Abstract
The wounding-induced reactive oxygen species (ROS) wave is accompanied by a systemic whole-plant redox response in Arabidopsis.
Dear Editor,
Reactive oxygen species (ROS) play a central role in the regulation of plant responses to different developmental signals, abiotic stresses, wounding, and pathogen attack (e.g. Fryer et al., 2003; Chang et al., 2004; Mhamdi and Van Breusegem, 2018; Kollist et al., 2019). ROS alter gene expression in response to many of these stimuli through a change in the redox state of different proteins (Mittler, 2017; Huang et al., 2019). In addition to triggering responses at the tissues directly subjected to abiotic stress, ROS regulate rapid whole-plant systemic responses at tissues not directly subjected to the stress, inducing a state of systemic acquired acclimation (SAA), or systemic wound response (SWR; e.g. Miller et al., 2009; Szechyńska-Hebda et al., 2010; Suzuki et al., 2013; Gilroy et al., 2016; Fichman et al., 2019; Zandalinas et al., 2019, 2020a). The ROS-dependent systemic signaling pathway mediating these processes, termed “the ROS wave” (Miller et al., 2009), is regulated by respiratory burst oxidase homolog proteins and mediated through the vascular bundles of plants (Zandalinas et al., 2020b). However, whether the ROS wave that spreads from the local tissues subjected to stress to the rest of the plant triggers alterations in redox levels, is mostly unknown.
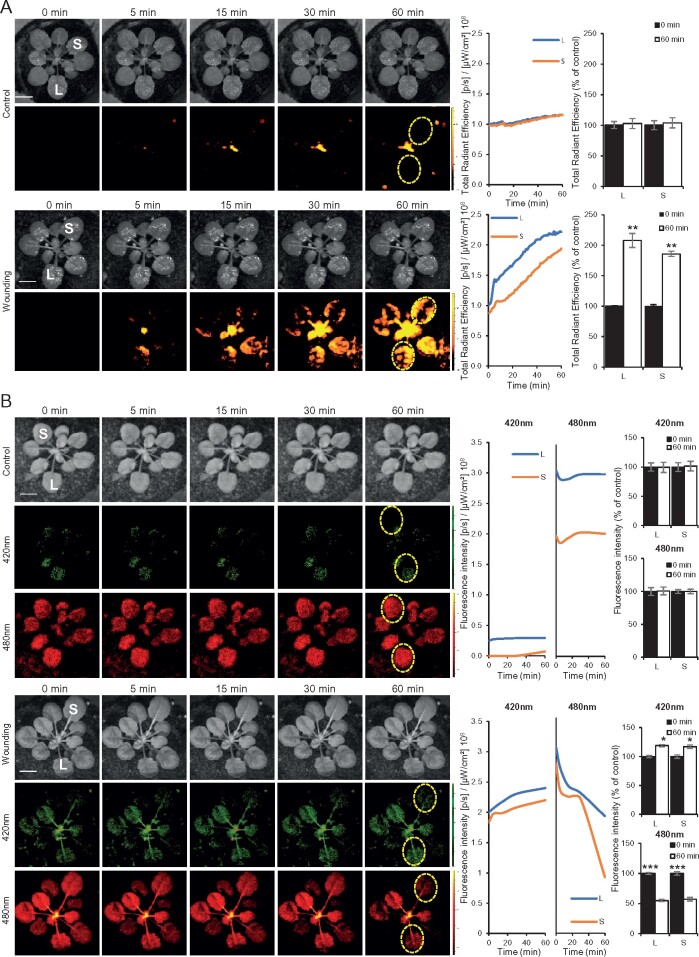
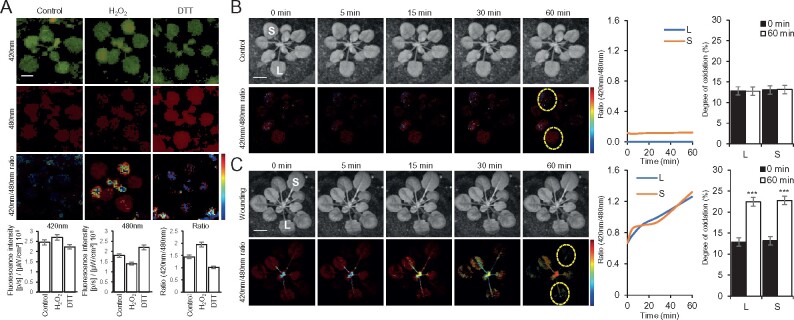
Genetically encoded reporters for cellular and glutathione redox changes and their application in plants have led to major advancements in the study of redox and ROS signaling in recent years (e.g. Hanson et al., 2004; Schwarzländer et al., 2008; Meyer and Dick, 2010; Rosenwasser et al., 2010; Beneloujaephajri et al., 2013; Schwarzländer et al., 2016; Exposito-Rodriguez et al., 2017; Lim et al., 2019; Nietzel et al., 2019; García-Quirós et al., 2020; Haber and Rosenwasser, 2020; Ugalde et al., 2020). These reporters have however been primarily used in conjunction with confocal microscopy, limiting their application to the detection of redox changes in specific cells, tissues, and organs (e.g. local responses to wounding; Beneloujaephajri et al., 2013). In contrast, whole-plant detection of redox changes in mature plants grown in soil has been limited. We recently developed a method for live whole-plant imaging of ROS levels in soil-grown plants and used it to study the ROS wave in wild-type plants and different mutants responding to different stimuli (e.g. Devireddy et al., 2020; Fichman et al., 2020; Zandalinas et al., 2020a). Although changes in ROS levels accompanied the systemic response of plants to different stimuli (Fichman et al., 2019), resulting in metabolic and transcriptomic changes that drove SAA or SWR (e.g. Suzuki et al., 2013; Zandalinas et al., 2019, 2020a), it is not known whether a systemic whole-plant redox response also accompanies this rapid signaling process. To test this possibility, we studied cytosolic reduction–oxidation sensitive green fluorescent protein 1 (roGFP1)-expressing plants (Jiang et al., 2006; Meyer et al., 2007; Schwarzländer et al., 2008; Supplementary Methods) subjected to a local injury stimuli (Figures 1, 2). We chose roGFP1 as a tool to measure glutathione redox levels because of its highly reduced state at the cytosol, which provides a low background in unstressed plants, and wounding as a local stimulus because treatments such as heat or high light stresses may alter roGFP1 activity. Wounding of a single leaf of an Arabidopsis (Arabidopsis thaliana) wild-type (Col-0) plant, grown in soil, resulted in the triggering of a systemic ROS wave response, imaged by the accumulation of oxidized 2′,7′-dichlorofluorescein (DCF) fumigated as H2DCFDA into whole plants (Figure 1, A; Fichman et al., 2019). Wounding of a single leaf of a cytosolic-expressing roGFP1 transgenic plant (Jiang et al., 2006) grown in soil resulted in local and systemic changes in the redox state of the roGFP1 probe, evident by raw changes in excitation/emission at 420/520 nm (for oxidized roGFP1) and excitation/emission of 480/520 nm (for reduced roGFP1) fluorescence (Figure 1, B and Supplementary Movie S1). To quantify these changes and to calculate degree of oxidized roGFP1 in local and systemic tissues in response to wounding, we next determined the levels of 420/520 nm and 480/520 nm fluorescence in whole plants fumigated with 5 mM hydrogen peroxide (H2O2; to induce roGFP1 oxidation), or 5 mM dithiothreitol (DTT; to induce roGFP1 reduction), for 15 min and calculated the ratio of 420/480 nm for these treatments (Figure 2, A), and used these ratios to determine the 420/480 nm ratio and degree of roGFP1 oxidation in local and systemic leaves of the control and wounded plants shown in Figure 1 (Figure 2, B and C, and Supplementary Movies S2, S3). Wounding of a single Arabidopsis leaf resulted in the oxidation of the cytosolic roGFP1 probe in both local and systemic leaves (Figures 1, B, 2, C and Supplementary Movies S1–S3), and these changes corresponded to the changes recorded with the H2DCFDA probe (Figure 1, A). Similar degrees of roGFP oxidation were recently reported for whole-plant imaging of chloroplastic roGFP2-expressing plants subjected to light stress (Haber and Rosenwasser, 2020), further supporting our findings and demonstrating that the degree of increase in roGFP oxidation in whole plants upon enhanced ROS accumulation is in the range of 5%–10% (Figure 2, C).
Figure 1.
Imaging of ROS and redox levels in whole plants subjected to wounding. A, Left: Representative time-lapse images of whole-plant ROS levels (indicated by DCF oxidation) in wild-type Arabidopsis (Col-0) plants, untreated (control, top), or wounded (bottom; applied to the local leaf only). Middle: Representative line graphs showing continuous measurements of ROS levels in local and systemic leaves over the entire course of the experiment (0–60 min; ROIs used to generate the line graphs are indicated with dashed yellow ovals on left). Right: Statistical analysis of ROS levels in local and systemic leaves at 0 and 60 min. B, Representative time-lapse images, and line and bar graphs, of roGFP1 fluorescence of transgenic plants overexpressing the roGFP1 protein at the cytosol (Jiang et al., 2006), untreated (top), or subjected to wounding applied to leaf L only (bottom; similar to the experimental set up shown in A). roGFP1 fluorescence was measured at excitation/emission (ex/em) of 420/520 nm for oxidized roGFP1 (middle, in green; oxidized), and at ex/em of 480/520 nm (bottom, in red; reduced). Experiments were conducted in three biological repeats, each with two technical repeats. Student’s t test, SE, N = 3, *P < 0.05, **P < 0.01, ***P < 0.005. Scale bar indicates 1 cm. ex/em, excitation/emission; L, local; S, systemic; and ROI, region of interest.
Figure 2.
Whole-plant ratiometric fluorescence measurements of cytosolic roGFP1-expressing plants subjected to wounding. A, Representative images of whole-plant roGFP1 florescence (top; 420 and 480 nm excitations are in green and red, respectively), and statistical analysis of 420, 480, and 420/480 nm ratio (bottom) of untreated cytosolic roGFP1 plants (Control), or cytosolic roGFP1 plants subjected to a 15 min fumigation with 5 mM hydrogen peroxide (H2O2), or 5 mM DTT. Ratios of florescence intensities obtained from the H2O2 and DTT treatments were used for calculating the degree of roGFP1 oxidation (similar to Schwarzländer et al., 2008) shown in B. B, Representative time-lapse images (left), line graphs of 420/480 nm ratio for the entire time course (ROIs used to generate the graphs are shown in the images as dotted yellow ovals; middle), and bar graphs showing the degree of roGFP1 oxidation for the 0- and 60-min time points in local (L) and systemic (S) leaves calculated using the normalization range obtained in A, generated for the control untreated plants shown in Figure 1, B, top panels. C, Same as in B, but for the wounded plants shown in Figure 1, B, bottom panels. Experiments were conducted in three biological repeats, each with two technical repeats. Student’s t test, SE, N = 3, *P < 0.05. Scale bar indicates 1 cm. L, local; S, systemic; ROI, region of interest.
Our findings that wounding of a single leaf is accompanied by a systemic wave of ROS production (Fichman et al., 2019; Figure 1, A), as well as a systemic change in cytosolic redox levels (a “redox wave”; Figures 1, B, 2 and Supplementary Movies S1–S3) could therefore provide an initial clue to how the systemic ROS wave response alters the levels of metabolites and transcripts in systemic tissues causing an enhanced state of SAA or SWR (Miller et al., 2009; Szechyńska-Hebda et al., 2010; Suzuki et al., 2013; Gilroy et al., 2016; Fichman et al., 2019; Zandalinas et al., 2019, 2020a, 2020b). Interestingly, the roGFP1 probe remained oxidized for at least 60 min following wounding (Figure 1, B). This finding is in agreement with the systemic ROS wave response to excess light stress remaining “on” for at least 3 h following the initial application of a 10-min high light stress treatment to the local leaf (,Devireddy et al., 2020,). Further studies are of course needed to identify the different regulatory proteins altered by the ROS and redox waves in response to a local stimulus and to tie their altered activity to the systemic response they cause. One potential regulator, recently identified during systemic responses to light stress, is MYB30 (,Fichman et al., 2020,). Because changes in MYB30 protein oxidation alter its DNA binding activity (,Tavares et al., 2014,), MYB30 could be a potential regulator that links changes in redox levels in systemic tissues to transcript expression and SAA (,Fichman et al., 2020,). In addition to demonstrating that the ROS wave is accompanied by a change in redox levels in local and systemic leaves, our study also demonstrates that at least some genetically encoded reporters can be imaged in whole-plants grown in soil, using a sufficiently sensitive apparatus (,Fichman et al., 2019,). This finding opens the way for further studies of ROS and redox signaling in whole plants grown in soil, and perhaps even to large-scale phenotyping studies.
Supplemental data
Supplementary Movie S1. Time-lapse video imaging of raw whole-plant changes in cytosolic roGFP1 fluorescence in response to wounding applied to the local leaf.
Supplementary Movie S2. Time-lapse video imaging of local and systemic changes in ratio of oxidized to reduced cytosolic roGFP1 in a control plant.
Supplementary Movie S3. Time-lapse video imaging of local and systemic changes in ratio of oxidized to reduced cytosolic roGFP1 in a wounded plant.
Supplementary Material
Acknowledgments
The roGFP1 seeds were obtained from the Arabidopsis Biological Resource Center (ABRC). The authors thank Professors Markus Schwarzländer and Andreas J. Meyer for critical comments, guidance, and advice. They apologize to all authors of papers not mentioned in this manuscript due to space limitations.
Funding
This work was supported by funding from the National Science Foundation (MCB-1936590, IOS-1932639, and IOS-1353886) and the University of Missouri.
Conflict of interest statement. The authors declare no conflict of interest.
References
- Beneloujaephajri E, Costa A, L’Haridon F, Métraux JP, Binda M (2013) Production of reactive oxygen species and wound-induced resistance in Arabidopsis thaliana against Botrytis cinereaare preceded and depend on a burst of calcium. BMC Plant Biol 13: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CCC, Ball L, Fryer MJ, Baker NR, Karpinski S, Mullineaux PM (2004) Induction of ascorbate peroxidase 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated with changes in photosynthesis. Plant J 38: 499–511 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Liscum E, Mittler R (2020) Phytochrome B is required for systemic stomatal responses and ROS signaling during light stress in Arabidopsis. Plant Physiol 184: 1563–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM (2017) Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat Commun 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y, Miller G, Mittler R (2019) Whole-plant live imaging of reactive oxygen species. Mol Plant 12: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Zandalinas SI, Sengupta S, Burks D, Myers RJ, Azad R, Mittler R (2020) MYB30 orchestrates systemic reactive oxygen signaling and plant acclimation. Plant Physiol 184: 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33: 691–705 [DOI] [PubMed] [Google Scholar]
- García-Quirós E, Alché JDD, Karpinska B, Foyer CH, Wilson Z (2020) Glutathione redox state plays a key role in flower development and pollen vigour. J Exp Bot 71: 730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171: 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber Z, Rosenwasser S (2020) Resolving the dynamics of photosynthetically produced ROS by high-resolution monitoring of chloroplastic EGSH in Arabidopsis. bioRxiv 2020.03.04.976092
- Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington J (2004) Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053 [DOI] [PubMed] [Google Scholar]
- Huang J, Willems P, Wei B, Tian C, Ferreira RB, Bodra N, Martínez Gache SA, Wahni K, Liu K, Vertommen D, et al. (2019) Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proc Natl Acad Sci U S A 116: 21256–21261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Schwarzer C, Lally E, Zhang S, Ruzin S, Machen T, Remington SJ, Feldman L (2006) Expression and characterization of a redox-sensing green fluorescent protein (reduction–oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol 141: 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R (2019) Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci 24: 25–37 [DOI] [PubMed] [Google Scholar]
- Lim SD, Kim S-H, Gilroy S, Cushman JC, Choi W-G (2019) Quantitative ROS bioreporters: a robust toolkit for studying biological roles of ROS in response to abiotic and biotic stresses. Physiol Plant 165: 356–368 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Dick TP (2010) Fluorescent protein-based redox probes. Antioxidants Redox Signal 13: 621–650 [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145: dev164376. [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R (2017) ROS are good. Trends Plant Sci 22: 11–19 [DOI] [PubMed] [Google Scholar]
- Nietzel T, Elsässer M, Ruberti C, Steinbeck J, Ugalde JM, Fuchs P, Wagner S, Ostermann L, Moseler A, Lemke P, et al. (2019) The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol 221: 1649–1664 [DOI] [PubMed] [Google Scholar]
- Rosenwasser S, Rot I, Meyer AJ, Feldman L, Jiang K, Friedman H (2010) A fluorometer-based method for monitoring oxidation of redox-sensitive GFP (roGFP) during development and extended dark stress. Physiol Plant 138: 493–502 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Dick TP, Meyer AJ, Morgan B (2016) Dissecting redox biology using fluorescent protein sensors. Antioxidants Redox Signal 117: 13810–13820 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ (2008) Confocal imaging of glutathione redox potential in living plant cells. J Microsc 231: 299–316 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal–spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Kruk J, Górecka M, Karpińska B, Karpiński S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares CP, Vernal J, Delena RA, Lamattina L, Cassia R, Terenzi H (2014) S-nitrosylation influences the structure and DNA binding activity of AtMYB30 transcription factor from Arabidopsis thaliana. Biochim Biophys Acta 1844: 810–817 [DOI] [PubMed] [Google Scholar]
- Ugalde JM, Fuchs P, Nietzel T, Cutolo EA, Vothknecht UC, Holuigue L, Schwarzländer M, Müller-Schüssele SJ, Meyer AJ (2020) Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments. bioRxiv 2020.07.20.212670 [DOI] [PMC free article] [PubMed]
- Zandalinas SI, Fichman Y, Devireddy AR, Sengupta S, Azad RK, Mittler R (2020a) Systemic signaling during abiotic stress combination in plants. Proc Natl Acad Sci U S A 117: 13810–13820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Mittler R (2020b) Vascular bundles mediate systemic reactive oxygen signaling during light stress in Arabidopsis. Plant Cell 32: 3425–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Sengupta S, Burks D, Azad RK, Mittler R (2019) Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J 98: 126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.