Abstract
Plants are aerobic organisms relying on oxygen to serve their energy needs. The amount of oxygen available to sustain plant growth can vary significantly due to environmental constraints or developmental programs. In particular, flooding stress, which negatively impacts crop productivity, is characterized by a decline in oxygen availability. Oxygen fluctuations result in an altered redox balance and the formation of reactive oxygen/nitrogen species (ROS/RNS) during the onset of hypoxia and upon re-oxygenation. In this update, we provide an overview of the current understanding of the impact of redox and ROS/RNS on low-oxygen signaling and adaptation. We first focus on the formation of ROS and RNS during low-oxygen conditions. Following this, we examine the impact of hypoxia on cellular and organellar redox systems. Finally, we describe how redox and ROS/RNS participate in signaling events during hypoxia through potential post-translational modifications (PTMs) of hypoxia-relevant proteins. The aim of this update is to define our current understanding of the field and to provide avenues for future research directions.
An analysis of the role of reactive oxygen species, reactive nitrogen species, and redox components in hypoxia signaling pathways and an outline of potential future research avenues.
Advances
A role for ROS, RNS, and redox signaling during hypoxia in plants is currently emerging, implying a complex cellular crosstalk that coordinates metabolic, physiological, and morphological responses.
Upon hypoxia, ROS, RNS, and redox component levels show highly dynamic behavior in plants as in animals. In both systems, their levels are tightly regulated through transcriptional, translational, and post-translational regulation.
The action of ethylene, as a key regulator of submergence responses in plants, is tightly linked to ROS and RNS homeostasis and signaling.
ROS and RNS sources and signaling during hypoxia
ROS and RNSlike nitric oxide (NO) are short-lived redox-active molecules with established roles in stress signaling. Both are implicated in the regulation of several physiological and developmental processes as well as in stress responses in plants (Huang et al., 2019; Gupta et al., 2020). Nevertheless, uncontrolled accumulation results in tissue damage and death. This double-edged sword effect of ROS and NO depends on the cellular location, timing, and concentration. ROS and NO are continuously formed and scavenged during normal cellular physiology; cells monitor cellular processes that generate ROS and NO and act upon a disturbance of their homeostasis (Schmidt and Schippers, 2015; Mittler, 2017). ROS and NO formation increase rapidly during various biotic and abiotic stresses. Their generation represents specific signals with the potential to activate downstream signaling components involved in stress adaptation. During submergence, these signals are implicated in the regulation of adaptive traits that enhance oxygen diffusion in the plant, like adventitious root growth and aerenchyma formation (Mühlenbock et al., 2007; Steffens et al., 2012).
ROS and RNS sources
ROS are produced continuously throughout the cell during normal cellular activity, especially at metabolically active sites like the chloroplast, mitochondrion, and peroxisome, as well as in the endoplasmic reticulum (ER) during oxidative protein folding (Schippers and Schmidt, 2016; Fichman and Mittler, 2020). Even though oxygen levels are strongly reduced or depleted during hypoxia and anoxia, a ROS burst has been detected under such conditions in both plant and animal cells (Gonzali et al., 2015; Yao et al., 2017). Like animal cells (Diebold et al., 2010; Smith et al., 2017), plant cells exposed to hypoxia respond with a ROS burst originating in the mitochondrion and at the plasma membrane (Baxter-Burrel et al., 2002; Chang et al., 2012). Furthermore, like animal cells, NO formation occurs during oxygen deprivation in plant mitochondria (Castello et al., 2006; Vishwakarma et al., 2018). Thus, both animal and plant cells respond similarly to low oxygen with respect to the location of ROS and RNS formation.
In addition to the plasma membrane and the mitochondrion, other organelles might affect ROS and NO formation during low-oxygen stress (Schmidt et al., 2018a). For instance, oxidative protein folding in the ER requires the regeneration of oxidized PROTEIN DISULFIDE ISOMERASE (PDI) by oxygen-dependent ER THIOL OXIDASES (EROs; Koritzinsky et al., 2013; Meyer et al., 2019), suggesting a shift in the redox balance of the ER under hypoxia. As EROs act in an oxygen-dependent manner, they might function as oxygen sensors in the ER. Peroxisomes are known as crossroads for many biochemical pathways and production sites for hydrogen peroxide (H2O2) and NO (Corpas et al., 2019). Although peroxisomes have the potential to be signaling hubs during hypoxia, their role in ROS/NO formation is not explored. Chloroplasts are prone to ROS formation at their photosystem complexes under fluctuating conditions (Dietz et al., 2016). Interestingly, low-oxygen stress-induced expression of PHOSPHATE STARVATION RESPONSE1 was suggested to depend on a chloroplast-derived retrograde signal (Klecker et al., 2014). Furthermore, the paraquat-resistant radical-induced cell death 1 (RCD1) mutant was implicated in the crosstalk between photosynthetic electron transfer and the mitochondrion by modulating the expression of genes responsive to mitochondrial dysfunction and hypoxia (Shapiguzov et al., 2020).
The observation that multiple organelles trigger ROS or NO bursts upon oxygen deprivation is highly intriguing. It indicates that oxygen affects many different processes within the cell, which requires a concerted action to deal with. Potentially, each organelle may have a different threshold at which the oxygen level becomes limiting. Such characteristics would allow for a gradual, structured response to oxygen deprivation. Under mild hypoxic stress, only one or a few compartments might initiate signaling, whereas under severe stress, signals from multiple organelles need to be integrated in order to initiate an appropriate response.
ROS and NO signaling under hypoxia
ROS and NO signaling mediate stress adaptation responses through the activation of downstream cascades, altering protein activity and promoting a transcriptional response (Schippers and Schmidt, 2016; Jahnová et al., 2019; Fichman and Mittler, 2020; Figure 1). Upon stress, cells can generate an active ROS or NO burst in an enzyme-dependent manner, or through electron leakage at their photosystems and electron transfer chains (ETCs). Here, we give an overview of proteins that contribute to ROS and NO signaling during hypoxia.
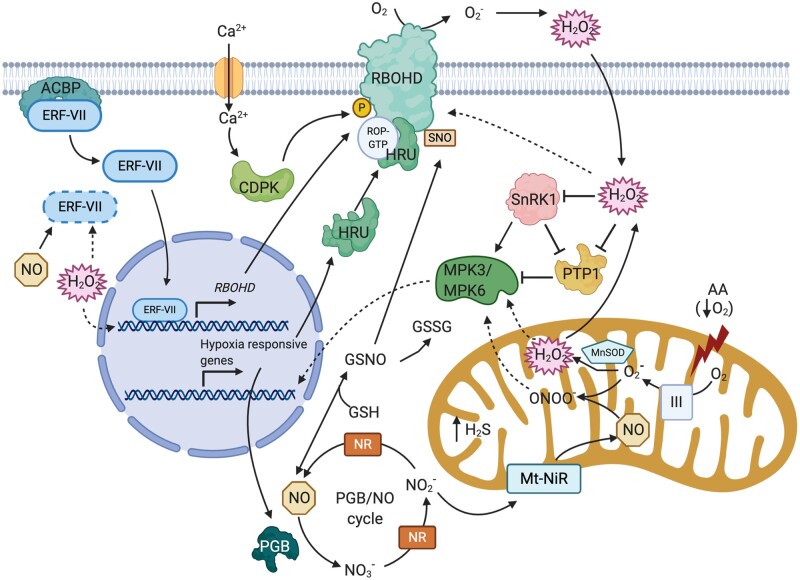
Figure 1.
ROS and NO signaling pathways during hypoxia. Hypoxia-induced production of ROS and NO occurs at several sites within the cell. Mitochondrial dysfunction during hypoxia generates ROS and can be pharmacologically mimicked by AA. The mitochondrial MnSOD mediates conversion to H2O2. Apoplastic ROS generation can be attributed to membrane-bound NADPH oxidases (RBOHD). RBOHD-mediated ROS production is modulated by HRU. HRU proteins exist as dimers in the cytosol and regulate ROS production via association with RBOHD in a protein complex including ROP-GTP. ROS production via RBOHD is tightly regulated via several PTMs. This includes: ROS-mediated PTM; NO-mediated S-nitrosylation; Phosphorylation by CDPKs. CDPKs are activated by hypoxia-mediated Ca2+ influxes. RBOHD can be synergistically activated via Ca2+ binding and CDPK-dependent phosphorylation. Cellular NO production during hypoxia can occur via reductive pathways. This includes cytosolic NRs, which generate NO from , and the Mt-NiR. Cellular NO levels are regulated primarily via removal by PGBs in a series of reactions known as the PGB–NO cycle. NO and ROS can affect hypoxia responses by influencing the stability of the oxygen labile group VII-ERF TFs. Under normoxic conditions, ERF-VIIs are either degraded via the N-degron pathway, or sequestered via association with Acyl-CoA-binding proteins. Hypoxia triggers ERFVII release and nuclear translocation to activate target gene (RBOHD, HRU, and PGB1) expression. NO and ROS could also influence hypoxia responses by regulating MPK activity. In Arabidopsis, MPK3/6 mediates hypoxia survival by an unknown mechanism. Their activation is triggered by mitochondrial stress signals, potentially either H2O2 or peroxynitrate (ONOO−) H2O2 could also regulate MPKs indirectly via Sucrose nonfermenting1 related Kinase (SnRK1) and a PTP. SnRK1 phosphorylates and activates MPK6, but SnRK1 activity is impaired by oxidative stress. PTP1 inhibits MPK3/6 but is itself inactivated by SnRK1 and also H2O2. The reaction of NO with GSH results in GSNO representing an important cellular storage form of NO. GSNO levels in turn are dependent on GSNOR activity. Figure created with BioRender.com.
NOX and ROS function in hypoxia signaling and adaptation
Plant NADPH oxidases (NOXs) or respiratory burst oxidase homologs (RBOHs) are transmembrane proteins that catalyze the production of superoxide () at the apoplast via the transfer of electrons from NAD(P)H to oxygen (Sagi and Fluhr, 2001). The subsequent dismutation of the product to the more stable H2O2 is considered to be essential for RBOH function in systemic signaling. Plant RBOHs typically possess six transmembrane domains, N-terminal calcium (Ca2+) binding (elongation factor) EF-hand motifs, and a C-terminal FAD and NAD(P)H-binding site (Marino et al., 2012). RBOH genes form a large multigene family with members displaying tissue- and development-specific expression and responding to various environmental stress signals.
RBOH-mediated ROS signaling is an integral part of plant responses to hypoxia, flooding, and re-oxygenation. RBOH expression is upregulated in shoots and roots of various species in response to hypoxia and flooding (Rajhi et al., 2011; Pucciariello et al., 2012; Yang and Hong, 2015, Yao et al., 2017; Yamauchi et al., 2017; Yeung et al., 2018). Among the hypoxia-inducible RBOH genes in Arabidopsis thaliana, RBOHD has been studied extensively. ROS production and survival are strongly reduced in rbohd knock-out mutants compared to wild-type following exposure to flooding or hypoxia/anoxia stress (Pucciariello et al., 2012; Chen et al., 2015; Yeung et al., 2018). Similarly, rbohf mutants display an impaired low-oxygen tolerance (Chen et al., 2015; Liu et al., 2017). RBOHD belongs to the so-called anaerobic core gene set (Mustroph et al., 2010), predominantly controlled by the low-oxygen-related group VII ethylene response factor (ERF-VII) transcription factors (TFs). RELATED TO AP-2.12 (RAP2.12) was shown to regulate RBOHD expression during hypoxia and ROS accumulation during flooding (Yao et al., 2017). Additionally, RBOHD activity is required for the induction of anaerobic core genes such as ALCOHOL DEHYDROGENASE 1 (ADH1), PYRUVATE DECARBOXYLASE 1 (PDC1), SUCROSE SYNTHASE 1, and ROS-scavenging enzymes during flooding (Yao et al., 2017). This suggests that the full activation of anaerobic core genes by ERF-VII TFs requires a ROS signal. This assumption is supported by experiments with a pAOX1a:LUC reporter in 35S:RAP2.12 protoplasts under aluminum stress, where the ability of RAP2.12 to activate the reporter is completely blocked upon diphenyleneiodonium chloride (DPI; a chemical inhibitor of RBOH activity) treatment. Still, how a ROS signal is transmitted to the RAP2.12 protein and if this involves PTMs remain to be answered.
Another link between RAP2.12 and ROS signaling is established via the transcriptional control of RAP2.12 over HYPOXIA-RESPONSIVE UNIVERSAL STRESS PROTEIN 1 (HRU1), which can interact with RBOHD (Gonzali et al., 2015). Mutating or altering HRU1 expression interferes with anoxia-induced H2O2 accumulation. Both in vivo and in vitro tests demonstrated HRU1 association with RBOHD and the hypoxia-activated GTP-ROP2. ROP2 is also required for ROS formation and survival under anoxia (Baxter-Burrel et al., 2002). This multi-protein interaction is considered to be an important element for the tight regulation of ROS accumulation during hypoxia (Gonzali et al., 2015). In this regard, it would be interesting to determine if HRU1 association is specific to RBOHD or includes other RBOHs as well.
RBOH-mediated ROS generation is a vital component of flooding-induced aerenchyma formation in several species (Rajhi et al., 2011; Yamauchi et al., 2014, 2017). Lysigenous aerenchyma consists of internal gas spaces that facilitate gas diffusion between the shoot and the root and result from programmed cell death of cortical cells. In rice (Oryza sativa), wheat (Triticum aestivum), and maize (Zea mays), waterlogging triggers increased expression of RBOH genes and ROS accumulation specifically in the root cortical cells, which in turn undergo programmed cell death to form aerenchyma tissue. Impairment of rice RbohH activity through pharmacological or genetic means block-inducible root aerenchyma formation (Yamauchi et al. 2017). Whereas increased RbohH expression during waterlogging is attributed to ethylene accumulation, ROS production requires Ca2+-dependent activation of RbohH by group-I Ca2+-dependent protein kinases (CDPKs). Ca2+ binding by the RBOH EF-hand domains upon a hypoxia-mediated Ca2+ influx and phosphorylation by the CDPKs are thought to synergistically activate RBOHs (Ogasawara et al., 2008; Yamauchi et al., 2017). Interestingly, Arabidopsis rbohd mutants display impaired Ca2+ increases during hypoxia, suggesting a role for RBOHD in modulating hypoxia-induced Ca2+ signaling (Wang et al. 2017). It has long been known that the Ca2+ wave and the ROS wave interact and amplify each other through the positive effects of Ca2+ on RBOH-mediated ROS production, and conversely through ROS modulation of Ca2+ channels (Fichman and Mittler, 2020).
In addition to phosphorylation by CDPKs, RBOH activity is also regulated by other PTMs. Notably, persulfidation of RBOHD was demonstrated to stimulate ROS production during abscisic acid-mediated stomatal closure (Shen et al., 2020). Persulfidation is a redox-based PTM effectuated by the gaseous signaling molecule hydrogen sulfide (H2S) and involves conversion of reactive cysteines (–SH) to persulfides (–SSH; Aroca et al., 2017; Filipovic and Jovanović, 2017). In mammals, H2S is an important signaling molecule during hypoxia (Peng et al., 2010). In plants, H2S alleviates hypoxia-induced cell death (Cheng et al., 2013) and promotes NO-induced hypoxia tolerance (Peng et al., 2016).
The mitochondrion as a ROS source under oxygen deficiency is thought to initiate stress adaptation responses. The response of several cytosolic parameters under hypoxia, including changes in ATP, Ca2+ levels, and the redox status, can be mimicked by pharmacologically inducing mitochondrial dysfunction with antimycin-A (AA; Wagner et al., 2019). Inhibition of complex III by AA interferes with the mitochondrial ETC and results in formation (Maxwell et al., 1999). AA also activates two mitogen-activated protein kinases (MPKs), MPK3 and MPK6, required for low-oxygen and re-oxygenation tolerance (Chang et al., 2012). It is likely that a mitochondrial ROS signal results in the activation of these MPKs under oxygen deficiency as both kinases are also activated upon exogenous H2O2 application (Kovtun et al., 2000).
Whereas controlled ROS production is essential for signaling during hypoxia and re-oxygenation, excessive ROS accumulation can result in cell death (Yeung et al., 2019). Therefore, ROS scavenging is an essential part of the stress survival response. In rice, the ERF-VII TF SUBMERGENCE 1A (SUB1A) has been implicated in several signaling and acclimation pathways under flooding. SUB1A activates a hibernation strategy to survive prolonged flooding periods (Fukao et al., 2011). Importantly, SUB1A upregulates the antioxidant and ROS scavenging systems to promote plant survival upon re-oxygenation. Also, in Arabidopsis, maintenance of ROS homeostasis is an important aspect of increasing tolerance to flooding stress (Yeung et al., 2018).
NO signaling during hypoxia
In animals and humans, NO and nitrite promote tolerance to oxygen deprivation (Fago and Jensen, 2015). Whereas in animals NO is generated through NO synthases (NOS), plants lack NOS enzymes but utilize several different oxidative and reductive NO biosynthesis pathways (Astier et al., 2018). Reductive pathways of NO production active under low-oxygen conditions include the cytosolic nitrate reductase (NR) pathway, mitochondrial NR (Mt-NiR) activity, and the plasma membrane-associated nitrite:NO reductase (NiNOR; Gupta et al., 2011). NO levels show dynamic changes and increase over time during flooding and hypoxia (Hebelstrup et al., 2012; Wany et al., 2017; Zhan et al., 2018). Enzymatic NO production appears to occur predominantly via the NAD(P)H-dependent reduction of nitrite by NR during low-oxygen stress (Chamizo-Ampudia et al., 2017). As NO overaccumulation is toxic, its levels are suppressed through the action of class-I plant PHYTOGLOBINS (PGBs), which can efficiently scavenge NO during hypoxia in an oxygen-dependent manner (Hebelstrup and Jensen, 2008). The very high affinity for oxygen (Km ∼4 nM) of class-1 PGBs allows them to function even under very low-oxygen concentrations (Smagghe et al., 2009). NO scavenging by PGBs results in the formation of nitrate and ferric hemoglobin using NADPH as an electron donor. The PGB–NO cycle allows for NAD+ regeneration during hypoxia and thereby could promote plant survival. However, it also causes acidification of the cell (Gupta et al., 2020).
In addition to cytosolic NR, the mitochondrial ETC (mETC) has been implicated in the reduction of nitrite to NO (Gupta et al., 2005). In both mammalian and yeast cells, mitochondrial NO production is ascribed to the pH-dependent action of CYTOCHROME C OXIDASE (Castello et al., 2006). The pH optima coincide with the typical intracellular pH drop (pH ∼6; Castello et al., 2006), which accompanies the onset of hypoxia (Wagner et al., 2019). Current evidence supports the notion that under anaerobic conditions, mETC functionality is maintained by nitrite (Stoimenova et al., 2007; Gupta et al., 2017). It is clear that mitochondrial reduction of nitrite to NO can act as a substitute for fermentation, recycling NADH, generating ATP, and decreasing ROS production. Still, the exact mechanism of mitochondrial NO production in plants requires more investigation.
NO can exert its signaling role by reacting with other redox-active molecules and protein residues. NO-mediated PTMs include metal nitrosylation, tyrosine nitration, and the most extensively studied S-nitrosylation (Lindermayr, 2018). In contrast to other PTMs, S-nitrosylation is considered to be nonenzymatic through the direct action of NO or S-nitrosoglutathione (GSNO) with target thiols (Jahnová et al., 2019). GSNO results from the reaction of NO with glutathione (GSH) and represents a storage form of NO that also mediates signaling as it can transfer its NO moiety to target proteins (Lindermayr, 2018). Under hypoxia, NO levels and the S-nitrosylation level of proteins rapidly increase (Hebelstrup et al., 2012), indicating that NO signaling affects protein functions under hypoxic conditions. Of interest, in this regard, is the modulation of RBOHD activity by S-nitrosylation during plant–pathogen interaction (Yun et al., 2011), which might also occur during hypoxia. NO is also implicated in regulating the stability of ERF-VII TFs (Gibbs et al., 2015; Vicente et al., 2017). Both oxygen and NO promote degradation of ERF-VII TFs through oxidation of the conserved penultimate cysteine residue, marking these proteins for degradation by the N-end degron pathway (Gibbs et al., 2011; Licausi et al., 2011). Arabidopsis seedlings treated with NO scavengers and the NR‐deficient nia1nia2 mutant exhibited an increased abundance of the ERFVIIs RAP2.3 and HRE2 (Gibbs et al., 2014). Potentially, during mild hypoxia, NO modulates ERFV-II levels, especially when considering that the oxygen-dependent enzymatic degradation by PLANT CYSTEINE OXIDASES (PCOs) is possibly inactive as PCOs are only active at a pH ˃ 6.5 (White et al., 2018). Recent technical advances have enabled the proteome-wide identification of S-nitrosylated proteins (Astier and Lindermayr, 2012). Parallel approaches on hypoxia- or flooding-stressed plants could identify specific NO targets involved in stress acclimation. Reversible and selective NO-mediated PTMs on target proteins during hypoxia might act as redox-responsive molecular switches. This could be of considerable functional relevance to trigger transient responses during hypoxia that can subsequently be reversed during re-oxygenation.
The level of GSNO, which represents the NO signaling strength, is enzymatically controlled by GSNO-REDUCTASE (GSNOR), which catalyzes the NADH-dependent reduction of GSNO to GSH disulfide (GSSG) and ammonium (Liu et al., 2001). Interestingly, GSNOR activity is modulated by oxidative PTMs and S-nitrosylation (Zhan et al., 2018). gsnor mutants, which possess excessive NO and S-nitrosylation, show perturbed stress responses. During hypoxia, GSNOR is S-nitrosylated, which leads to the exposure of an autophagy motif resulting in selective degradation (Zhan et al., 2018). Degradation of GSNOR during hypoxia might explain the accumulation of NO and GSNO under these conditions. Moreover, elimination of the S‐nitrosylation site in GSNOR abolishes the positive effect of NO on hypoxic germination of Arabidopsis seeds, indicating that the NO‐dependent PTM of GSNOR is a physiologically relevant process that contributes to the hypoxic response (Zhan et al., 2018).
Mutants of GSNOR have previously been identified as paraquat-resistant 2 (par2), as these mutants are less sensitive to the -inducing herbicide paraquat (Chen et al., 2009). The exact mechanism by which par2 mutants prevent cell death upon paraquat treatment is unknown. However, NO readily reacts with to form peroxynitrite, thereby avoiding H2O2 formation, which might prevent cell death. Peroxynitrite itself can cause S-nitrosylation (Fernando et al., 2019). Although this mainly occurs on tyrosine (Astier and Lindermayr, 2012), peroxynitrite can also interact with cysteine residues and transition metals. One of the best-characterized examples of protein modification via metal nitrosylation in plants is hemoglobin/PGB.
Another interesting aspect of S-nitrosylation is its documented role in the regulation of ethylene signaling, an important hormonal regulator of flooding responses (Sasidharan and Voesenek, 2015). Ethylene–NO crosstalk is complex and involves bilateral regulation and feedback loops. Whereas ethylene is reported to reduce endogenous NO levels by enhancing PGB-mediated scavenging (Hartman et al., 2019), NO itself can regulate ethylene metabolism. A decrease in ethylene production is associated with S-nitrosylation of several proteins involved in ethylene biosynthesis (Lindermayr et al., 2006). Accordingly, NO-associated PTMs are speculated to play a role in hyponastic leaf movement (Hebelstrup et al., 2012) and aerenchyma formation (Wany et al., 2017), both ethylene-mediated, flood-adaptive traits. A detailed biochemical investigation of how PTMs like S-nitrosylation or metal nitrosylation influence the activity of important flooding regulators such as hemoglobin or ethylene biosynthetic genes is needed to reveal whether these modifications inhibit or activate these proteins.
Cellular redox systems and low-oxygen stress
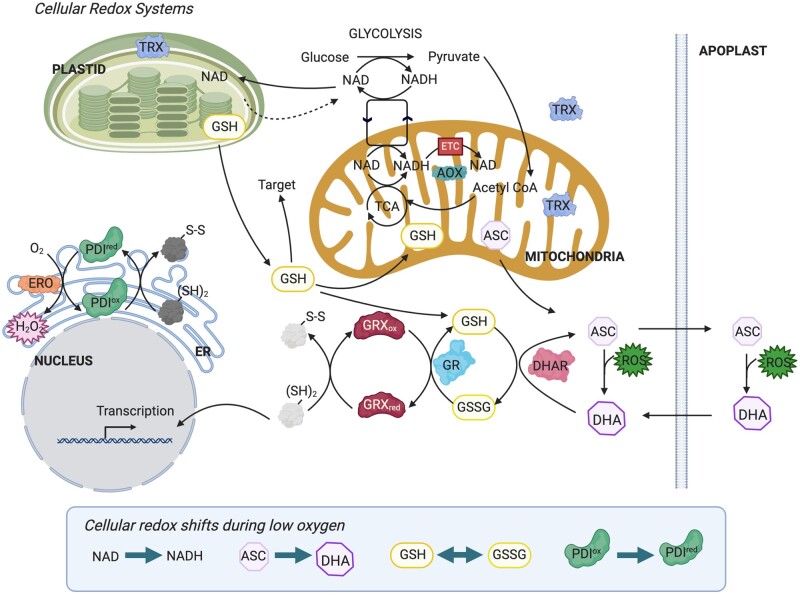
Upon oxygen limitation, a plant cell switches its metabolism from aerobic to fermentative pathways (Ismond et al., 2003). Metabolism relies on redox chemistry through oxidation or reduction of redox-active compounds involved in numerous metabolic reactions (Geigenberger and Fernie, 2014). The pyridine nucleotide coenzymes nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP) are essential to plant metabolism, playing a central role in glycolysis, fermentation, tricarboxylic acid (TCA) cycle, oxidative pentose phosphate pathway, and respiratory electron transport (Noctor, 2006). These ubiquitous coenzymes act as redox couples, with NAD+/NADP+ and NADH/NADPH representing the oxidized and reduced forms, respectively. The ratio of oxidized to reduced form is an indicator of the cellular redox status having a major impact on plant metabolism and signaling (Gakière et al., 2018). During the initial phase of hypoxia, the cytosolic NAD pool becomes more reduced in plants (Kennedy et al., 1992; Schmidt et al., 2018b; Wagner et al., 2019). NADH accumulation is thus a direct consequence of oxygen limitation. Moreover, NADH is known to inhibit PDC and TCA cycle dehydrogenases, which might trigger metabolic switching (Gakière et al., 2018). Recently, it was demonstrated that upon hypoxia, bifurcation of the TCA cycle occurs, indicating major metabolic reprogramming (António et al., 2016). Once fermentative pathways are activated, the NAD pool again becomes more oxidized (Wagner et al., 2019). The transient reductive or redox stress caused by NADH accumulation needs to be recognized and acted upon by the cell. This NADH increase is potentially directly sensed through single Cystathionine β-Synthase Domain-containing proteins, which regulate thioredoxin (TRX) activity in plants (Yoo et al., 2011). Thus, upon hypoxia, the NADH/NAD ratio rapidly increases, indicating a redox shift that triggers an adaptive response to restore the NADH/NAD ratio (Figure 2).
Figure 2.
Major cellular redox systems and the direction of redox shifts during hypoxia. Initial NAD biosynthesis steps occur in plastids, whereas the final step takes place in the cytosol. During hypoxia, a shift toward NADH results in inhibition of the TCA cycle. GSH is synthesized in plastids and GSH is readily transported throughout all cellular compartments. GSH powers enzymes, like GRXs or peroxidases, and acts as a ROS scavenger. Oxidized glutathione (GSSG) is readily converted back to GSH by GR. GRX proteins control oxidative modifications on target proteins at cysteine residues. In a similar fashion, TRXs also modulate redox modification on target proteins in a NAD(P)H-dependent manner. ASC synthesis is performed in the mitochondria and relies on respiratory activity. ASC reacts directly with ROS to form the corresponding oxidized product DHA. DHA is reduced in a GSH-dependent manner through DHA reductase back to ASC. Upon oxygen deprivation, the ASC pool is readily oxidized, which affects multiple enzymes and the cellular capacity for detoxifying ROS. The ER represents a rather oxidative environment due to oxidative protein folding by PDI. Upon oxygen limitation, it is expected that the reduced form of PDI accumulates as its oxidation requires the activity of the oxygen-dependent ERO proteins. Figure created with BioRender.com.
Another redox-active compound, ascorbate (ASC), is known in the mammalian field as an essential cofactor for PROLYL-HYDROXYLASES (PDHs), which destabilize the key transcriptional regulator HYPOXIA-INDUCIBLE FACTOR 1 (HIF1; Appelhoff et al., 2004). ASC oxidation during hypoxia in mammalian cells results in decreased PDH activity and subsequent HIF1 stabilization. Although ASC can directly act as a ROS scavenger, its cellular action is mainly coupled to peroxide metabolism through specific enzymes (e.g. peroxidases; Foyer and Noctor, 2011). In contrast to GSH or NAD, there is no indication that the ratio between reduced ASC and its oxidized form [dehydroascorbate (DHA)] represents a cellular read-out initiating signaling. This is mainly due to spatial separation of apoplastic DHA and ASC pools (Green and Fry, 2005; Figure 2). Nevertheless, a depletion of the ASC reservoir will greatly impair ROS scavenging ability, resulting in a more oxidized cellular environment. Interestingly, upon 2 h of anoxia, Arabidopsis cell cultures show a ≥90% oxidation of the ASC pool (Paradiso et al., 2016), indicating a more oxidized cellular environment under stress. Although the ASC biosynthesis pathway is known, submergence tolerance of associated mutants remains untested. After 2 h of re-oxygenation, the ASC pool is readily reduced and the total amount nearly doubles (Paradiso et al., 2016). In Arabidopsis, an increase in ASC and re-oxygenation tolerance following submergence is regulated by the jasmonate-controlled MYC2 TF (Yuan et al., 2017). MYC2 overexpression improves submergence tolerance partly through the induction of ASC biosynthesis genes like VITAMIN C DEFECTIVE 1 (VTC1). Accordingly, VTC1 overexpression in the myc2 background restores re-oxygenation tolerance. The decrease in ASC levels during submergence and its rapid restoration during recovery is linked in rice to submergence tolerance (Kawano et al., 2002).
GSH is an essential redox metabolite that can be oxidized by ROS and thereby functions as antioxidant to ameliorate oxidative stress (Noctor et al., 2012; Figure 2). Oxidized GSH is recycled by NADPH‐dependent GSH REDUCTASE, such that in the absence of stress, the cellular GSH pool is maintained in a highly reduced state (Mhamdi et al., 2010). Furthermore, GSH accumulates in cells up to millimolar concentrations, indicating the ability of the system to buffer the redox state of the cell (Noctor et al., 2012). Interestingly, in human cell lines, it was shown that the redox state of the GSH pool affects the activity of HIF1, that is, a more reduced GSH state mitigates the effect of the TF whereas a more oxidized pool promotes HIF1 activity (Tajima et al., 2009; Yi et al., 2019). During anoxia exposure of an Arabidopsis cell suspension, the GSH pool showed a transient decline in size after 2 h of treatment but returned to pre-stress levels thereafter while maintaining its redox state (Paradiso et al., 2016). Re-oxygenation strongly increased the GSH pool, which is indicative of a cellular response toward oxidative stress (Paradiso et al., 2016). Interestingly, GSH biosynthesis during re-oxygenation is promoted by MYC2 and sufficient to protect the plant from oxidative stress during this phase (Yuan et al., 2017).
GSH TRANSFERASES (GSTs) are multifunctional enzymes that usually promote the nucleophilic attack of the cysteine thiol group of the tripeptide GSH on molecules having electrophilic carbon, sulfur, or oxygen atoms (Edwards et al., 2000). Arabidopsis contains 54 GST genes, grouped into seven distinct classes in plants (Dixon et al., 2009). Among the phi class (GSTF), GSTF4, GSTF6, GSTF8, GSTF10, GSTF11, GSTF12, GSTF13, and GSTF14 are differentially expressed after 4 h of hypoxia (Christianson et al., 2009). Interestingly, GSTF6 promotes pathogen defense through camalexin biosynthesis and was constitutively upregulated in the prt6 mutant defective in the N-degron pathway (Vicente et al., 2019). Furthermore, GSTF8 is a common marker for pathogen stress, and GSTF11 and GSTF12 promote anthocyanin biosynthesis (Kitamura et al., 2004). Whereas zeta and theta class members are not hypoxia-inducible, among the tau class (GSTU), 20 out of 28 members are hypoxia responsive (Christianson et al., 2009). As GSTs are hypoxia regulated, they likely play an extensive role in metabolic reprogramming and modulating protein functions during hypoxia via glutathionylation.
Another family of GSH-dependent enzymes is glutaredoxins (GRXs), which act as oxidoreductases controlling the redox state of thiol groups in proteins or small compounds (Gutsche et al., 2015). A link between a hypoxic niche and plant development is exemplified by the maize mutant male–sterile-converted anther 1 (MSCA1; Kelliher and Walbot, 2012). MSCA1 encodes a GRX, essential for maintaining an unknown redox-sensitive protein in a reduced state during de novo germinal cell specification within anthers. To promote a reduced environment, the anther creates a hypoxic surrounding to divert carbon away from mitochondrial respiration and into alternative pathways to avoid ROS formation (Kelliher and Walbot, 2014). Transcriptome analysis in diverse species during hypoxia indicates differential regulation of GRX genes (Christianson et al., 2009; Safavi-Rizi et al., 2020). Next to GRXs, TRXs are major regulators of the redox state of target proteins and molecules (Meyer et al., 2008; Delorme-Hinoux et al., 2016). TRX is a major regulator of the mitochondrial TCA cycle by modulating the redox-state of associated enzymes (Daloso et al., 2015). The TRX-controlled redox network in plants connects plastidial and mitochondrial metabolism to ensure optimal performance of the plant under fluctuating environmental conditions (Geigenberger et al., 2017).
Organellar redox system
Mitochondria represent the site of aerobic metabolism providing a steady flow of ATP to drive cellular operations. Energy requirements fluctuate during development or in response to environmental conditions (Møller et al., 2020). In response to rapid developmental or stress-induced fluctuations in oxygen levels, corresponding adaptation of mitochondrial metabolism needs to be swift. Interestingly, oxygen fluctuations provoke a mitochondrial ROS burst, which in turn might initiate further ROS release (Zandalinas and Mittler, 2018). Although the phenomenon of a mitochondrial ROS burst in plants is just emerging (Nie et al., 2015), it is well established in mammalian systems where acute hypoxia triggers an burst at complex I (Brand, 2016; Hernansanz-Agustín et al., 2017). In rice, formation at complex I was suggested to trigger adaptive responses during stress, including the upregulation of ALTERNATIVE OXIDASE (Li et al., 2013). In Arabidopsis, a hypoxia-triggered ROS burst attributed to the mitochondria results in activation of an MPK cascade (Chang et al., 2012).
How the mitochondrial ROS signal is sensed and converted into a (post-)transcriptional response is far from being understood. Potentially, the burst is decoded into an H2O2 signal through the action of MANGANESE-DEPENDENT SUPEROXIDE DISMUTASE (MnSOD; Figure 2). Interestingly, reduction of mitochondrial MnSOD activity alters both the TCA cycle flux and mitochondrial ROS homeostasis (Morgan et al., 2008). Measurements of the redox status using a fluorescent reporter revealed that knocking down MnSOD causes an oxidized environment in the mitochondrion, whereas the cytosolic redox state remains unaffected. Whether the altered TCA cycle flux is due to oxidative stress or altered redox signaling remains unexplored. Mitochondrial levels might also be directly sensed by mitochondrial proteins containing transition metals, like the TCA cycle enzymes aconitase and fumarase (Moeder et al., 2007; Daloso et al., 2015). In such a scenario, the ROS signal is converted into a metabolic signal which might trigger adaptive responses. Alternatively, is converted into H2O2 that, through its action on protein thiols, transforms the ROS signal into a proteinaceous signal.
The role of the ER in ROS formation under stress is poorly explored. The ER represents an oxidized environment under normoxic conditions due to oxidative protein folding and H2O2 formation by the oxygen-dependent ERO proteins (Meyer et al., 2019). An impaired ERO activity during hypoxia would concomitantly result in an altered redox state of the ER. ER protein-folding homeostasis and particularly disulfide bond formation is highly sensitive to an altered redox balance, whereby both reducing and oxidizing reagents disturb protein folding and cause ER stress (Malhotra and Kaufmann, 2007). Under hypoxia stress, it is expected that inhibition of the putative oxygen-sensing ERO proteins will result in an unfolded protein response capable of signaling function. However, no systematic studies in this direction have been performed in plants.
Potential role for oxidative and redox PTMs under hypoxia
In addition to the ROS- and NO-induced PTMs described before, here we present examples of proteins that undergo redox regulation via PTMs, which might also be relevant to hypoxic stress regulation.
Metabolism-related enzymes
The sensitivity of TCA cycle and glycolytic/fermentative enzymes toward redox changes raises the exciting possibility that redox perturbations may immediately act on metabolic adjustment mechanisms under hypoxia. During hypoxia, the TCA intermediates fumarate and malate decline (António et al., 2016), suggesting inactivation of both fumarase and succinate dehydrogenases. Interestingly, the activity of both enzymes is regulated by the mitochondrial TRX system (Daloso et al., 2015). Thus, redox-regulation of the TCA cycle during hypoxia might contribute to metabolic switching. Fermentative enzymes also undergo oxidative modifications. ADH, catalyzing the last step of ethanol fermentation, is inhibited by H2O2 through oxidation of critical cysteine residues (Dumont et al., 2018). NADH-binding decreases the sensitivity of ADH toward H2O2. This observation suggests that during hypoxia, ADH inactivation is prevented by high NADH levels, whereas upon re-oxygenation, it can be rapidly inactivated through H2O2. Translating the current knowledge regarding redox regulation of metabolic enzymes to hypoxia-specific studies will be highly rewarding. For instance, the role of redox-dependent metabolic switching during hypoxia could be tested by using redox-sensitive or insensitive fumarase or succinate dehydrogenase transgenic lines.
N-degron enzymes
The role of the N-degron pathway in regulating ERF-VII stability through oxidation of the penultimate cysteine by PCOs is well explored (Gibbs et al., 2015; White et al., 2018). As PCOs are oxygen-dependent and pH-sensitive, oxidation of the penultimate cysteine residue of target proteins is impaired during hypoxia. This suggests that oxidation and degradation of ERF-VIIs are initiated mainly upon re-oxygenation. However, considering NO involvement in ERF-VII degradation, it still is not fully clear why ERF-VII proteins are stable under hypoxic conditions despite NO accumulation. During a flooding event, early accumulation of ethylene is implicated in stabilizing ERF-VIIs before the onset of hypoxia. This is associated with NO scavenging by ethylene-enhanced PGB levels in Arabidopsis (Hartman et al., 2019). Several ERF-VIIs like HRE1 and HRE2 are strongly transcribed and de novo synthesized during hypoxia. Targeting these proteins for degradation by the N-degron pathway requires removal of methionine to expose the penultimate cysteine residue. Methionine removal is catalyzed by METHIONINE AMINOPEPTIDASES (MAPs), which recently were shown to be modified by H2O2in planta (Waszczak et al., 2014). Oxidative modification decreases MAP activity in human cell lines under hypoxic conditions (Chiu et al., 2014). In such a scenario, de novo ERF-VII synthesis during hypoxia prevents methionine removal and avoids targeting to the N-degron pathway.
Kinases
Phosphorylation represents a rapid and common way to propagate signaling events to downstream effectors under many stresses. The kinases MPK3, MPK4, and MPK6 are activated upon oxygen deprivation in Arabidopsis (Chang et al., 2012; Figure 2). MPK signaling is regulated by oxidative modifications. De-repression of MPK signaling relies on inactivation of PROTEIN TYROSINE PHOSPHATASE 1 (PTP1). Oxidative conditions inactivate PTP1 via thiol oxidation and thereby inhibit dephosphorylation of MPK3 and MPK6 (Gupta and Luan, 2003; Waszczak et al., 2014). S-sulfenylation of MPK4 upon H2O2 treatment stimulates its kinase activity (Waszczak et al., 2014; Huang et al., 2019). Thus, redox signaling can work as a double-edged sword, inactivating repressors, and promoting activators of stress signaling. SNF1-RELATED PROTEIN KINASE 1 (SnRK1) is an important component of energy sensing under hypoxia with a direct link to sugar starvation under stress. Interestingly, H2O2 causes oxidation of a cysteine residue and thereby inactivates SnRK1 (Wurzinger et al., 2017). Potentially, under hypoxia, modulation of SnRK1 activity enables fine-tuning of the energy metabolism to promote plant survival. Still, the role of many kinases and their regulation by redox-dependent modification during hypoxia awaits discovery.
TFs
TF activity can be modulated through PTMs. Thus far, thiol-dependent regulation of TFs is poorly explored; however, current examples encourage further research on this topic as it represents an important regulatory aspect for plants to deal with rapid environmental fluctuations.
Heat-shock factors (HSFs) are TFs that act under heat stress and also participate in hypoxia signaling. Specifically, HSFA1a, HSFA1b, and HSFA2 positively regulate plant performance under anoxia (Banti et al., 2010). From a redox perspective, HSFA1a is especially interesting as oxidation of its regulatory thiol results in its activation (Liu et al., 2013). In addition, HSF2A, which participates in hypoxia signaling, is transcriptionally induced by H2O2 (Miller and Mittler, 2006).
Next to N-degron-mediated degradation of ERF-VIIs, another ERF, RAP2.4, is also subject to redox-dependent regulation. RAP2.4 activates the expression of a chloroplast-localized 2-Cys peroxiredoxin-A in a redox-dependent manner (Shaikhali et al., 2008). As the cysteines targeted in RAP2.4 are not conserved, it remains unclear if other RAP members are also targeted by oxidative modifications.
In addition to a TF itself being a target of redox modifications, interacting proteins with a regulatory role might also be redox-controlled. One such regulatory protein known to interact with a multitude of TFs, including those acting under stress, is RCD1. RCD1 contains several cysteine residues rendering it redox sensitive. Oxidative conditions, as they occur for example, under stress, result in dimerization of RCD1 proteins, probably affecting its activity. RCD1 interacts with ANAC017, which was recently shown to regulate submergence tolerance (Bui et al., 2020; Shapiguzov et al., 2020). Thus, RCD1 represents a potential hub for redox signals during hypoxia that controls the activity of different TFs.
Conclusions
Our understanding of low-oxygen signaling has seen major advances in recent years. Next to the identification of additional transcriptional regulators, further proteins are being identified and characterized as relevant to low-oxygen stress. Thereby, a complex signaling network is being uncovered that acts upon signals from different organelles, which are integrated and translated into an appropriate adaptation response. Still, little is known regarding the nature and impact of redox and ROS signals during hypoxia in plants and many questions remain (see Outstanding questions).
Considering that organelles and cellular compartments have different oxygen requirements and different levels of redox homeostasis, it is extremely tempting to suggest that these organelles and compartments perceive and signal low-oxygen stress at different concentrations. Yet, the nature of this complex signaling network is far from being understood, especially due to a lack of knowledge on primary signaling events, those that trigger the signaling cascades. ROS and NO are potent signaling molecules; however, when and where they are formed during hypoxia in plants is still not clear. This can be resolved by using specific and sensitive sensors, either genetically encoded or by using micro-electrode systems. Recent developments with genetically encoded sensors allow for organelle-specific reporters (Voon et al., 2018; Nietzel et al., 2019). The adaptation of such tools will enable more accurate monitoring of spatial and temporal redox-based changes during flooding or hypoxia. In addition, in the context of flooding, it is essential to know how other flooding signals like ethylene and sugars might influence this redox output and signaling. However, these experiments are still technically very challenging and in part restricted by the tools currently available.
The next step forward in resolving the integrated stress signaling during low oxygen is the study that focuses on the initial phase of oxygen depletion at a time-scale of minutes. Currently, no studies have resolved the kinetics of the triggering events leading to low-oxygen signaling. Such studies should include the parallel analysis of metabolites, ROS, NO, thiol-oxidation, redox, and transcript levels, and also organelle-specific changes. Such an approach will not only resolve the order of events, that is, which organelle or organelles are affected first, but can also reveal which signals (redox, metabolite, or small chemicals) are fed into the low-oxygen signaling network to provoke the adaptation response.
Finally, many proteins with a link to low-oxygen stress and redox regulation, as mentioned above, remain uncharacterized in the context of hypoxia stress. Functional genomics studies will greatly contribute to a better understanding of these individual components and their role during stress signaling.
The pursuit of these research avenues in the future will no doubt provide exciting new insights into the complex signaling networks integrating hypoxia and redox sensing and signaling. An in-depth understanding of redox-based regulation of hypoxia and flood-adaptive responses is essential if results are to be used toward improving stress resilience of relevant plant species.
Outstanding questions
How do cells integrate ROS and NO signals, with different cellular origins, to modulate hypoxic stress responses?
Through which components do RBOH-derived signals initiate transcriptional reprogramming during hypoxia?
How do oxidative PTMs contribute to cellular metabolic switching during hypoxia and re-oxygenation? What is the role of the TRX system during hypoxia or submergence stress?
What is the causal and temporal relationship between peroxynitrite formation and cellular reprogramming during hypoxia?
Which components are involved in mitochondrial sensing and signaling during hypoxia and re-oxygenation in plants?
What are the spatial and temporal dynamics of ROS, NO, and other redox-mediated changes during hypoxia and/or re-oxygenation?
How does positive feedback crosstalk between Ca2+ and RBOH-mediated ROS production function during hypoxia?
Funding
R.S.: The Netherlands Organization for Scientific Research (NWO), grants TTW 14700, 016.VIDI.171.006 and 867.15.031. J.H.M.S.: IPK Gatersleben, R.R.S.: Bielefeld University.
Conflict of interest statement. The authors declare no conflict of interest.
Rashmi Sasidharan, Jos H M Schippers and Romy R Schmidt authors contributed to conceiving and writing the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Romy R. Schmidt (romy.schmidt@uni-bielefeld.de).
References
- António C, Päpke C, Rocha M, Diab H, Limami AM, Obata T, Fernie AR, van Dongen JT (2016) Regulation of primary metabolism in response to low oxygen availability as revealed by carbon and nitrogen isotope redistribution. Plant Physiol 170: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465 [DOI] [PubMed] [Google Scholar]
- Aroca A, Benito JM, Gotor C, Romero LC (2017) Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot 68: 4915–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69: 3401–3411 [DOI] [PubMed] [Google Scholar]
- Astier J, Lindermayr C (2012) Nitric oxide-dependent posttranslational modification in plants: an update. Int J Mol Sci 13: 15193–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banti V, Mafessoni F, Loreti E, Alpi A, Perata P (2010) The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol 152: 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028. [DOI] [PubMed] [Google Scholar]
- Brand MD (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100: 14–31 [DOI] [PubMed] [Google Scholar]
- Bui LT, Shukla V, Giorgi FM, Trivellini A, Perata P, Licausi F, Giuntoli B (2020) Differential submergence tolerance between juvenile and adult Arabidopsis plants involves the ANAC017 transcription factor. Plant J 104: 979–994 [DOI] [PubMed] [Google Scholar]
- Castello PR, David PS, McClure T, Crook Z, Poyton RO (2006) Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab 3: 277–287 [DOI] [PubMed] [Google Scholar]
- Chamizo-Ampudia A, Sanz-Luque E, Llamas A, Galvan A, Fernandez E (2017) Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci 22: 163–174 [DOI] [PubMed] [Google Scholar]
- Chang R, Jang CJ, Branco-Price C, Nghiem P, Bailey-Serres J (2012) Transient MPK6 activation in response to oxygen deprivation and re-oxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol Biol 78: 109–122 [DOI] [PubMed] [Google Scholar]
- Chen L, Liao B, Qi H, Xie LJ, Huang L, Tan WJ, Zhai N, Yuan LB, Zhou Y, Yu LJ, et al. (2015) Autophagy contributes to regulation of the hypoxia response during submergence in Arabidopsis thaliana. Autophagy 11: 2233–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Sun S, Wang C, Li Y, Liang Y, An F, Li C, Dong H, Yang X, Zhang J, Zuo J (2009) The Arabidopsis PARAQUAT RESISTANT2 gene encodes an S-nitrosoglutathione reductase that is a key regulator of cell death. Cell Res 19: 1377–1387 [DOI] [PubMed] [Google Scholar]
- Cheng W, Zhang L, Jiao C, Su M, Yang T, Zhou L, Peng R, Wang R, Wang C (2013) Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol Biochem 70: 278–286 [DOI] [PubMed] [Google Scholar]
- Chiu J,, Wong JW, Hogg PJ (2014) Redox regulation of methionine aminopeptidase 2 activity. J Biol Chem 289: 15035–15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Wilson IW, Llewellyn DJ, Dennis ES (2009) The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol 149: 1724–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Del Río LA, Palma JM (2019) Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J Integr Plant Biol 61: 803–816 [DOI] [PubMed] [Google Scholar]
- Daloso DM, Müller K, Obata T, Florian A, Tohge T, Bottcher A,, Riondet C, Bariat L, Carrari F, Nunes-Nesi A, et al. (2015) Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proc Natl Acad Sci USA 112: E1392–E1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Hinoux V, Bangash SA, Meyer AJ, Reichheld JP (2016) Nuclear thiol redox systems in plants. Plant Sci 243: 84–95 [DOI] [PubMed] [Google Scholar]
- Diebold I, Petry A, Hess J, Görlach A (2010) The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Turkan I, Krieger-Liszkay A (2016) Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol 171: 1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60: 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont S, Bykova NV, Khaou A, Besserour Y, Dorval M, Rivoal J (2018) Arabidopsis thaliana alcohol dehydrogenase is differently affected by several redox modifications. PLoS One 13: e0204530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Fago A, Jensen FB (2015) Hypoxia tolerance, nitric oxide, and nitrite: lessons from extreme animals. Physiology 30: 116–126 [DOI] [PubMed] [Google Scholar]
- Fernando V, Zheng X, Walia Y, Sharma V, Letson J, Furuta S (2019) S-Nitrosylation: an emerging paradigm of redox signaling. Antioxidants (Basel) 8: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y, Mittler R (2020) Rapid systemic signaling during abiotic and biotic stresses: is the ROS wave master of all trades? Plant J 102: 887–896 [DOI] [PubMed] [Google Scholar]
- Filipovic MR, Jovanović VM (2017) More than just an intermediate: hydrogen sulfide signaling in plants. J Exp Bot 68: 4733–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23: 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakière B, Fernie AR, Pétriacq P (2018) More to NAD+ than meets the eye: a regulator of metabolic pools and gene expression in Arabidopsis. Free Radic Biol Med 122: 86–95 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR (2014) Metabolic control of redox and redox control of metabolism in plants. Antioxid Redox Signal 21: 1389–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Thormählen I, Daloso DM, Fernie AR (2017) The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci 22: 249–262 [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, Holdsworth MJ (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Md Isa N, Movahedi M, Lozano-Juste J, Mendiondo GM, Berckhan S, Marín-de la Rosa N, Vicente Conde J, Sousa Correia C, Pearce SP, et al. (2014) Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol Cell 53: 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Conde JV, Berckhan S, Prasad G,, Mendiondo GM, Holdsworth MJ (2015) Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress responses in plants. Plant Physiol 169: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Cardarelli F, Novi G, Parlanti S, Pucciariello C, Bassolino L, Banti V, Licausi F, Perata P (2015) Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat Plants 1: 1–9 [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature 433: 83–87 [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56: 2601–2609 [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16: 160–168 [DOI] [PubMed] [Google Scholar]
- Gupta R, Luan S (2003) Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol 132: 1149–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KJ, Lee CP, Ratcliffe RG (2017) Nitrite protects mitochondrial structure and function under hypoxia. Plant Cell Physiol 58: 175–183 [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Mur LA, Wany A, Kumari A, Fernie AR, Ratcliffe RG (2020) The role of nitrite and nitric oxide under low oxygen conditions in plants. New Phytol 225: 1143–1151 [DOI] [PubMed] [Google Scholar]
- Gutsche N, Thurow C, Zachgo S, Gatz C (2015) Plant-specific CC-type glutaredoxins: functions in developmental processes and stress responses. Biol Chem 396: 495–509 [DOI] [PubMed] [Google Scholar]
- Hartman S, Liu Z, van Veen H, Vicente J, Reinen E, Martopawiro S, Zhang H, van Dongen N, Bosman F, Bassel GW, et al. (2019) Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat Commun 10: 4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebelstrup KH, Jensen EO (2008) Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana. Planta 227: 917–927 [DOI] [PubMed] [Google Scholar]
- Hebelstrup KH, van Zanten M, Mandon J, Voesenek LA, Harren FJ, Cristescu SM, Møller IM, Mur LA (2012) Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana. J Exp Bot 63: 5581–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernansanz-Agustín P, Ramos E, Navarro E, Parada E, Sánchez-López N, Peláez-Aguado L, Cabrera-García JD, Tello D, Buendia I, Marina A,. et al. (2017) Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol 12: 1040–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismond KP, Dolferus R, de Pauw M, Dennis ES, Good AG (2003) Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol 132: 1292–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnová J, Luhová L, Petřivalský M (2019) S-nitrosoglutathione reductase—the master regulator of protein S-nitrosation in plant NO signaling. Plants 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano N, Ella E, Ito O, Yamauchi Y, Tanaka K (2002) Metabolic changes in rice seedlings with different submergence tolerance after desubmergence. Environ 47: 195–203 [Google Scholar]
- Kelliher T, Walbot V (2012) Hypoxia triggers meiotic fate acquisition in maize. Science 337: 345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher T, Walbot V (2014) Maize germinal cell initials accommodate hypoxia and precociously express meiotic genes. Plant J 77: 639–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Rumpho ME, Fox TC (1992) Anaerobic metabolism in plants. Plant Physiol 100: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37: 104–114 [DOI] [PubMed] [Google Scholar]
- Klecker M, Gasch P, Peisker H, Dörmann P, Schlicke H, Grimm B, Mustroph A (2014) A shoot-specific hypoxic response of Arabidopsis sheds light on the role of the phosphate-responsive transcription factor PHOSPHATE STARVATION RESPONSE1. Plant Physiol 165: 774–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzinsky M, Levitin F, van den Beucken T, Rumantir RA, Harding NJ, Chu KC, Boutros PC, Braakman I, Wouters BG (2013) Two phases of disulfide bond formation have differing requirements for oxygen. J Cell Biol 203: 615–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Liang DD, Li J, Duan YB, Li H, Yang YC, Qin RY, Li L, Wei PC, Yang JB (2013) Unravelling mitochondrial retrograde regulation in the abiotic stress induction of rice ALTERNATIVE OXIDASE 1 genes. Plant Cell Environ 36: 775–788 [DOI] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Lindermayr C, Saalbach G, Bahnweg G, Durner J (2006) Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J Biol Chem 281: 4285–4291 [DOI] [PubMed] [Google Scholar]
- Lindermayr C (2018) Crosstalk between reactive oxygen species and nitric oxide in plants: key role of S-nitrosoglutathione reductase. Free Radic Biol Med 122: 110–115 [DOI] [PubMed] [Google Scholar]
- Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS (2001) A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410: 490–494 [DOI] [PubMed] [Google Scholar]
- Liu B, Sun L, Ma L, Hao FS (2017) Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep 36: 947–957 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang C, Chen J, Guo L, Li X, Li W, Yu Z, Deng J, Zhang P, Zhang K, Zhang L (2013) Arabidopsis heat shock factor HsfA1a directly senses heat stress, pH changes, and hydrogen peroxide via the engagement of redox state. Plant Physiol Biochem 64: 92–98 [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293 [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17: 9–15 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y, Siala W, Bashandy T, Riondet C, Vignols F, Reichheld JP (2008) Glutaredoxins and thioredoxins in plants. Biochim Biophys Acta 1783: 589–600 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Riemer J, Rouhier N (2019) Oxidative protein folding: state-of-the-art and current avenues of research in plants. New Phytol 221: 1230–1246 [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou JP,. et al. (2010) Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Mittler R (2006) Could heat shock factors function as hydrogen peroxide sensors in plants? Ann Bot 98: 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2017) ROS are good. Trends Plant Sci 22: 11–19 [DOI] [PubMed] [Google Scholar]
- Moeder W, Del Pozo O, Navarre DA, Martin GB, Klessig DF (2007) Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol Biol 63: 273–287 [DOI] [PubMed] [Google Scholar]
- Møller IM, Igamberdiev AU, Bykova NV, Finkemeier I, Rasmusson AG, Schwarzländer M (2020) Matrix redox physiology governs the regulation of plant mitochondrial metabolism through posttranslational protein modifications. Plant Cell 32: 573–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Lehmann M, Schwarzländer M, Baxter CJ, Sienkiewicz-Porzucek A, Williams TC, Schauer N, Fernie AR, Fricker MD, Ratcliffe RG, et al. (2008) Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol 147: 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenbock P, Plaszczyca M, Plaszczyca M, Mellerowicz E, Karpinski S (2007) Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 19: 3819–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J (2010) Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol 152: 1484–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie S, Yue H, Zhou J, Xing D (2015) Mitochondrial-derived reactive oxygen species play a vital role in the salicylic acid signaling pathway in Arabidopsis thaliana. PLoS One 10: e0119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietzel T, Elsässer M, Ruberti C, Steinbeck J, Ugalde JM, Fuchs P, Wagner S, Ostermann L, Moseler A, Lemke P, et al. (2019) The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol 221: 1649–1664 [DOI] [PubMed] [Google Scholar]
- Noctor G (2006) Metabolic signaling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29: 409–425 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, Han YI, Neukermans J, Marquez‐Garcia BE, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35: 454–484 [DOI] [PubMed] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283: 8885–8892 [DOI] [PubMed] [Google Scholar]
- Paradiso A, Caretto S, Leone A, Bove A, Nisi R, De Gara L (2016) ROS production and scavenging under anoxia and re-oxygenation in Arabidopsis cells: a balance between redox signaling and impairment. Front Plant Sci 7: 1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR (2010) H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107: 10719–10724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, Bian Z, Zhou L, Cheng W, Hai N, Yang C, Yang T, Wang X, Wang C (2016) Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep 35: 2325–2340 [DOI] [PubMed] [Google Scholar]
- Pucciariello C, Parlanti S, Banti V, Novi G, Perata P (2012) Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol 159: 184–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, et al. (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190: 351–368 [DOI] [PubMed] [Google Scholar]
- Safavi-Rizi V, Herde M, Stöhr C (2020) RNA-Seq reveals novel genes and pathways associated with hypoxia duration and tolerance in tomato root. Sci Rep 10: 1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan R, Voesenek LA (2015) Ethylene-mediated acclimations to flooding stress. Plant Physiol 169: 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schippers JHM (2015) ROS-mediated redox signaling during cell differentiation in plants. Biochim Biophys Acta 1850: 1497–1508 [DOI] [PubMed] [Google Scholar]
- Schmidt RR, Weits DA, Feulner CFJ, van Dongen JT (2018a) Oxygen sensing and integrative stress signaling in plants. Plant Physiol 176: 1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RR, Fulda M, Paul MV, Anders M, Plum F, Weits DA, Kosmacz M, Larson TR, Graham IA, Beemster GTS, et al. (2018b) Low-oxygen response is triggered by an ATP-dependent shift in oleoyl-CoA in Arabidopsis. Proc Natl Acad Sci USA 115: E12101–E12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers JHM, Schmidt R (2016) The role of ROS and redox signaling during the initial cellular response to abiotic stress. Redox State as a Central Regulator of Plant-Cell Stress Responses. Springer, Cham, Switzerland, pp 253–273 [Google Scholar]
- Shaikhali J, Heiber I, Seidel T, Ströher E, Hiltscher H, Birkmann S, Dietz KJ, Baier M (2008) The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiguzov A, Nikkanen L, Fitzpatrick D, Vainonen JP, Gossens R, Alseekh S, Aarabi F, Tiwari A, Blokhina O, Panzarová K, et al. (2020) Dissecting the interaction of photosynthetic electron transfer with mitochondrial signaling and hypoxic response in the Arabidopsis rcd1 mutant. Philos Trans R Soc Lond B Biol Sci 375: 20190413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhang J, Zhou M, Zhou H, Cui B, Gotor C, Romero LC, Fu L, Yang J, Foyer CH, et al. (2020) Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 32: 1000–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagghe BJ, Hoy JA, Percifield R, Kundu S, Hargrove MS, Sarath G, Hilbert JL, Watts RA, Dennis ES, Peacock WJ, et al. (2009) Review: correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 91: 1083–1096 [DOI] [PubMed] [Google Scholar]
- Smith KA, Waypa GB, Schumacker PT (2017) Redox signaling during hypoxia in mammalian cells. Redox Biol 13: 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Kovalev A, Gorb SN, Sauter M (2012) Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell 24: 3296–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD (2007) Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 226: 465–474 [DOI] [PubMed] [Google Scholar]
- Tajima M, Kurashima Y, Sugiyama K, Ogura T, Sakagami H (2009) The redox state of glutathione regulates the hypoxic induction of HIF-1. Eur J Pharmacol 606: 45–49 [DOI] [PubMed] [Google Scholar]
- Vicente J, Mendiondo GM, Movahedi M, Peirats-Llobet M,, Juan YT, Shen YY, Dambire C, Smart K, Rodriguez PL, Charng YY, et al. (2017) The Cys-Arg/N-end rule pathway is a general sensor of abiotic stress in flowering plants. Curr Biol 27: 3183–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J, Mendiondo GM, Pauwels J, Pastor V, Izquierdo Y, Naumann C, Movahedi M, Rooney D, Gibbs DJ, Smart K, et al. (2019) Distinct branches of the N-end rule pathway modulate the plant immune response. New Phytol 221: 988–1000 [DOI] [PubMed] [Google Scholar]
- Vishwakarma A, Kumari A, Mur LA, Gupta KJ (2018) A discrete role for alternative oxidase under hypoxia to increase nitric oxide and drive energy production. Free Radic Biol Med 122: 40–51 [DOI] [PubMed] [Google Scholar]
- Voon CP, Guan X, Sun Y, Sahu A, Chan MN, Gardeström P, Wagner S, Fuchs P, Nietzel T, Versaw WK, et al. (2018) ATP compartmentation in plastids and cytosol of Arabidopsis thaliana revealed by fluorescent protein sensing. Proc Natl Acad Sci USA 115: E10778–E10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Steinbeck J, Fuchs P, Lichtenauer S, Elsässer M, Schippers JHM, Nietzel T, Ruberti C, Van Aken O, Meyer AJ, et al. (2019) Multiparametric real-time sensing of cytosolic physiology links hypoxia responses to mitochondrial electron transport. New Phytol 224: 1668–1684 [DOI] [PubMed] [Google Scholar]
- Wang F, Chen ZH, Liu X, Colmer TD, Shabala L, Salih A, Zhou M, Shabala S (2017) Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J Exp Bot 68: 3191–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wany A, Kumari A, Gupta KJ (2017) Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ 40: 3002–3017 [DOI] [PubMed] [Google Scholar]
- Waszczak C, Akter S, Eeckhout D, Persiau G, Wahni K, Bodra N, Van Molle I, De Smet B, Vertommen D, Gevaert K, et al. (2014) Sulfenome mining in Arabidopsis thaliana. Proc Natl Acad Sci USA 111: 11545–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MD, Kamps JJAG, East S, Taylor Kearney LJ, Flashman E (2018) The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J Biol Chem 293: 11786–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzinger B, Mair A, Fischer-Schrader K, Nukarinen E, Roustan V, Weckwerth W, Teige M (2017) Redox state-dependent modulation of plant SnRK1 kinase activity differs from AMPK regulation in animals. FEBS Lett 591: 3625–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65: 261–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Yoshioka M, Fukazawa A, Mori H, Nishizawa NK, Tsutsumi N, Yoshioka H, Nakazono M (2017) An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 29: 775–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Hong CP (2015) The NADPH oxidase Rboh D is involved in primary hypoxia signaling and modulates expression of hypoxia-inducible genes under hypoxic stress. Environ 115: 63–72 [Google Scholar]
- Yao Y, He RJ, Xie QL, Zhao XH, Deng XM, He JB, Song L, He J, Marchant A, Chen XY, et al. (2017) ETHYLENE RESPONSE FACTOR 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)‐dependent mechanism in response to different stresses in Arabidopsis. New Phytol 213: 1667–1681 [DOI] [PubMed] [Google Scholar]
- Yeung E, van Veen H, Vashisht D, Sobral Paiva AL, Hummel M, Rankenberg T, Steffens B, Steffen-Heins A, Sauter M, de Vries M, et al. (2018) A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana. Proc Natl Acad Sci USA 115: E6085–E6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E, Bailey-Serres J, Sasidharan R (2019) After the deluge: plant revival post-flooding. Trends Plant Sci 24: 443–454 [DOI] [PubMed] [Google Scholar]
- Yi Z, Jiang L, Zhao L, Zhou M, Ni Y, Yang Y, Yang H, Yang L, Zhang Q, Kuang Y, et al. (2019) Glutathione peroxidase 3 (GPX3) suppresses the growth of melanoma cells through reactive oxygen species (ROS)‐dependent stabilization of hypoxia‐inducible factor 1‐α and 2‐α. J Cell Biochem 120: 19124–19136 [DOI] [PubMed] [Google Scholar]
- Yoo KS, Ok SH, Jeong BC, Jung KW, Cui MH, Hyoung S, Lee MR, Song HK,, Shin JS (2011) Single cystathionine β-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell 23: 3577–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LB, Dai YS, Xie LJ, Yu LJ, Zhou Y, Lai YX, Yang YC, Xu L, Chen QF, Xiao S (2017) Jasmonate regulates plant responses to postsubmergence reoxygenation through transcriptional activation of antioxidant synthesis. Plant Physiol 173: 1864–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, et al. (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268 [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Mittler R (2018) ROS-induced ROS release in plant and animal cells. Free Radic Biol Med 122: 21–27 [DOI] [PubMed] [Google Scholar]
- Zhan N, Wang C, Chen L, Yang H, Feng J, Gong X, Ren B, Wu R, Mu J, Li Y, et al. (2018) S-nitrosylation targets GSNO reductase for selective autophagy during hypoxia responses in plants. Mol Cell 71: 142–154 [DOI] [PubMed] [Google Scholar]