Abstract
Genetically encoded biosensors pave the way for understanding plant redox dynamics and energy metabolism on cellular and subcellular levels.
ADVANCES
Methodological advances in fluorescent protein-based in vivo biosensing have been instrumental for several paradigm shifts in our understanding of cell physiology, metabolism and signaling.
An increasing number of genetically encoded biosensors has been used to dissect the dynamics of several distinct redox couples and energy physiology in plants.
In vivo monitoring using biosensors has pioneered the simultaneous read-out of different physiological parameters in different subcellular locations by parallelized plate reader-based, multiwell fluorimetry, or expression strategies for multiple sensors in parallel.
Sensing dynamic changes in hydrogen peroxide levels is possible with sensors of the HyPer family, or roGFP fusion variants with a thiol peroxidase.
Peredox and SoNar family sensors enable direct visualization of NADH/NAD+, while iNAP family sensors respond to NADPH concentration in plants.
Sensor variants with different sensitivity ranges enable use of the most appropriate variant for the specific in vivo environment or experimental scope.
Integration of redox regulation into plant energy metabolism
In eukaryotic cells, cell compartments fulfill different yet complementary functions, while they are linked by the cellular metabolic network. In addition, plants possess photosynthetic and non-photosynthetic tissues, which most visibly differ by the types of plastids they contain. In photosynthetic tissues, plants can shift flexibly from photo-autotrophic to heterotrophic metabolism, depending on the presence of light, which increases the challenge of maintaining metabolic control. During active photosynthesis, chloroplasts produce ATP, reduce equivalents in the light reactions and fix carbon in the Calvin–Benson–Bassham (CBB) cycle. The discovery of thioredoxins (TRXs) as light-dependent Cys-redox switch operators for multiple enzymes in carbon assimilation by Wolosiuk and Buchanan (1977) established a hotspot of Cys-based redox regulation, which is directly connected to the availability of photosynthesis-derived reductant. Maintaining photosynthetic efficiency additionally relies on active adjustment of the ATP:NADPH ratio by mitochondria acting as electron sinks, as well as the recycling of 2-phosphoglycolate during photorespiration including multiple reaction steps in peroxisomes and mitochondria. In the dark and heterotrophic tissues, mitochondria make a major contribution to energy metabolism by respiration.
Generally, pools of metabolites and reducing equivalents are separated between compartments and selectively linked via specific transport across membranes. While stromal and cytosolic pools of reduced glutathione (GSH) are connected by chloroquine-resistance transporter-like transporters (CLTs; Maughan et al., 2010), the oxidized form of glutathione, glutathione disulfide (GSSG), cannot be transported and requires local reducing systems in both the cytosol and the stroma (Marty et al., 2009; Marty et al., 2019). Similarly, NAD+ can be transported across the membranes of the plastids, mitochondria, and peroxisomes (van Roermund et al., 2016; Gakière et al., 2018; Souza Chaves et al., 2019; Feitosa-Araujo et al., 2020), while NADH is not directly transported across membranes necessitating the indirect transport of reducing equivalents via metabolite shuttles (Hoefnagel et al., 1998; Selinski and Scheibe, 2018). Hydrogen peroxide (H2O2) transport across specific membranes may be facilitated by certain aquaporins (Smirnoff and Arnaud, 2019).
Thus, intracellular boundaries with selective connectivity are necessary for metabolism of eukaryotic cells with specific membrane contact sites between different compartments additionally emerging as local exchange and signaling hubs (Pérez-Sancho et al., 2016; Baillie et al., 2020). Reactive oxygen species (ROS) serve as important signaling components in plant development and stress responses (Waszczak et al., 2018; Smirnoff and Arnaud, 2019). ROS can be specifically and locally generated by enzymes, or are formed under stress conditions, especially in the chloroplasts and mitochondria, which harbor electron transport chains that can release specific ROS at a high rate. ROS production and scavenging are tightly controlled inside cell compartments that differ in their inventory of redox-active proteins present. Those proteins further differ in their coupling to different oxidation and reduction systems.
Classical experimental approaches to address stress responses are frequently based on tissue extraction followed by chromatography- and/or mass spectrometry-based analytical techniques, which make it difficult to separate out individual tissue types, single cells, or even individual subcellular compartments. That has led to a situation in which we still do not systematically understand the diversity of metabolism and physiology between different tissues, cells, and cell compartments in many cases. Genetically encoded biosensors partly address this shortcoming since they are based on fluorescent proteins (FPs) that change their fluorescence properties in response to specific stimuli (Kostyuk et al., 2020). Thus, genetically encoded biosensors allow probing the dynamics of specific metabolites and physiological parameters at high resolution and across all scales of plant structures. The spatial resolution as ensured by precise genetic expression and/or targeting, in combination with dynamic monitoring and adjustable sensitivity, has started to provide detailed insights into plant physiology and introduced shifts in several long-standing paradigms of redox biology and energy metabolism (Meyer et al., 2021).
Photochemical and enzymatic principles for sensor design
The functionality of genetically encoded biosensors is based on different physicochemical properties of FPs (Figure 1). To date, several available sensor constructs have been adapted for plants (names in green boxes in Figure 1) and provide insights into the in vivo Cys-redox and energy dynamics in plant cells.
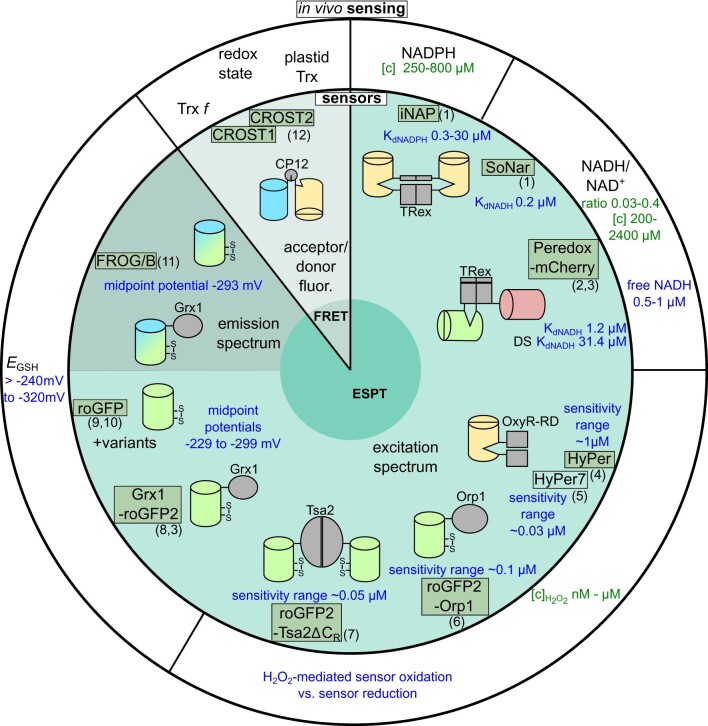
Figure 1.
Overview of available sensors for redox-related physiological parameters. Most available redox sensors are designed to exploit excited state proton transfer (ESPT). An exception is the thioredoxin (TRX)-sensor CROST, which exploits Förster resonance energy transfer (FRET) between two fluorescent proteins. While most of these sensors are ratiometric by excitation, the recent addition FROG/B shows a redox-dependent change in its emission properties. The ESPT is influenced by physical constraints on the ß-barrel structure, which are mediated by an inter-strand disulfide in the case of roGFP and FROG/B, or by a fusion partner linked to the N- and C-terminus of a circularly permuted (cp) fluorescent protein (indicated by an indented ß-barrel). Specificity of sensors for their respective analytes is mediated by the fused sensing domain (in gray with names indicated) that specifically binds a ligand substrate (in iNAP, SoNar, Peredox), or specifically forms (HyPer) or transfers a disulfide bond to the sensor (roGFP2-Orp1, roGFP2-Tsa2ΔCR). In the FRET-based CROST, part of the TRX-dependent chloroplast protein 12 (CP12) is used as a sensing domain between the FRET-pair. CP12 changes its conformation upon oxidation/reduction and thus leads to a change in energy transfer between the two attached FPs. Names of sensors used in photosynthetic organisms to date are in green boxes, references are given as numbers in brackets: (1) Lim et al. (2020), (2) Steinbeck et al. (2020), (3) Wagner et al. (2019), (4) Costa et al. (2010), (5) Pak et al. (2020), (6) Nietzel et al. (2019), (7) Niemeyer et al. (2020), (8) Gutscher et al. (2008), (9) Meyer et al. (2007), (10) Schwarzländer et al. (2008), (11) Sugiura et al., (2020) and (12) Sugiura et al. (2019). Blue text color shows sensor parameters and physiological parameters estimated based on sensor data. Green text color shows physiological concentrations observed with alternative techniques. Ranges of experimentally observed pool sizes for NADPH+/NADP+ and NADH+/NAD+ and their ratios in plants (chloroplasts, mitochondria, cytosol) are from Wigge et al. (1993) and Igamberdiev and Gardeström (2003). Sensitivities towards H2O2in vitro (minimal concentration to elicit detectable response) are from Morgan et al. (2016), Nietzel et al. (2019), and Pak et al. (2020). No absolute H2O2 concentrations can be measured in vivo: sensor redox state is determined by rates of H2O2-mediated sensor oxidation and sensor reduction mediated by cellular reducing systems (see Box 3). For a review on H2O2 in plants, see Smirnoff and Arnaud (2019). For midpoint potentials of roGFP variants, see Meyer and Dick (2010).
Excitation-based sensors
Dual-wavelength excitation in FP variants is caused by the ionization state of the chromophore that can be shifted between protonated and deprotonated forms. The equilibrium between the two states is governed by an internal hydrogen bond network that permits proton transfer to neighboring amino acids when the chromophore is excited (Brejc et al., 1997; Jung et al., 2005). Interference with the efficiency of this excited state proton transfer (ESPT) through structural changes in the FP ß-barrel leads to changes in excitation properties and hence has opened the door to engineering biosensors for excitation ratio analysis. Sensitivity of the ESPT to the cellular environment can be realized, e.g., by engineering an inter-strand disulfide into the ß-barrel structure of FPs. For a sufficiently strong effect on the ESPT, only two possible positions linking ß-strands 7 and 10 by a disulfide have been identified: C147-C204 and C149-C202. Both disulfides have been exploited in the redox-sensitive yellow fluorescent protein (rxYFP) and the redox-sensitive green fluorescent protein (roGFP) variants 1 to 6 (Ostergaard et al., 2001; Dooley et al., 2004; Hanson et al., 2004). The most commonly used variant to date is roGFP2, which offers photostability, pH-insensitivity (not for emission intensities of the individual excitations, but at the level of the ratio), and a large dynamic range resulting in good signal-to-noise values (Meyer et al., 2007; Schwarzländer et al., 2008; Ugalde et al., 2020). roGFP1 was the first roGFP variant to be reported in a plant context (Jiang et al., 2006) and offers the advantage of a more negative midpoint potential, but it is prone to artifacts by photoconversion (Schwarzländer et al., 2008). roGFP3-6 may offer specialized applications, such as exploration of redox shifts towards more reducing potentials by roGFP3, but although those variants were introduced already in 2004 (Hanson et al., 2004) their use in planta has not yet been reported.
Circular permutation of the FP sequence and fusion of the newly established N- and C-termini to a sensing domain provides another possibility for creating a metastable hydrogen bond network around the chromophore (Baird et al., 1999; Zapata-Hommer and Griesbeck, 2003). Regarding redox-related sensors, circular permuted FPs (cpFPs) have been exploited, e.g., for different variants of the H2O2-sensitive HyPer-family (Zheng, 1998; Belousov et al., 2006; Pak et al., 2020), the NADH/NAD+ sensors Peredox (Hung et al., 2011), SoNar (Zhao et al., 2015) and for the NADPH/NADP+ sensor iNAP (Tao et al., 2017; Figure 1). In cpFPs, the newly introduced N- and C-termini leave a cleft in the ß-barrel, which renders the chromophore responsive to conformational changes and concomitantly influences the ESPT. Changes in excitation properties are thus linked to conformational changes induced by substrate-binding to the fused sensing domains. At the same time, the engineered cleft can leave the cpFP chromophore more accessible to protons from the solvent, which introduces the danger of artefactual pH sensitivity from the medium (Schwarzländer et al., 2014).
Emission-based sensors
In addition to changes in the excitation properties, changes in the ESPT can also lead to changes in emission properties of FPs because the protonated chromophore of GFP emits blue light at excitation while only the deprotonated form fluoresces in green. Further modification of amino acids in proximity to the hydrogen bond network may impact the efficiency of the ESPT and thus causes a clamp of the protonated state of the chromophore and a concomitant change in emission wavelength. This principle has been used to develop the Ca2+ sensor protein GEM-GECO1 (Zhao et al., 2011) and, most recently, a newly designed redox-sensitive protein called FROG/B (Sugiura et al., 2020). For the latter, a redox-sensitive probe was created by limiting the efficiency of the ESPT by mutations in combination with an inter-strand disulfide bond linking ß-strands 7 and 10. In this case, the probe is always excited at 400 nm but emits fluorescence predominantly at ∼450 nm (blue) in the reduced state and at ∼510 nm (green) when oxidized.
Förster resonance energy transfer (FRET) between a pair of fluorophores is used in another class of emission-based ratiometric sensors, by linking the FRET pair through a sensing domain that undergoes conformational changes upon oxidation/reduction or substrate binding. This concept of radiation-free energy transfer from a donor FP to an acceptor FP has been used to design the TRX-dependent sensor CROST (Sugiura et al., 2019) and ATeam as a sensor for MgATP2- (Imamura et al., 2009). A similar sensor concept has been implemented in Redoxfluor by integrating a tandem repeat of a redox-sensitive fragment of the yeast transcription factor Yap1 between the FPs Cerulean and Citrine (Yano et al., 2010). This probe has only a low dynamic range and has not yet been used in plants, but exemplifies how further redox-sensitive constructs may be generated in the future. FRET can be detected via several readouts, of which the donor-to-acceptor fluorescence emission intensity ratio has been the most widely used for biosensors, even though fluorescence lifetime imaging (FRET-FLIM) has been gaining popularity in related applications (Xing et al., 2016; Algar et al., 2019).
Sensor specificity
Sensor specificity is conferred by the fused sensing domain that is dependent on the oxidation and reduction reactions or the binding and dissociation of the ligand, respectively. As the exposed inter-strand disulfide on roGFP and FROG/B is reduced and oxidized preferentially via glutaredoxin (GRX)-mediated catalysis, these sensors equilibrate with the glutathione redox potential (EGSH) in vivo when GRX activity is present (Meyer et al., 2007; Gutscher et al., 2008; Schwarzländer et al., 2008; Sugiura et al., 2020; Figure 1; Box 1). Different variants of roGFPs are available, covering a range of midpoint potentials from –229 mV (roGFP1-iL) to –299 mV (roGFP3; Meyer and Dick, 2010). RoGFP may be used in the free form for sensing of EGSH as long as dithiol GRXs (Trnka et al., 2020) are present at sufficient catalytic capacity in the respective compartment (Gutscher et al., 2008). However, in practice roGFP is mainly used as fusion to human Grx1, to ensure comparable kinetic characteristics and circumvent potential problems that may be caused by the abundance and kinetic properties of endogenous GRX in different species, cell types, and subcellular compartments. To date, there are no reports to indicate problems of roGFPs or human Grx1 expression in plants. An exception has been the inability to target Gxr1-roGFP2 to mitochondria that was overcome by an inverted design of the sensor construct (Albrecht et al., 2014). However, mitochondrial roGFP2-Grx1 lines still show a slight developmental phenotype (De Col et al., 2017), and silencing of the sensor has been occasionally observed.
Box 1.
The roGFP2 is a sensor for the glutathione redox potential
Glutaredoxins (GRX) catalyze the reversible electron transfer between glutathione (GSH) and roGFP2 (Meyer et al., 2007; Gutscher et al., 2008; Trnka et al., 2020). In the absence of any other known oxidoreductase or thiol oxidase efficiently interacting with roGFP2, this catalyzed bidirectional electron transfer leads to thermodynamic equilibration of the redox potentials EGSH and EroGFP2 described by the respective Nernst equations. The Nernst equations (see Box 1 Figure) include the midpoint potentials of glutathione (–240 mV) and roGFP2 (–280 mV) at pH 7.
Fusion of human Grx1 to either the N- or the C-terminus of roGFP2 guarantees the availability of a functional GRX in close proximity to the engineered disulfide of roGFP2 and improves kinetic responses and reliability of the readouts (Gutscher et al., 2008; Albrecht et al., 2014).
Steady-state measurements in nonstressed wild-type plants have consistently shown that roGFP2 is largely reduced in the cytosol, plastid stroma, the mitochondrial matrix, and peroxisomes (Schwarzländer et al., 2008). With the degree of oxidation (OxD) of roGFP2 approaching 0% under these circumstances, this implies that the probe is well outside its usable dynamic range of 10%–90% OxD. Hence, the redox potential of glutathione (EGSH) would be –310 mV or even more negative, at pH 7. As the redox potential of thiols is affected by pH, the actual redox potential would be even more negative under more alkaline local pH conditions. EGSH necessarily varies with changing amounts of total glutathione and the degree of oxidation of GSSG. Irrespective of the exact physiological concentration, which is in the low millimolar range (Meyer et al., 2001), a redox potential of –310 mV or less implies that the OxD of GSH must be very low leaving effectively only nanomolar amounts of GSSG. This implies GSH:GSSG ratios up to 250.000:1. With this, roGFP2 becomes extremely sensitive to changes in GSSG and thus can be used as a sensor for oxidative events in planta. C, cysteine; Chr, chromophore.

In addition to GRX-mediated oxidation, a different oxidation specificity for roGFP can also be achieved by fusion to different redox-active fusion partners, which may effectively act as thiol oxidase. This strategy has been exploited by fusion of the yeast peroxidase Orp1 (Gutscher et al., 2009) and a mutated version of the 2-Cys peroxiredoxin Tsa2 (Tsa2ΔCR) from yeast (Morgan et al., 2016). Both proteins render roGFP2 oxidation particularly sensitive to H2O2. While roGFP2-Orp1 has been used in Arabidopsis (Arabidopsis thaliana; Nietzel et al., 2019; Ugalde et al., 2021), roGFP2-Tsa2ΔCR has been tested in Chlamydomonas reinhardtii (Niemeyer et al., 2020). In cpFP- and FRET-based sensors, the specificity depends on the properties of the fused sensing domain, consisting of different well-characterized ligand-binding or redox-sensitive protein domains (Figure 1).
Assessing sensor characteristics
In vitro and in vivo sensor responses require careful assessment in terms of specificity, range of sensitivity for the analyte and spectroscopic dynamic range. It is a fundamental – and in most cases justified – assumption that the biochemical and physicochemical sensor behavior is similar in vitro and in vivo, but this hypothesis requires critical testing for each individual sensor for validation. If possible, the in vivo dynamic range should be determined by in situ calibration of the sensor, i.e., complete saturation at both ends of the titration curve and compared side-by-side to the in vitro calibration. For thiol/disulfide-based redox sensors, this means that full oxidation and reduction need to be achieved as sensor calibration and spectroscopic dynamic range is important for sensor data interpretation and comparability (see Box 2).
Box 2.
Sensor calibration and spectroscopic dynamic range
If technically possible, the maximal response of a biosensor in either direction should always be assessed in vivo, because cell morphology and particularly differential absorption of either excitation or emission wavelengths bear the risk of causing misinterpretation of sensor data. For a Cys-based redox sensor, membrane-permeable reductants and oxidants can be used, such as dithiothreitol (DTT), diamide, or 2,2′-dipyridyldisulfide (DPS). As an alternative, H2O2 is often used to achieve full oxidation. However, H2O2 is not specific for thiols and may cause other damage or interfere with imaging due to formation of O2 bubbles released from endogenous activities, such as catalase. The complete in vivo reduction (red.) and oxidation (ox.) of the sensor determines its maximal and minimal observable intensity ratios (0% oxidized vs. 100% oxidized) and their difference, the spectroscopic dynamic range (DR, δ; see Figure panels A and B). Dynamic sensor readouts can only carry meaning if the data points of physiological (phys.) measurements are within the DR. When a graphical representation of a ratio readout on a pseudo-color scale is used, the calibration represents the minimal and maximal values of the color scale. After calibration, ratio values can be transformed into degree of oxidation values (OxD; Meyer et al., 2007; Fricker, 2016). As the absolute ratio values can vary depending on the microscopy- or plate reader-setup used, measurements carried out in different laboratories become comparable based on OxD values.
OxD values can be further converted to absolute redox potentials expressed in mV if the midpoint potential (E0) of the sensor is known (Meyer et al., 2007; Fricker, 2016) (Figure panel C). As redox potentials in mV are dependent on pH (Schwarzländer et al., 2008; Meyer and Dick, 2010), reporting of the assumed compartment-specific pH value is crucial for comparison with literature values. Because each conversion step brings in another level of noise and uncertainty, the log(ratio) is most robust to assess dynamic changes. The near linear range that can be used for meaningful physiological measurements in roGFPs is the midpoint potential of the sensor ±30 mV (equivalent to OxD 10%–90%, Schwarzländer et al., 2008) (Figure panel A)).

Live monitoring of plant redox and energy physiology
H2O2
Of different chemical compounds generated in the cells that are collectively referred to as ROS, H2O2 is particularly stable and can diffuse within cell compartments or may be translocated across membranes (Waszczak et al., 2018; Smirnoff and Arnaud, 2019). The local steady-state concentration of H2O2 depends on the rate of generation and the rate of scavenging. Chemical dyes for H2O2 are frequently unspecific and provide an accumulative response over time requiring the measurement of their accumulation rate in order to deduce dynamic changes in ROS concentrations (Smirnoff and Arnaud, 2019). In contrast, genetically encoded FP-based probes enable direct access to dynamic information in vivo. Local H2O2 dynamics in response to exogenous H2O2, H2S, light stress, or the elicitation of an immune response have been assessed using HyPer, roGFP2-Tsa2ΔCR or roGFP2-Orp1 probes in the cytosol, the peroxisomes and the mitochondrial matrix as well as the plastid stroma and thylakoid lumen (Costa et al., 2010; Caplan et al., 2015; Exposito-Rodriguez et al., 2017; Scuffi et al., 2018; Nietzel et al., 2019; Niemeyer et al., 2020; Ugalde et al., 2021; Waadt et al., 2020). Key findings include that redox dynamics in different compartments are indeed linked: an elicitor-induced oxidative burst in the apoplast has a time-shifted impact on the cytosolic redox environment (Nietzel et al., 2019, Ugalde et al., 2020). Light stress in chloroplasts triggers redox changes in the cytosol in Arabidopsis and C. reinhardtii (Exposito-Rodriguez et al., 2017; Niemeyer et al., 2020; Ugalde et al., 2020a), while a response in the nucleus has only be observed in tobacco epidermis cells (Exposito-Rodriguez et al., 2017). The observations that parallel sensor responses in the stroma and the nucleus were independent of cytosolic ascorbate peroxidase (cAPX) expression levels and that chloroplasts contact nuclei in tobacco epidermis via stromules (Caplan et al., 2015; Exposito-Rodriguez et al., 2017) suggest that membrane contact sites may play a direct role in H2O2 and/or glutathione exchange between the chloroplast stroma and the nucleoplasm. The dynamic cell-biological linkage between specific organelles represents an intriguing possibility to by-pass high-capacity H2O2 scavenging systems, as present in the cytosol, to mediate direct inter-compartment ROS-signal transduction. The H2O2 sensor oxidation steady state is dependent on the rate of oxidation by H2O2 and the rate of reduction via the endogenous reduction system that the sensor relies on for rereduction in a specific cell compartment (Nietzel et al., 2019; Box 3). In C. reinhardtii under light stress, plastid-generated H2O2 did elicit a sensor response in the cytosol but not in other compartments, suggesting that the cytosol acts as an effective antioxidant barrier (Niemeyer et al., 2020). Notably, weakening the scavenging system in one compartment (such as cytosolic glutathione reductase (GR)) had an impact on sensor responses in other compartments (such as roGFP2-Orp1 in the mitochondrial matrix) in Arabidopsis (Nietzel et al., 2019), indicating a protective role of scavenging capacity in one compartment regarding other subcellular compartments. Thus, the cytosolic response and potential downstream responses in gene expression (such as induction of cAPX; Exposito-Rodriguez et al., 2017) likely depend on local ROS concentrations in different subcellular locations and capacities of several scavenging systems. Differential contributions of different organelles to ROS-related transcriptional events during dark-induced senescence have been reported (Rosenwasser et al., 2011).
Future challenges include the expansion of H2O2-sensing to additional subcellular compartments and the use of additional sensor variants with properties that match specific physiological requirements (such as the pH-stable HyPer7; Pak et al., 2020). Key questions include which diffusion and transport pathways for H2O2 originate from different subcellular localizations and how specificity of ROS-signaling is underpinned by the precise spatial and temporal distribution of H2O2 in the cell. While oxidation of H2O2-sensors is directly dependent on H2O2, the subcellular pools of reducing power are also linked, as scavenging and repair enzymes use GSH or TRXs for rereduction. As a consequence, the scavenging of ROS can also result in changes of local EGSH, especially when ROS generation is higher than the rate of GSSG rereduction or in mutants lacking GR (Nietzel et al., 2019; Box 3).
Box 3.
Sensor oxidation and reduction rates in vivo
The steady state of sensor oxidation is dependent on oxidation and reduction rates in vivo (see Box 3 Figure). For the H2O2 sensors roGFP2-Orp1 (A) and HyPer family (based on circularly permuted YFP (cpYFP; B) sensors, the oxidation rate is dependent on H2O2 levels, whereas the reduction rate is dependent on the cellular reducing system resolving the formed disulfide. Both roGFP2 and OxyR-RD (redox domain of hydrogen peroxide-inducible genes activator from E. coli) disulfides are reduced by glutathione (GSH) via glutaredoxins (GRX), generating glutathione disulfide (GSSG) (Zheng, 1998; Nietzel et al., 2019). Thus, sensor readouts should be carefully interpreted regarding steady state of sensor oxidation as well as changes in oxidation and reduction rates. For instance, even with specific fusion partners, the rereduction of roGFP2 is dependent on GRXs and the glutathione redox potential EGSH and only the relative dynamic change of roGFP2-Orp1 oxidation state over time monitors H2O2 dynamics (Nietzel et al., 2019).

GSH/GSSG
Glutathione is the most abundant low-molecular-weight thiol in plant cells with concentrations in the low millimolar range (Meyer et al., 2001). Glutathione constitutes an important redox couple in the cell, since two molecules of GSH can be oxidized to form GSSG. The constant cycling between reduction and oxidation by glutathione is most prominently apparent in the glutathione-ascorbate-cycle, in which the regeneration of ascorbate is linked to the oxidation of GSH (Foyer and Halliwell, 1976). Similarly, GSH is also required as an electron donor for glutathione S-transferases (GSTs) exhibiting glutathione peroxidase activities for detoxification of organic peroxides and H2O2 (Dixon et al., 2009). The relative in- and efflux of electrons into and out of the glutathione pool give rise to the steady-state EGSH. Under non-stress conditions, the maintenance of a high NADPH/NADP+ ratio by sufficiently high electron flux into the NADP pool allows GRs to remove GSSG almost completely through reduction to GSH. Early biochemical considerations already led to the conclusion that GR activity would leave only low nM concentrations of GSSG (Veech et al., 1969). Such low amounts of GSSG in the presence of millimolar GSH would lead to highly negative redox potentials of –300 mV and less. While analytical techniques that rely on tissue and cell disruption could not confirm this prediction and found less-reducing EGSH values in extracts (Fey et al., 2005; Queval et al., 2008), roGFP2-based measurements in the cytosol, the mitochondria, the peroxisomes, and the plastids of plant cells confirmed a highly reducing EGSHin vivo by observing roGFP2 as almost completely reduced (Meyer et al., 2007; Schwarzländer et al., 2008). With a midpoint potential of –280 mV, the sigmoidal titration curve of roGFP2 allows resolving redox potentials along the near-linear part of the curve (see Boxes 1 and 2). A largely reduced state of roGFP2 hence indicates EGSH values of –310 mV (assuming pH 7.0; –340 mV at pH 7.5) or even more negative. In a 2 mM glutathione solution at pH 7.0, a redox potential of –320 mV is established by the presence of only 8 nM GSSG and a GSSG:GSH ratio of 1:250.000. These values are orders of magnitude lower than GSSG:GSH ratios in the order of 1:10 to 1:100 (Mhamdi et al., 2010b; Han et al., 2013; Schnaubelt et al., 2015) typically found in plant tissue extracts. The large difference is caused by two sources of unavoidable experimental errors: (i) mixing of the contents of different cell compartments with different EGSH during plant extraction (note that, for instance, ER EGSH is much more oxidizing than cytosolic EGSH; Schwarzländer et al., 2016) and (ii) peroxidative conversion of GSH to GSSG during deproteinization (Hansen and Winther, 2009). While analytical measurement of total GSH and GSSG content may still provide some information about the stress level plants are exposed to, the values can provide only very little information about the physiologically relevant situation in individual compartments and dynamic responses of plant cells to acute stress situations.
The introduction of roGFP-based measurements has allowed capturing true glutathione redox physiology in plants because it enabled monitoring dynamic and local EGSH changes in individual subcellular compartments (Meyer et al., 2007; Schwarzländer et al., 2008). RoGFP2 with a midpoint potential of –280 mV has been shown to be the most suitable probe variant to monitor steady-state EGSH values and dynamic oxidative changes in response to external triggers. Additionally, roGFP2-based spectroscopic measurements in plant mitochondria at different states of respiratory activity were validated by iodo-TMT-based redox proteomics and quantification of the shift in oxidation of the redox-active Cys-peptides (Nietzel et al., 2020).
Very low total amounts of glutathione or very high degrees of glutathione oxidation are bound to decrease EGSH values and would thus drive EroGFP2 to values outside the linear range of the probe. Due to a lack in GR and oxidative pressure generated by the oxidative protein folding machinery in the secretory pathway, a steep gradient in EGSH exists across the ER membrane (Schwarzländer et al., 2008; Meyer et al., 2019). This gradient can be exploited to investigate the topology of membrane proteins by fusing roGFP2 to either the N- or the C-termini and directly observing its orientation towards the cytosol or the lumen (Brach et al., 2009). For dynamic measurements in an environment with a less negative EGSH, probe variants with less negative midpoint potentials may be used to again match the suitable dynamic range of the probe with the actual EGSH. A roGFP2 variant, roGFP2iL (midpoint potential –238 mV) has been generated and successfully used in the cytosol of the GSH-deficient mutant rml1, which has less than 5% of WT GSH (Aller et al., 2013). The same probe also holds the promise of being suitable for analyses in the ER lumen. Whether roGFP2 variants can be used in the apoplast remains to be tested. The two major caveats in this case may be an acidic pH causing quenching of GFP fluorescence and the lack of reducing power, which is required for roGFPs to attain their oxidation-sensitive form. An interesting approach to circumvent these limitations was recently shown by using bacteria expressing roGFP2 as a biomarker for wash fluids from the surface of flowers and fruits (Liu et al., 2020).
Glutathione, the cell cycle, and plant development
Gradual depletion of glutathione either pharmacologically through inhibition of biosynthesis or genetically through mutation of the first enzyme in the biosynthetic pathway for GSH, glutamate-cysteine ligase (GSH1), leads to dwarfism or even an almost complete arrest of the cell cycle (Vernoux et al., 2000; Shanmugam et al., 2012). Conversely, genetic restriction of GSH turnover leads to maintenance of GSH even under sulfur deficiency and hence improved root growth under these conditions (Joshi et al., 2019). The apparent link between GSH content and growth has fostered speculations about redox control of the cell cycle. Generally, the cell cycle in eukaryotic cells is synchronized with the metabolic cycle of the cells with S phase and M phase occurring only during the reductive phase of metabolism and G1 in the oxidative phase (Chiu and Dawes, 2012). Based on direct measurements with Grx1-roGFP2, it has been claimed that EGSH differs between the cytosol and the nucleus in dividing root cells with EGSH in the nucleoplasm being slightly more negative than in the cytosol (Diaz-Vivancos et al., 2015). In non-dividing cells, no such differences have been observed (Schwarzländer et al., 2008; Marty et al., 2009). It is not clear, however, how gradients in EGSH across the nuclear envelope could be established because the nuclear pores allow free diffusion of GSH and GSSG and they are large enough to enable the presence of GR in the nucleoplasm (Marty et al., 2009). Redox cycling in synchronized cells, with EGSH monitored with Grx1-roGFP2 in both cytosol and nucleus becoming more oxidized during the G1 phase of the cell cycle and more reduced during S- and M-phases, also suggests effective redox coupling between both compartments (de Simone et al., 2017).
Tissue-specific readouts have been observed with sensors for different physiological parameters (De Col et al., 2017; Steinbeck et al., 2020). Similarly, roGFP2 indicates different redox potentials for different stages of flower development in the partially glutathione-deficient mutant cad2 (García-Quirós et al., 2020). The findings suggest that the flowers of cad2 mutants have a lower capacity to keep the cellular glutathione pool reduced and maintain high GSH/GSSG ratios but the full biological significance of this observation remains unclear.
The importance of glutathione reductase
If the total glutathione pool is depleted, the amount of GSSG decreases proportionally because GRs can re-establish the equilibrium between GSH and GSSG (Meyer et al., 2007; García-Quirós et al., 2020). A lack of GR1 in the cytosol causes a shift of EGSH towards less negative values by 20–40 mV (Marty et al., 2009). The actual EGSH in the cytosol of gr1 null mutants is thus well within the linear response range of roGFP2 (Box 2) and hence the sensor responds almost instantaneously with a pronounced change in fluorescence to an induced oxidation of the glutathione pool (Marty et al., 2009; Nietzel et al., 2019). With the lack of reductive capacity for removal of GSSG, the respective changes in roGFP2 fluorescence ratios exhibit a larger amplitude and longer duration in the gr1 background (Nietzel et al., 2019; Ugalde et al., 2020). The fact that gr1 mutants are fully viable with no or only a very minor phenotype (Marty et al., 2009; Mhamdi et al., 2010a) makes gr1 a highly suitable model for detection of stress-induced redox signals. This has been exploited in detection of cytosolic oxidation induced by osmotic shock or bacterial elicitors, which could only be detected in gr1 but not in wild-type plants (Bangash et al., 2019; Nietzel et al., 2019; Ugalde et al., 2020). The reason that EGSH in gr1 does not completely collapse is due to the presence of TRXs and NADPH-dependent TRX reductases (NTRs), which together provide a semi-efficient backup system for GSSG reduction (Marty et al., 2009). The absence of clear cell cycle aberrations in gr1 despite the less reducing EGSH poses question on the significance of changes in EGSH of ±30 mV for progression during the cell cycle. These questions have not yet been addressed in more detail and provide a lead for future work.
A second GR isoform, AtGR2 in Arabidopsis (Marty et al., 2019) and PpGR1 in the model moss Physcomitrium (Physcomitrella) patens (Müller-Schüssele et al., 2020), is dual-targeted to mitochondria and plastids. Deletion of GR2 in Arabidopsis is embryo lethal due to an essential role of GR2 in plastids during very early stages of embryo development well before the photosynthetic apparatus becomes functional (Marty et al., 2019). The cause of lethality is yet to be explored but it is very likely that GSSG accumulates in the stroma with no backup system for reduction or export of GSSG from plastids in place. In mitochondria, GR2 can be deleted because the mitochondrial ABC-transporter ATM3 may export some GSSG (Schaedler et al., 2014). More importantly, however, are the dual-targeted cytosolic-mitochondrial NTRs A and B that, similar to their role in the cytosol, are responsible for reducing GSSG together with mitochondrial TRXs (Marty et al., 2019). Despite this reductive power acting on GSSG, roGFP2-Grx1 targeted to the matrix of mitochondria lacking GR2 indicates a less reducing EGSH resulting in a pronounced EGSH gradient across the mitochondrial envelope. The lack of GR2 in the mitochondrial matrix is associated with enhanced respiration and deregulated TCA cycle dynamics, which suggests a decreased resource efficiency of energy metabolism (Nietzel et al., 2020). In pronounced contrast to Arabidopsis, the lack of stromal GR in P. patens does not cause lethality (Müller-Schüssele et al., 2020). Grx1-roGFP2 measurements clearly indicate a pronounced oxidation in the stroma, which results in decreasing photosynthetic efficiency with increasing light intensities.
Light dependency of EGSH in plastids
Probing EGSH in different photosynthetic species has revealed that the stromal EGSH is not as stable as in other cell compartments, but responds to changes in light conditions and to light/dark and dark/light transitions in a remarkably dynamic manner (Haber et al., 2021; Hipsch et al., 2020; Müller-Schüssele et al., 2020; Sugiura et al., 2020). FROG/B was used to probe light-dependent EGSH dynamics in vegetative cells and heterocysts in Anabaena and showed EGSH-dependent reduction of the sensor in the light and oxidation in the dark (Sugiura et al., 2020). In plants, EGSH responded dynamically within seconds and a similar range (changes of about 30 mV) to light and darkness in P. patens (Müller-Schüssele et al., 2020). These dynamic changes occurred in the presence of a GR and could, in theory, be based on changes in GSSG or GSH levels with rapid changes of the low levels of GSSG being the more likely candidate. Notably, stromal EGSH dynamics were recently also reported in a diurnal light and dark regime in Arabidopsis (Haber et al., 2021), pointing to a general underlying mechanism leading to an oxidative shift of stromal EGSH in the dark. The biological relevance of stromal EGSH dynamics requires further investigation to unravel cause-and-effect relationships and to test the potential consequences for photosynthetic function and regulation.
Thioredoxin
Reducing power for cellular thiol-based scavenging and repair systems stems from either GSH or TRXs. Reducing equivalents such as NADH and NADPH (see the following paragraph) or electrons from photosynthetic electron transport are used by different reductases to regulate the oxidation state of TRXs (Geigenberger et al., 2017). Electron flux through the light reactions is directly linked to metabolic flux through the photosynthetic carbon reactions via TRX-operated thiol switches. Thus, the dynamic monitoring of TRX redox state is highly desirable to establish an in vivo understanding of photosynthetic regulation but was to date mainly realized by Western blotting of protein extracts (Yoshida et al., 2014) or in vitro assays with reconstituted redox relays between different proteins (Yoshida and Hisabori, 2017). Recently, two different TRX redox sensors were constructed. Trx1-rxRFP is an intensiometric TRX sensor based on the fusion of a circularly permuted redox-sensitive RFP to human cytoplasmic Trx1 (Fan et al., 2017). While Trx1-rxRFP has not yet been successfully used in plants, the FRET-based biosensor CROST was generated specifically for use in photosynthetic organisms, using the stromal TRX target protein CP12 from Arabidopsis (Sugiura et al., 2019). CP12 is specific to photosynthetic organisms and undergoes disulfide-based conformational changes upon oxidation/reduction, forming a complex with the two CBB cycle enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and phosphoribulokinase (PRK) in its oxidized form (Wedel et al., 1997). In the GAPDH/CP12/PRK complex, the CBB cycle enzymes are inactive and protected from oxidation but can be rapidly reactivated via TRX f and m isoforms (Marri et al., 2009; Marri et al., 2014). Sugiura and colleagues (Sugiura et al., 2019) used a partial sequence containing one disulfide of CP12 from two different species (A. thaliana CP12-2 in CROST1 and the cyanobacterium Anabaena spec. CP12 in CROST2) to create a FRET-based ratiometric sensor linking a variant of mTurquoise and cp173-mVenus as FRET pair. While the sensor response of CROST1 was specific to AtTRX-f1 in vitro, CROST2 interacted with TRX f, m, x, y, z from several tested species. As the ratio of cpmVenus/mTurquoise in CROST sensors was shown to be responsive to pH-changes (decrease of cpmVenus fluorescence with lower pH) in the physiological range of the plastid stroma (Sugiura et al., 2019), the sensor response in vivo will be influenced by pH changes by a decrease of dynamic range. However, in vivo experiments in A. thaliana leaf chloroplasts confirmed a decrease of FRET in the light that was dependent on electron flux through the light reactions and an increase of FRET in the dark in the range of 10 min. The in vivo dynamic sensor responses of CROST1 and CROST2 confirm the dynamics of CBB redox regulation already described by biochemical and gas-exchange methods (Stitt et al., 1989; Yoshida et al., 2014). CROST sensors are thus a promising starting point to further dissect TRX-dependent redox dynamics in vivo in different photosynthetic model species.
Reducing equivalents and ATP
Cellular physiology can only be upheld by the biosynthesis of ATP to maintain a high adenylate charge that in turn drives many metabolic and biosynthetic enzymes. Similarly, reducing equivalents of appropriate potential in the form of NAD(P)H are needed to maintain metabolism and redox homeostasis in an aerobic environment. In plants, phosphorylation of ADP and Pi to ATP is driven by chemiosmosis in the mitochondria and in chloroplasts in the light. Photosynthetic light reactions additionally reduce NADP+ to NADPH, while NAD+ is reduced to NADH mainly in catabolism through glycolysis and the TCA cycle. Thus, ATP (adenylate charge), NADPH/NADP+, and NADH/NAD+ levels differ between the different compartments, depending on light conditions and metabolic mode (Stitt et al., 1982; Wigge et al., 1993; Igamberdiev and Gardeström, 2003; Gardeström and Igamberdiev, 2016). However, the transfer of reducing equivalents and phosphorylation potential between compartments is crucial and relies on metabolite shuttles such as the malate valve (Selinski and Scheibe, 2018) or the triose phosphate shuttle, or direct transport of ATP and ADP via ADP/ATP carriers (Hoefnagel et al., 1998; Haferkamp et al., 2011).
The FRET-based ATeam sensor consisting of CFP and YFP fused to the epsilon subunit of ATP synthase from Bacillus subtilis as sensing domain for MgATP2- (Imamura et al., 2009; Kotera et al., 2010) has already been used in plants (Hatsugai et al., 2012; De Col et al., 2017; Voon et al., 2018, Elsässer et al., 2020). With the help of the sensor-variant ATeam 1.03-nD/nA, mitochondria were confirmed as the major suppliers of ATP to the cytosol using mitochondrial inhibitors and monitoring cytosolic sensor readout, as well as in an ex situ assay using isolated mitochondria (De Col et al., 2017). Dynamic and tissue-specific energy charge of the cytosol was pinpointed by a lower level of MgATP2- in non-green tissues, a decrease after wounding and under low oxygen conditions (De Col et al., 2017; Wagner et al., 2019) as well as by monitoring the restart of mitochondrial function during seed imbibition and germination (Nietzel et al., 2020). MgATP2- concentration in the plastid stroma was found to be lower than in the cytosol and responsive to light, with both pools being independent of each other, except for young seedlings where ATP is imported efficiently into the stroma via chloroplast nucleotide transporters (Voon et al., 2018; Elsässer et al., 2020).
It needs to be emphasized that ATeam is specific for MgATP2- concentration, which is the bioactive form of ATP for most enzymes, but not adenylate charge (i.e. the ratio between ATP, ADP, and AMP). That means that changes in energetic status can only be inferred under the assumption that the total size of the adenylate pool remains stable. That assumption is justified for rapid transitions, such as onset hypoxia within minutes; by contrast, different MgATP2- concentrations between different cells, tissues, or even plants lines may indicate either different adenylate charge or different adenylate pool sizes (or a combination of both). Further the MgATP2--complex requires sufficient Mg2+ availability meaning that the sensor may be an attractive option to probe for intracellular Mg2+ (rather than ATP) changes at limiting Mg2+ availability. To measure ATP/ADP, other sensor variants have been engineered, which show significant pH sensitivity, however, and their use has not been reported for plants so far (Berg et al., 2009; Tantama et al., 2013).
Sensing of reducing equivalents has become accessible through the Thermus aquaticus Rex (T-Rex) as NADH/NAD+ sensing module that has been used to design the cpFP-based sensors Peredox and SoNar (Hung et al., 2011; Zhao et al., 2015) as well as the NADPH sensing SoNar variant iNAP (see Figure 1; Tao et al., 2017). Recently, all three sensor types have been used to probe metabolic flexibility and the linkage of NAD(P)H pools between different compartments (Elsässer et al., 2020; Lim et al., 2020; Steinbeck et al., 2020). Experiments with different light/dark regimes in combination with chemical inhibition of light reactions, respiration, or photorespiration revealed tight and dynamic metabolic coupling between cytosol and organelles. After illumination, stromal NADPH concentration and NADH/NAD+ ratio increased, with a concomitant increase in cytosolic NADH/NAD+ ratios. These observations confirm the functionality of reducing equivalent interconversion and export to the cytosol via metabolite shuttles (e.g. malate/oxaloacetate; Elsässer et al., 2020). Cytosolic NADH/NAD+ ratios were also influenced by catabolic activities: sucrose feeding increased NADH/NAD+ ratios by fueling glycolysis; blocking mitochondrial respiration by inhibitors or low oxygen increased NADH/NAD+ ratios, revealing mitochondria as governors of cytosolic NAD redox homeostasis (Steinbeck et al., 2020).
Similar to cytosolic MgATP2- levels, imaging of whole Arabidopsis seedlings revealed differences in NADH/NAD+ ratios between cells and tissues, most strikingly between shoot and root, with root cells exhibiting particularly high NADH/NAD+ ratios (Steinbeck et al., 2020). At etiolation, the NAD pool in root cells showed a much more oxidized state, indicating profound metabolic reprogramming associated with photomorphogenesis even in the non-aerial organs.
These studies initialized the in vivo imaging of ATP and NAD(P) redox status in plant cells and have already revealed important principles of the in vivo dynamics of metabolic coupling of chloroplasts and mitochondria to the cytosol. This will pave the way for further studies of how photosynthetic and respiratory status influences the whole plant physiology. Surprisingly, leaf cytosolic NADH/NAD+ ratios increased transiently and markedly in response to elicitor-exposure (Steinbeck et al., 2020), indicating that in vivo biosensing allows the exploration of previously unknown modes of crosstalk between central redox metabolism and plant immunity.
Limits and improvement of sensors
Sensor design/in vitro limits
Whether a genetically encoded biosensor is suitable to address a specific biological question needs to be carefully assessed in each individual case considering the inherent biochemical and physicochemical characteristics of the sensor.
The thermodynamic behavior of a sensor is determined by the midpoint potential of the redox-sensitive disulfide as well as the ability to interact with other redox-active proteins. For sensors that do not react with, but reversibly bind the analyte, the equivalent to midpoint potential is the binding/dissociation curve as determined by Km and Hill coefficient (measure of cooperativity when more than one substrate-binding site is present at a sensor).
Binding and dissociation kinetics of ligands determine the kinetic behavior. For the roGFP-based sensors, the relative kinetics of the sensor with different interaction partners (GRX, TRX, H2O2, etc.) sets the specificity of the sensor (Meyer et al., 2007; Nietzel et al., 2019). In addition, the achievable temporal resolution of in vivo measurements is limited by oxidation/reduction rates or on- and off-rates of analyte binding in vivo. While those rates are typically fast and allow detection of physiological changes in the order of seconds or even milliseconds, binding and dissociation rates may be limiting for measuring rapid NAD redox dynamics using Peredox (Steinbeck et al., 2020).
The spectroscopic dynamic range of a sensor designates its maximal possible spectral response, usually determined in an in vitro calibration (Box 2) on purified sensor protein and sets the limits for sensor use. It is important to note that the dynamic range is wavelength-specific meaning that different experimental approaches that use slightly different excitation and/or emission windows will deliver different maximal spectroscopic dynamic ranges (Ugalde et al., 2021).
Sensor characteristics should match the expected range/changes of the coupled redox pool in the used in vivo system (see Figure 1). For instance, EGSH in the plant cytosol leads to nearly complete roGFP2 reduction, limiting its responsiveness to reduction of the EGSH and the monitoring of reductive changes. To cover the EGSH range that is represented in different cell compartments, several roGFP variants have been engineered with different midpoint potentials (Meyer and Dick, 2010; Aller et al., 2013). However, there is currently no sensor with a midpoint potential to match the most reducing (e.g. cytosol; EGSH ∼320 mV) and most oxidizing (e.g. ER ∼208 mV) cell compartments (Schwarzländer et al., 2016). Development of such sensor variants will be desirable for fully dynamic measurements in both directions (oxidation and reductions) in the future. Analogously, analyte-binding sensors, such as the NADH/NAD+ sensor Peredox, can become saturated if their affinity is too high in the context of the physiological concentrations of the bound compounds, NAD+ and NADH. Although the affinity of Peredox has turned out well-matched with the physiological NAD+ and NADH concentrations in the cytosol of Arabidopsis leaves, protein engineering of the ligand-binding pocket by targeted mutagenesis has allowed to adjust the measurement range for situations of highly reduced NAD pools (as observed in root tissues or after specific metabolic stimuli) by lowering NADH binding affinity (Peredox-mCherry DS; Steinbeck et al., 2020). For iNap affinity variants have been generated to cover a large physiological range of NADPH (Tao et al., 2017), and in recent work different affinity variants have been selected as suitable for dynamic measurements in the cytosol (iNap1) and the chloroplast stroma and peroxisomes (iNap4) of Arabidopsis cotyledons (Lim et al., 2020). Since the exact compartment-specific analyte concentrations are often not known or only rough estimations exist based on analytical approaches, empirical in vivo testing of different sensor variants is generally advisable.
Biosensors may respond to different parameters, in addition to the desired parameter, and if those parameters change, in vivo artifacts can arise. A particularly widespread additional sensitivity is that to pH. In vitro assessment is required to estimate robustness of sensor responses. Most GFP-variants are quenched at low pH and circularly permuted fluorescent proteins, in particular, can be responsive to pH changes in the physiological range, due to direct protonation of the exposed chromophore (Schwarzländer et al., 2014). This behavior can lead to misinterpretation of sensor responses in vivo, and – if present – deserves appropriate caution. pH-dependence as a side-specificity in biosensors must be suppressed or compensated for, or carefully considered when interpreting the measurement results. Significant efforts have been made to address this problem and multiple different approaches have been chosen.
The readout of roGFP2 is pH-insensitive, because the pH sensitivity of roGFP2 fluorescence is proportional across its spectrum and is canceled out by ratioing two excitation wavelengths. For HyPer sensors, a corresponding H2O2-insensitive Cys mutant, SypHer (Poburko et al., 2011), needs to be used as parallel pH control while in HyPer7 pH-dependence has been largely overcome by mutagenesis, resulting in pH-robustness of the ratiometric readout (Pak et al., 2020). The pH sensitivity of SoNar and iNap sensors has been addressed by using a control construct that cannot bind to the nucleotides and allows pH correction (Zhao et al., 2015; Tao et al., 2017). The strategy of pH correction has recently also been adopted in plants (Lim et al., 2020). Since a control construct is only available for one of the sensor affinity variants and the correction is only valid for the nucleotide-free state of the sensor, it remains unclear what degree of accuracy the pH correction can achieve. In the case of Peredox, the cpFP variant cpT-Sapphire with a low pKa was chosen and the initial pH-sensitive sensor sequence was mutagenized, and a pH-insensitive variant was selected largely circumventing the danger of pH artifacts (Hung et al., 2011; Steinbeck et al., 2020).
Applying those corrections is critical in plants where metabolic changes often coincide with pH changes (Behera et al., 2018; Wagner et al., 2019; Lim et al., 2020). However, due to identical spectroscopic properties of the control constructs, the control measurements can only be carried out in separate biological material, which increases required sample sizes and may average out physiological meaningful responses. Co-expressing a pH sensor of different colors in the same sample is possible and allows more refined correction and has recently been done for HyPer in tobacco leaves using the pH sensor pHRed (Exposito-Rodriguez et al., 2017). Yet it is rare that both sensors share identical pH responses (spectroscopic dynamic range, pKa) and hence the accurate correction remains technically demanding. Hence, if available, pH-inert sensor variants are generally preferable for in planta analyses.
Limits to meaningful biosensor usage in planta
Sensor responses in vivo can differ from the sensor responses monitored in vitro. An in vivo calibration (Box 2) can determine the in vivo dynamic range that may be affected by interfering autofluorescence, chromatic artifacts, or interactions with the local (protein) environment. While disulfide-based sensors can be calibrated by membrane-permeable oxidants and reductants, the in vivo calibration of metabolite sensors is often not feasible. Metabolite levels, such as MgATP2-, cannot be easily driven to the necessary extremes in all living plant tissues, and the feasibility of permeabilization of the plasma membrane to apply saturating concentrations externally, like often employed in mammalian cells or yeast, remains to be tested case by case (Loro et al., 2016). If an in vivo calibration is not possible because the necessary values lie outside the experimentally reachable range in cells (such as for NAD(P)H/NAD(P)+, MgATP2- or pH) the in vitro calibration of purified sensor protein and/or calibration of the sensor in plant tissue extracts (as recently done for pH calibration by Wagner et al., 2019) can be taken as an empirical reference point for the sensor response in vivo.
In addition, sensor concentrations cannot be controlled in vivo, which introduces challenges regarding data normalization and comparability of intensiometric sensor data, e.g., between different cells or treatments. Sensors exhibiting changes into opposite directions in either the excitation or the emission properties or sensors containing two different fluorophores enable ratiometric analysis as a prerequisite for a robust and quantitative in vivo readout. Ratiometric sensor behavior comes with the advantage of internal normalization, i.e., the signal ratio is independent of the amount of sensor (expression level, field of view) and this principle applies to both chemical dyes and protein-based sensors (Grynkiewicz et al., 1985; Remington, 2011). In Peredox-mCherry, an engineered domain consisting of circular-permuted tSapphire and two NAD(H)-binding TRex domains responds to the NADH/NAD+-ratio via changes in emission intensity without a change in the opposite direction in another emission range (intensiometric sensor; Figure 1). Here, the fusion of the intensiometric sensor module to mCherry enables a ratiometric readout (Hung et al., 2011). Along the same rationale, a versatile Matryoshka-platform for an indifferent reference FP with a long emission wavelength nested in a circular permuted reporter FP with the respective sensor domain has been developed (Ast et al., 2017).
Importantly, sensor use in plants is also limited by the ability to target sensors to all subcellular compartments. While it has often been speculated that sensor-analyte interaction may affect local cell physiology (which is unavoidable to a degree and indeed appears to account for growth phenotypes of Arabidopsis lines expressing specific hormone biosensors in the cytosol; Waadt et al., 2014), the observation of similar biosensor-related phenotypes independent of sensor specificity has raised the hypothesis that the expression of large organelle-targeted fusion protein constructs may compromise organelle import (De Col et al., 2017). Thus, either transgenic lines with developmental phenotypes, such as dwarfism as for mitochondrial ATeam (De Col et al., 2017), or no transgenic lines were obtained, such as for mitochondrial Peredox-mCherry (Steinbeck et al., 2020), mitochondrial and peroxisomal SoNar as well as mitochondrial iNAP (Lim et al., 2020). This illustrates the necessity to carefully document arising phenotypes of transgenic fluorescent sensor lines.
Similarly, redox sensors are not yet available for all compartments. To date, dynamic apoplastic redox sensing is of high interest to plant development, signaling, and stress responses (Franck et al., 2018; Smirnoff and Arnaud, 2019), but no apoplastic redox sensor line is available yet. While this is not a problem of protein targeting, prerequisites for apoplastic redox sensing are robustness of fluorescence signal towards low pH and a sufficiently oxidizing midpoint potential to match apoplastic physiology. Further, dynamic Cys-based redox sensing in the apoplast would rely on the availability of an extracellular reducing system for Cys, the existence of which is unknown to date.
There are additional technical limitations when working with plant-based systems, e.g., combining the readout of sensor fluorescence with illumination regimes to monitor plant physiology under active photosynthesis. To date, this problem has been solved by using custom illumination setups for confocal microscopes or camera-based systems (Voon et al., 2018; Sugiura et al., 2019; Elsässer et al., 2020; Haber et al., 2021; Hipsch et al., 2020; Lim et al., 2020; Müller-Schüssele et al., 2020; Steinbeck et al., 2020) or circumvented by fixing the sensor redox state using cell-permeable thiol-modifying agents (Niemeyer et al., 2020).
A general consideration for the interpretation of biosensor data is that they can only capture the steady state of a parameter, and not the in- and efflux that set this steady state. For instance, a stable MgATP2- concentration may be detected, despite MgATP2- being generated and consumed at high rate. Steady-state changes can only be observed through biosensing if the balance between generation and consumption is shifted and it remains generally unknown whether a change in the steady state is due to a change in the influx, the efflux, or both. In the case of redox sensors, this means that electron flux cannot be measured. Redox sensors, such as those from the roGFP or HyPer families, that are based on the specific oxidation of Cys via a fusion partner, the steady-state oxidation level is read out, which is dependent on both, the rate of oxidation and the rate of rereduction. Thus, the specific oxidation and the capacity of the linked reduction systems (e.g. TRX or GRX/EGSH) determine the sensor steady-state oxidation level in vivo (Box 3).
Concluding remarks
The use of genetically encoded biosensors in plants has already allowed for the observation of hitherto unknown dynamics and the specification of links between redox pools previously know or hypothesized based on data from complementary techniques. This area of plant research is currently in an explorative phase, driving development of new hypotheses and concepts on plant metabolic flexibility and information transfer between subcellular compartments (see Outstanding Questions). After fundamental questions into the characteristics of the sensors applied to plants are answered and the first conclusions regarding the dynamics of energy metabolism of plants are drawn, future reverse genetics studies in transgenic sensor lines will give insights into what molecules are transferred between compartments and how. Further, expansion of sensor uses to more model species and crops is starting. This development and the increasing technical possibilities to gain systemic overview of plant responses to environmental challenges in whole plant camera-based systems (Fichman et al., 2019; Haber et al., 2021; Zandalinas et al., 2020) will certainly pave the way to use biosensors on an organismic level. While sensor use is limited by sensor characteristics and the fact that only a low number of fluorescent readouts can be combined at the same time in the same cell, the field is developing towards combining multiple sensors (Kostyuk et al., 2018; Waadt et al., 2020) and towards in vivo multiplexing of physiological parameters (Nietzel et al., 2019; Wagner et al., 2019), opening the field of systemic dynamic sensing of plant physiology.
OUTSTANDING QUESTIONS
Which redox pools are linked between subcellular compartments?
How dynamic is this linkage in changing environmental/metabolic conditions?
Precisely which molecules are transferred between compartments, and how?
How is this additional layer of communication between cell compartments integrated with known signaling pathways?
What is the interplay between redox pools in different tissues, and at the whole plant level?
Acknowledgments
We are grateful to Dr. Stephan Wagner for providing a structural model of roGFP2.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (DFG) through the Research Training Group GRK 2064 “Water use efficiency and drought stress responses: From Arabidopsis to Barley” (A.J.M., M.S., and S.J.M.-S.), grants ME1567/9-1/2 and SCHW719/7-1 within the Priority Program SPP1710 “Dynamics of thiol-based redox switches in cellular physiology” (A.J.M. and M.S.) and grant MU 4137/1-1 (S.J.M.-S.).
S.J.M.-S., M.S., and A.J.M. wrote the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Stefanie J. Müller-Schüssele (stefanie.mueller@uni-bonn.de).
References
- Albrecht SC, Sobotta MC, Bausewein D, Aller I, Hell R, Dick TP, Meyer AJ (2014) Redesign of genetically encoded biosensors for monitoring mitochondrial redox status in a broad range of model eukaryotes. J Biomol Screen 19: 379–386 [DOI] [PubMed] [Google Scholar]
- Algar WR, Hildebrandt N, Vogel SS, Medintz IL (2019) FRET as a biomolecular research tool – understanding its potential while avoiding pitfalls. Nat Methods 16: 815–829 [DOI] [PubMed] [Google Scholar]
- Aller I, Rouhier N, Meyer AJ (2013) Development of roGFP2-derived redox probes for measurement of the glutathione redox potential in the cytosol of severely glutathione-deficient rml1 seedlings. Front Plant Sci 4: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ast C, Foret J, Oltrogge LM, De Michele R, Kleist TJ, Ho C-H, Frommer WB (2017) Ratiometric Matryoshka biosensors from a nested cassette of green- and orange-emitting fluorescent proteins. Nat Commun 8: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie AL, Falz A-L, Müller-Schüssele SJ, Sparkes I (2020) It started with a kiss: Monitoring organelle interactions and identifying membrane contact site components in plants. Front Plant Sci 11: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird GS, Zacharias DA, Tsien RY (1999) Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci USA 96: 11241–11246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangash SAK, Müller-Schüssele SJ, Solbach D, Jansen M, Fiorani F, Schwarzländer M, Kopriva S, Meyer AJ (2019) Low-glutathione mutants are impaired in growth but do not show an increased sensitivity to moderate water deficit. PloS One 14: e0220589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S, Xu Z, Luoni L, Bonza MC, Doccula FG, De Michelis MI, Morris RJ, Schwarzländer M, Costa A (2018) Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 30: 2704–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S (2006) Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286 [DOI] [PubMed] [Google Scholar]
- Berg J, Hung YP, Yellen G (2009) A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat Methods 6: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach T, Soyk S, Müller C, Hinz G, Hell R, Brandizzi F, Meyer AJ (2009) Non-invasive topology analysis of membrane proteins in the secretory pathway. Plant J 57: 534–541 [DOI] [PubMed] [Google Scholar]
- Brejc K, Sixma TK, Kitts PA, Kain SR, Tsien RY, Ormo M, Remington SJ (1997) Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc Natl Acad Sci USA 94: 2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Kumar AS,, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J, Dawes IW (2012) Redox control of cell proliferation. Trends Cell Biol 22: 592–601 [DOI] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Schiavo FL (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system: Ca2+ stimulates H2O2 scavenging in plant. Plant J 62: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Col V, Fuchs P, Nietzel T, Elsässer M, Voon CP, Candeo A, Seeliger I, Fricker MD, Grefen C, Møller IM, et al. (2017) ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology. eLife 6: e26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Vivancos P, de Simone A, Kiddle G, Foyer CH (2015) Glutathione – linking cell proliferation to oxidative stress. Free Radic Biol Med 89: 1154–1164 [DOI] [PubMed] [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60: 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY (2004) Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 279: 22284–22293 [DOI] [PubMed] [Google Scholar]
- Elsässer M, Feitosa-Araujo E, Lichtenauer S, Wagner S, Fuchs P, Giese J, Kotnik F, Hippler M, Meyer AJ, Maurino VG, et al. (2020) Photosynthetic activity triggers pH and NAD redox signatures across different plant cell compartments. bioRxiv doi: 10.1101/2020.10.31.363051
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM (2017) Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat Commun 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Makar M, Wang MX, Ai H-W (2017) Monitoring thioredoxin redox with a genetically encoded red fluorescent biosensor. Nat Chem Biol 13: 1045–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitosa-Araujo E, de Souza Chaves I, Florian A, da Fonseca-Pereira P, Condori Apfata JA, Heyneke E, Medeiros DB, Pires MV, Mettler-Altmann T, Neuhaus HE, et al. (2020) Downregulation of a mitochondrial NAD+ transporter (NDT2) alters seed production and germination in Arabidopsis. Plant Cell Physiol 61: 897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey V, Wagner R, Braütigam K, Wirtz M, Hell R, Dietzmann A, Leister D, Oelmüller R, Pfannschmidt T (2005) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280: 5318–5328 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Miller G, Mittler R (2019) Whole-plant live imaging of reactive oxygen species. Mol Plant 12: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 133: 21–25 [DOI] [PubMed] [Google Scholar]
- Franck CM, Westermann J, Boisson-Dernier A (2018) Plant malectin-like receptor kinases: From cell wall integrity to immunity and beyond. Annu Rev Plant Biol 69: 301–328 [DOI] [PubMed] [Google Scholar]
- Fricker MD (2016) Quantitative redox imaging software. Antioxid Redox Signal 24: 752–762 [DOI] [PubMed] [Google Scholar]
- Gakière B, Hao J, de Bont L, Pétriacq P, Nunes-Nesi A, Fernie AR (2018) NAD+ biosynthesis and signaling in plants. Crit Rev Plant Sci 37: 259–307 [Google Scholar]
- García-Quirós E, Alché J, de D, Karpinska B, Foyer CH (2020) Glutathione redox state plays a key role in flower development and pollen vigour. J Exp Bot 71: 730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeström P, Igamberdiev AU (2016) The origin of cytosolic ATP in photosynthetic cells. Physiol Plant 157: 367–379 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Thormählen I, Daloso DM, Fernie AR (2017) The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci 22: 249–262 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450 [PubMed] [Google Scholar]
- Gutscher M, Pauleau A-L, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP (2008) Real-time imaging of the intracellular glutathione redox potential. Nat Methods 5: 553–559 [DOI] [PubMed] [Google Scholar]
- Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP (2009) Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem 284: 31532–31540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber Z, Lampl, N., Meyer, A. J., Zelinger, E., Hipsch, M., Rosenwasser S (2021) Resolving diurnal dynamics of the chloroplastic glutathione redox state in Arabidopsis reveals its photosynthetically-derived oxidation. Plant Cell doi: 10.1093/plcell/koab068 [DOI] [PMC free article] [PubMed]
- Haferkamp I, Fernie AR, Neuhaus HE (2011) Adenine nucleotide transport in plants: much more than a mitochondrial issue. Trends Plant Sci 16: 507–515 [DOI] [PubMed] [Google Scholar]
- Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G (2013) Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal 18: 2106–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RE, Winther JR (2009) An introduction to methods for analyzing thiols and disulfides: reactions, reagents, and practical considerations. Anal Biochem 394: 147–158 [DOI] [PubMed] [Google Scholar]
- Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ (2004) Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053 [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Perez Koldenkova V, Imamura H, Noji H, Nagai T (2012) Changes in cytosolic ATP levels and intracellular morphology during bacteria-induced hypersensitive cell death as revealed by real-time fluorescence microscopy imaging. Plant Cell Physiol 53: 1768–1775 [DOI] [PubMed] [Google Scholar]
- Hipsch M, Lampl N, Zelinger E, Barda O, Rosenwasser S (2020) Sensing stress responses in potato with whole-plant redox imaging. bioRxiv doi: 10.1101/2020.11.26.386573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT (1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta BBA - Bioenerg 1366: 235–255 [Google Scholar]
- Hung YP, Albeck JG, Tantama M, Yellen G (2011) Imaging cytosolic NADH-NAD+ redox state with a genetically encoded fluorescent biosensor. Cell Metab 14: 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igamberdiev AU, Gardeström P (2003) Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in mitochondria and cytosol of pea leaves. Biochim Biophys Acta BBA Bioenerg 1606: 117–125 [DOI] [PubMed] [Google Scholar]
- Imamura H, Nhat KPH, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H (2009) Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA 106: 15651–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Schwarzer C, Lally E, Zhang S, Ruzin S, Machen T, Remington SJ, Feldman L (2006) Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol 141: 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NC, Meyer AJ, Bangash SAK, Zheng Z-L, Leustek T (2019) Arabidopsis γ-glutamylcyclotransferase affects glutathione content and root system architecture during sulfur starvation. New Phytol 221: 1387–1397 [DOI] [PubMed] [Google Scholar]
- Jung G, Wiehler J, Zumbusch A (2005) The photophysics of green fluorescent protein: Influence of the key amino acids at positions 65, 203, and 222. Biophys J 88: 1932–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera I, Iwasaki T, Imamura H, Noji H, Nagai T (2010) Reversible dimerization of Aequorea victoria fluorescent proteins increases the dynamic range of FRET-based indicators. ACS Chem Biol 5: 215–222 [DOI] [PubMed] [Google Scholar]
- Kostyuk AI, Panova AS, Bilan DS, Belousov VV (2018) Redox biosensors in a context of multiparameter imaging. Free Radic Biol Med 128: 23–39 [DOI] [PubMed] [Google Scholar]
- Kostyuk AI, Panova AS, Kokova AD, Kotova DA, Maltsev DI, Podgorny OV, Belousov VV, Bilan DS (2020) In vivo imaging with genetically encoded redox biosensors. IJMS 21: 8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-L, Voon CP, Guan X, Yang Y, Gardeström P, Lim BL (2020) In planta study of photosynthesis and photorespiration using NADPH and NADH/NAD+ fluorescent protein sensors. Nat Commun 11: 3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-H, Yaghmour MA, Lee M-H, Gradziel TM, Leveau JHJ, Bostock RM (2020) An roGFP2-based bacterial bioreporter for redox sensing of plant surfaces. Phytopathology 110: 297–308 [DOI] [PubMed] [Google Scholar]
- Loro G, Wagner S, Doccula FG, Behera S, Weinl S, Kudla J, Schwarzländer M, Costa A, Zottini M (2016) Chloroplast-specific in vivo Ca2+ imaging using Yellow Cameleon fluorescent protein sensors reveals organelle-autonomous Ca2+ signatures in the stroma. Plant Physiol 171: 2317–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marri L, Thieulin-Pardo G, Lebrun R, Puppo R, Zaffagnini M, Trost P, Gontero B, Sparla F (2014) CP12-mediated protection of Calvin-Benson cycle enzymes from oxidative stress. Biochimie 97: 228–237 [DOI] [PubMed] [Google Scholar]
- Marri L, Zaffagnini M, Collin V, Issakidis-Bourguet E, Lemaire SD, Pupillo P, Sparla F, Miginiac-Maslow M, Trost P (2009) Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Mol Plant 2: 259–269 [DOI] [PubMed] [Google Scholar]
- Marty L, Bausewein D, Müller C, Bangash SAK, Moseler A, Schwarzländer M, Müller-Schüssele SJ, Zechmann B, Riondet C, Balk J, et al. (2019) Arabidopsis glutathione reductase 2 is indispensable in plastids, while mitochondrial glutathione is safeguarded by additional reduction and transport systems. New Phytol 224: 1569–1584 [DOI] [PubMed] [Google Scholar]
- Marty L, Siala W, Schwarzlander M, Fricker MD, Wirtz M, Sweetlove LJ, Meyer Y, Meyer AJ, Reichheld J-P, Hell R (2009) The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc Natl Acad Sci USA 106: 9109–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan SC, Pasternak M, Cairns N, Kiddle G, Brach T, Jarvis R, Haas F, Nieuwland J, Lim B, Müller C, et al. (2010) Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci U S A 107: 2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot J-P, Hell R (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Dick TP (2010) Fluorescent protein-based redox probes. Antioxid Redox Signal 13: 621–650 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, May MJ, Fricker M (2001) Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J 27: 67–78 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Riemer J, Rouhier N (2019) Oxidative protein folding: State‐of‐the‐art and current avenues of research in plants. New Phytol 221: 1230–1246 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Dreyer A, Ugalde JM, Feitosa-Araujo E, Dietz K-J, Schwarzländer M (2021) Shifting paradigms and novel players in Cys-based redox regulation and ROS signaling in plants – and where to go next. Biol Chem 402: 399–423 [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou J-P, et al. (2010a) Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010b) Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61: 4197–4220 [DOI] [PubMed] [Google Scholar]
- Morgan B, Van Laer K, Owusu TNE, Ezeriņa D, Pastor-Flores D, Amponsah PS, Tursch A, Dick TP (2016) Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat Chem Biol 12: 437–443 [DOI] [PubMed] [Google Scholar]
- Müller‐Schüssele SJ, Wang R, Gütle DD, Romer J, Rodriguez‐Franco M, Scholz M, Buchert F, Lüth VM, Kopriva S, Dörmann P, et al. (2020) Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. Plant J 103: 1140–1154 [DOI] [PubMed] [Google Scholar]
- Niemeyer J, Scheuring D, Oestreicher J, Morgan B, Schroda M (2020) Real-time monitoring of subcellular H2O2 distribution in Chlamydomonas reinhardtii. bioRxiv doi: 10.1101/2020.11.13.382085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietzel T, Elsässer M, Ruberti C, Steinbeck J, Ugalde JM, Fuchs P, Wagner S, Ostermann L, Moseler A, Lemke P, et al. (2019) The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol 221: 1649–1664 [DOI] [PubMed] [Google Scholar]
- Nietzel T, Mostertz J, Ruberti C, Née G, Fuchs P, Wagner S, Moseler A, Müller-Schüssele SJ, Benamar A, Poschet G, et al. (2020) Redox-mediated kick-start of mitochondrial energy metabolism drives resource-efficient seed germination. Proc Natl Acad Sci USA 117: 741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H, Henriksen A, Hansen FG, Winther JR (2001) Shedding light on disulfide bond formation: Engineering a redox switch in green fluorescent protein. EMBO J 20: 5853–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak VV, Ezeriņa D, Lyublinskaya OG, Pedre B, Tyurin-Kuzmin PA, Mishina NM, Thauvin M, Young D, Wahni K, Martínez Gache SA, et al. (2020) Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab 31: 642–653.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sancho J, Tilsner J, Samuels AL, Botella MA, Bayer EM, Rosado A (2016) Stitching organelles: organization and function of specialized membrane contact sites in plants. Trends Cell Biol 26: 705–717 [DOI] [PubMed] [Google Scholar]
- Poburko D, Santo-Domingo J, Demaurex N (2011) Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem 286: 11672–11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Hager J, Gakiere B, Noctor G (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J Exp Bot 59: 135–146 [DOI] [PubMed] [Google Scholar]
- Remington SJ (2011) Green fluorescent protein: A perspective. Protein Sci 20: 1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund CWT, Schroers MG, Wiese J, Facchinelli F, Kurz S, Wilkinson S, Charton L, Wanders RJA, Waterham HR, Weber APM, et al. (2016) The peroxisomal NAD carrier from Arabidopsis imports NAD in exchange with AMP. Plant Physiol 171: 2127–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser S, Rot I, Sollner E, Meyer AJ, Smith Y, Leviatan N, Fluhr R, Friedman H (2011) Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol 156: 185–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedler TA, Thornton JD, Kruse I, Schwarzländer M, Meyer AJ, van Veen HW, Balk J (2014) A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J Biol Chem 289: 23264–23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaubelt D, Queval G, Dong Y, Diaz-Vivancos P, Makgopa ME, Howell G, De Simone A, Bai J, Hannah MA, Foyer CH (2015) Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant Cell Environ 38: 266–279 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Dick TP, Meyer AJ, Morgan B (2016) Dissecting redox biology using fluorescent protein sensors. Antioxid Redox Signal 24: 680–712 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ (2008) Confocal imaging of glutathione redox potential in living plant cells. J Microsc 231: 299–316 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Wagner S, Ermakova YG, Belousov VV, Radi R, Beckman JS, Buettner GR, Demaurex N, Duchen MR, Forman HJ, et al. (2014) The “mitoflash” probe cpYFP does not respond to superoxide. Nature 514: E12–E14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuffi D, Nietzel T, Di Fino LM, Meyer AJ, Lamattina L, Schwarzländer M, Laxalt AM, García-Mata C (2018) Hydrogen sulfide increases production of NADPH oxidase-dependent hydrogen peroxide and phospholipase D-derived phosphatidic acid in guard cell signaling. Plant Physiol 176: 2532–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski J, Scheibe R (2018) Malate valves: old shuttles with new perspectives. Plant Biol 21 (Suppl 1): 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V, Tsednee M, Yeh K-C (2012) Zinc tolerance induced by iron 1 reveals the importance of glutathione in the cross-homeostasis between zinc and iron in Arabidopsis thaliana. Plant J 69: 1006–1017 [DOI] [PubMed] [Google Scholar]
- de Simone A, Hubbard R, de la Torre NV, Velappan Y, Wilson M, Considine MJ, Soppe WJJ, Foyer CH (2017) Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis thaliana. Antioxid Redox Signal 27: 1505–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221: 1197–1214 [DOI] [PubMed] [Google Scholar]
- Souza Chaves I, Feitosa-Araújo E, Florian A, Medeiros DB, Fonseca‐Pereira P, Charton L, Heyneke E, Apfata JAC, Pires MV, Mettler‐Altmann T, et al. (2019) The mitochondrial NAD+ transporter (NDT1) plays important roles in cellular NAD+ homeostasis in Arabidopsis thaliana. Plant J 100: 487–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck J, Fuchs P, Negroni YL, Elsässer M, Lichtenauer S, Stockdreher Y, Feitosa Araújo E, Kroll JB, Niemeier J-O, Humberg C, et al. (2020) In vivo NADH/NAD+ biosensing reveals the dynamics of cytosolic redox metabolism in plants. Plant Cell 32: 3324–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RMcC, Heldt HW (1982) Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol 70: 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Scheibe R, Feil R (1989) Response of photosynthetic electron transport and carbon metabolism to a sudden decrease of irradiance in the saturating or the limiting range. Biochim Biophys Acta BBA Bioenerg 973: 241–249 [Google Scholar]
- Sugiura K, Mihara S, Fu N, Hisabori T (2020) Real-time monitoring of the in vivo redox state transition using the ratiometric redox state sensor protein FROG/B. Proc Natl Acad Sci USA 117: 16019–16026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Yokochi Y, Fu N, Fukaya Y, Yoshida K, Mihara S, Hisabori T (2019) The thioredoxin (Trx) redox state sensor protein can visualize Trx activities in the light/dark response in chloroplasts. J Biol Chem 294: 12091–12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama M, Martínez-François JR, Mongeon R, Yellen G (2013) Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun 4: 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Zhao Y, Chu H, Wang A, Zhu J, Chen X, Zou Y, Shi M, Liu R, Su N, et al. (2017) Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat Methods 14: 720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnka D, Engelke AD, Gellert M, Moseler A, Hossain MF, Lindenberg TT, Pedroletti L, Odermatt B, de Souza JV, Bronowska AK, et al. (2020) Molecular basis for the distinct functions of redox-active and FeS-transferring glutaredoxins. Nat Commun 11: 3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde JM, Fuchs P, Nietzel T, Cutolo E, Vothknecht UC, Holuigue L, Schwarzländer M, Müller-Schüssele SJ, , Meyer AJ (2021) Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments. Plant Physiol 186: 125–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde JM, Fecker L, Schwarzländer M, Müller-Schüssele SJ, Meyer AJ (2020) Live monitoring of ROS-induced cytosolic redox changes with roGFP2-based sensors in plants. bioRxiv doi: 10.1101/2020.12.21.423768 [DOI] [PubMed] [Google Scholar]
- Veech RL, Eggleston LV, Krebs HA (1969) The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J 115: 609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC,, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al. (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon CP, Guan X, Sun Y, Sahu A, Chan MN, Gardeström P, Wagner S, Fuchs P, Nietzel T, Versaw WK, et al. (2018) ATP compartmentation in plastids and cytosol of Arabidopsis thaliana revealed by fluorescent protein sensing. Proc Natl Acad Sci USA 115: E10778–E10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Hitomi K, Nishimura N, Hitomi C, Adams SR, Getzoff ED, Schroeder JI (2014) FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 3: e01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Köster P, Andrés Z, Waadt C, Bradamante G, Lampou K, Kudla J, Schumacher K (2020) Dual-reporting transcriptionally linked genetically encoded fluorescent indicators resolve the spatiotemporal coordination of cytosolic abscisic acid and second messenger dynamics in Arabidopsis. Plant Cell 32: 2582–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Steinbeck J, Fuchs P, Lichtenauer S, Elsässer M, Schippers JHM, Nietzel T, Ruberti C, Van Aken O, Meyer AJ, et al. (2019) Multiparametric real‐time sensing of cytosolic physiology links hypoxia responses to mitochondrial electron transport. New Phytol 224: 1668. [DOI] [PubMed] [Google Scholar]
- Waszczak C, Carmody M, Kangasjärvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69: 209–236 [DOI] [PubMed] [Google Scholar]
- Wedel N, Soll J, Paap BK (1997) CP12 provides a new mode of light regulation of Calvin cycle activity in higher plants. Proc Natl Acad Sci USA 94: 10479–10484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge B, Kromer S, Gardestrom P (1993) The redox levels and subcellular distribution of pyridine nucleotides in illuminated barley leaf protoplasts studied by rapid fractionation. Physiol Plant 88: 10–18 [Google Scholar]
- Wolosiuk RA, Buchanan BB (1977) Thioredoxin and glutathione regulate photosynthesis in chloroplasts. Nature 266: 565–567 [Google Scholar]
- Xing S, Wallmeroth N, Berendzen KW, Grefen C (2016) Techniques for the analysis of protein-protein interactions in vivo. Plant Physiol 171: 727–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Oku M, Akeyama N, Itoyama A, Yurimoto H, Kuge S, Fujiki Y, Sakai Y (2010) A novel fluorescent sensor protein for visualization of redox states in the cytoplasm and in peroxisomes. Mol Cell Biol 30: 3758–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hisabori T (2017) Distinct electron transfer from ferredoxin-thioredoxin reductase to multiple thioredoxin isoforms in chloroplasts. Biochem J 474:1347–1360 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Matsuoka Y, Hara S, Konno H, Hisabori T (2014) Distinct redox behaviors of chloroplast thiol enzymes and their relationships with photosynthetic electron transport in Arabidopsis thaliana. Plant Cell Physiol 55: 1415–1425 [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Mittler R (2020) Vascular bundles mediate systemic reactive oxygen signaling during light stress in Arabidopsis. Plant Cell 32: 3425–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Hommer O, Griesbeck O (2003) Efficiently folding and circularly permuted variants of the Sapphire mutant of GFP. BMC Biotechnol 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang Y-F, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, et al. (2011) An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hu Q, Cheng F, Su N, Wang A, Zou Y, Hu H, Chen X, Zhou H-M, Huang X, et al. (2015) SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab 21: 777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279: 1718–1722 [DOI] [PubMed] [Google Scholar]