Abstract

We report a practical fluorescent sensor device for the trace amount detection of hydrogen peroxide vapor. In this paper, we have significantly improved the performance of fluorescence analysis for the detection of peroxides by solving the problems of packaging and storage of active materials and transferring the chemical experiment phenomenon to the actual project output. The fluorescent sensor molecule, test substrates, mixing methods, and the way to improve the life time are carefully studied. Combined with the design of circuit and programming, a field-test prototype was designed for peroxide explosives and its performance and algorithm were screened and optimized. In the detection of traces of H2O2 generated by ultraviolet separation or leaked as inherent impurities, the high-efficiency and rapid detection of peroxide-based explosives is achieved. The detection limit of H2O2 is expected to reach 2 ppb, and the response time can reach <0.5 s.
1. Introduction
With the emergence of new explosives and the development of improvised explosive devices, the accidental explosions and terrorism attacks have seriously threatened the national security as well as the safety and property of the people.1 In the last decades, the management of energetic materials and effective detection of bombs thus have attracted great attention from many countries, especially for those with long borders, dense population, and many transportation hubs and ports. Compared to the conventional explosive detection methods, the vapor detection has proven to be a promising way suitable for nondestructive remote explosive monitoring. For example, the vapor detection of hydrogen peroxide (H2O2) implies many applications in industrial and biorelated monitoring and moreover will provide a new way for detecting the peroxide-based improvised explosives such as triacetone triperoxide (TATP), diacetone diperoxide (DADP), and hexamethylene triperoxide diamine, from which H2O2 is considered as a signature compound.2
As a representative of peroxide explosives, TATP was first synthesized by the German scholar Richard Wolffenstein in 1895. However, this explosive has not been used due to its poor stability. TATP is soluble in a series of organic solvents such as toluene, acetone, and ethanol, but insoluble in water.3−7 During the synthesis of organic peroxide closed-loop trimer TATP, its dimer DADP is produced as a byproduct of the reaction.8 The byproduct DADP is less stable than the main product TATP.9,10 This increases the instability of the reaction product to a certain extent. Because peroxide explosives generally contain more than one peroxide group (−O–O−), this type of group is prone to photolysis under the irradiation of ultraviolet and visible light to generate H2O2, and so, steam detection of peroxide explosives is realized.
However, vapor detection of H2O2, particularly at the trace level (ppb), remains challenging for conventional sensor techniques.11−14 The challenge lies in the combined difficulty of molecular design and materials engineering to produce a sensor system that not only enables strong binding with H2O2 (for efficient vapor sampling) but also expedient, selective reaction with H2O2 to transduce the readable signal.15 Although few papers reported on chemical sensors such as colorimetric sensors16−21 that can be employed for vapor detection of H2O2, the reported sensors either suffer from the long response time (>10 min) or complicated instrument alignments (e.g., involving laser and cooled charge-coupled device) or other deficiencies.
In recent years, the application of fluorescence turn-on (or enhanced) molecular sensors in explosive detection has received increasing attention.22 Therefore, the researchers have employed fluorescence analysis to detect H2O2 vapor efficiently and quickly. Xu23 et al. used a fluorescent probe molecule to react with H2O2 vapor generated by the decomposition of peroxide explosives and realized the detection of H2O2 vapor through the change of the fluorescence spectra. Under H2O2 vapor concentration of 1 ppm, the response time could reach no more than 0.5 s. Fan24 et al. investigated fluorenyl boronate ester chromophore-based thin films for the detection of TATP via H2O2 from the decomposed product. An25 et al. constructed a low-cost, portable, reusable, and visible paper-based fluorescence sensor for sensitive detection of TATP by steam sampling. Zhang26 reported two new fluorescent compounds, DB-WCZ and DB-W, which were served as turn-off-type fluorescent film probes to H2O2 vapor. Therefore, the fluorescence analysis method reveals the advantages of high sensitivity, good selectivity, simple operation, and easy portability, which makes it one of the future development trends in the field of explosive detection. However, most of the current studies are still on the laboratory level. For example, their detection limits and sensitivity response times are usually obtained by data fitting, and there is no real practical exploration.
Therefore, we are working to fabricate a practical fluorescent device for trace amount detection of H2O2 vapor, which is expected to find broad applications in the areas of security, environment, and biological monitoring. C6NIB is a naphthalimide-based fluorescence turn-on sensor, which can be fabricated into a porous matrix to enable trace vapor detection of H2O2 down to a few parts per billion. The sensor is extremely selective to H2O2 with no response to other common reagents even at orders of magnitude higher vapor concentrations. However, there are still many problems to hinder it to be fabricated into a practical, expedient, and reliable instrument suitable for trace vapor detection of H2O2 (detection limit down to the low ppb level). The challenges mainly focus on the lifetime of test papers and engineering design to bring all the sophisticated testing parts into a compact chamber.
In this paper, we have significantly improved the performance of fluorescence analysis for the detection of peroxides by solving the problems of packaging and storage of active materials and transferring the chemical experiment phenomenon to the actual project output. First, the performance the C6NIB fluorescent molecule is optimized to be suitable toward engineering applications. Our work combines molecular design and engineering technology and integrates detection parts and devices. In the past, laboratory testing was an instant reaction of the test paper without considering the storage problem and the problem of testing efficiency changing with storage time. Due to the high sensitivity of the test paper, it will be slowly oxidized in the air and the simple packaging of plastic bags will shorten the life of the test paper. In this paper, the vacuum tester is used to seal the test paper to prevent it from contacting the air to a certain extent and the life is improved. Also, we convert the fluorescence change of the test strip before and after the reaction into an electrical signal, which determines the existence of peroxides according to the electrical signal change and sets an alarm threshold for the simple output by careful coding. Really exploring the performance of fluorescence analysis for the detection of peroxides from practical applications has increased the practical value of previous fluorescence analysis studies.
2. Experimental Section
2.1. Screening of the Test Paper Matrix
First, we screened the preparation process conditions of the test substrates used in the project. Many test substrates that commonly used in the laboratory were considered, such as the filter paper, cotton, gauze, glass-based silica gel plate, aluminum foil-based silica gel plate, and polyester-based silica gel plate. The purpose of this step is to look for a balanced performance between the fluorescent response and material processability. The reaction effect is shown in Figure 1. The results showed that the filter paper, cotton, and gauze did not meet the expected experimental phenomenon. The pristine blue fluorescent lights were immediately turned to yellow, which was the characteristic emission wavelength of C6NIO (Figure 1a,b). Because the manufacturers normally used peroxide chemicals to bleach the filter paper, cotton, and gauze, this fast transformation from C6NIB to C6NIO was probably due to the residual peroxide bleaches in substrates. Also, this phenomenon implied that the chemical sensor we used in this work was extremely sensitive to trace the amount of peroxide compounds. Then, we found that all types of the silica gel plates could give pristine weak blue-violet emission in the absence of H2O2 vapor and with a significant increase in the yellow-green response after contacting H2O2 vapor. However, the glass substrate was heavy and hard to be folded and cut, making it impossible to be fabricated into light soft materials (Figure 1c). Although polyester boards could be folded and cut and have obvious phenomena before and after the reaction, the limited cohesive affinity between the substrates and silica gels lead to the exfoliation of the silica gel together with the active sensor materials. Additionally, the background emission of the polyester substrate under UV light was also blue-violet (Figure 1e), which might interfere with the experimental phenomenon. The combined problems hindered further engineering applications of the polyester substrate. Compared with the glass and polyester substrates, the aluminum foil-based silica gel plate demonstrates the best performance in both fluorescent response and material processability (Figure 1d). The aluminum foil was soft and light weighted and stuck firmly with the silica gel. Moreover, the fluorescent background was satisfyingly low, and C6NIB can maintain stable for very long time without H2O2 vapor. Therefore, the aluminum foil-based silica gel board is ultimately used as the test substrate in this work.
Figure 1.
Reaction effect diagram on (a) two different filter papers, (b) cotton and gauze, (c) glass-based silica gel plate, (d) aluminum foil-based silica gel plate, and (e) polyester-based silica gel plate. (f) Comparison of polyester-based and aluminum foil-based substrates.
2.2. Testing of Material Mixing Methods
To fabricate the optimized test substrate, we used an organic base, tetrabutylammonium hydroxide (TBAH), to produce the basic reaction condition. C6NIB was proven stable in the fluorescence spectra within the experimental time, but the fluorescence turn-on reaction of C6NIB was found to be dependent on the way of mixing TBAH. Therefore, we studied the different mixing methods of these two materials. The reaction effect is shown in Supporting Information Figure S7. The preliminary results show that the three drop methods have similar results. In comparison, mixing C6NIB and TBAH together illustrates a more uniform spot, while the other two methods increase difficulties to generate even films because two compounds have to be added sequentially.
For further quantitative analysis, the excitation wavelength of 458 nm is used for the test paper of different mixing methods (C6NIB/TBAH = 2:1, C6NIB concentration is 2 mmol/L). The solid-state fluorescence emission spectra were tested before and after the H2O2 solution was introduced to quantitatively investigate the effect of the mixing methods on the reaction performance of the test substrates (Figure 2).
Figure 2.
Fluorescent spectra of (a) C6NIB first, (b) TBAH first, and (c) C6NIB and TBAH together. (d) Comparison of the emission peak growth multiples of different mixing methods.
The increase of the emission peak is calculated at the wavelength of 522 nm. The results show that the growth of the peaks varies with changed mixing methods. The mixing method of adding C6NIB and then TBAH dropwise has the largest increment in the absorption peak in 5 and 15 s. Similarly, adding TBAH and then C6NIB can also achieve the best performance, while the direct mixing can only give half the value. However, in the case where the two substances are added separately, it is impossible to ensure that the positions of dropwise addition and diffusion are completely coincident. Considering the practical application and low-cost fabrication, we select the direct mixing method because of its acceptable performance and much easier operation.
2.3. Research on Concentration, Ratio, and Excitation Wavelength
The concentration, ratio, and excitation wavelength were screened and explored. While maintaining the fixed ratio of C6NIB and TBAH, the concentration of C6NIB was changed, and a preliminary qualitative investigation was made on its concentration change law. It is shown in Supporting Information Figure S8.
Qualitative analysis of concentration shows that under different ratios, the same reaction performance is exhibited with the change of C6NIB concentration. Before the H2O2 gas was introduced, as the concentration decreased, the blue-violet light gradually weakened. When the solubility was too small, even the blue-violet light was not seen; after the H2O2 gas was introduced, the greater the concentration, the more obviously the blue-violet changed to yellow-green, the faster the reaction during the H2O2 gas flow. Therefore, a series of single-substance C6NIB solutions with different concentrations were prepared, and solid-state fluorescence spectroscopy was performed on test papers of different concentrations using an excitation wavelength of 458 nm. The curve of the absorption peak growth multiple at 522 nm within 5 and 15 s with concentration was made to quantitatively investigate the effect of the concentration of a single substance C6NIB on the reaction performance, and the optimal concentration was selected accordingly (Figure 3).
Figure 3.

Curves of the increase of the emission peak with C6NIB concentration.
The test results show that the absorption peak growth multiple increases first and then decreases with increasing concentration. When the concentration is too large, the surface of the test (the test paper is sealed for 6 h) has obviously turned yellow, and it will not react with H2O2 gas. If the concentration is too small, the reaction performance is poor. When the concentration of C6NIB was 0.5 mmol/L, the absorption fold increase of the absorption peak reached the maximum within 5 and 15 s, that is, the optimal concentration of single substance C6NIB was initially determined to be 0.5 mmol/L.
Under the condition that the C6NIB concentration was kept at 0.5 mmol/L, the ratio of C6NIB to TBAH was changed to investigate the effect of the ratio on the reaction performance. It is shown in Supporting Information Figure S9.
The qualitative reaction results show that from left to right, the blue-violet color becomes lighter and deeper before the gas is introduced. After passing in the gas, the left half becomes yellow-green, the phenomenon is obvious, and the right half is basically unchanged. The closer it is to the left end, the faster is the response speed. In the case of the same concentration and ratio, further quantitative investigations were conducted. The test paper for the solid-state fluorescence spectrum was tested when H2O2 gas is introduced for 0, 5, and 15 s. The change of the absorption peak growth factor was calculated when H2O2 gas is introduced for 5 and 15 s, and a graph of the growth factor was plotted. Then, the conclusions were analyzed to find the best match.
The test results show that under a certain C6NIB concentration, the absorption peak multiple fluctuates with the change in the ratio of C6NIB to TBAH (Figure 4). Compared with the test paper with different proportions of TBAH, the response of single substance C6NIB is relatively good. When C6NIB/TBAH = 1:2, the effect is particularly prominent, which is used as the best ratio.
Figure 4.

Curves of the increase of the emission peak with the ratio of C6NIB to TBAH.
With the selected concentration and ratio, the absorption spectra of the same test strip before and after the H2O2 gas was tested were tested using excitation wavelengths of 365, 415, and 458 nm (Figure 5). The peak of the absorption peak corresponding to different excitation wavelengths was found, and the growth multiples under the absorption peaks before and after the H2O2 is introduced at different excitation wavelengths were calculated (Table 1).
Figure 5.
Fluorescence spectra measured at (a) 365, (b) 415, and (c) 458 nm.
Table 1. Analysis of the Influence of Different Excitation Wavelengths.
The experiment is performed under the condition that other conditions are the same and only the excitation wavelength is changed. The results show that when the same test strip is tested at different excitation wavelengths, the absorption peaks before and after the reaction occur at different wavelengths. When the excitation wavelength changes from 365 to 458 nm, the peak wavelength gradually red-shifts. The same test paper is tested at different excitation wavelengths, and the absorption peak growth times before and after the H2O2 gas is passed are different. When the 458 nm excitation wave is detected, the absorption peak growth times are the largest.
2.4. Test Paper Storage Life Tracking
Based on known conditions, the storage life of the test paper is tracked. First, under the laboratory conditions, qualitative observation experiments were performed on test papers with the same concentration, the same ratio, the same storage method, and different storage times before and after the H2O2 was introduced. It is shown in Supporting Information Figure S10.
The laboratory qualitatively observed that the test paper was partially oxidized after several days of storage, and the reaction rate became slower. The test strip 1 month ago had been completely oxidized, and no reaction phenomenon was observed even when H2O2 was added again. However, the degree to which the test paper is oxidized, that is, the change in the life decay amount with storage time is unknown. Therefore, test papers with a ratio of C6NIB to TBAH of 2:1 and C6NIB concentrations of 2, 1, and 0.67 mmol/L were prepared each day, and a solid-state fluorescence spectrum was measured after 9 days to investigate the life decay law.
The laboratory observed that the test paper, which was placed naturally without any protection measures, could not be used after 2 days. Using plastic wrap to wrap, the test paper in a plastic bag can effectively extend its service life. On this basis, a vacuum packaging machine is used to further improve the storage method in order to better extend its service life.
Taking 2 mmol/L as an example, the scatter plots of two different packaging methods and the graphs of exponential fitting based on this are given at the bottom for analysis and comparison (Figure 6). See the Supporting Information for the result analysis diagrams of other concentrations and time periods.
Figure 6.
(a) 2 mmol/L, 5 s. (b) 2 mmol/L, 10 s.
It can be seen from the fitted curve diagram that the absorption peak growth multiple has a single change relationship with time, which conforms to the exponential curve. With the extension of the storage time, the increase of the absorption peak under the VM condition is greater than that under the PB condition. The long-term preservation effect of VM is better, which greatly improves the service life of the test paper. Enable the fluorescence detection substance to go out of the laboratory and get a certain application in engineering practice.
3. Results and Discussion
3.1. Production of the Test Prototype
The fluorescence detection system for peroxide explosives studied in this paper mainly includes the flow field shaping control of the fluorescence reaction of gaseous chemical substances, the precise optical path structure design, the high stability modulation light source drive, the detection of weak fluorescence signals, the design of multichannel isolated power supply, and the integration of the system master control. On the basis of establishing a reliable flow-field simulation model, the electrical and structural design of the excitation light source and fluorescence detector is completed.
In the structural design, the peroxide explosive test prototype system includes a fluorescence-generating device, a fluorescence-generating device-fixing device, a fluorescence-receiving device, a fluorescence-receiving device-fixing device, a detection gas chamber device, a fluorescence detection sensor-carrying device, a fixing bracket, and a handle and shell. The fluorescence-generating device is fixed on the fixing device of the fluorescence-generating device and then installed on the fixing bracket; the fluorescence-receiving device is fixed on the fixing device of the fluorescence-receiving device and then installed on the fixing bracket; the detection gas chamber structure is fixed on the fixing bracket and the fluorescence detection sensor-carrying device is matched with it; the device shell is connected with the fluorescence-receiving device-fixing device and the fixing bracket, and the handle is fixed on the shell. This project is combined with device design and circuit design. A prototype that can detect H2O2 gas has been preliminarily designed, and its appearance is shown below (Figure 7).
Figure 7.
Schematic diagram of the internal workflow of the testing prototype (upper). Schematic diagram of (a) front panel, (b) rear panel, and (c) upper panel and test strip base (below).
3.2. Combination of the Test Prototype and Test Paper
The prototype is designed with a self-cleaning function to clean any residual H2O2 gas. The test strips are disposable and need to be replaced with new ones before each test. When H2O2 gas is detected, the buzzer sounds long alarm; when it is not detected, the prototype remains the same under normal working conditions. Combining reading software, mapping software, and testing prototypes, we can preliminarily observe the reaction of the test substrate and the working status of the device during the testing process. In the experiment, a 458 nm light source was used to perform an immediate reaction on the test strip, and the reaction was performed after a period of time for comparison to explore the working life of the test strip.
We then investigate the lifetime of the test substrate by two ways. First, we tested its stability under the long-time irradiation to examine the photobleaching effect (Figure 8). The original response of the freshly made sensor materials shows 3.3% signal changing within first 5 s under 5% H2O2 vapor. After 3 h of 458 nm light to irradiate the sensor material, although the reaction performance decreased to 2.8% within 5 s under 5% H2O2 vapor, the phenomenon was still obvious enough to analyze the detection progress, which was in line with the expected goal. It is to be noted that we found a 10 s delay time in this process, which might be due to the slow vapor diffusion and long pipe design.
Figure 8.
(a) Instant response. (b) Reaction after 3 h of light.
Next, we further tested the reaction performance of the test paper after a certain period of storage to examine the oxygen and environmental effects (Figure 9). The response of the 5% H2O2 vapor within 5 s slightly dropped to 2.5% after 1 week of storage, which demonstrate that the long-time storage may affect the response efficiency. Similarly, the response of the 5% H2O2 vapor within 5 s furtherly decreased to 1.9% after 2 weeks of storage, which also meets our expectation. The data show that the test paper can maintain most of their performance after 2 weeks because of our storage method. The storage life of the test paper is good in actual tests and meets the expected goals.
Figure 9.
Response effect maps after (a) 1 week of storage and (b) 2 weeks of storage.
3.3. Anti-interference Performance Test
The common solvents in the laboratory, acetonitrile, acetone, petroleum ether, and ethyl acetate, and a certain brand of perfume were compared with H2O2 to determine the anti-interference performance of the test paper. The experimental results are shown in Figure 10. The results show that our substrate has good anti-interference performance.
Figure 10.
Test substrate anti-interference performance test chart.
3.4. Commissioning of the Testing Prototype
Based on a large number of experiments and analysis of data, the prototype is debugged. The alarm threshold is set for peroxide detection, and the crude data in the test are processed and combined with image processing for subsequent analysis. The schematic diagram of the prototype test after debugging is as follows.
In Figure 11a, the red line represents the coarse data, and the yellow line represents the processed data, which is also the original data. The data collection method may be to collect one every other segment. In Figure 11b, the red line is the average of 1000 points; the purple line is an alarm line; the buzzer will alarm when the purple line fluctuates; the blue line is the data collected at intervals; and when the blue line intersects with the purple line and continues to decline, the buzzer will alarm. The yellow line is another way of judging, but a large amount of experimental data show that the blue line is better than the yellow line; so, the criterion represented by the blue line is ultimately adopted.
Figure 11.
Schematic test of the prototype after debugging, (a) raw data collected by the prototype and (b) guidelines for setting the prototype alarm domain.
The schematic diagram of the specific detection process is shown in Figure 12. A breakthrough from laboratory mechanism exploration to practical application of engineering was realized. Finally, 30% H2O2 solution was diluted with ethanol 5200 times, which can be detected within 30 s. At this time, the H2O2 concentration is 0.046 mg/mL (13 ppb), which is equivalent to the concentration of H2O2 produced by the complete decomposition of 0.1 mg/mL TATP. The detection limit that can be achieved in the experiment is less than 2 ppb, and the detection time is greater than 70 s. When 30% H2O2 was directly used for detection, the detection time was less than 0.5 s.
Figure 12.
Detection flow chart.
4. Conclusions
In summary, we have screened the test paper substrate, finally selected the aluminum foil-based silica gel plate to initially prepare the reaction test paper, screened the process conditions, and carried out qualitative and quantitative tests on the material mixing method. The optimal concentration of C6NIB in the test paper, the ratio of C6NIB to TBAH, excitation wavelength, test paper storage life, and test paper anti-interference performance were tested.
By designing the peroxide explosive fluorescence detection system and the prototype, we carried out the peroxide explosive fluorescence detection experiment. The influencing factors of the fluorescence detection sensor, including excitation light source wavelength, illumination time, detection substance concentration, detection substance composition, inhalation fan switch, H2O2 reagent concentration, other organic solvents, sensor aging, and so forth were analyzed. We gradually determined the optimized parameters of the fluorescence detection sensor and the parameter settings of the prototype. Finally, the detection capability of the prototype of the peroxide explosive fluorescence detector developed in this paper was verified through fluorescence detection experiments. The prototype can realize fast, efficient, and accurate detection of peroxide explosives with a certain accuracy.
This work initially realized the transition from chemical molecules to engineering applications. This paper mainly focuses on the practical application of engineering and explores the preparation, packaging, storage, and detection of the reaction test paper to a certain extent. A breakthrough has been achieved from laboratory mechanism exploration to practical engineering application. Eventually, the detection limit for H2O2 gas is 2 ppb, which is better than the laboratory data fitting results to a certain extent.
5. Synthesis of the Substance C6NIB
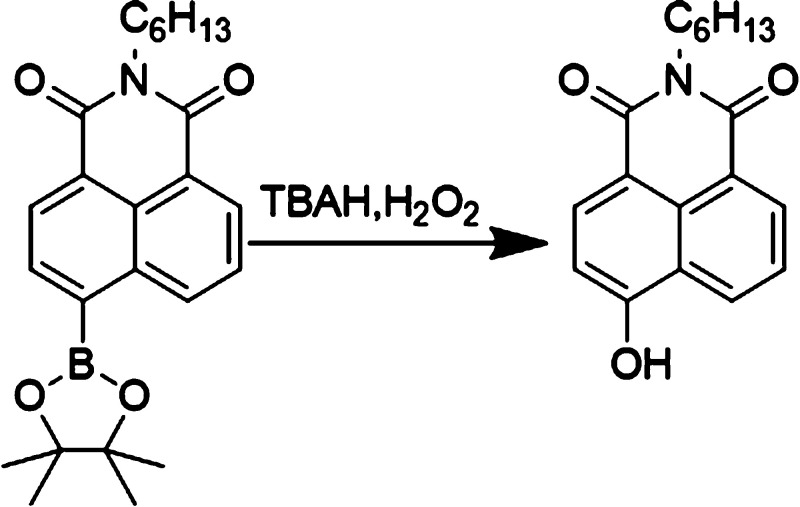
The synthesis of the substance C6NIB used for detection was performed following the modified steps in the literature.20 As shown in Scheme 1, the synthesis contains two steps. 4-Bromo-1,8-naphthalic anhydride, hexylamine, and triethylamine were added into anhydrous ethanol and refluxed to obtain the intermediate. Afterward, the intermediate was mixed with anhydrous potassium acetate, bis(pinacolato)diboron, [PdCl2(dppf)], and dppf in dioxane. The product was then obtained as a white powder. In this study, C6NIB is only weakly fluorescent in the UV region, where the quantum yield is only 0.6% under basic conditions. However, upon reaction with H2O2, the aryl boranate group of C6NIB is transformed to phenol. The weak blue-fluorescent naphthalimide backbone transition was converted to the electron donor–acceptor (push–pull) C6NIO structure, which turns on the charge transfer transition and fluorescent emission in the longer wavelength band. Because the pristine C6NIB molecule is close to zero emission in the charge transfer band, an extremely high turn-on ratio will be obtained if the reaction with H2O2 is monitored in the long wavelength domain (Scheme 2).
Scheme 1. Synthesis Route of Sensor Molecule C6NIB.
Scheme 2. Mechanism of the Reaction between C6NIB and H2O2.
5.1. Materials and General Instrumentations
All raw materials and reagents were obtained from commercial suppliers (Alfa Aesar, Aldrich, Weiss). C6NIB refers to the steps in Xu’s article for synthesis and subsequent processing and verifies it with NMR. The 1H NMR spectra and 13C NMR spectra were recorded using a Bruker Avance 500 MHz superconducting nuclear magnetic resonance spectrometer.
Both the aluminum foil-based silicone plate and the vacuum laminator were purchased from commercial manufacturers and used as received. C6NIB is dissolved with chloroform as the solvent, and TBAH is dissolved with ethanol as the solvent. The experimental reagents (the concentration of C6NIB and the ratio of C6NIB to TBAH) are all measured with a pipette and are prepared in a 20 mL centrifuge bottle, and the additional solvent is ethanol.
The aluminum foil-based silica gel plate is cut into a size of 20 mm × 20 mm as the test paper base, and a pipette is used to suck 30 μL of liquid in the center of the test paper base and let it to spread evenly and dry to complete the test paper preparation. The sealing method of the plastic wrap and disposable plastic bag mentioned in the article is to wrap the prepared test paper in the plastic wrap, then put it into a disposable plastic bag, and store it at room temperature away from light. The abovementioned storage method of the cling film plus disposable plastic bag and vacuum laminator is to wrap the prepared test paper with the cling film, then put it in a disposable plastic bag, then put it in a vacuum packaging bag, use vacuum plastic sealing, and store it at room temperature away from light.
The fluorescence emission spectrum was tested using a RF6000 fluorescence spectrophotometer.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 21801016), the State Key Laboratory of Explosion Science and Technology (no. ZDKT19-01), and Beijing Institute of Technology Research Fund Program for Young Scholars.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00471.
Materials and general instrumentations, basic information and parameter settings of the fluorescence spectrometer, original data and subsequent processing instructions of the concentration and ratio test, and raw data and data processing of the life tracking experiment (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Oxley J. C.; Smith J. L.; Shinde K.; Moran J. Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using a Gas Chromatography Headspace Technique. Propellants, Explos., Pyrotech. 2005, 30, 127–130. 10.1002/prep.200400094. [DOI] [Google Scholar]
- Dunn L.; Al Obaidly H. S. A.; Khalil S. E. Development and validation of fast liquid chromatography high-resolution mass spectrometric (LC-APCI-QToF-MS) methods for the analysis of hexamethylene triperoxide diamine (HMTD) and triacetone triperoxide (TATP). Forensic Chem. 2018, 10, 5–14. 10.1016/j.forc.2018.06.003. [DOI] [Google Scholar]
- Dubnikova F.; Kosloff R.; Almog J.; Zeiri Y.; Boese R.; Itzhaky H.; Alt A.; Keinan E. Decomposition of Triacetone Triperoxide Is an Entropic Explosion. J. Am. Chem. Soc. 2005, 127, 1146–1159. 10.1021/ja0464903. [DOI] [PubMed] [Google Scholar]
- Schulte-Ladbeck R.; Kolla P.; Karst U. Trace Analysis of Peroxide-Based Explosives. Anal. Chem. 2003, 75, 731–735. 10.1021/ac020392n. [DOI] [PubMed] [Google Scholar]
- Scott A. M.; Petrova T.; Hill F.; Leszczynski J. Density functional theory study of interactions of cyclotrimethylene trinitramine (RDX) and triacetone triperoxide (TATP) with metal–organic framework (IRMOF-1(Be)). Struct. Chem. 2012, 23, 1143–1154. 10.1007/s11224-011-9936-3. [DOI] [Google Scholar]
- Widmer L.; Watson S.; Schlatter K.; Crowson A. Development of an LC/MS method for the trace analysis of triacetone triperoxide (TATP). Analyst 2002, 127, 1627–1632. 10.1039/b208350g. [DOI] [PubMed] [Google Scholar]
- Burks R. M.; Hage D. S.; Chemistry B. Current trends in the detection of peroxide-based explosives. Anal. Bioanal. Chem. 2009, 395, 301–313. 10.1007/s00216-009-2968-5. [DOI] [PubMed] [Google Scholar]
- Sinditskii V. P.; Kolesov V. I.; Egorshev V. Y.; Patrikeev D. I.; Dorofeeva O. V. Thermochemistry of cyclic acetone peroxides. Thermochim. Acta 2014, 585, 10–15. 10.1016/j.tca.2014.03.046. [DOI] [Google Scholar]
- Li Z.; Bassett W. P.; Askim J. R.; Suslick K. S. Differentiation among peroxide explosives with an optoelectronic nose. Chem. Commun. 2015, 51, 15312–15315. 10.1039/c5cc06221g. [DOI] [PubMed] [Google Scholar]
- Guo C.; Persons J.; Jeffrey N.; Woodford J. N.; Gerard S. Gas-Phase Infrared and NMR Investigation of the Conformers of Diacetone Diperoxide (DADP). J. Phys. Chem. A 2015, 119, 10221–10228. 10.1021/acs.jpca.5b07074. [DOI] [PubMed] [Google Scholar]
- Dubnikova F.; Kosloff R.; Almog J.; Zeiri Y.; Boese R.; Itzhaky H.; Alt A.; Keinan E. Decomposition of Triacetone Triperoxide Is an Entropic Explosion. J. Am. Chem. Soc. 2005, 127, 1146–1159. 10.1021/ja0464903. [DOI] [PubMed] [Google Scholar]
- Schulte-Ladbeck R.; Kolla P.; Karst U. Trace Analysis of Peroxide-Based Explosives. Anal. Chem. 2003, 75, 731–735. 10.1021/ac020392n. [DOI] [PubMed] [Google Scholar]
- Scott A. M.; Petrova T.; Hill F.; Leszczynski J. Density functional theory study of interactions of cyclotrimethylene trinitramine (RDX) and triacetone triperoxide (TATP) with metal–organic framework (IRMOF-1(Be)). Struct. Chem. 2012, 23, 1143–1154. 10.1007/s11224-011-9936-3. [DOI] [Google Scholar]
- Widmer L.; Watson S.; Schlatter K.; Crowson A. Development of an LC/MS method for the trace analysis of triacetone triperoxide (TATP). Analyst 2002, 127, 1627–1632. 10.1039/b208350g. [DOI] [PubMed] [Google Scholar]
- Burks R. M.; Hage D. S.; Chemistry B. Current trends in the detection of peroxide-based explosives. Anal. Bioanal. Chem. 2009, 395, 301–313. 10.1007/s00216-009-2968-5. [DOI] [PubMed] [Google Scholar]
- Lin H.; Suslick K. S. A Colorimetric Sensor Array for Detection of Triacetone Triperoxide Vapor. J. Am. Chem. Soc. 2010, 132, 15519–15521. 10.1021/ja107419t. [DOI] [PubMed] [Google Scholar]
- Üzer A.; Durmazel S.; Erçağ E.; Apak R. Determination of hydrogen peroxide and triacetone triperoxide (TATP) with a silver nanoparticles—based turn-on colorimetric sensor. Sens. Actuators, B 2017, 247, 98–107. 10.1016/j.snb.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Gökdere B.; Üzer A.; Durmazel S.; Erçağ E.; Apak R. Titanium dioxide nanoparticles–based colorimetric sensors for determination of hydrogen peroxide and triacetone triperoxide (TATP). Talanta 2019, 202, 402–410. 10.1016/j.talanta.2019.04.071. [DOI] [PubMed] [Google Scholar]
- Cao W.; Ju P.; Wang Z.; Zhang Y.; Zhai X.; Jiang F.; Sun C. Colorimetric detection of H2O2 based on the enhanced peroxidase mimetic activity of nanoparticles decorated Ce2(WO4)3 nanosheets. Spectrochim. Acta, Part A 2020, 239, 118499. 10.1016/j.saa.2020.118499. [DOI] [PubMed] [Google Scholar]
- Cao X.; Yang H.; Wei Q.; Yang Y. Fast colorimetric sensing of H2O2 and glutathione based on Pt deposited on NiCo layered double hydroxide with double peroxidase-/oxidase-like activity. Inorg. Chem. Commun. 2021, 123, 108331. 10.1016/j.inoche.2020.108331. [DOI] [Google Scholar]
- Şen F. B.; Bener M.; Bekdeşer B.; Apak R. Redox-based colorimetric sensing of H2O2 after removal of antioxidants with ABTS radical oxidation. Spectrochim. Acta, Part A 2021, 248, 119266. 10.1016/j.saa.2020.119266. [DOI] [PubMed] [Google Scholar]
- Sinditskii V. P.; Kolesov V. I.; Egorshev V. Y.; Patrikeev D. I.; Dorofeeva O. V. Thermochemistry of cyclic acetone peroxides. Thermochim. Acta 2014, 585, 10–15. 10.1016/j.tca.2014.03.046. [DOI] [Google Scholar]
- Xu M.; Han J.-M.; Wang C.; Yang X.; Pei J.; Zang L. Fluorescence Ratiometric Sensor for Trace Vapor Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2014, 6, 8708–8714. 10.1021/am501502v. [DOI] [PubMed] [Google Scholar]
- Fan S.; Lai J.; Burn P. L.; Shaw P. E. Solid-State Fluorescence-based Sensing of TATP via Hydrogen Peroxide Detection. ACS Sens. 2019, 4, 134–142. 10.1021/acssensors.8b01029. [DOI] [PubMed] [Google Scholar]
- An Y.; Xu X.; Liu K.; An X.; Shang C.; Wang G.; Liu T.; Li H.; Peng H.; Fang Y. Fast, sensitive, selective and reversible fluorescence monitoring of TATP in a vapor phase. Chem. Commun. 2019, 55, 941–944. 10.1039/c8cc08399a. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Feng Y.; Zhang Z.; Zhang M. Highly Efficient Fluorescent Film Probe of Hydrogen Peroxide Vapor. Microchem. J. 2020, 158, 105290. 10.1016/j.microc.2020.105290. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.