reactions were carried out with alcohol (2.0 mmol) and 2-aminobenzamide (1.0 mmol) in the presence of 70% aqueous TBHP (3.2 equiv, 3.2 mmol = 440 μL) illuminated under a 40 W white LED lamp for 12 h at 25 °C.
Scheme 3. Preparative Synthesis of 2-Phenylquinazolin-4(3H)-one.
From the above studies, it could be clearly ascertained that the formation of free radicals through the decomposition of TBHP was greatly influenced by visible light irradiation as no other photocatalyst was present in the medium. To confirm the hypothesis, we performed a fluorescence-based experiment where the free radical generation from TBHP under visible light irradiation was studied using terephthalic acid as the probe molecule. As shown in Figure 2, terephthalic acid preferentially reacts with •OH radicals to form a highly fluorescent product (i.e., 2-hydroxy terephthalic acid).39 Terephthalic acid itself is weakly fluorescent; however, upon irradiation of visible light with TBHP, the fluorescence intensity dramatically increased with a maximum at 425 nm that was enhanced with time. For comparison, we also performed a fluorimetric experiment at an elevated temperature (90 °C) instead of visible light irradiation. Although the fluorescence intensity due to the formation of 2-hydroxy terephthalic acid significantly increased with time, it was much lower as compared to that under visible light irradiation. This result clearly suggests that visible light irradiation had a much more pronounced influence on the decomposition of TBHP resulting in faster generation of •OH radicals as compared to thermal conditions, as clearly evident from the comparative fluorescence intensity of 2-hydroxy terephthalic acid.
Figure 2.
(a) Formation of fluorescent 2-hydroxy terephthalic acid by hydroxyl radical, (b) fluorescence spectra of an aqueous solution of terephthalic acid and TBHP under visible light irradiation and thermal conditions (90 °C) showing the emission of 2-hydroxy terephthalic acid, and (c) time-dependent fluorescence changes at 425 nm due to the oxidation of terephthalic acid by TBHP under visible light irradiation and under thermal conditions (90 °C).
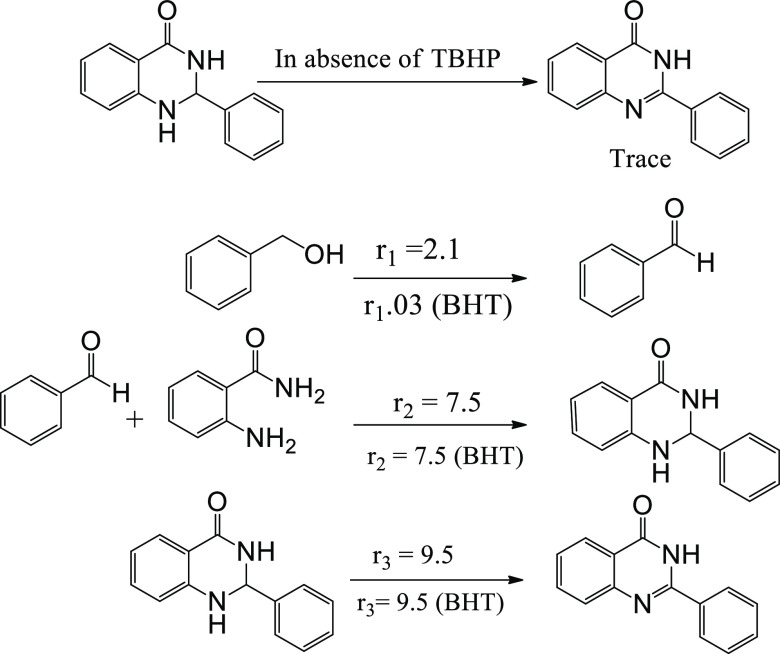
A few controlled experiments were performed to understand the participation of various free radicals in the reaction. The reaction was significantly inhibited in the presence of a radical scavenger, butylated hydroxytoluene (BHT). This result confirms the involvement of free radicals in the reaction mechanism. On the other hand, addition of a •OH radical scavenger, tert-butyl alcohol (TBA), in the reaction medium had no effect on the conversion. Similarly, addition of p-benzoquinone (BQ) as an •O2– scavenger has no impact on the reaction (Figure 3). From these studies, it could be inferred that •OH or •O2– radicals were not involved in the reaction mechanism and probably alkoxy free radicals were responsible for the oxidation of alcohols to aldehydes, which were formed as an intermediate.
Figure 3.
(a) Control experiments demonstrating the effect of various free radical scavengers on the visible light-mediated quinazolinone synthesis: butylated hydroxytoluene (BHT), p-benzoquinone (BQ, •O2– scavenger), and tert-butyl alcohol (TBA, •OH radical scavenger). (b) Effect of free radical scavengers on the product yield during the visible light-mediated quinazolinone synthesis.
Control experiments were further carried out to gain insight into the reaction mechanism (Scheme 4). While the visible light-mediated reaction of benzaldehyde and 2-aminobenzamide in the presence of TBHP as the oxidant yielded quinazolinone as the exclusive product, only dihydroquinazolinone was obtained as the major product in the absence of TBHP. Therefore, it can be concluded that benzaldehyde and dihydroquinazolinone could be the intermediates in the model coupling reaction of benzyl alcohol and 2-aminobenzamide. TBHP not only acted as an oxidant for the conversion of alcohol to aldehyde but also participated in the oxidation of dihydroquinazolinone to yield the final products.
Scheme 4. Control Experiments with Benzaldehyde and 2-Aminobenzamide as Starting Materials.
Further, to confirm the involvement of the free radical species, we performed the model reaction in a stepwise manner and calculated their reaction rates (r1, r2, r3). The reaction rate (r1) of oxidation from alcohol to aldehyde was merely affected by the presence of a radical scavenger such as BHT, whereas the reaction rates of condensation of aldehyde and 2-aminobenzamide (r2) and oxidation of dihydroquinazolinone (r3) were unaffected, thus eliminating the involvement of any radical process. Similarly, p-benzoquinone (BQ) as an •O2– scavenger has no impact on the reaction. These results suggest that the oxidation of the O–H bonds in the present system could not be associated with •OH or •O2– radicals.
Based on the control experiments, a probable reaction sequence for the quinazolinone synthesis is shown in Scheme 5. In the first step, which is the rate-determining step, oxidation of benzyl alcohol to its corresponding aldehyde takes place through the involvement of visible light-induced tert-BuO• radicals generated from TBHP. The benzaldehyde then reacts with 2-aminobenzamide and undergoes intramolecular cyclization to generate dihydroquinazolinone. Finally, oxidation of dihydroquinazolinone by TBHP results in the final product quinazolinone.
Scheme 5. Proposed Mechanism for Quinazolinone Synthesis upon Visible Light Irradiation.
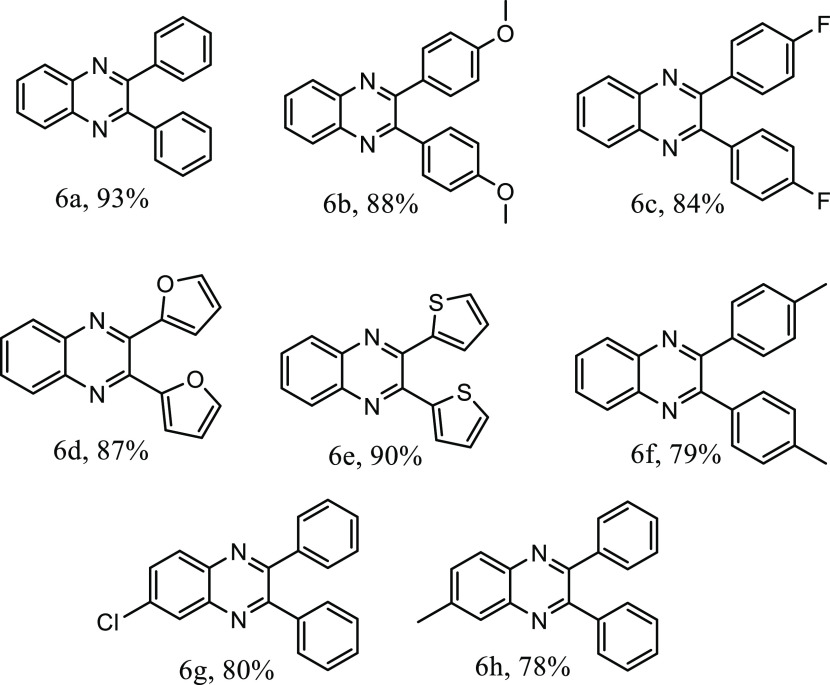
The methodology could be further extended toward the synthesis of another important class of bioactive moieties, quinoxalines, through the sequential oxidation of aryl-substituted α-hydroxy ketones, followed by condensation with aryl 1,2-diamine using TBHP as the oxidant under visible light irradiation in water. Both electron-withdrawing and electron-donating substituents on the aromatic ring afforded the corresponding quinoxaline product with good yield (79–93%, Table 3). Further, heterocyclic furan and thiophene groups could be introduced in the moiety under visible light irradiation with excellent product yield (87 and 90%, respectively; Table 3, 6a–6h).
Table 3. Visible Light-Mediated Synthesis of Quinoxaline in the Presence of TBHPa.
Unless otherwise specified, all of the reactions were carried out with α-hydroxyl ketone (1.0 mmol, 226 mg) and diamine (1.0 mmol,108 mg) in the presence of 70% aqueous TBHP (3.2 equiv, 3.2 mmol = 440 μL) illuminated under a 40 W white LED lamp for 8 h at 25 °C.
Conclusions
In summary, TBHP decomposes faster when irradiated with visible light to generate free radicals. This phenomenon can be successfully applied for a straightforward synthesis of N-heterocyclic moieties such as quinazolinone and quinoxaline in water using alcohols as the starting materials under mild reaction conditions. This protocol that involves no metal or external photocatalysts can be utilized for green synthesis of important bioactive molecules through radical chemistry.
Experimental Section
General Information
1H and 13C NMR spectra were recorded using Bruker Advance (III) 400 and 100 MHz spectrometers, respectively. Data for 1H NMR spectra are reported as a chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), coupling constant (J Hz), and integration, and assignment data for 13C NMR spectra are reported as a chemical shift. Emission spectra were obtained using a fluoromax-4p fluorimeter (HoribaYovin, model: FM-100).
Materials
tert-Butyl hydroperoxide (TBHP), hydrogen peroxide, and all other chemicals were purchased from Sigma-Aldrich, India, or Merck, India, and used without further purification. We used Millipore water (ultrapure level) throughout the experiments.
Photomediated Synthesis of Quinazolinone
In a typical reaction, 2.0 mmol (208 μL) of the alcohol substrate, 1.0 mmol (136 mg) of anthranilamide, 3.2 equiv of a 70% aqueous TBHP (440 μL) solution, and 4 mL of H2O were taken in a reaction vial and the mixture was exposed to visible light using a 40 W white LED lamp for 12 h, using a homemade photoreactor system. Magnetic stirring was performed throughout the reaction. The temperature of the reaction was maintained at 28 °C. The progress of the reaction was monitored using TLC and ethyl acetate and hexane as the eluent. After completion of the reaction, the resulting mixture was extracted with ethyl acetate (3 × 20 mL) and washed with water (1 × 15 mL). The organic layer was dried over anhydrous sodium sulfate and evaporated under reduced pressure to obtain the residue. The residue was purified using silica gel column chromatography (100–200 mesh) where a mixture of hexane and ethyl acetate was used as the eluent. The isolated products were analyzed using NMR. The conversion and selectivity of the obtained products were confirmed by 1H NMR.
Photomediated Synthesis of Quinoxalines
For the quinoxaline synthesis, 1.0 mmol (226 mg) of α-hydroxy ketone, 1.0 mmol of diamine (108 mg), and 3.2 equiv of a 70% aqueous TBHP (440 μL) solution were taken in a vial containing 4 mL of water and subjected to visible light illumination using a 40 W LED lamp for 8 h. The progress of the reaction was monitored using TLC and ethyl acetate and hexane as the eluent. After completion of the reaction, the resulting mixture was extracted with ethyl acetate (3 × 20 mL) and washed with water (1 × 15 mL). The organic layer was dried over anhydrous sodium sulfate and evaporated under reduced pressure to obtain the residue. The residue was purified using silica gel column chromatography (100–200 mesh) where a mixture of hexane and ethyl acetate was used as the eluent.
Characterization of the Isolated Intermediates
2-Phenyl-2,3-dihydroquinazolin-4(1H)-one
Colorless solid, 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 7.76 Hz, 1H), 7.60 (m, 2H), 7.44 (m, 3H), 7.33 (t, J = 7.52 Hz, 1H), 6.90 (t, J = 7.76 Hz, 1H), 6.67 (d, J = 8.04 Hz, 1H), 5.90 (s, 1H), 5.88 (br, 1H, NH), 4.35 (br, 1H, NH), 13C NMR (100 MHz, DMSO-d6): δ 164.0, 148.3, 142.1, 133.8, 128.9, 128.8, 127.8, 127.3, 117.6, 115.4, 114.8, 67.0.
Benzaldehyde
Colorless liquid; 1H NMR (400 MHz, CDCl3) δ 10.02 (s, 1H), 8.13 (d, J = 7.3 Hz. 2H), 7.89 (d, J = 7.2 Hz, 2H), 7.61 (t, J = 7.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 192.4, 136.3, 134.4, 130.1, 129.7, 128.9, 128.4.
Characterization Data of Quinazolinones and Quinoxalines
2-Phenylquinazolin-4(3H)-one (3aa)
Colorless solid; 1H NMR (CDCl3, 400 MHz): δ = 11.30 (br, s, 1H), 8.35–8.33 (d, J = 7.68, 1H), 8.24–8.22 (m, 2H), 7.86–7.79 (m, 2H), 7.60–7.59 (m, 3H), 7.53–7.50 (m, 1H); 13C NMR (CDCl3, 100 MHz): δ = 163.7, 151.7, 149.9, 134.9, 132.9, 131.7, 129.1, 128.0, 127.3, 126.8, 126.4, 120.9.
2-(4-Chlorophenyl)quinazolin-4(3H)-one (3ab)
1H NMR (DMSO-d6, 400 MHz): δ = 12.61 (br, s, 1H), 8.21–8.19 (d, J = 8.48 Hz, 2H), 8.17–8.15 (d, J = 7.72 Hz, 1H), 7.87–7.83 (t, J = 7.64 Hz, 1H), 7.76–7.74 (d, J = 8.08 Hz, 1H), 7.64–7.62 (d, J = 8.4 Hz, 2H), 7.56–7.52 (t, J = 7.44 Hz, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 162.6, 151.9, 148.9, 136.8, 135.1, 132.0, 130.0, 129.2, 128.0, 127.3, 126.3, 121.5.
2-(4-Bromophenyl)quinazolin-4(3H)-one (3ac)
1H NMR (DMSO-d6, 400 MHz): δ = 12.63 (br, s, 1H), 8.19–8.14 (m, 3H), 7.89–7.85 (t, J = 7.04 Hz, 1H), 7.80–7.76 (m, 3H), 7.58–7.54 (t, J = 7.6 Hz, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 162.1, 151.9, 135.7, 135.2, 132.4, 132.1, 130.3, 128.2, 127.4, 126.2, 125.9, 125.7, 121.5.
2-(4-Fluorophenyl)quinazolin-4(3H)-one (3ad)
1H NMR (DMSO-d6, 400 MHz): δ = 12.59 (br, s, 1H), 8.20–8.15 (m, 3H), 7.88–7.82 (t, J = 7.04 Hz, 1H), 7.81–7.78 (m, 3H), 7.60–7.56 (t, J = 7.6 Hz, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 162.3, 151.7, 135.5, 135.2, 132.5, 132.3, 130.1, 128.1, 127.4, 126.1, 126.0, 125.8, 121.5.
2-(2-Nitrophenyl)quinazolin-4(3H)-one (3ae)
1H NMR (DMSO-d6, 400 MHz): δ = 12.83 (br, s, 1H), 8.23–8.18 (t, J = 8.24 Hz, 2H), 7.94–7.82 (m, J = 7.28 Hz, 4H), 7.67–7.65 (d, J = 8.04 Hz, 1H), 7.60–7.57 (t, J = 7.78 Hz, 1H);13C NMR (DMSO-d6, 100 MHz): δ = 162.0, 152.1, 149.0, 148.8, 135.1, 134.4, 132.0, 129.6, 127.8, 127.6, 126.3, 125.0, 121.6.
2-(p-Tolyl)quinazolin-4(3H)-one (3af)
1H NMR (CDCl3, 400 MHz): δ = 11.34 (br, s, 1H), 8.26–8.25 (d, J = 7.52 Hz, 1H), 8.07–8.05 (d, J = 8.28 Hz, 2H), 7.76–7.72 (m, 2H), 7.44–7.41 (t, J = 7.76 Hz, 1H), 7.32–7.30 (d, J = 8.0 Hz, 2H); 2.45 (s 3H);13C NMR (CDCl3, 100 MHz): δ = 164.1, 151.7, 149.5, 142.2, 135.1, 130.2, 129.9, 129.8, 129.1, 127.9, 127.4, 126.6, 126.4, 120.7, 21.5.
2-(4-Methoxyphenyl)quinazolin-4(3H)-one (3ag)
1H NMR (CDCl3, 400 MHz): δ = 10.74 (br, s, 1H), 8.25–8.23 (d, J = 7.52 Hz, 1H), 8.09–8.07 (d, J = 8.0 Hz, 2H),7.73 (m, 2H), 7.41 (m, 1H), 7.01–6.99 (d, J = 8.04 Hz, 2H), 3.84 (s, 3H); 13C NMR (DMSO-d6, 100 MHz): δ = 163.4, 152.1, 148.2, 135.9, 134.5, 129.9, 128.7, 127.3, 127.1, 126.5, 121.3.
(E)-2-Styrylquinazolin-4(3H)-one (3ah)
1H NMR (DMSO-d6, 400 MHz): δ = 11.49 (br, s, 1H), 7.28–7.26 (d, J = 7.4 Hz, 1H), 7.13–7.09 (d, J = 16.0 Hz, 1H), 6.97–6.95 (t, J = 7.08 Hz, 1H), 6.83 (m, 3H), 6.62 (m, 4H), 6.19–6.15 (d, J = 16.0 Hz, 1H);13C NMR (DMSO-d6, 100 MHz): δ = 161.7, 159.8, 151.6, 148.9, 138.3, 135.1, 129.7, 129.0, 127.5, 126.1, 125.6, 121.3.
2-(Pyridin-2-yl)quinazolin-4(3H)-one (3ai)
1H NMR (DMSO-d6, 400 MHz): δ = 11.80 (br, s, 1H),8.75–8.73 (m, 1H), 8.46–8.42 (m, 1H), 8.19–8.15 (m, 1H), 8.09–8.03 (m,1H), 7.89–7.76 (m, 2H), 7.67–7.63 (m, 1H), 7.59–7.53 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 160.80, 149.9, 149.0, 148.6, 148.4, 138.0, 134.7, 127.7, 127.3, 126.5, 126.1, 122.2, 122.0.
2-(Furan-2-yl)quinazolin-4(3H)-one (3aj)
1H NMR (DMSO-d6, 400 MHz): δ = 12.49 (br, s, 1H), 8.12–8.10 (d, J = 8.04 Hz, 1H), 7.99 (m, 1H), 7.82–7.78 (t, J = 8.52 Hz, 1H), 7.69–7.67 (d, J = 8.0 Hz, 1H), 7.62–7.61 (d, J = 3.52 Hz, 1H), 7.50–7.46 (t, J = 7.04 Hz, 1H), 6.74–6.73 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 161.6, 148.7, 146.6, 146.1, 144.0, 134.7, 127.3, 126.5, 125.9, 121.1, 114.5, 112.5.
2-(Thiophen-2-yl)quinazolin-4(3H)-one (3ak)
1H NMR (DMSO-d6, 400 MHz): δ = 12.64 (br, s, 1H), 8.23–8.21 (d, J = 4.76 Hz, 1H), 8.12–8.10 (d, J = 7.8 Hz, 1H),7.86–7.85 (d, J = 5.76 Hz, 1H), 7.81–7.77 (t, J = 8.52 Hz, 1H), 7.65–7.63 (d, J = 8.0 Hz, 1H), 7.50–7.46 (t, J = 8.04 Hz, 1H), 7.24–7.21 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 161.8, 148.7, 147.8, 137.4, 134.7, 132.2, 129.4, 128.5, 127.0, 126.3, 125.9, 120.9.
6-Chloro-2-phenylquinazolin-4(3H)-one (3ba)
1H NMR (DMSO-d6, 400 MHz): δ = 12.69 (br, s, 1H), 8.16–8.14 (m, 2H), 8.07 (s, 1H), 7.86–7.84 (m, 1H), 7.77–7.75 (m, 1H), 7.61–7.54 (m, 3H).
6-Chloro-2-(p-tolyl)quinazolin-4(3H)-one (3bb)
1H NMR (DMSO-d6, 400): δ = 11.40 (br, s, 1H), 8.32–8.30 (d, J = 7.52 Hz, 1H), 8.12–8.10 (s, J = 8.28 Hz, 1H), 7.82–7.76 (m, 2H), 7.50–7.46 (t, J = 7.76 Hz, 1H), 7.37–7.35 (d, J = 8.0 Hz, 2H), 2.89 (s, 3H); 13C NMR (DMSO-d6, 100 MHz): δ = 164.3, 151.1, 149.4, 142.2, 134.9, 130.4, 129.8, 129.5, 129.1, 127.7, 127.3, 126.7, 126.1, 120.4, 21.1.
6-Chloro-2-(4-methoxyphenyl)quinazolin-4(3H)-one (3bc)
1H NMR (DMSO-d6, 400 MHz): δ = 12.59 (br, s, 1H), 8.20–8.18 (d, J = 8.8 Hz, 2H), 8.09 (s, 1H), 7.90–7.83 (m, 2H), 7.74–7.72 (d, J = 8.76 Hz, 1H), 3.34 (s,3H); 13C NMR (DMSO-d6, 100 MHz): δ = 164.9, 151.8, 148.3, 136.1, 134.5, 129.7, 128.8, 127.4, 127.0, 126.2, 121.2.
6-Chloro-2-(4-fluorophenyl)quinazolin-4(3H)-one (3bd)
1H NMR (DMSO-d6, 400 MHz): δ = 11.90 (br, s, 1H), 7.42–7.38 (m, 2H), 7.25 (s, 1H), 7.04–7.02 (dd, J = 8.72 Hz, J = 2.36 Hz, 1H), 6.93–6.91 (d, J = 8.72 Hz, 1H), 6.58–6.54 (t, J = 8.76 Hz,2H); 13C NMR (DMSO-d6, 100 MHz): δ = 162.4, 152.6, 148.5, 135.9, 134.8, 129.7, 128.5, 127.8, 127.2, 125.4, 121.2.
(E)-6-Chloro-2-(4-styrylphenyl)quinazolin-4(3H)-one (3be)
1H NMR (DMSO-d6, 400 MHz): δ = 12.51 (br, s, 1H), 8.05–8.04 (s, 1H), 7.99–7.95 (t, J = 9.8, 1H), 7.85–7.82 (dd, J = 8.76 Hz, J = 2.48, 1H), 7.72–7.66 (m, 3H), 7.53–7.41 (m, 5H), 7.03–6.99 (d, J = 16.0 Hz, 1H); 13C NMR (DMSO-d6, 100 MHz): δ = 163.8, 159.6, 151.1, 149.3, 138.6, 135.2, 129.7, 129.3, 127.5, 126.4, 125.5, 121.0.
6-Chloro-2-(pyridin-2-yl) quinazolin-4(3H)-one (3bf)
1H NMR (CDCl3, 400 MHz): δ = 10.99 (br, s, 1H), 8.66–8.65 (d, J = 4.76 Hz, 1H), 8.55–8.53 (d, J = 8.04.76 Hz 1H), 8.29 (s, 1H), 7.93–7.88 (m,1H), 7.76–7.68 (m, 2H), 7.50–7.46 (m, 1H); 13C NMR (CDCl3, 100 MHz): δ = 160.78, 149.7, 149.4, 148.6, 148.3, 138.1, 134.6, 127.5, 127.3, 126.4, 126.0, 122.1, 122.2.
6-Chloro-2-(furan-2-yl)quinazolin-4(3H)-one (3bg)
1H NMR (CDCl3, 400 MHz): δ = 11.12 (br, s, 1H), 7.95 (s, 1H), 7.75 (d, J = 7.38 Hz, 1H),7.74 (d, J = 7.26 Hz, 1H), 7.45 (d, J = 7.26 Hz, 1H), 6.52 (t, J = 6.30 Hz, 1H),7.25 (d, J = 6.30 Hz, 1H); 13C NMR (CDCl3, 100 MHz): δ = 161.0, 156.2, 142.2, 141.7, 133.5, 132.9, 132.2, 127.7, 122.2, 109.9, 109.4.
2,3-Diphenylquinoxaline (6a)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.11–8.09 (m, 2H), 7.11–7.68 (m, 2H), 7.45–7.43 (d, J =7.42, 4H), 7.30–7.14 (m, 6H);13C NMR (CDCl3, 100 MHz): δ = 153.5, 141.2, 139.1, 129.9, 129.8 129.2, 128.8, 128.3.
2,3-Bis(4-methoxyphenyl)quinoxaline (6b)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.13–8.10 (m, 2H), 7.73–7.71 (m, 2H), 7.50 (d, J = 8.56 Hz, 4H), 6.88 (d, J = 8.52 Hz, 4H), 3.83 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ = 164.5, 159.8, 152.7, 140.7, 132.0, 131.4, 130.9, 129.2, 128.7, 113.4, 55.0.
2,3-Bis(4-fluorophenyl)quinoxaline (6c)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.16–8.14 (m, 2H), 7.91–7.77 (m, 2H), 7.52–7.48 (m, 4H), 7.07–7.03 (m, 4H);13C NMR (CDCl3, 100 MHz): δ = 164.5, 162.0, 152.2, 141.2, 135.1, 131.9, 130.2, 129.2, 115.7, 115.5.
2,3-Di(furan-2-yl)quinoxaline (6d)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.14–8.12 (m, 2H), 7.77–7.73 (m, 2H), 7.64–7.62 (m, 4H), 6.66 (m,2H), 6.56–6.55 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ = 151.6, 145.4, 142.1, 140.9, 131.3, 129.0, 112.8, 111.6
2,3-Di(thiophen-2-yl)quinoxaline (6e)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.08–8.05 (m, 2H), 7.73–7.70 (m, 2H), 7.49–7.48 (d, J = 5.0, 2H), 7.24–7.23 (m, 2H), 7.04–7.02 (t, J = 5, 2H); 13C NMR (CDCl3, 100 MHz): δ = 135.6, 134.6, 128.1, 127.8, 126.7, 126.2, 125.9, 124.8.
2,3-Di-p-tolylquinoxaline (6f)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 815–8.13 (m, 2H), 7.74–7.72 (m, 2H), 7.43–7.41 (d, J = 8.04, 4H), 7.15–7.13 (d, J = 7.8, 2H); 13C NMR (CDCl3, 100 MHz): δ = 153.2, 140.9, 138.5, 136.1, 129.4, 128.8, 128.7, 21.1.
6-Chloro-2,3-diphenylquinoxaline (6g)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.02–8.01 (m, 3H), 7.94 (m, 1H), 7.61–7.48 (m, 8H), 7.25–7.22 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ = 164.9, 161.6, 154.4, 152.8, 142.3, 138.9, 138.1, 129.8, 129.6, 129.3, 128.8, 128.3, 120.6, 113.1.
6-Methyl-2,3-diphenylquinoxaline (6h)
Colorless solid, 1H NMR (CDCl3, 400 MHz): δ = 8.07–8.04 (d, J = 8.52, 1H), 7.94 (m, 1H), 7.61–7.58 (dd, J = 8.8, J = 1.76, 1H), 7.35–7.29 (m, 6H); 13C NMR (CDCl3, 100 MHz): δ = 153.1, 151.3, 141.7, 140.6, 138.8, 131.4, 129.7, 128.1, 127.8, 127.1, 126.8, 126.5.
Acknowledgments
The authors acknowledge IIT Indore, SIC IIT Indore, SAIF, NEHU, Shillong, IIT Kanpur, and IIT Bombay for research funding and instrumentation facilities. D.S. thanks the Ministry of Education and S.J. thanks DST, Government of India, for their fellowships.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00211.
NMR characterization data and spectra of the synthesized compounds (Figures S1–S36) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Horton D. A.; Bourne G. T.; Smythe M. L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- Huang C.; Fu Y.; Fu H.; Jiang Y.; Zhao Y. Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives. Chem. Commun. 2008, 6333–6335. 10.1039/b814011a. [DOI] [PubMed] [Google Scholar]
- Pereira J. A.; Pessoa A. M.; Cordeiro M. N. D. S.; Fernandes R.; Prudencio C.; Noronha J. P.; Vieira M. Quinoxaline, its derivatives and applications: A State of the Art review. Eur. J. Med. Chem. 2015, 97, 664–672. 10.1016/j.ejmech.2014.06.058. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S.; Barak D. S.; Batra S. TBHP as Methyl Source under Metal-Free Aerobic Conditions To Synthesize Quinazolin-4(3H)-ones and Quinazolines by Oxidative Amination of C(sp3)–H Bond. Eur. J. Org. Chem. 2018, 2784–2794. 10.1002/ejoc.201800495. [DOI] [Google Scholar]
- Kshirsagar U. A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. 10.1039/c5ob01379h. [DOI] [PubMed] [Google Scholar]
- Abdel-Jalil R. J.; Aldoqum H. M.; Ayoub M. T.; Voelter W. Synthesis and antitumor activity of 2-aryl-7-fluoro-6-(4-methyl-1-piperazinyl)-4(3H)quinazolinones. Heterocycles 2005, 65, 2061–2070. 10.3987/COM-05-10387. [DOI] [Google Scholar]
- Mitobe Y.; Ito S.; Mizutani T.; Nagase T.; Sato N.; Tokita S. Development of a selective and potent radioactive ligand for histamine H3 receptors: A compound potentially useful for receptor occupancy studies. Bioorg. Med. Chem. Lett. 2009, 19, 4075–4078. 10.1016/j.bmcl.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Wang M.; Zhang C.; Zhang Z.; Lu J.; Wang F. A cascade synthesis of quinazolinones and quinazolines using α-MnO2 catalyst and tertbutyl hydroperoxide (TBHP) as oxidant. Chem. Commun. 2015, 51, 9205–9207. 10.1039/C5CC02785C. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Lv M.; Liu J.; Li Y.; Xu Q.; Zhang X.; Cao H. Efficient Synthesis of Quinazolinones by Transition-Metal-Free Direct Aerobic Oxidative Cascade Annulation of Alcohols with o-Aminoarylnitriles. ChemSusChem. 2019, 12, 3043–304. 10.1002/cssc.201900265. [DOI] [PubMed] [Google Scholar]
- Wang X.; He D.; Huang Y.; Fan Q.; Wu W.; Jiang H. Copper-Catalyzed Synthesis of Substituted Quinazolines from Benzonitriles and 2-Ethynylanilines via Carbon–Carbon Bond Cleavage Using Molecular Oxygen. J. Org. Chem. 2018, 83, 5458–5466. 10.1021/acs.joc.8b00378. [DOI] [PubMed] [Google Scholar]
- Chakraborty G.; Sikari R.; Das S.; Mondal R.; Sinha S.; Banerjee S.; Paul N. D. Dehydrogenative Synthesis of Quinolines, 2-Aminoquinolines, and Quinazolines Using Singlet Diradical Ni(II)-Catalysts. J. Org. Chem. 2019, 84, 2626–2641. 10.1021/acs.joc.8b03070. [DOI] [PubMed] [Google Scholar]
- Wang C.; Li S.; Liu H.; Jiang Y.; Fu H. Copper-Catalyzed Synthesis of Quinazoline Derivatives via Ullmann-Type Coupling and Aerobic Oxidation. J. Org. Chem. 2010, 75, 7936–7938. 10.1021/jo101685d. [DOI] [PubMed] [Google Scholar]
- Chen M.; Zhang M.; Xiong B.; Tan Z.; Lv W.; Jiang H. A Novel Ruthenium-Catalyzed Dehydrogenative Synthesis of 2-Arylquinazolines from 2-Aminoaryl Methanols and Benzonitriles. Org. Lett. 2014, 16, 6028–6031. 10.1021/ol503052s. [DOI] [PubMed] [Google Scholar]
- Watson A. J. A.; Maxwell A. C.; Williams J. M. J. Ruthenium-catalysed oxidative synthesis of heterocycles from alcohols. Org. Biomol. Chem. 2012, 10, 240–243. 10.1039/c1ob06516e. [DOI] [PubMed] [Google Scholar]
- Hikawa H.; Ino Y.; Suzuki H.; Yokoyama Y. Pd-Catalyzed Benzylic C-H Amidation with Benzyl Alcohols in Water: A Strategy To Construct Quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. 10.1021/jo301282n. [DOI] [PubMed] [Google Scholar]
- Chakrabarti K.; Maji M.; Kundu S. Cooperative Iridium Complex Catalyzed Synthesis of Quinoxalines, Benzimidazoles and Quinazolines in Water. Green Chem. 2019, 21, 1999–2004. 10.1039/c8gc03744b. [DOI] [Google Scholar]
- Hu B.-Q.; Cui J.; Wang L.-X.; Tang Y.-L.; Yang L. Metal-free synthesis of quinazolinones without any additives in water. RSC Adv. 2016, 6, 43950–43953. 10.1039/C6RA05777B. [DOI] [Google Scholar]
- Mohammed S.; Vishwakarma R. A.; Bharate S. B. Iodine Catalyzed Oxidative Synthesis of Quinazolin-4(3H)-ones and Pyrazolo[4,3-d]pyrimidin-7(6H)-ones via Amination of sp3 C-H Bond. J. Org. Chem. 2015, 80, 6915–6921. 10.1021/acs.joc.5b00989. [DOI] [PubMed] [Google Scholar]
- Das S.; Sinha S.; Samanta D.; Mondal R.; Chakraborty G.; Brandao P.; Paul N. D. Metal–Ligand Cooperative Approach To Achieve Dehydrogenative Functionalization of Alcohols to Quinolines and Quinazolin-4(3H)-ones under Mild Aerobic Conditions. J. Org. Chem. 2019, 84, 10160–10171. 10.1021/acs.joc.9b01343. [DOI] [PubMed] [Google Scholar]
- Qiu D.; Wang Y.; Lu D.; Zhou L.; Zeng Q. Potassium hydroxide-promoted transition-metal-free synthesis of 4(3H)-quinazolinones. Monatsh Chem. 2015, 146, 1343–1347. 10.1007/s00706-015-1434-7. [DOI] [Google Scholar]
- Cheng R.; Guo T.; Zhang-Negrerie D.; Du Y.; Zhao K. One-Pot Synthesis of Quinazolinones from Anthranilamides and Aldehydes via p-Toluenesulfonic Acid Catalyzed Cyclocondensation and Phenyliodine Diacetate Mediated Oxidative Dehydrogenation. Synthesis 2013, 45, 2998–3006. 10.1055/s-0033-1338521. [DOI] [Google Scholar]
- Zhou J.; Fang J. One-Pot Synthesis of Quinazolinones via Iridium-Catalyzed Hydrogen Transfers. J. Org. Chem. 2011, 76, 7730–7736. 10.1021/jo201054k. [DOI] [PubMed] [Google Scholar]
- Schley N. D.; Dobereiner G. E.; Crabtree R. H. Oxidative Synthesis of Amides and Pyrroles via Dehydrogenative Alcohol Oxidation by Ruthenium Diphosphine Diamine Complexes. Organometallics 2011, 30, 4174–4179. 10.1021/om2004755. [DOI] [Google Scholar]
- He H.; Pei B.-J.; Lee A. W. M. Metal free oxidation of alkyl substituted aromatics with aqueous tert-butyl hydroperoxide under microwave irradiation. Green Chem. 2009, 11, 1857–1861. 10.1039/b916265h. [DOI] [Google Scholar]
- Sharif M.; Opalach J.; Langer P.; Beller M.; Wu X.-F. Oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols and aldehydes. RSC Adv. 2014, 4, 8–17. 10.1039/c3ra45765f. [DOI] [Google Scholar]
- Majumdar B.; Sarma D.; Jain S.; Sarma T. K. One-Pot Magnetic Iron Oxide–Carbon Nanodot Composite Catalyzed Cyclooxidative Aqueous Tandem Synthesis of Quinazolinones in the Presence of tert-Butyl Hydroperoxide. ACS Omega 2018, 3, 13711–13719. 10.1021/acsomega.8b01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo L.; Pagire S. K.; Reiser O.; Koenig B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis?. Angew. Chem., Int. Ed. 2018, 57, 10034–10072. 10.1002/anie.201709766. [DOI] [PubMed] [Google Scholar]
- Chen J. -R.; Hu X. -Q.; Lu L. -Q.; Xiao W.-J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923. 10.1021/acs.accounts.6b00254. [DOI] [PubMed] [Google Scholar]
- Ravelli D.; Fagnoni M.; Albini A. Photoorganocatalysis. What for?. Chem. Soc. Rev. 2013, 42, 97–113. 10.1039/c2cs35250h. [DOI] [PubMed] [Google Scholar]
- Nicewicz D. A.; Nguyen T. M. Recent Applications of Organic Dyes as Photoredox Catalysts in Organic Synthesis. ACS Catal. 2014, 4, 355–360. 10.1021/cs400956a. [DOI] [Google Scholar]
- Lang X.; Chen X.; Zhao J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. 10.1039/c3cs60188a. [DOI] [PubMed] [Google Scholar]
- Hari D. P.; König B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014, 50, 6688–6699. 10.1039/c4cc00751d. [DOI] [PubMed] [Google Scholar]
- Sun J.; Tao T.; Xu D.; Cao H.; Konga Q.; Wang X.; Liu Y.; Zhao J.; Wang Y.; Pan Y. Metal-free oxidative cyclization of 2-amino-benzamides, 2-aminobenzenesulfonamide or 2-(aminomethyl) anilines with primary alcohols for the synthesis of quinazolinones and their analogues. Tetrahedron Lett. 2018, 59, 2099–2102. 10.1016/j.tetlet.2018.04.054. [DOI] [Google Scholar]
- Reddy M. B.; Prasanth K.; Anandhan R. Visible-light induced copper (i)-catalyzed oxidative cyclization of o-aminobenzamides with methanol and ethanol via HAT. Org. Biomol. Chem. 2020, 18, 9601–9605. 10.1039/D0OB02234A. [DOI] [PubMed] [Google Scholar]
- Xia Q.; Shi Z.; Yuan J.; Bian Q.; Xu Y.; Liu B.; Huang Y.; Yang X.; Xu H. Visible-Light-Enabled Selective Oxidation of Primary Alcohols through Hydrogen-Atom Transfer and its Application in the Synthesis of Quinazolinones. Asian J. Org. Chem. 2019, 8, 1933–1941. 10.1002/ajoc.201900491. [DOI] [Google Scholar]
- Yadav A. K.; Yadav L. D. S. N-Hydroxyphthalimide: a new photoredox catalyst for [4+1] radical cyclization of N-methylanilines with isocyanides. Chem. Commun. 2016, 52, 10621–10624. 10.1039/c6cc04846c. [DOI] [PubMed] [Google Scholar]
- Tan J.; Zheng T.; Yu Y.; Xu K. TBHP-promoted direct oxidation reaction of benzylic Csp3–H bonds to ketones. RSC Adv. 2017, 7, 15176–15180. 10.1039/c7ra00352h. [DOI] [Google Scholar]
- Wu J.; Liu Y.; Ma X.; Liu P.; Gu C.; Dai B. Metal-free oxidation of secondary benzylic alcohols using aqueous TBHP. Synth. Commun. 2016, 46, 1747–1758. 10.1080/00397911.2016.1223307. [DOI] [Google Scholar]
- Wang X.; Hou C.; Qiu W.; Ke Y.; Xu Q.; Liu X. -Y.; Lin Y. Protein-Directed Synthesis of Bifunctional Adsorbent-Catalytic Hemin-Graphene Nanosheets for Highly Efficient Removal of Dye Pollutants via Synergistic Adsorption and Degradation. ACS Appl. Mater. Interfaces 2017, 9, 684–692. 10.1021/acsami.6b12495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.