Abstract
Programmed cell death (PCD) and apoptosis have key functions in development and disease resistance in diverse organisms; however, the induction of necrosis remains poorly understood. Here, we identified a semi-dominant mutant allele that causes the necrotic death of the entire seedling (DES) of wheat (Triticum aestivum L.) in the absence of any pathogen or external stimulus. Positional cloning of the lethal allele mDES1 revealed that this premature death via necrosis was caused by a point mutation from Asp to Asn at amino acid 441 in a nucleotide-binding leucine-rich repeat protein containing nucleotide-binding domain and leucine-rich repeats. The overexpression of mDES1 triggered necrosis and PCD in transgenic plants. However, transgenic wheat harboring truncated wild-type DES1 proteins produced through gene editing that exhibited no significant developmental defects. The point mutation in mDES1 did not cause changes in this protein in the oligomeric state, but mDES1 failed to interact with replication protein A leading to abnormal mitotic cell division. DES1 is an ortholog of Sr35, which recognizes a Puccinia graminis f. sp. tritici stem rust disease effector in wheat, but mDES1 gained function as a direct inducer of plant death. These findings shed light on the intersection of necrosis, apoptosis, and autoimmunity in plants.
An R-gene in hexaploid wheat causes premature death of the entire seedling in the absence of any pathogen or external stimulus.
Introduction
The major types of cell death in multicellular organisms include apoptosis and necrosis (Fink and Cookson, 2005; Kroemer et al., 2009). Apoptosis is a form of programmed cell death (PCD) induced by pathogens or involved in cell turnover in healthy tissues in multicellular organisms, including animals and humans (Kerr et al., 1972). PCD is well known in plant science. When a pathogen attacks a plant, plant immunity, once activated, initiates a PCD-like cell death called the hypersensitive response (HR) at the site of infection to limit the spread of the pathogen (Chisholm et al., 2006; Jones and Dangl, 2006; Takken et al., 2006; Coll et al., 2011; Maekawa et al., 2011; Wu et al., 2017; Kourelis and van der Hoorn, 2018).
The PCD response is triggered by the direct or indirect recognition of effectors from the invading pathogen by plant nucleotide-binding leucine-rich repeat (NLR) proteins, which comprise two major types based on their N-terminal domains, coiled-coil (CC) domain, and Toll/interleukin-1 receptor (TIR) domain . The CC-type is subdivided into several subclasses, including the CC-RPW8 (RNL) class (Zhong and Cheng, 2016). A CC-type NLR protein contains CC, nucleotide-binding (NB)-ARC, and leucine-rich repeat (LRR) domains. The unique ARC domain is known as the NB adaptor, which is shared by human homolog apoptotic protease-activating factor 1 (Apaf1), which activates caspase-3 and apoptosis (Zou et al., 1997; van der Biezen and Jones, 1998; Tao et al., 2000; Tornero et al., 2002), resistance (R) proteins in plants, and cell death protein 4 (CED-4), which causes cell death in the nematode Caenorhabditis elegans (Yuan and Horvitz, 1992), Among the approximately 20 cloned genes conferring resistance against wheat (Triticum aestivum L.) diseases, most encode NLR proteins. Sr35, a typical NLR protein in wheat, is produced in response to stem rust disease or Puccinia graminis f. sp. tritici (Pgt) race UG99 or TTKSK (Saintenac et al., 2013). Sr35 interacts with the effector AvrSr35 (the gene product of the pathogen) to elicit severe PCD as an immune response (Salcedo et al., 2017).
Compared to apoptosis, necrosis remains poorly understood in plants. The term necrosis is used to describe a form of non-PCD in humans and animals caused by external factors, such as injury or trauma, resulting in the unregulated degradation of cells and premature death. Necrosis is a commonly used term in wheat because synthetic wheat and hybrid wheat frequently exhibit premature death regardless of external factors. For example, when diploid T. tauschii (2n = 4×= 14, DD) is crossed with tetraploid wheat (T. turgidum L., 2n = 2× = 28, AABB) to generate synthetic hexaploid wheat (T. aestivum L., 2n = 2× = 42, AABBDD), the F1 hybrids are usually completely lethal or semi-lethal, resulting in necrosis (Tsunewaki, 1992; Tomar and Singh, 1998; Chu et al., 2006). Hybrid necrosis, a process characterized by the death of the whole hybrid plant, is also frequently observed in F1 hybrids between two common wheat cultivars and between tetraploid wheat and common wheat in an attempt to induce heterosis in wheat (Tsunewaki, 1992). Hybrid necrosis has become a serious barrier, preventing the combination of desirable traits from different wheat genotypes and limiting the utilization of heterosis from specific parental lines in wheat (Bizimungu et al. 1998).
Genes responsible for hybrid necrosis in Arabidopsis thaliana have been cloned, including DANGEROUS MIX (DM) genes DM1 (Bomblies et al., 2007) and DM2 (Chae et al., 2014), both of which encode NLR proteins. The incompatible combinations of the two genes from different genetic backgrounds cause hybrid necrosis due to their epistatic interactions (Bomblies and Weigel, 2007). DM10 is a singleton NLR gene that is also able to cause hybrid necrosis in Arabidopsis (Barragan et al., 2020). NLRs are also involved in hybrid necrosis in lettuce (Lactuca sativa; Jeuken et al., 2009), tomato (Solanum lycopersicum; Krüger et al., 2002), cotton (Gossypium hirsutum; Deng et al., 2019), and rice (Oryza sativa; Yamamoto et al., 2010; Chen et al., 2013). However, the genes causing hybrid necrosis in wheat remain enigmatic.
A lethal mutation is often induced in a gene that is essential for plant development, but the homozygous dominant or recessive state of the lethal gene causes the termination of viable biological activity and failed seed reproduction, limiting the cloning and utilization of the lethal gene (Golling et al., 2002; Meinke et al., 2008; Lloyd et al., 2015). In the current study, we discovered a mutant exhibiting whole-plant death 1–2 weeks after planting in the absence of any pathogen or external stimulus. We cloned the semi-dominant lethal allele that automatically activates necrotic death in the mutant and identified a point mutation in an NLR protein that causes seedling death of the entire seedling (DES), shedding light on this crucial process.
Results
Discovery and cloning of a lethal allele involved in spontaneous death of the entire wheat seedling
In our study of the E3 gene (TraesCS3B01G019600.1), we serendipitously identified a mutant plant. We cloned E3 into the pMCG161 vector that produces double-stranded RNA for RNA interference (RNAi) and transformed the construct into the spring wheat cultivar ‘Sumai3’ to characterize its function. When we grew a T1 population of Sumai3 harboring the E3-RNAi construct in the greenhouse, where the temperature and photoperiod were well controlled and nutrients were sufficiently supplied, we observed the segregation of wild-type, intermediate, and dead plants at the seedling stage. A typical T1 plant with the intermediate phenotype had reduced viability and productivity compared to the wild-type (Supplemental Figure 1, a). The dead plants and plants with the intermediate phenotype were not associated with the E3-RNAi. This unexpected result suggested that the dead plants harbored a homozygous allele in a gene that had been mutated during transformation or tissue culture. We crossed a nontransgenic T1 plant with an intermediate phenotype with wild-type Sumai3 to confirm the genetic model for the gene/allele, resulting in the segregation of plant lethality/viability in the BC1F1 and BC1F2 populations (Supplemental Figure 1, b–e).
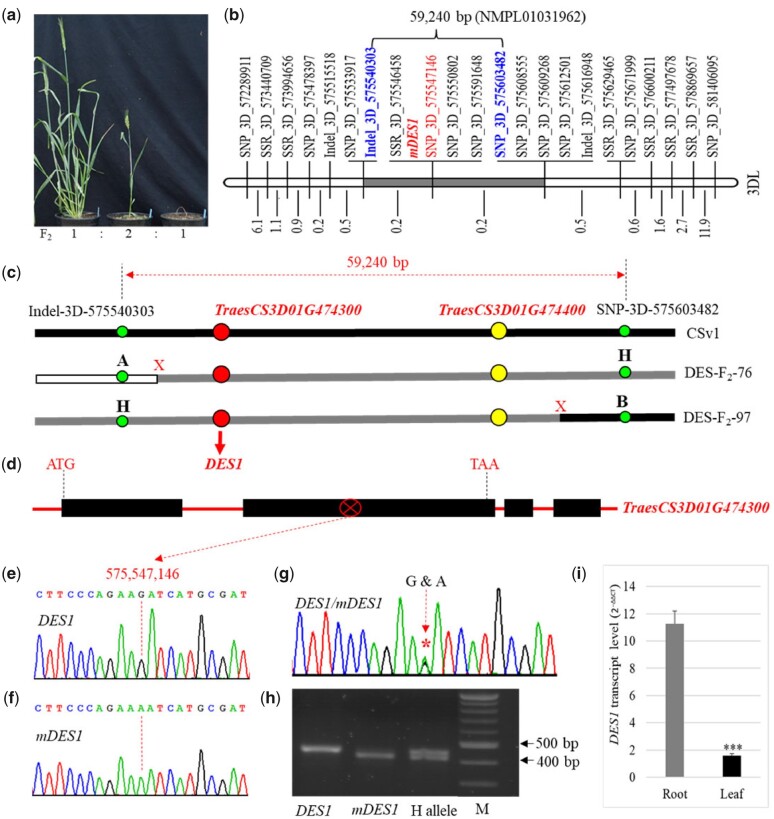
To map and clone the mDES1 allele, we crossed an intermediate Sumai3 plant with the spring wheat cultivar ‘Bobwhite’. The resulting F1 plants showed a 1:1 segregation ratio for Bobwhite wild-type to intermediate phenotypes (Supplemental Figure 1, f). This result indicates that mDES1 also exerts its function in the Bobwhite genetic background. We used six intermediate F1 plants from a Sumai3 mDES1-line x Bobwhite cross to generate a population of 104 individual F2 plants, including 21 that showed the wild-type phenotype, 56 that showed semi lethality, and 27 that showed lethality and died 10–14 d after planting (Supplemental Figure 1, g). The segregation ratio of 1:2:1 fit the single semi-dominant gene model (χ21:2:1 = 1.308, P = 5.99). The Sumai3 mDES1 line × Bobwhite F2 population showed clear segregation of the visible phenotypes (Figure 1, a), facilitating the cloning of mDES1.
Figure 1.
Mapping and cloning of mDES1. A, Phenotypes of F2 plants in the Sumai3 mDES1-line × Bobwhite population. B, Initial genetic map of mDES1 on chromosome 3DL. The numbers on the left indicate the genetic distance between two neighboring markers. GBS markers are indicated on the right side of the chromosome; the code numbers for these markers represent their physical locations on the chromosome based on the Chinese Spring genomic sequence released by IWGSC (2018). NMPL01031962 is GenBank accession number for T. aestivum cultivar Chinese Spring 005316F. C, Physical distance of the two closest markers flanking mDES1: Indel_3D_575540303 and SNP_3D_575603482. The flanking markers were identified in two critical crossovers, as indicated in recombinant #76 and #97. Two candidate genes, TraesCS3D01G474300 and TraesCS3D01G474400, were identified in the targeted region between the two flanking markers. D, Gene structure of TraesCS3D01G474300. E, Partial sequence of the homozygous DES1 allele. F, Partial sequence of homozygous mDES1 allele. G, Partial sequence of heterozygous DES1/mDES1 alleles. The SNP G/A is found in the mDES1 allele. H, Diagnostic PCR marker for allelic variation between DES1 and mDES1. M is a DNA ladder marker. I, DES1 expression in roots and leaves of wild-type Sumai3 at the seedling stage. The transcript level was determined by using RT-qPCR-DES1-F1 and RT-qPCR-DES1-R1, and the house-keeping ACTIN gene was used as an endogenous control.
We cloned mDES1 using a positional cloning approach. Specifically, we generated genotyping-by-sequencing (GBS) markers to genetically locate mDES1 in an initial mapping population (Figure 1, B), developed markers in the interval region (Supplemental Figure 2), and phenotyped and genotyped critical recombinant plants screened from a cloning population of 326 F2:3 plants (Figure 1, C; Supplemental Figure 3, a). Finally, mDES1 was narrowed down to a 59,240-bp region, which contains only two candidate genes, TraesCS3D01G474300 and TraesCS3D01G474400, according to the IWGSC RefSeq v1.0 database (Figure 1, C; Supplemental Figure 3, b).
Subsequently, we sequenced the target region from the wild-type and mDES1-line of Sumai3 and identified only one single nucleotide polymorphism (SNP), which occurred in exon 2 of TraesCS3D01G474300, a gene consisting of 4 exons and 3 introns (Figure 1, D; Supplemental Figure 4), making it the only candidate gene for mDES1. The ‘G’ nucleotide in wild-type Sumai3 (Figure 1, E) was mutated to ‘A’ at the same position in the mDES1-line (Figure 1, F). We detected the heterozygous G/A nucleotide at the mutation site in the sequence of lines with both the alleles (Figure 1, G). TraesCS3D01G474300 was annotated as a gene encoding an NB-LRR-like resistance protein of 958 amino acids (aa) in the IWGSC database. The G/A SNP resulted in a nonsynonymous mutation in the TraesCS3D01G474300 protein from ‘Asp’ (aspartate, D) encoded by “GAT” in wild-type DES1 to “Asn” (asparagine, N) encoded by “AAT” at the same position in mDES1. We developed a diagnostic marker (Figure 1, h) to confirm that the SNP resulted from a mutation event and not from a sequencing error.
TraesCS3D01G474300 is expressed at much higher levels in roots than in leaves (Figure 1, i; P = 5.467E-07). The wild-type DES1 protein shares the highest identity (87%) with CNL9 or Sr35, which is encoded by a gene on chromosome 3A of diploid T. monococcum (GenBank accession number AGP75918). DES1 is orthologous to Sr35, which initiates plant immune responses in wheat through PCD in response to UG99 or TTKSK, a race of the fungus Puccinia graminis f. sp. tritici (Pgt; Saintenac et al., 2013). Multiple protein sequence alignment indicated that the Asp at position 441 in DES1 is conserved among 44 paralogous and orthologous proteins from diploid, tetraploid, and hexaploid wheat, as well as other cereal species, but it was mutated to Asn at the same position in mDES1 (Supplemental Figure 5).
Validation of mDES1 via transgenic plants
To determine if the D441N point mutation in mDES1 is directly involved in the auto-activated death of the entire wheat plant, we transformed the mDES1 gene into wild-type Bobwhite wheat. We cloned mDES1 cDNA into the pMDC32 vector under the control of the Ubiquitin promoter (Liu et al., 2017) and transformed this construct into wheat by micro-projectile bombardment. We obtained two independent transgenic T0 plants (mDES1-OE17 and mDES1-OE20). RT-qPCR showed that mDES1 was expressed in the transgenic T0 plants ( Supplemental Figure 6). The T0 plants showed semi-lethal phenotypes but did not die (Figure 2A) because the positive transgenic plants in the T0 generation should have been hemizygous for mDES1 and homozygous for wild-type DES1. The wild-type and dead plants in the T1 populations at the seedling stage co-segregated with the absence and presence of the introduced mDES1, respectively (Figure 2B), demonstrating the lethal effect of mDES1 on the entire wheat plant in the absence of a pathogen or external stimulus.
Figure 2.
Validation of DES1/mDES1 function in transgenic wheat. A, Transgenic T0 wheat plant (mDES1-OE20) expressing mDES1 in culture tubes. Plant on the right is positive transgenic (+), while plant on the left is nontransgenic plant (−). B, Transgenic T1 wheat plants expressing mDES1 in MS medium, wild-type (− −) on the left, homozygous transgenic plant (+ +), and heterozygous plant (− +) on the middle. C, Tobacco leaves were agro-infiltrated with constructs expressing DES1 and mDES1. Representative leaves were imaged 2, 3, and 4 d after agro-infiltration. HR-like cell death appeared in the infiltration area of mDES1, auto-activating PCD 2 d after infiltration. D, DES1 and mDES1 constructs in the pEG101-YFP vector backbone were transformed into tobacco protoplasts using PEG-calcium-mediated transfection method. Protoplast suspensions in wells were imaged 24 h after incubation in the dark before suspension (on the left) and full suspension (on the right). Most of the protoplasts expressing DES1 were still alive. The protoplasts expressing mDES1 were dead and aggregated together showing green chunks.
We used a transient expression system in Nicotiana benthamiana leaves to determine whether wheat mDES1 would induce cell death in the infiltrated area or would auto-activate PCD. DES1 and mDES1 were respectively cloned into the pEG101-yellow fluorescent protein (YFP) vector and the constructs were Agroinfiltrated into N. benthamiana leaves. We then examined the ability of DES1 and mDES1 to induce cell death at the infiltration site. When DES1-YFP was infiltrated into N. benthamiana leaves, YFP signal was observed under BX-51 fluorescence microscope 4 d after infiltration. However, no signal was observed when mDES1-YFP was infiltrated into N. benthamiana leaves, suggesting that mDES1 induced local cell death (Figure 2C). We transformed N. benthamiana protoplasts with the DES1 and mDES1 constructs. After 24 h of incubation in the dark, most protoplasts that were transformed with the construct expressing DES1 were still alive, but protoplasts transformed with the construct expressing mDES1 were dead and aggregated together, appearing as green clusters (Figure 2D). These results indicate that wheat mDES1 could induce cell death in the infiltrated leaf areas and the protoplast of N. benthamiana.
Effects of edited DES1 on plant development
To determine whether the functional loss of DES1 caused necrosis of the entire wheat seedling, we selected a 20-bp unique sequence in the DES1 gene as the binding site of Cas9 for CRISPR-Cas9-mediated gene editing. Two editing events were obtained: DES-ED152 (with a 6-bp deletion in the gRNA site) and DES-ED271 (with a 1-bp insertion in the gRNA site; Supplemental Figure 7a). The editing event in DES-ED152 resulted in the loss of two aa at positions 390 and 391, whereas the editing event in DES-ED271 caused a frame shift, resulting in the production of a 392-aa truncated protein (Supplemental Figure 7b). However, transgenic plants harboring the edited DES-ED152 (Supplemental Figure 7c) and DES-ED271 (Supplemental Figure 7d) proteins developed normally and advanced to maturity. Immunoblot analysis on the total proteins with anti-DES1/mDES1 antibody showed the presence of the edited protein in DES-ED152 but not in DES-ED271 (Supplemental Figure 7e), because the truncated DES-ED271 protein lacks the sequence that is recognized by the antibody (Supplemental Figure 7f). Therefore, the necrosis of the entire wheat seedling did not occur because of DES1 loss-of-function. Instead, it occurred because of mDES1 gain-of-function.
Structural characteristics of DES1 protein and its interacting protein
We investigated whether mDES1 protein had structural changes that would cause whole-plant death. The mutated residue Asn in mDES1 occurs in the ARC domain, which is located between the NBS and LRR domains (Figure 3A). Based on the available structures of NLR proteins, we carried out homology modeling of DES1/mDES1. First, we used CED-4 protein structure (PDB code 4m9y) as a template and found that the ARC domain in DES1/mDES1 is similar to that in CED-4. The crystal structure of CED-4 appeared as an octameric apoptosome consisting of eight CED-4 molecules organized as a tetramer of an asymmetric dimer, forming a funnel-shaped structure (Qi et al., 2010). Based on our modeling, we predicted that D441 in DES1 forms a salt bridge with the K (Lys) at position 365 in the SEEILK segment (Supplemental Figure 8a) and mDES1 lost the salt bridge. When a cryo-electron microscopy structure from the Arabidopsis NLR protein ZAR1 (HOPZ-ACTIVATED RESISTANCE 1, GenBank accession number NP_190664) became available (Wang et al., 2019a, 2019b), we repeated our homology modeling using the wheel-like pentameric ZAR1 resistosome structure as a template (PDB code, 6j5t). This modeling confirmed our prediction of a salt bridge between K365 and D441 in DES1 (Figure 3B). The calculated distance between K365 and D441 was 2.9-Å (Figure 3C). Notably, the equivalent position in the structure of ZAR1 protein also has a salt bridge [K356 in the helical domain (HD) and E427 in the winged-helix domain (WHD)] (Supplemental Figure 8b).
Figure 3.
Structural models of DES1/mDES proteins. A, Schematic protein domains of DES1/mDES. DES1 is the wild-type, mDES1 contains a mutation at position 441 from Asp (D) to Asn (N), and mDES2 contains a mutation at position 365 from Lys (K) to Ala (A). These proteins consist of an N-terminus CC domain, central NB domain and ARC domain (HD) and (WHD), and a C-terminus LRR domain. Numbers indicate aa positions. B, DES1 is modeled using the cryo-electron microscopy structure of a single protein molecule of Arabidopsis ZAR1as template. Domains are colored in scheme: CC (green), NBD (yellow), HD (cyan), WHD (orange), LRR (light blue). Locations of D441 and K365 in DES1 are marked with arrows. C, A D-K salt bridge between D441 and K365 in DES1 was indicated with an arrow. D and E, Gel filtration chromatographs of DES1 proteins from both wild-type Sumai3 (D) and mDES1 from homozygous mDES1 plants (E). The collected protein fractions were analyzed by SDS–PAGE followed by Western blotting with DES1 antibody. Fractions were collected from 13 to 23 and their elution volumes were calculated from 7.5 to 12.5 ml. M is a molecular weight marker for proteins. The peak of fractions and calculated elution volume are highlighted in red.
Interactions between the HD and WHD in NLR proteins are critical for conformational changes and therefore the functions of these proteins (Wang et al., 2019a, 2019b). We hypothesized that the mutation in mDES1 may cause structural perturbations in DES1. We performed structure-guided biochemical analysis of DES1 and mDES1 to test if the oligomeric state of mDES1 changed due to the loss of the salt bridge. Expressed DES1 and mDES1 proteins in bacteria were aggregated, inhibiting further analysis of their molecular weights. We therefore extracted total proteins from wheat plants (Supplemental Figure 9) and examined the molecular weights of DES1 and mDES1 using size exclusion chromatography (SEC). The eluted proteins were examined using anti-DES1/mDES1 antibody (Figure 3, D and E). The estimated molecular weight of both DES1 and mDES1 from the gel filtration analysis was 737.1-kDa (Supplemental Figure 9, a–c), corresponding to a homo-heptamer with 103.9-kDa protomers. This is different from the oligomeric states of CED-4 (octomer) and ZAR1 (pentamer). The measured DES1 and mDES1 could also be a heptameric oligomer or a complex with other interacting proteins, but our data did not reveal measurable difference in their molecular weights.
Interactions of DES1 with RPA32
To determine if DES1 interacts with any essential protein or if mDES1 acquired the ability to interact with a death-related protein, we used DES1/mDES1 as baits to screen yeast two-hybrid (Y2H) libraries generated from wheat. When DES1 was used as bait, two independent positive clones were identified from the Y2H library. Sequencing of the two clones revealed that they harbored the same gene, TraesCS6A01G220800 on chromosome 6A, encoding 32-kDa replication protein A (RPA32; Supplemental Figure 10). However, when mDES1 was used as bait, no positive clones were obtained from the same Y2H library. Comparative Y2H interaction studies confirmed that RPA32 interacts with DES1 but not with mDES1 in the Y2H system (Figure 4A).
Figure 4.
Interaction of DES1 and RPA32 proteins in yeast and BiFC analysis. A, Interaction of DES1 and RPA32 proteins in yeast. All co-transformed cells were cultured on plates with yeast minimal media and synthetic defined premixes (SD), which lacked 2 aa (TL: −Trp/−Leu) on the left or 4 aa (TLHA: −Trp/−Leu/−His/−Ade) on the right. Colony solutions diluted with different folds (1, 10, 100, 1,000) were inoculated on the same plate for each protein/protein pair. Only cells co-transformed with DES1 in the BD vector and RPA in the AD vector that are highlighted in red were able to grow on the SD-TLHA plate. B–E, Sub-cellular localization and interaction analyses of DES1 and RPA32 proteins in tobacco leaves. For subcellular localization, DES1-YFP (B) and RPA32-YFP (C) proteins were expressed in tobacco and images were captured were cut 4 d after infiltration. For protein interactions, RPA32-YN and DES1-YC (D) or RPA32-YN and mDES1-YC (E) were co-expressed in tobacco and images were captured between 36 and 48 h after infiltration. Infiltrated leaf portions were stained with DAPI (nuclear marker) and FM4-64 (plasma membrane marker). Images were captured in a fluorescent microscope with recommended filters. Scale bar = 100 μm. f–h, Expression analyses of RPA32-YN and DES1-YC/mDES1-YC proteins. The Western blots of total proteins from the same infiltrated leaves used in BiFC analysis were stained with Coomassie blue (F) and examined with anti-RPA32 antibody (G) and anti-DES1 antibody (H). CK is a control from the total proteins from un-infiltrated leaves. M indicates protein markers. Arrow points to the proteins detected by the antibodies. Star indicates tobacco protein detected by the anti-DES1 antibody.
We confirmed the differential interactions of DES1 and mDES1 with RPA32 in living cells via transient expression in N. benthamiana leaves (Li et al., 2013). The genes encoding DES1, mDES1, and RPA32 were, respectively, cloned into the pEG101-YFP vector to analyze the proteins in N. benthamiana leaves 4 d after Agroinfiltration. When DES1 was expressed in N. benthamiana leaves, enriched fluorescent signals were detected (Figure 4B). Expression of mDES1 caused complete cell death at the infiltration site. When RPA32 was expressed in N. benthamiana leaves, enriched fluorescent signals were detected (Figure 4C).
Next, we analyzed protein interactions of DES1/mDES1 with RPA32 by bimolecular fluorescence complementation (BiFC). DES1 and mDES1 were, respectively, fused to the C-terminal aa region (aa 1–174) of YFP in the pEG201-YC vector, whereas RPA32 was fused to the N-terminal aa portion (aa175–239) of YFP in the pEG202-YN vector. N. benthamiana leaves Agroinfiltrated separately with these constructs did not produce any YFP signal (Supplemental Figure 11). When DES1-YC and RPA32-YN were simultaneously expressed in the same cell, enriched fluorescent signals were observed, indicating that DES1 and RPA32 interacted very strongly (Figure 4D). However, when mDES1-YC and RPA32-YN were simultaneously expressed in the same cell, no fluorescent signals were detected (Figure 4E). These results indicate that RPA32 interacts with DES1 but not with mDES1 in the transient expression system in N. benthamiana. Western blot analysis was performed to examine the expression levels of RPA32-YN, DES1-YC, or mDES1-YC one the infiltrated leaves used in the microscopic study (Figure 4F–H). The anti-RPA32 and anti-DES1 antibodies were used to detect protein expression. In addition to RPA32 (Figure 4G), expression of both DES1 and mDES1 proteins (Figure 4H) were detected in the infiltrated leaves. These results indicate that the lack of interaction between RPA32 and mDES1 was not due to a lack of protein expression.
Effects of artificial mutant mDES2
To further test the significance of the predicted D441:K365 salt bridge in DES1, we created the artificial mutant mDES2 by substituting K365 to alanine (Ala, A; Figure 3A). We hypothesized that the K365A mutation would disrupt the predicted salt bridge and cause a similar phenotype to that of mDES1. We first tested the effect of mDES2 on cell death. When a construct expressing mDES2 was infiltrated into N. benthamiana leaves, cell death was observed at the infiltrated site 4 d after infiltration (Supplemental Figure 12a). Protoplasts transformed with the construct expressing mDES2 were also dead and aggregated together (Supplemental Figure 12b). We generated mDES2-overexpressing transgenic T0 Bobwhite plants. The mDES2 transgenic plants did not die, but their growth was severely inhibited (Supplemental Figure 12c). The K365A mutation also disrupted the interaction of DES1 with RPA32 in yeast cells (Supplemental Figure 12d).
Abnormal cell division and effects of hormones on mDES1 plants
We selected plants carrying the homozygous DES1 allele and the homozygous mDES1 allele. Unlike DES1 plants, when grown on medium, the mDES1 plants showed no root branching (Figure 5A), formed shrunken cells in the tip and elongation zone (Figure 5B), and produced an exterior sheath with a thickened cell wall or cellular layer at the root tip (Figure 5C). Furthermore, compared to DES1 plants, the root tip cells of mDES1 plants showed defects in cell division, including the formation of bridged chromosomes (Figure 5D) and lagging chromosomes during anaphase (Figure 5E), resulting in decreased chromosome numbers in root-tip cells (Figure 4F; Supplemental Figure 13). These results indicate that the spontaneous death of the mDES1 plants is associated with defects in cell division.
Figure 5.
Histological sections of the roots of DES1/mDES1 plants. A, Roots of DES1/mDES1 plants grown in MS medium. B, The root tip and elongation zone. The images were taken 18 d after planting. C, Cells in the root tips. The slide was stained with acetocarmine, and the images were taken 10 d after planting. D, Root section showing bridged chromosomes (marked by arrow) in an mDES1 plant at anaphase. E, Root section showing lagging chromosomes (marked by arrow) in an mDES1 plant at telophase. F, Root cells undergoing mitosis have reduced chromosome numbers in mDES1 plants. Scale bar = 200 μm (B and C) or 10 μm (D–F).
Finally, we attempted to rescue plants carrying the homozygous mDES1 allele using different plant hormones and environmental factors, but the plant-death phenotype of spontaneous mutant mDES1 plants could not be rescued (Supplemental Figure 14).
Discussion
In this study, we successfully obtained a naturally occurring mDES1 mutant, which carries a heterozygous allele that can be continuously maintained to enable cloning of the lethal allele. The behavior of the mDES1 mutant is similar to wheat hybrid necrosis because in both cases, seedlings undergo premature death, but it differs from non-PCD resulting from human and animal necrosis because the mutant is not injured or damaged. The infiltration of N. benthamiana leaves with Agrobacterium expressing mDES1 caused local cells at the infiltration site to die, demonstrating the interaction of necrosis and PCD caused by a single wheat NLR protein.
A lethal gene is often identified by examining a mutant that is induced through DNA insertion or chemical methods that results in death of the plant; such genes are essential for plant development (Golling et al., 2002; Meinke et al., 2008; Lloyd et al., 2015). However, the wild-type DES1 in hexaploid wheat is not an essential gene. When wild-type DES1 was damaged by gene editing, the resulting transgenic wheat plants showed no significant defects in development. In addition, tetraploid wheat does not contain the DES1 gene that is on chromosome 3D in hexaploid wheat, yet tetraploid wheat plants exist in nature. The mDES1 protein may lose its negative regulation, resulting in auto-activation that causes cell death and ultimately seedling lethality.
mDES1 behaves as a semi-dominant allele with the death of entire seedling as its only phenotype. The availability of the surviving mutant facilitated the cloning of mDES1. We observed various associated phenotypes during seedling death. The most striking feature of the dying seedling was that no branching roots were observed. In addition, the root tissue of the mutant exhibited many morphological and cytological differences. Plants from inter-generic crosses between tetraploid wheat (AABB) and the D genome progenitor Aegilops tauschii (DD) exhibit autoimmune responses and mitotic abnormalities at low temperature (type II necrosis), suggesting that the repression of cell division at the shoot apical meristem is one reason for hybrid necrosis (Mizuno et al., 2011). The finding that RPA32 interacts with DES1 but not with mDES1 is exciting. RPA32 proteins are involved in regulating meristem development in Arabidopsis (Xia et al., 2006), but their functions in wheat are unknown. To date, no RPA protein was reported to interact with any NLR protein in plants or other organisms. It is not known whether cell division in the mutant was affected directly by auto-activated mDES1 or by the disrupted interaction with RPA32. Further work is needed to reveal the functional mechanisms of the DES1–RPA32 protein complex in cell division.
NLRs are essential components of the immune systems of plants and animals (Takken et al., 2006). A traditional NLR protein exists in an inactive state prior to signaling (Qi et al., 2010). ZAR1 undergoes a similar activation process to form resistosomes (Wang et al., 2019b). The interaction between the HD and WHD domains of ZAR1 is critical for maintaining the conformation of this protein in the inactive-active states (Wang et al., 2019b). In the inactive state, the HD/WHD interdomain association tethers the N-terminal CC domain. In the active state, conformational changes (caused by interaction with other proteins) release the CC, which leads to the oligomerization and activation of this protein. CED-4 is also maintained in an inactive state and must undergo an activation process. The freed CED-4 dimers then form tetramers, which function as death-inducing apoptosomes (Spector et al., 1997; Rodriguez et al., 1999; Chen et al., 2000; Yan et al., 2005; Qi et al., 2010). A subclass of plant NLR proteins also contain the TIR domain that plays an essential role in degrading nicotinamide adenine dinucleotide in its oxidized form (NAD+), which functions in plant cell death signaling pathways (Horsefield et al., 2019; Wan et al., 2019).
The SEC analysis coupled with antibodies to detect DES1/mDES1 revealed a 730-kDa complex of the NLR proteins. Both the inactive DES1 and auto-active mDES1 are in the same size complex, suggesting the oligomeric state itself may not directly affect the NLR signaling. However, it is likely the DES1 and mDES1 could adopt different structural conformations that are caused by the disruption of the bridging salt bridge between D441 in the WHD and K365 in the HD. The structural changes may lead to their direct protein–protein interactions with partners, which is a key part of the activation process. In fact, we found that mDES1 abolished its interaction with RPA32. However, further work is needed to reveal the functional mechanisms of the DES1–RPA32 protein complex in cell division and plant death.
The Methionine–Histidine–Aspartate (MHD) motif is a highly conserved motif in NLRs that is located at the C terminus of the NB-ARC domain. The mutation of either of the two residues (H and D) of in MHD motif results in auto-activation of the NLR protein, as the loss of a subdomain interaction and conformational change lead to ATP binding (Tameling et al., 2006; van Ooijen et al., 2008). Besides the MHD motif, other mutations in the NB-ARC domain also lead to the auto-activation of some NLRs. For example, the chs2 (S389F) and ssi4 (G422R) mutations of Arabidopsis (Shirano et al., 2002) and nls1-1D (S367N) and nls1-2D (S366T) of rice (Tang et al., 2011) induce the auto-activation of particular NLRs, leading to enhanced disease resistance. mDES1 is the first auto-activation mutation with a lethal phenotype that has been observed in hexaploid wheat. The mutation D441N in mDES1 is not present in the previously characterized MHD motif. The corresponding sequences to the conserved MHD motif are VHD in DES1 at positions 501–503, which is 59 aa downstream of the mDES1 point mutation D441N (Supplemental Figure 8c). These findings provide additional information about subdomain interactions in the NB-ARC domains.
DES1/mDES1 is orthologous to Sr35, which triggers the immune response to stem rust disease caused by Ug99, resulting in PCD at the site of infection (Saintenac et al., 2013). When Sr35 interacts with the pathogen effector protein AvrSr35, the plant displays severe PCD, which functions as an immune response (Salcedo et al., 2017). As mDES1 is orthologous to Sr35, this protein causes necrosis that is distinguishable from PCD caused by Sr35. Hybrid necrosis in wheat was first described in the 1940s (Caldwell and Compton, 1943). Subsequently, a series of classical studies revealed that this phenomenon is genetically controlled by three Ne1 alleles and five Ne2 alleles (Hermsen, 1963; Mirua et al., 1992; Chu et al., 2006). A recent mapping study showed that Ne2m is linked to Lr13, a resistance gene against leaf rust in wheat (Zhang et al., 2016). Two NLR proteins, DM1 and DM2, cause hybrid necrosis in Arabidopsis (Bomblies et al., 2007; Chae et al., 2014). During the processing of cloning the necrosis genes in synthetic and hybrid wheat, any NLR genes in targeted regions should be functionally investigated. As an inducer of cell death, mDES1 could be utilized as a terminator of seed production. Perhaps, this gene could be also linked to a promoter that drives its spatial and temporal expression in pollen to generate an engineered wheat plant with male sterility for use in development of hybrid wheat.
Materials and methods
Mapping of mDES1
A mutant plant among the E3 RNAi transgenic Sumai3 wheat (Triticum aestivum L.) progeny (Sumai3 E3-T1-39-65) lacked the E3 RNAi construct but showed an intermediate phenotype. The mDES1 plant was backcrossed with wild-type Sumai3, and the resulting F1 plants were self-pollinated to generate an F2 population, which was used to test for the presence of a lethal allele as a single genetic factor. The segregation of phenotypes in F1 and F2 offspring plants derived from wild-type Sumai3 x mutant Sumai3 suggested that the premature death of the T1 plants is controlled by a single gene with a semi-dominant effect for lethality, regardless of any abiotic and biotic stress. The lethal allele in the Sumai3 mutant is not fully dominant, because a fully dominant lethal allele kills the individual in the homozygous or heterozygous state. In a single dominant gene model in Mendelian genetics, F1 plants die, and the segregation ratio would be 3:1 for normal to dead plants in an F2 population.
A Sumai3 mDES1 plant that showed intermediate phenotypes was also crossed with Bobwhite to generate F1 plants, which were self-pollinated to develop an F2 population for mapping the mDES1 lethal allele. A Sumai3 mDES1 x Bobwhite F1 hybrid was backcrossed with Bobwhite to generate a BC1F1 population, which was subjected to phenotypic analysis. In addition to the initial mapping population of Sumai3 mDES1 x Bobwhite F2 plants, some of these F2 plants carrying the heterozygous mDES1 allele were used to generate F3 plants for screening crossovers in the targeted mDES1 region.
The F1, BC1F1, and F2 mapping and cloning populations were grown in a greenhouse at constant temperatures of 20–25°C under a 16/8-h light/dark cycle throughout the study. All plants were grown in pots containing commercial soil with sufficient nutrients (Sun Gro Horticulture Canada Ltd., Agawam, MA, USA). The phenotypes were visibly scored and placed into three categories: plants that grew normally, plants that spontaneously died, and plants with intermediate phenotypes.
Positional cloning of mDES1
The mapping population of 89 Sumai3 mDES1 x Bobwhite F2 plants was genotyped using GBS markers developed for this study (Supplemental Table 1). The IWGSC genome sequence was used to determine the physical locations of the GBS markers on the chromosomes.
A diagnostic marker was developed for the SNP between DES1 and mDES1. The forward primer DES-M-F2 and reverse primer DES-M-R2 were designed, and the resulting 431-bp PCR products were digested with the restriction enzyme Bgl II (New England BioLabs, MA, USA). The homozygous wild-type DES1 allele generated a 395-bp visible fragment and a 36-bp fragment that ran off of an agarose gel, whereas the homozygous mDES1 allele generated an undigested 431-bp fragment, and the heterozygous alleles generated both 431- and 395-bp plus 36-bp fragments.
Transgenic wheat overexpressing mDES1
The mDES1 sequence was fused into the pMDC32 vector under the control of the maize Ubiquitin promoter (Liu et al., 2017). The ubiquitin-mDES1-pMDC32 construct was transformed into Bobwhite embryos by microprojectile bombardment as described previously (Liu et al., 2017). Bobwhite was used as the host plant because mDES1 was able to exert its function in this genetic background, as observed in the mapping population.
Reverse transcription quantitative PCR (RT-qPCR) was performed to determine the transcript levels of DES1/mDES1 using the primers DES1-M-F1 and DES1-M-R1 (Supplemental Table 2). Total RNA was extracted from leaves and RT-qPCR analysis of gene expression was performed as previously described (Li et al., 2013). Gene transcript levels were calculated by the 2–ΔΔCT method, where CT is the threshold cycle. The endogenous ACTIN gene was used as a control. Six repeats were performed on each plant. Statistical comparison of the average transcript levels was performed using two-tailed Student’s t test to determine the significance level, with “***” indicating P < 0.001.
Editing of DES1 in transgenic wheat
The sgRNA Scorer 1.0.33 program was used to design a DES1 sgRNA (Supplemental Table 2) lacking potential off-target matches, which was cloned into pBUN421 under TaU3 promoter within the BsaI site for genome editing (Wang et al., 2018). The gene-editing construct was also transformed into Bobwhite.
Characteristics of DES1/mDES1 proteins
The TraesCS3D01G474300 protein sequence was used as a query for BLAST analysis against the GenBank databases. Proteins that showed the highest identity to TraesCS3D01G474300 in crops included those encoded by homoeologous genes, orthologous genes, and paralogous genes in wheat of different ploidy levels and orthologous proteins in barley (Hordeum vulgare), rice, and maize (Zea mays; Supplemental Figure 6).
The DES1/mDES1 protein structures were modeled and saved as a PyMol session file based on CED-4, which assumes an octameric crystal structure, forming a funnel-shaped apoptosome (pdb code 4m9y) and ZAR1, which forms a wheel-like pentameric ZAR1 resistosome, as revealed by cryo-electron microscopy (pdb code, 6j5t). Gel filtration chromatography was used to analyze the molecular weights of DES1 and mDES1. The cDNAs for the full-length proteins were cloned into the pSUMO vector to produce a SUMO fusion protein with 6×HIS-tag (SUMO-tag), and the constructs were expressed in Escherichia coli (BL21 DE3; Li et al., 2013). Immunoblot analysis was performed as previously described (Earley et al., 2006). Primary antibodies (1:2,000 dilution) anti-6×HIS (SAB4600048; Sigma-Aldrich) were used to detect DES1-SUMO or mDES1-SUMO, and anti-Mouse IgG-Alkaline Phosphatase antibody (A3562; Sigma-Aldrich) was used as the secondary antibody for DES1-SUMO and mDES1-SUMO protein. However, since the expressed DES1 and mDES1 proteins formed aggregates, their molecular weights and oligomeric states could not precisely be determined. Therefore, proteins were extracted from wheat plants to determine the molecular weight or oligomeric state of DES1/mDES1.
The total proteins were extracted from wheat plants as previously described (Rubio et al., 2005). Leaf and root tissues (0.4 g) from plants carrying the homozygous DES1 or mDES1 allele were homogenized in liquid nitrogen. The samples were combined with 0.8 ml extraction buffer containing 50-mM Tris–HCl (pH 7.5), 150-mM NaCl, 10% (v/v) glycerol, 0.1% Nonidet P-40, 1-mM DTT, 1-mM Phenylmethylsulfonyl fluoride (PMSF), and 1× protease inhibitor cocktail (Roche, Indianapolis, IN, USA). The protein extracts were centrifuged twice at 4°C for 10 min each, and protein concentration in the supernatant was determined by Bradford assay.
SEC was performed with 400 µl of total protein at a concentration of 2 mg/mL in buffer containing 50-mM Tris–HCl (pH 7.5), 150-mM NaCl, 10% (v/v) glycerol, 0.1% (v/v) Nonidet P-40, 1-mM DTT, 1-mM PMSF, and 1× protease inhibitor cocktail (Roche). The protein sample was injected into a Superdex 200 10/300 GL column in an ATKA FPLC system (GE, Pittsburgh, PA, USA) in buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 10% (v/v) glyceroll, 2mM DTT) at a flow rate of 0.4 mL/min at 4°C. The A280 was monitored for each eluted fraction and plotted. Gel filtration standard (Bio-Rad, Hercules, CA, USA) was also injected into the column at a flow rate of 0.4 ml/min at 4°C. The eluted fractions were collected and analyzed by SDS–polyacrylamide gel electrophoresis (PAGE).
The proteins on SDS–PAGE gels were blotted onto polyvinylidene fluoride membranes (Millipore; Sigma-Aldrich). The blots were probed with primary antibody anti-DES (1:2,000 dilution, ABclonal, Woburn, MA, USA) for 8 h. The primary antibody was generated against DES1 protein, (VSQGEANDQEVDEAKAAVR) at positions 887–995 (Product number E9259). After washing, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1: 4,000 dilution; ABclonal, Woburn, MA, USA). The membranes were washed three times and signal developed with Clarity Western ECL substrate (Bio-Rad). Digital images were taken using a FluorChem System (ProteinSimple, San Jose, CA, USA).
The peak of the eluted fraction of both DES1 and mDES1 was identified, and the corresponding elusion volume was calculated. The molecular weight of DES1 and mDES1 in the eluted fraction was estimated based on a new standard protein curve. The oligomeric state of the proteins was predicted based on the weight of a single molecule.
Identification of interacting proteins with DES1
DES1 and mDES1 were used as baits to screen the Jagger prey Y2H library constructed in a previous study (Cao and Yan, 2013). The cDNAs encoding the complete DES1/mDES1 proteins were cloned into the DNA-binding domain of the pGBKT7 vector used for Y2H library screening. The positive colonies were further confirmed using the co-transformation as per the previously reported protocol (Li et al., 2013). Searches of the sequences of the cDNA clones in the IWGSC database were performed to identify the chromosomal locations in the wheat genome.
Effects of DES1/mDES1 in N. benthamiana protoplasts
DES1 and mDES1 cDNAs were separately cloned into the pEarleyGate101 vector (pEG101) containing with the YFP gene; the primers used for cloning are listed in Supplemental Table 2. Nicotiana benthamiana was grown at 26°C, and leaves from 3-week-old plants were used for protoplast isolation using a based on the method described for Arabidopsis mesophyll protoplast isolation (Li et al., 2013). The DES–YFP protein constructs were transformed into protoplasts prepared from N. benthamiana leaves using PEG-calcium-mediated transfection method. The transformed protoplasts were re-suspended in 250–μL W5 solution, transferred into 24-well plates, and incubated in the dark at room temperature for 18–20 h prior to imaging.
Protein subcellular localization and interaction assays
Constructs expressing DES1 and mDES1 in the pEG101 vector backbone were also used to analyze the subcellular localizations of the encoded proteins in N. benthamiana leaves, and RPA32 was cloned into the pEG101 vector for the same type of analysis. A. tumefaciens cells (GV3101) carrying the pEG101 constructs were co-infiltrated into N. benthamiana leaves together with p19 strain as previously described (Li et al., 2013). In brief, Agrobacteria containing DES1 and RPA32 expression vectors were grown in LB media and the pellet was suspended in 10 mM MgCl2 to an OD600 of 1.5. Equal volumes of these individual cultures were first mixed and incubated (4 h) separately with p19 culture (OD600 of 1.0). Equal volume of RPA32 and p19 culture was mixed with DES1 and p19 or mDES1 and p19 just before infiltration. Images were taken 1 or 4 d after infiltration on BX-51 fluorescence microscope (Olympus) equipped with Olympus DP71 digital camera. For staining plasma membrane and nucleus, styryl dye FM4-64 (Invitrogen) at a concentration of 100 μg/mL in water and DAPI (4′,6-diamidino-2-phenylindole) at a concentration of 2 μg/mL in PBS buffer were used, respectively. The 35 μg total proteins extracted from uninfiltrated and infiltrated leaves were used to analyze protein expression levels using Western blotting with anti-RPA32 antibody (TQPQVTANASTWNQAPPPN, Abclonal, E9264) and the anti-DES1 antibody used in the SEC analysis.
In vivo protein interactions were analyzed by BiFC. RPA32 in pDONR207 was fused to the N-terminal portion (aa 1–174) of YFP in the pEarleyGate201-YN vector (RPA32-YN) to examine in vivo interactions with DES1 or mDES1 that had been fused to the C-terminal portion (aa 175–239) of YFP in the pEarleyGate202-YC vector (DES1-YC or mDES1-YC). The interactions of the DES1/mDSE1 proteins in the pEG202-YC constructs and RPA32 in the pEG201-YN construct were tested in leaf discs (live N. benthamiana cells), which were imaged as previously reported (Li et al., 2013).
Histological sectioning of root cells
Histological sections of root cells and mitotic analyses were performed as described previously (He et al., 2017). Seeds of normal DES1 plants and mDES1 mutant plants were germinated at 25°C on MS medium for 2 d, transferred to 4°C for approximately 24 h, and returned to 25°C. Roots 1–2 cm in length were sectioned, incubated in ice water for approximately 24 h, and fixed in Carnoy’s solution. After fixation, the root tips were stained and squashed in carbol fuchsin, and the root tips and mitotic chromosomes were observed under an optical microscope.
Effects of plant hormones and environmental factors on mDES1 plants
Plants carrying homozygous and heterozygous alleles of DES1/mDES1 were grown on MS medium containing 10−6 and 10−7 mol/kg indole-3-acetic acid (IAA; added before planting) to test the effects of IAA on plants of different genotypes. In a separate experiment, 0.5 or 1.0 mg/L cytokinin was added to the medium to test the effects of cytokinin. In addition, the DES1/mDES1 plants were tested for shade avoidance effects in the greenhouse.
All primer sets used for SNP markers, gene cloning for transgenic wheat production, and analysis of protein interactions are listed in Supplemental Table 2.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: DES1 (KAF7040047) and RPA32 (KAF7079387).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Discovery and mapping of a lethal allele.
Supplemental Figure S2. Development of molecular markers for fine mapping of the mDES1 locus.
Supplemental Figure S3. Fine genetic map and physical map of the mDES1 locus.
Supplemental Figure S4. Sequences and gene structures of DES1/mDES1.
Supplemental Figure S5. Multiple sequence alignment of DES1 and mDES1 with homologous proteins.
Supplemental Figure S6. Identification of over-expressed mDES1 in transgenic wheat.
Supplemental Figure S7. Effects of edited DES1 on plant growth and development.
Supplemental Figure S8. Protein sequences and modeling of DES1/mDES1 based on CED-4 and ZAR1.
Supplemental Figure S9. Molecular weight analysis of DES1 and mDES1.
Supplemental Figure S10. Sequences of RPA32.
Supplemental Figure S11. Construct controls for BiFC analysis.
Supplemental Figure S12. Effects of mDES2.
Supplemental Figure S13. Fewer chromosomes in root cells of mDES1 plants.
Supplemental Figure S14. Effects of hormones on mDES1 plants.
Supplemental Table S1. Detailed information about GBS markers and PCR products.
Supplemental Table S2. Primers used in this study.
Funding
This project was supported by the Agriculture and Food Research Initiative Competitive Grants (2019-67013-29198) from the USDA National Institute of Food and Agriculture (NIFA). This project was also supported by the Oklahoma Agricultural Experiment Station at Oklahoma State University under projects: OKL03107 (L.Y.) and OKL03060 (J.D.). H.J. was supported by the 111 Project (B08025).
Conflict of interest statement. The authors declare that they have no competing interests.
Supplementary Material
H.J., S.X., and L.L. performed experimental procedures and analyzed results. M.F. contributed to Y2H library screening and transgenic experiments. T.L. contributed to the protein interaction experiments. R.N. contributed to analyses of histological sections of root cells. B.C. contributed to genotyping and phenotyping. Z.M. provided supervision to H.J., S.X., and M.F. S.P. and J.P. contributed to protein studies and data analysis. B.C., Z.M., and J.P. provided valuable analyses and discussion. L.Y. conceived the idea, designed experiments, and interpreted results. H.J. and L.Y. wrote the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Liuling Yan (liuling.yan@okstate.edu).
References
- Barragan AC, Collenberg M, Wang J, Lee RRQ, Cher WY, Rabanal FA, Ashkenazy H, Weigel D, Chae E (2020) A truncated singleton NLR of recent origin causes hybrid necrosis in Arabidopsis thaliana. Molecular Biology and Evolution doi: 10.1093/molbev/msaa245 [DOI] [PMC free article] [PubMed]
- Bizimungu B, Collin J, Comean A, St-Pierre CA (1998) Hybrid necrosis as a barrier to gene transfer in hexaploid winter wheat × Triticale Crosses. Can J PlantSci 72: 239–244 [Google Scholar]
- Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, Weigel D (2007) Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol 5: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K, Weigel D (2007) Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nat Rev Genet 8: 382–393 [DOI] [PubMed] [Google Scholar]
- Caldwell RM, Compton LE (1943) Complementary lethal genes in wheat causing a progressive lethal necrosis of seedlings. J Hered 34: 67–70 [Google Scholar]
- Chae E, Bomblies K, Kim S-T, Karelina D, Zaidem M, Ossowski S, Martín-Pizarro C, Laitinen RAE, Rowan BA, Tenenboim H, et al. (2014) Species-wide genetic incompatibility analysis identifies immune genes as hot spots of deleterious epistasis. Cell 159: 1341–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Yan L (2013) Construction of a high-quality yeast two-hybrid (Y2H) library and its application in identification of interacting proteins with key vernalization regulator TaVRN-A1 in wheat. BMC Res Notes 6: 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hersh BM, Conradt B, Zhou Z, Riemer D, Gruenbau Y, Horvitz HR (2000) Translocation of C elegans CED-4 to nuclear membranes during programmed cell death. Science 287: 1485–1489 [DOI] [PubMed] [Google Scholar]
- Chen C, Chen H, Shan J-X, Zhu M-Z, Shi M, Gao J-P, Lin H-X (2013) Genetic and physiological analysis of a novel type of interspecific hybrid weakness in rice. Mol Plant 6: 716–28 [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 124: 803–814 [DOI] [PubMed] [Google Scholar]
- Chu C-G, Faris JD, Friesen TL, Xu SS (2006). Molecular mapping of hybrid necrosis genes Ne1 and Ne2 in hexaploid wheat using microsatellite markers. Theor Appl Genet 112: 1374–1381 [DOI] [PubMed] [Google Scholar]
- Coll NS, Epple P, Dangl JL (2011) Programmed cell death in the plant immune system. Cell Death Differ 18: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Fang L, Zhu X, Zhou B, Zhang T (2019) A CC-NBS-LRR gene induces hybrid lethality in cotton. J Exp Bot 70: 5145–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S, et al. (2002) Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet 31: 135–140 [DOI] [PubMed] [Google Scholar]
- He F, Xing P, Bao Y, Ren M, Liu S, Wang Y, Li X, Wang H (2017) Chromosome pairing in hybrid progeny between Triticum aestivum and Elytrigia elongata. Front Plant Sci 8: 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsen JGT (1963) Hybrid necrosis as a problem for the wheat breeder. Euphytica 12: 1–16 [Google Scholar]
- Horsefield S, Burdett H, Zhang X, Manik MK, Shi Y, Chen J, Qi T, Gilley J, Lai JS, Rank MX (2019) NAD+ cleavage activity by animal and plant TIR domains in cell death pathways. Science 365: 793–799 [DOI] [PubMed] [Google Scholar]
- IWGSC. (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361: eaar7191. [DOI] [PubMed] [Google Scholar]
- Jeuken MJW, Zhang NW, McHale LK, Pelgrom K, den Boer E, Lindhout P, Michelmore RW, Visser RGF, Niks RE (2009) Rin4 causes hybrid necrosis and race-specific resistance in an interspecific lettuce hybrid. Plant Cell 21: 3368–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourelis J, van der Hoorn RAL (2018) Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 30: 285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 16: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang S, Mulder L, Jones JDG (2002) A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296: 744–747 [DOI] [PubMed] [Google Scholar]
- Li G, Yu M, Fang T, Cao S, Carver BF, Yan L (2013) Vernalization requirement duration in winter wheat is controlled by TaVRN-A1 at the protein level. Plant J 76: 742–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lei L, Miao F, Powers C, Zhang X, Deng J, Tadege M, Carver BF, Yan L (2017) The STENOFOLIA gene from Medicago alters leaf width, flowering time and chlorophyll content in transgenic wheat. Plant Biotech J 16: 186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JP, Seddon AE, Moghe GD, Simenc MC, Shiu S-H (2015) Characteristics of plant essential genes allow for within- and between-species prediction of lethal mutant Phenotypes. Plant Cell 27: 2133–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Kufer TA, Schulze-Lefert P (2011) NLR functions in plant and animal immune systems: So far and yet so close. Nat Immunol 12: 817–826 [DOI] [PubMed] [Google Scholar]
- Meinke D, Muralla R, Sweeney C, Dickerman A (2008) Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci 13: 483–491 [DOI] [PubMed] [Google Scholar]
- Mirua H, Parker BB, Snape JW (1992) The location of major genes and associated quantitative trait loci on chromosome arm 5BL of wheat. Theor Appl Genet 85: 197–204 [DOI] [PubMed] [Google Scholar]
- Mizuno N, Shitsukawa N, Hosogi N, Park P, Takumi S (2011) Autoimmune response and repression of mitotic cell division occur in inter-specific crosses between tetraploid wheat and Aegilops tauschii Coss. that show low temperature-induced hybrid necrosis. Plant J 68: 114–128 [DOI] [PubMed] [Google Scholar]
- Qi S, Pang Y, Hu Q, Liu Q, Li H, Zhou Y, He T, Liang Q, Liu Y, Yuan X, et al. (2010) Crystal structure of the Caenorhabditis elegans Apoptosome reveals an octameric assembly of CED-4. Cell 141: 446–457 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Oliver H, Zou H, Chen P, Wang X, Abrams JM (1999) Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat Cell Biol 1: 272–279 [DOI] [PubMed] [Google Scholar]
- Rubio V, Shen Y, Saijo Y, Liu Y, Gusmaroli G, Dinesh-Kumar SP, Deng XW (2005) An alternative tandem affinity purification strategy applied to Arabidopsis protein complex isolation. Plant J 21: 767–778 [DOI] [PubMed] [Google Scholar]
- Saintenac C, Zhang W, Salcedo A, Rouse MN, Trick HN, Akhunov E, Dubcovsky J (2013) Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 341: 783–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo A, Rutter W, Wang S, Akhunova A, Bolus S, Chao S, Anderson N, Fernandez M, Soto D, Rouse M, et al. (2017) Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science 358: 1604–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirano Y, Kachroo P, Shah J, Klessig DF (2002) A gain-of-function mutation in an Arabidopsis Toll Interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 14: 3149–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector MS, Desnoyer S, Hoeppner DJ, Hengartner MO (1997) Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature 385: 653–656 [DOI] [PubMed] [Google Scholar]
- Takken FL, Albrecht M, Tameling WI (2006) Resistance proteins: molecular switches of plant defense. Curr Opin Plant Biol 9: 383–390 [DOI] [PubMed] [Google Scholar]
- Tameling WIL, Vossen JH, Albrecht M, Lengauer T, Berden JA, Haring MA, Cornelissen BJC, Takken FLW (2006) Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol 140: 1233–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Yuan F, Leister RT, Ausubel FM, Katagiri F (2000) Mutational analysis of the Arabidopsis nucleotide binding site-leucine-rich repeat resistance gene RPS2. Plant Cell 12: 2541–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Zhu X, Wang Y, Liu L, Xu B, Li F, Fang J, Chu C (2011) Semi-dominant mutations in the CC-NB-LRR-type R gene, NLS1, lead to constitutive activation of defense responses in rice. Plant J 66: 996–1007 [DOI] [PubMed] [Google Scholar]
- Tomar SMS, Singh B (1998) Hybrid chlorosis in wheat-rye crosses. Euphytica 99: 1–4 [Google Scholar]
- Tornero P, Chao RA, Luthin WN, Goff SA, Dangl JL (2002) Large-scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 14: 435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunewaki K (1992) Aneuploid analysis of hybrid necrosis and hybrid chlorosis in tetraploid wheats using the D-genome chromosome substitution lines of durum wheat. Genome 35: 594–601 [Google Scholar]
- van der Biezen EA, Jones JD (1998) The NB-ARC domain: A novel signaling motif shared by plant resistance gene products and regulators of cell death in animals. Curr Biol 8: R226–R227 [DOI] [PubMed] [Google Scholar]
- van Ooijen G, Mayr G, Kasiem MMA, Albrecht M, Cornelissen BJC, Takken FLW (2008) Structure-function analysis of the NB-ARC domain of plant disease resistance proteins. J Exp Bot 59: 1383–1397 [DOI] [PubMed] [Google Scholar]
- Wan L, Essuman K, Anderson RG, Sasaki Y, Monteiro F, Chung EH, Nishimura ES, DiAntonio A, Milbrandt J, Dangl JL, et al. (2019) TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 365: 799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang J, Hu M, Wu S, Qi J, Wang G, Han Z, Qi Y, Gao N, Wang H-W, Zhou J-M, Chai J (2019a) Ligand-triggered allosteric ADP release primes a plant NLR complex. Science 364: eaav5868. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu M, Wang J, Qi J, Han Z, Wang G, Qi Y, Wang H-W, Zhou J-M, Chai J (2019b) Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364: eaav5870. [DOI] [PubMed] [Google Scholar]
- Wang W, Pan Q, He F, Akhunova A, Chao S, Trick H, Akhunov E (2018) Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J 1: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Abd-El-Haliem A, Bozkurt TO, Belhaj K, Terauchi R, Vossen JH, Kamoun S (2017) NLR network mediates immunity to diverse plant pathogens. Proc Natl Acad Sci USA 114: 8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Wang J, Liu C, Wang Y, Wang Y, Zhai J, Liu J, Hong X, Cao X, Zhu JK, Gong Z (2006). ROR1/RPA2A, a putative replication protein A2, functions in epigenetic gene silencing and in regulation of meristem development in Arabidopsis. Plant Cell 18: 85–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Takashi T, Morinaka Y, Lin S, Wu J, Matsumoto T, Kitano H, Matsuoka M, Ashikari M (2010) Gain of deleterious function causes an autoimmune response and Bateson-Dobzhansky-Muller incompatibility in rice. Mol Genet Genomics 283: 305–315 [DOI] [PubMed] [Google Scholar]
- Yan N, Chai J, Lee ES, Gu L, Liu Q, He J, Wu JW, Kokel D, Li H, Hao Q, Xue D, Shi Y (2005) Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature 437: 831–837 [DOI] [PubMed] [Google Scholar]
- Yuan J, Horvitz HR (1992) The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development 116: 309–320 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Cheng ZM (2016) A unique RPW8-encoding class of genes that originated in early land plants and evolved through domain fission, fusion, and duplication. Sci Rep 6: 32923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hiebert CW, McIntosh RA, McCallum BD, Thomas JB, Hoxha S, Singh D, Bansal U (2016) The relationship of leaf rust resistance gene Lr13 and hybrid necrosis gene Ne2m on wheat chromosome 2BS. Theor Appl Genet 129: 485–493 [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.