Abstract
This cross-sectional study aims to investigate the relationship between the simplified olecranon, simplified digital, and distal radius and ulna (DRU) classifications, and whether they can aid in more comprehensive maturity assessment together. Left hand and wrist and lateral elbow radiographs from pediatric patients were assessed using the three skeletal maturity indices. The association between maturity indices was investigated using Goodman and Kruskal’s gamma, and by mapping of individual grades based on chronological age. Specific maturity grades, at which peak height velocity (PHV) occurs as previously identified, were based upon to explore how the three systems interact. A total of 114 patients (63.2% girls) were studied. Correlations and associations between the three maturity parameters were significant (all at P < 0.001). Mapping revealed uneven spans and coverage of different periods by each index. Olecranon stage 1 coincided with R3 (for girls), R4 (for boys), U3, and SS1. Olecranon stage 5 occurred as early as R7, U6, and SS4. Upon elbow fusion, the simplified digital (SS5–SS8) and DRU (R8–R11 and U7–U9) classifications can be used for assessment until maturity. The inter-relationship of the simplified hand, wrist, and olecranon methods indicates their combined use. DRU grades can be used in growth periods which are less well covered. Prepubertal and growth acceleration phase of pubertal growth spurt can best be assessed by both the simplified olecranon (stages 1–3) and DRU classifications (R1–R5 and U1–U4). All three indices are required during PHV. For post-PHV, DRU (R8–R11 and U7–U9) and simplified digital method (SS5–SS8) complement each other for assessment until skeletal maturity.
Keywords: bone, distal radius and ulna, olecranon, skeletal maturity
Introduction
Assessment of skeletal maturity is essential in pediatric orthopedics when making decisions for timely initiation and completion of treatments; this is especially so for growth-modulated interventions of the lower limbs and spine [1,2]. Examples include decision-making for limb lengthening surgery [3], pediatric anterior cruciate ligament reconstruction [4], and the timing of brace initiation and weaning for idiopathic scoliosis [5–7]. Growth potential is therefore a key parameter for predicting disease progression and for treatment planning [3,8–11].
Various skeletal maturity indices are available for the assessment of growth. The Risser sign [12], based on the ossification of the iliac apophysis, has been used as a prognostic indication for evaluating growth [13], but is often criticized for its poor sensitivity during the acceleration phase of pubertal growth [14]. Another useful radiological guide for the pubertal growth period is Sauvegrain’s method and its contemporary simplified olecranon method [15,16]. The hand and wrist [17] are also very useful for bone age assessment, and classical methods such as the Greulich and Pyle method [18], and the Tanner and Whitehouse method, are widely used for assessment of skeletal maturity [19,20]. However, due to their complexity and flaws in sensitivity, simplified maturity parameters are becoming more popular. Of note are the distal radius and ulna (DRU) classification [21] and the simplified digital method [10] which are widely used in Asia and in North America, respectively. Tanner–Whitehouse III descriptors were used to develop the simplified digital method.
Most clinicians utilize more than one maturity parameter to better delineate and pinpoint the pubertal growth period. The use of multiple parameters is likely to provide more accurate growth prediction and treatment planning. As compared to the classical methods, simplified maturity indices may allow bone age assessment to be done faster with comparable accuracy. The commonly used simplified olecranon method, simplified digital method, and the DRU classification have each tested with the capabilities to predict peak height velocity (PHV) accurately [22–24], but how these three maturity parameters can be applied in combination have not been explored. Therefore, this study aims to investigate the relationship between the simplified olecranon method, the simplified digital method, and the DRU classification, and to explore how the three indices can be used in conjunction to provide a more comprehensive assessment of skeletal maturity.
Materials and methods
Study design and patient recruitment
This was a cross-sectional study of pediatric patients attending a tertiary orthopedic pediatric clinic during the period of 2011–2012. Inclusion criteria were pediatric patients who presented with: (1) orthopedic conditions requiring skeletal maturity assessment including idiopathic limb-length discrepancies, (2) idiopathic scoliosis, or (3) sequelae of epiphyseal trauma of the lower limb. Standardized anteroposterior left hand and wrist radiographs and lateral elbow radiographs must be available for assessment. Patients with congenital disorders, or a history of systemic illnesses or endocrinopathies were excluded. Patient profiles including gender and date of birth were recorded. Ethics approval was obtained from the local ethics committee and parental informed consent was gained.
Radiographic assessment of skeletal maturity
Radiographic assessment of skeletal maturity was performed by a total of nine raters [three raters per group] based on their years of experience in using radiographic maturity parameters: experienced (≥10 years), intermediate (5 to <10 years), and beginners (<5 years). Each rater performed skeletal maturity assessments independently using the simplified olecranon method, simplified digital method, and DRU classification, with the definition of each grade given and any corresponding images available as found in their previous publications [22,23,25]. The simplified olecranon method categorizes radiographic appearance of the apophysis at the olecranon into five stages: two ossification nuclei, a half-moon image, a rectangular shape, the beginning of fusion, and complete fusion [23]. The DRU classification consists of 11 radius grades (R1–R11) and nine ulnar grades (U1–U9) [25], whereas the simplified digital method consists of eight stages (stages 1–8, hereafter referred to as SS1–SS8) [22]. All radiographs were deidentified, so that raters were blind to all patient particulars and demographics. The images were distributed by an independent investigator who was not a rater, and the image order was randomized for each assessment. Two assessments were performed by each rater, with the second assessment completed four weeks after initial reading.
Statistical analysis
Descriptive statistics including chronological age were presented as mean ± standard deviation (SD), whereas range and median scores were presented for maturity grading. Since gender was blinded to avoid bias, the morphological sequence of the five distinct stages of olecranon development was termed as grades 1–5, and allowed for half grades. These grades were then converted to the corresponding bone age per gender after measurements.
The reliability and reproducibility of using these skeletal maturity indices together were assessed by the intraclass correlation for the first and second assessment. The associations of the three skeletal maturity indices were evaluated according to the rater’s experience level, using Goodman and Kruskal’s gamma with Bonferroni correction. A gamma coefficient of 0.00–0.24 indicates no relationship between two maturity indices, with 0.25–0.49, 0.50–0.74, and 0.75–1.00 indicating the respective weak, moderate, and strong relationships [26,27]. The use of Goodman and Kruskal’s gamma, G, a proportional reduction in error measure [28], allowed the estimation of the effect that one grading system had on the prediction of another.
Further comparison between skeletal maturity indices was performed by the mapping of each maturity index against chronological age, through calculating the mean age for each grade. Mapping utilized the chronological age as a common scale as it was demonstrated to have a positive, linear relationship with skeletal age for both genders [14], and it was a common variable for the indices.
With reference to the existing knowledge of each skeletal maturity index, the PHV was used as the important growth landmark to formulate a scheme of utilizing the three skeletal maturity indices in combination. Previous studies have suggested that PHV occurs at olecranon stage 4 [29], R6 U5 [24], and SS3 [30], whereas the end of the PHV is marked by the complete olecranon apophyseal fusion at stage 5 [31]. These pubertal growth time-points are used to mark the relevance of each maturity parameter. This allows us to identify the grades which are available for maturity assessment prior to reaching PHV and for the decelerating growth phase post-PHV.
Data analyses were conducted using SPSS Windows 23.0 (IBM SPSS Inc., Chicago, Illinois, USA). A P value of less than 0.05 was considered statistically significant.
Results
A total of 114 patients were recruited, including 72 girls (63.2%) and 42 boys (36.8%). The mean chronological age for girls and boys was 13.1 ± 1.6 years (ranging from 9.9 to 17.0 years) and 14.5 ± 1.3 years (ranging from 11.0 to 17.4 years), respectively. Table 1 shows the skeletal maturity profile of the study population, with skeletal maturity indices graded with no more than one grade discrepancy among the three experience groups, except the simplified digital method graded by beginners for boys (median grade of SS6 rated by beginners versus SS4 rated by intermediate and experienced raters).
Table 1.
Average ratings of the maturity parameters according to raters’ level of experience
| Maturity index | Overall (n = 114) | Boys (n = 42) | Girls (n = 72) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Experienced | Intermediate | Beginner | Experienced | Intermediate | Beginner | Experienced | Intermediate | Beginner | |
| Skeletal age, years, mean ± SD | |||||||||
| Olecranon | 13.3 ± 1.1 | 13.3 ± 1.0 | 13.3 ± 1.1 | 14.4 ± 0.7 | 14.4 ± 0.7 | 14.3 ± 0.7 | 12.6 ± 0.6 | 12.6 ± 0.5 | 12.6 ± 0.6 |
| Median grade, minimum to maximum, range | |||||||||
| Olecranon with coded grading | 5 1–5, 4 |
5 1–5, 4 |
5 1–5, 4 |
4 1–5, 4 |
4 1–5, 4 |
4 1–5, 4 |
5 1–5, 4 |
5 1–5, 4 |
5 1–5, 4 |
| Simplified digital method | 5 1–8, 7 |
5 1–8, 7 |
6 1–8, 7 |
4 1–8, 7 |
4 1–8, 7 |
6 1–8, 7 |
5 1–8, 7 |
6 1–8, 7 |
6 1–8, 7 |
| DRU classification | |||||||||
| Radius grade | 8 4–11, 7 |
8 4–11, 7 |
8 4–11, 7 |
7 5–11, 6 |
8 4–11, 7 |
8 5–11, 6 |
8 4–11, 7 |
8 4–11, 7 |
9 4–11, 7 |
| Ulnar grade | 6 3–9, 6 |
7 4–9, 5 |
7 3–9, 6 |
6 4–9, 5 |
6 4–9, 5 |
7 3–9, 6 |
6 3–9, 6 |
7 4–9, 5 |
7 4–9, 5 |
DRU, distal radius and ulna; SD: standard deviation.
Table 2 presents the reliability of skeletal maturity assessment using the three grading systems, which demonstrated significant correlations (all at P < 0.001) with each other (given that raters’ experience was controlled). Olecranon was strongly correlated with DRU and the digital method. Individual radius and ulnar grades were strongly and moderately correlated to the digital method. Good reproducibility was indicated with comparable gamma coefficients for both first and second assessments. In accordance with the raters’ experience level, strong overall correlations between indices were found, except beginners who could only achieve moderate correlations between radius and ulna with digital grades, and for ulna and olecranon stages for boys (Table 2). The three maturity indices were found to have strong associations (all at P < 0.001), with G being 0.839, 0.812, and 0.778 between the olecranon and the digital, radius and ulnar grades, respectively. The associations were relatively weaker between the digital method and the radius grades (G: 0.772), and with the ulnar grades (G: 0.743).
Table 2.
Correlation between the maturity parameters
| Between | Assessmenta | Boys | Girls | ||
|---|---|---|---|---|---|
| G | P value | G | P value | ||
| Conditional correlationb | |||||
| Radius and Olecranon | First | 0.81 | <0.001c | 0.83 | <0.001c |
| Second | 0.82 | <0.001c | 0.85 | <0.001c | |
| Overall | 0.81 | <0.001c | 0.84 | <0.001c | |
| Radius and Digital | First | 0.77 | <0.001c | 0.74 | <0.001c |
| Second | 0.74 | <0.001c | 0.79 | <0.001c | |
| Overall | 0.76 | <0.001c | 0.76 | <0.001c | |
| Ulna and Olecranon | First | 0.78 | <0.001c | 0.82 | <0.001c |
| Second | 0.81 | <0.001c | 0.84 | <0.001c | |
| Overall | 0.79 | <0.001c | 0.83 | <0.001c | |
| Ulna and Digital | First | 0.76 | <0.001c | 0.73 | <0.001c |
| Second | 0.76 | <0.001c | 0.77 | <0.001c | |
| Overall | 0.75 | <0.001c | 0.74 | <0.001c | |
| Olecranon and Digital | First | 0.84 | <0.001c | 0.85 | <0.001c |
| Second | 0.79 | <0.001c | 0.87 | <0.001c | |
| Overall | 0.81 | <0.001c | 0.86 | <0.001c | |
| Correlation according to level of experience for overall (first and second) assessment | |||||
| Radius and Olecranon | Beginner | 0.78 | <0.001c | 0.77 | <0.001c |
| Intermediate | 0.89 | <0.001c | 0.88 | <0.001c | |
| Experienced | 0.76 | <0.001c | 0.88 | <0.001c | |
| Radius and Digital | Beginner | 0.64 | <0.001c | 0.62 | <0.001c |
| Intermediate | 0.89 | <0.001c | 0.88 | <0.001c | |
| Experienced | 0.74 | <0.001c | 0.78 | <0.001c | |
| Ulna and Olecranon | Beginner | 0.68 | <0.001c | 0.75 | <0.001c |
| Intermediate | 0.89 | <0.001c | 0.87 | <0.001c | |
| Experienced | 0.82 | <0.001c | 0.88 | <0.001c | |
| Ulna and Digital | Beginner | 0.63 | <0.001c | 0.60 | <0.001c |
| Intermediate | 0.85 | <0.001c | 0.84 | <0.001c | |
| Experienced | 0.80 | <0.001c | 0.78 | <0.001c | |
| Olecranon and Digital | Beginner | 0.74 | <0.001c | 0.77 | <0.001c |
| Intermediate | 0.86 | <0.001c | 0.90 | <0.001c | |
| Experienced | 0.84 | <0.001c | 0.90 | <0.001c | |
G, gamma’s coefficient.
Using Goodman and Kruskal’s gamma with Bonferroni correction.
When experience of the rater was introduced as a control.
Statistical significance at P < 0.05.
In addition, Table 3 provides the distribution and the rate at which each specific grade of the three indices coincides. For the third olecranon stage (12.0 years for girls and 14.0 years for boys), there was no corresponding individual radius, ulnar, or digital grades with ≥25% prevalence. For SS4, no ulnar grade held for ≥25% of the girl cases, whereas no DRU grades held for ≥25% for boys. For SS5, it did not correspond to any DRU grade for ≥15% of the cases for both genders.
Table 3.
Three-way cross-tabulation of prevalence rates, expressed as percentages in rows, for specific grades of the three skeletal maturity indices in the study population
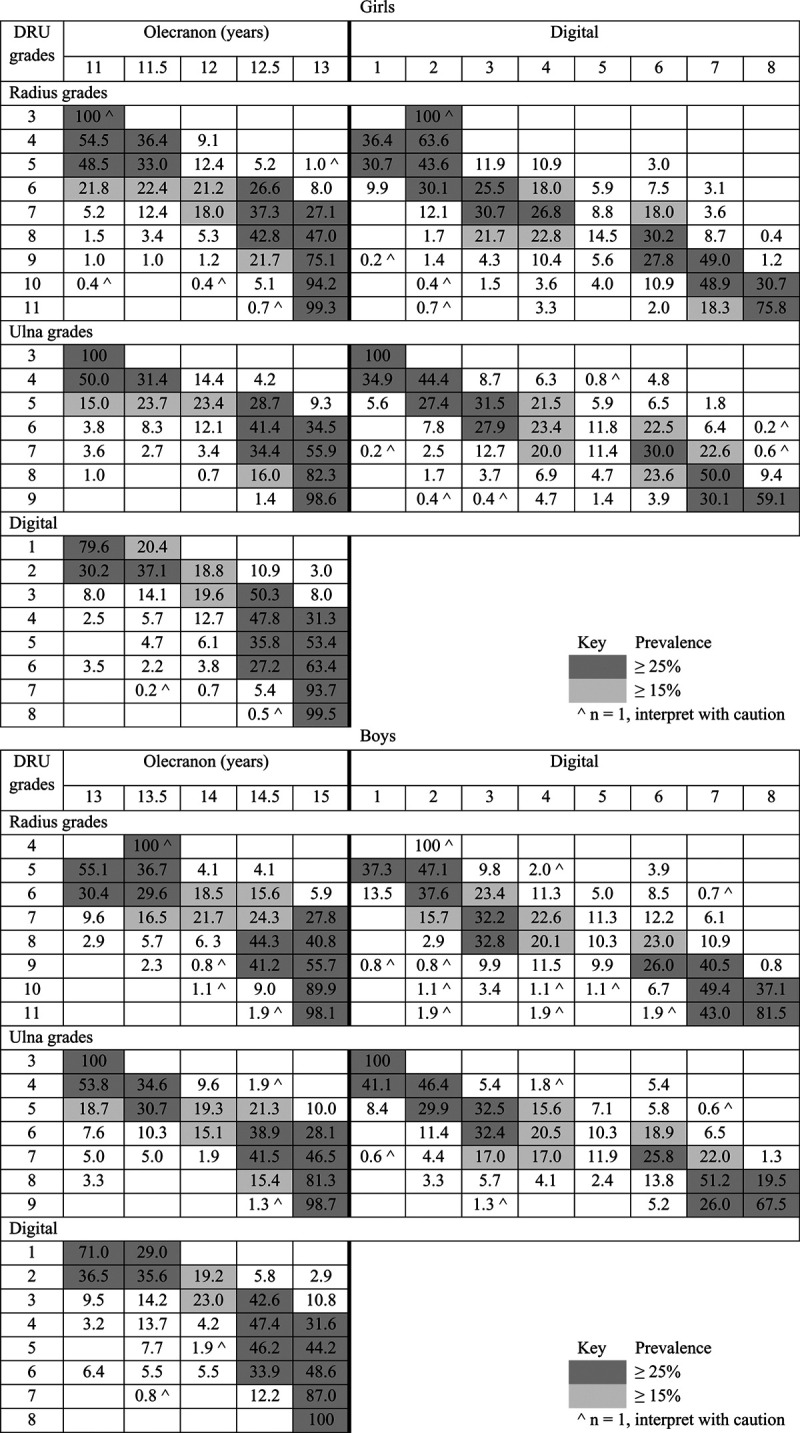
Mapping of each maturity grade on the common scale of chronological age (mean age, Table 4) revealed uneven spans between specific grades of each index (Figs. 1 and 2), particularly for the SS1 and SS2, SS4–SS6 for girls, and SS4–SS6, R4, R9, and R10, and U4 and U5 for boys. The DRU grades depicted the lowest and the highest mean age for girls at 10.1 and 15.2 years (Fig. 1), and boys were graded as early as U3 at 11.8 years (Fig. 2).
Table 4.
Skeletal maturity indices with mean age, age range, and frequency for each individual grade
| Girls (n = 1296 assessments from nine raters and with two assessments per rater) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olecranon | Mean age (years) | Range | % | Radius | Mean age (years) | Range | % | Ulna | Mean age (years) | Range | % | Digital | Mean age (years) | Range | % |
| 1 | 10.5 | 1.4 | 4.8 | R3* | 11.3 | 0.0 | 0.1 | U3 | 10.1 | 0.5 | 0.2 | SS1 | 10.2 | 1.1 | 2.2 |
| 1.5 | 11.1 | 0.3 | 0.3 | R4 | 10.9 | 1.4 | 0.8 | U4 | 10.8 | 3.4 | 5.4 | SS2 | 11.3 | 4.4 | 7.6 |
| 2 | 11.2 | 2.3 | 5.3 | R5 | 10.9 | 3.2 | 3.9 | U5 | 11.7 | 4.5 | 14.4 | SS3 | 11.8 | 4.0 | 12.7 |
| 2.5 | 11.1 | 1.9 | 0.3 | R6 | 11.7 | 4.6 | 14.0 | U6 | 12.4 | 4.3 | 18.4 | SS4 | 12.5 | 5.4 | 17.1 |
| 3 | 11.4 | 3.1 | 7.2 | R7 | 12.2 | 4.6 | 14.7 | U7 | 13.1 | 6.8 | 24.3 | SS5 | 12.8 | 5.8 | 7.4 |
| 3.5 | 11.6 | 2.3 | 0.5 | R8 | 12.8 | 6.6 | 22.8 | U8 | 13.8 | 6.6 | 21.8 | SS6 | 13.0 | 6.6 | 20.3 |
| 4 | 12.2 | 5.2 | 22.2 | R9 | 13.7 | 6.6 | 21.8 | U9 | 14.9 | 4.6 | 15.6 | SS7 | 14.2 | 6.0 | 22.8 |
| 4.5 | 12.4 | 3.2 | 2.4 | R10 | 14.4 | 6.8 | 14.3 | SS8 | 15.1 | 4.6 | 10.0 | ||||
| 5 | 14.1 | 5.6 | 56.9 | R11 | 15.2 | 4.3 | 7.6 | ||||||||
| Boys (n = 756 assessments from nine raters and with two assessments per rater) | |||||||||||||||
| Olecranon | Mean age (years) | Range | % | Radius | Mean age (years) | Range | % | Ulna | Mean age (years) | Range | % | Digital | Mean age (years) | Range | % |
| 1 | 13.0 | 3.5 | 11.0 | R4* | 13.3 | 0.0 | 0.1 | U3 | 11.8 | 0.0 | 0.2 | SS1 | 12.6 | 2.9 | 5.2 |
| 1.5 | 13.3 | 0.0 | 1.2 | R5 | 12.8 | 4.4 | 6.7 | U4 | 12.6 | 4.4 | 7.4 | SS2 | 13.2 | 4.9 | 0.1 |
| 2 | 13.5 | 4.2 | 10.8 | R6 | 13.4 | 5.2 | 18.7 | U5 | 13.7 | 5.2 | 20.4 | SS3 | 14.2 | 4.6 | 13.6 |
| 2.5 | 12.7 | 2.9 | 0.4 | R7 | 14.2 | 5.2 | 15.2 | U6 | 14.3 | 5.2 | 24.5 | SS4 | 14.1 | 5.0 | 19.6 |
| 3 | 13.5 | 4.4 | 8.4 | R8 | 14.6 | 3.0 | 23.0 | U7 | 14.7 | 3.0 | 21.0 | SS5 | 14.6 | 2.6 | 12.6 |
| 3.5 | 14.2 | 0.0 | 1.1 | R9 | 15.5 | 3.5 | 17.3 | U8 | 15.3 | 4.2 | 16.3 | SS6 | 14.7 | 4.6 | 6.9 |
| 4 | 14.4 | 5.0 | 24.2 | R10 | 15.4 | 3.8 | 11.8 | U9 | 16.0 | 3.1 | 10.2 | SS7 | 15.5 | 3.3 | 14.4 |
| 4.5 | 14.7 | 1.8 | 3.3 | R11 | 16.3 | 2.6 | 7.1 | SS8 | 16.1 | 2.8 | 17.3 | ||||
| 5 | 15.6 | 4.2 | 39.6 | ||||||||||||
%, frequency.
To be interpreted with caution as n = 1 for that particular grade/mean age.
Fig. 1.
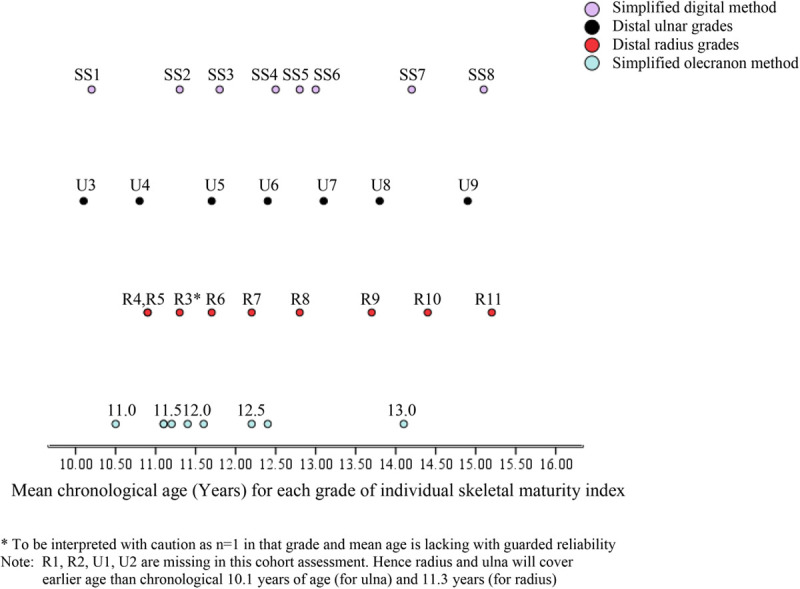
Mapping of DRU, simplified olecranon, and simplified digital methods by chronological age (girls). DRU, distal radius and ulna.
Fig. 2.
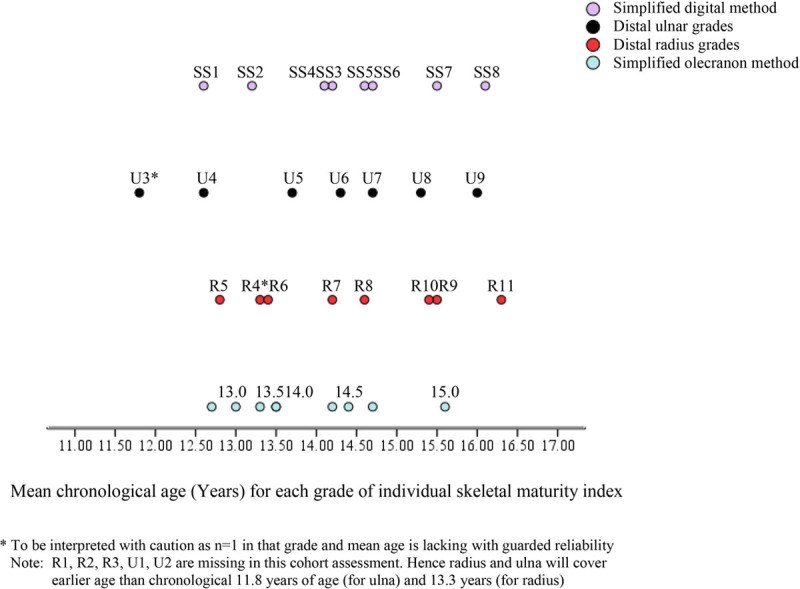
Mapping of DRU, simplified olecranon and simplified digital methods by chronological age (boys). DRU, distal radius and ulna.
Based on the relationship between PHV and the skeletal maturity system each established in the current literature, the concurrently occurring maturity grades were placed into the context of growth stages. With the uneven span between grades as revealed through mapping and the low rate of corresponding specific grades among maturity indices, a scheme of the recommended combined use of these maturity indices was developed (Fig. 3).
Fig. 3.
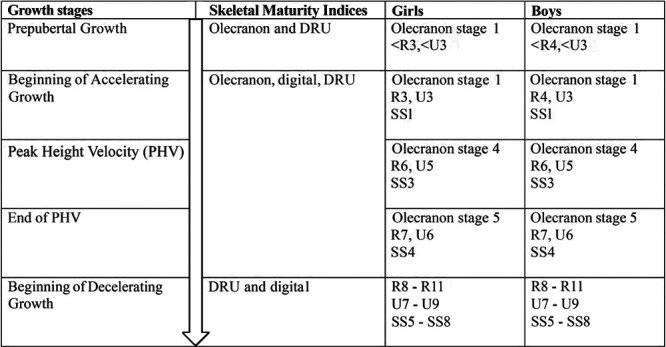
Summary of the recommended combinations of skeletal maturity grading with key stages of growth.
Discussion
Each skeletal maturity index has its characteristic and sensitivity for a particular period of pubertal growth. As any one maturity index cannot cover the entire growth period comprehensively, multiple skeletal maturity measures are frequently employed for more accurate assessment. The Greulich and Pyle method, the Tanner and Whitehouse method, and the Risser sign are commonly used together for pediatric patients in daily practice worldwide [32]. Dimeglio previously found that Risser 0 can be subdivided into two periods with open or closed triradiate cartilage, and the Risser sign can be split according to the apophyseal closure of the greater trochanter. For the Greulich and Pyle method, it was found to correlated well with the Sauvegrain’s method which can complement the Greulich and Pyle Atlas, especially for assessing bone age at 6-month intervals [33]. The Greulich and Pyle method and the Risser sign can be very useful when clinicians understand their limitations [31]. However, simplified maturity indices have increased popularity as they allow faster and equally precise bone age estimation. This study investigates specifically the relationships between the simplified olecranon, simplified digital and DRU classifications, and how they can be used together. Our findings reveal that these three skeletal maturity indices can complement each other. The PHV can be assessed with the combination of the three systems. Importantly, the simplified olecranon method and the DRU classification can be used to optimize maturity assessment of the growth acceleration phase, whereas the DRU classification and simplified digital method can be used in conjunction for assessment during the decelerating growth period until skeletal maturity.
It is essential to examine how the three skeletal maturity indices interact in order to understand whether and how their combined use can provide a more comprehensive coverage of pubertal growth. An example of such interaction is suggested by Canavese et al. [34] for the simplified olecranon method, which is capable of providing accurate information during the acceleration phase of the pubertal growth spurt prior to Risser 1, as it complements Risser 0 and triradiate cartilage fusion [23]. In our study, the first olecranon stage coincided predominantly with R3 (girls), R4 (boys), U3, and SS1 (both genders). DRU was the only classification with several early grades (R1, R2, R3 for boys, and U1 and U2) not represented in this cohort. As the youngest age in this study was 9.9 years for girls and 11.0 years for boys, the DRU classification can further identify those maturity statuses in even younger patients who cannot be assessed using the simplified olecranon and digital methods (Fig. 3). In fact, this corresponds to the period prior to the pubertal growth spurt, which usually occurs between 9.0 and 10.0 years of age for girls and between 11.0 and 12.0 years of age for boys [35]. Thus, the olecranon and DRU systems can be used in conjunction for effective coverage of both prepubertal and acceleration phases of pubertal growth. For example, the olecranon can be a modifier of DRU and further classifies R6 U5 when the olecranon is beginning to fuse or is completing fusion. This may not be possible with the digital grades, as the coverage of SS1–SS3 is too wide.
All three maturity indices overlap for the PHV. Previous studies have demonstrated PHV strongly correlated with each index at olecranon stage 4 [29], R6 U5 [24], and SS3 [30]. Olecranon apophyseal fusion completes at the end of PHV [31]. At this stage, the simplified olecranon method has reached its limit and it is impossible to estimate how much time has elapsed since complete fusion and PHV (Fig. 4). Despite the simplified olecranon method being the simplest scheme with the least number of grades, it cannot be used for assessing later growth stages. Therefore, it is crucial to utilize other maturity indices for further growth assessment (especially near the completion of olecranon fusion), so that the period of PHV until its end can be refined. For instance, if olecranon stage 4 is presented with the DRU advancing from R6 to R7 or U5 to U6, assessment can be continued without disruption beyond PHV to the end of growth. It is worth noting that the time span for SS2 advancing to SS3 is about one year [10]; thus, the DRU may have an advantage of more precise assessments when used in conjunction with the olecranon for patients entering PHV. However, for the end of PHV, the DRU and digital grades are more useful.
Fig. 4.
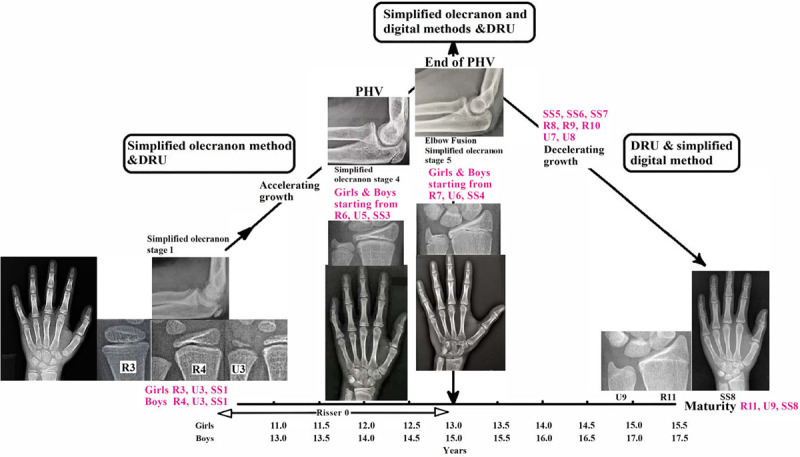
Schematic diagram of the interaction of DRU classification, simplified olecranon and simplified digital methods over the prepubertal, accelerating growth, PHV, immediately post-PHV, and decelerating growth. DRU, distal radius and ulna; PHV, peak height velocity.
For the deceleration growth phase post-PHV, the use of both DRU and digital grades is recommended. The first appearance of olecranon stage 5 for both genders was found as early as R7, U6, and SS4 (which occurred in >25% of the cases). Once the olecranon apophysis is fused, the use of SS5–SS8, together with R8–R11 and U7–U9, can provide maturity assessment immediately post-PHV until skeletal maturity (Fig. 4). Digital stages were previously found to be more useful after the pubertal growth spurt [34]. Closer examination reveals that digital grades have an uneven span of SS1–SS3, a clustering of SS4–SS6, with a wide span between SS6 and SS7 for both genders (Figs. 1 and 2). This is in agreement with existing findings of digital stages at irregular, nonlinear intervals [10,36]. SS4–SS6 progresses rapidly for each stage within 6 months of the previous stage or within one-year span, as compared to the long span between SS2 and SS3 [10,36]. Therefore, combining the use of the DRU and digital methods is recommended. SS7 corresponds to four radius (R8–R11) and three ulnar (U7–U9) grades, whereas SS8 corresponds to R10, R11, U8 (boys), or U9. Not only can these two systems complement each other, it suggests that the ulnar appearance further refines the radius grading.
The main limitation of this study is the unequal sample size for girls and boys. Nevertheless, the need to examine genders separately may not be necessary as the growth speed of individuals is similar at PHV regardless of gender [30,36]. Due to its cross-sectional nature, skeletal maturity indices were examined independent of growth parameters such as standing and sitting height. It is therefore necessary for future validation and examination of accuracy and precision of the combined use of these skeletal maturity indices based on prospectively collected data. The validity of using these simplified skeletal maturity parameters should also be studied in the context of the decision-making process for growth-sensitive interventions at specific combination of maturity grades. Moreover, application of the combined use of these three maturity indices needs to be investigated, particularly for patients with specific pathology of limb lengthening or growth plates in the long bones.
In clinical practice, clinicians use more than one skeletal maturity index, with repeated measurement and data such as secondary sexual characteristics and bodily growth, to improve accuracy of growth assessment for treatment decision-making. This study provides practical recommendations on how the three skeletal maturity measures of interests: the simplified hand, wrist, and olecranon maturity systems can complement each other. Insights are shared on how to utilize the DRU classification to contribute for the period that is not well covered by simplified digital and simplified olecranon methods. Given the good reliability and reproducibility, our findings suggest that using the three simplified maturity indices together can possibly give a comprehensive coverage of the pubertal growth period. The use of both simplified olecranon method and DRU classification can provide sufficient coverage of the prepubertal and growth acceleration phase, whereas the DRU and simplified digital methods are more suitable for assessment beyond PHV for the decelerating growth phase until skeletal maturity. The crucial PHV can be assessed with the combination of the three systems complementing each other.
Acknowledgements
Sanming Project of Medicine in Shenzhen (CN) (SZSM201612055).
We thank Drs. Siddharth N. Aiyer, Zhen Bian, Kevin C.H. Fok, Jewel T. Sadiang-abay, Ai-Min Wu, Colin S.Y. Yung for reading the images.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Joseph B, Nayagam S, Loder RT, Torode I. Paediatric Orthopaedics: A System of Decision-Making. New York, United States of America: Taylor and Francis Group; 2009. [Google Scholar]
- 2.Gottliebsen M, Shiguetomi-Medina JM, Rahbek O, Møller-Madsen B. Guided growth: mechanism and reversibility of modulation. J Child Orthop. 2016; 10:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenawey M, Abbas G, Ali F. Paediatric Orthopaedics: An Evidence-Based Approach to Clinical Questions. Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 4.Peterson DC, Ayeni OR. Pediatric anterior cruciate ligament reconstruction outcomes. Curr Rev Musculoskelet Med. 2016; 9:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung JPY, Luk KD. Managing the pediatric spine: growth assessment. Asian Spine J. 2017; 11:804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivett L, Rothberg A, Stewart A, Berkowitz R. Application of different measures of skeletal maturity in initiating weaning from a brace for scoliosis: two case reports. J Med Case Rep. 2009; 3:6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karol LA, Virostek D, Felton K, Jo C, Butler L. The effect of the risser stage on bracing outcome in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2016; 98:1253–1259. [DOI] [PubMed] [Google Scholar]
- 8.Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000; 82:1432–1446. [DOI] [PubMed] [Google Scholar]
- 9.Cheung JPY, Cheung PWH, Samartzis D, Luk KD. APSS-ASJ best clinical research award: predictability of curve progression in adolescent idiopathic scoliosis using the distal radius and ulna classification. Asian Spine J. 2018; 12:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders JO, Khoury JG, Kishan S, Browne RH, Mooney JF, III, Arnold KD, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008; 90:540–553. [DOI] [PubMed] [Google Scholar]
- 11.Cheung JPY, Cheung PWH, Samartzis D, Luk KD. Curve progression in adolescent idiopathic scoliosis does not match skeletal growth. Clinical orthopaedics related research. 2018; 476:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand RA. 50 years ago in CORR: the iliac apophysis: an invaluable sign in the management of scoliosis Joseph C. Risser MD CORR 1958;11:111-119. Clin Orthop Relat Res. 2008; 466:1516–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busscher I, Wapstra FH, Veldhuizen AG. Predicting growth and curve progression in the individual patient with adolescent idiopathic scoliosis: design of a prospective longitudinal cohort study. BMC Musculoskelet Disord. 2010; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opinya G. Bone age and maturity in association with anthropometric measurements of children and adolescents aged six to seventeen years in a high fluoride area. American Journal of Medicine and Medical Sciences. 2018; 8:178–190. [Google Scholar]
- 15.Sauvegrain J, Nahum H, Bronstein H. Study of bone maturation of the elbow. Ann Radiol (Paris). 1962; 5:542–550. [PubMed] [Google Scholar]
- 16.Diméglio A, Charles YP, Daures JP, de Rosa V, Kaboré B. Accuracy of the sauvegrain method in determining skeletal age during puberty. J Bone Joint Surg Am. 2005; 87:1689–1696. [DOI] [PubMed] [Google Scholar]
- 17.Hägg U, Taranger J. Maturation indicators and the pubertal growth spurt. Am J Orthod. 1982; 82:299–309. [DOI] [PubMed] [Google Scholar]
- 18.Greulich W, Pyle I. Radiographic Atlas of Skeletal Development of the Hand and Wrist. London: Stanford University Press; 1959. [Google Scholar]
- 19.Bull RK, Edwards PD, Kemp PM, Fry S, Hughes IA. Bone age assessment: a large scale comparison of the Greulich and Pyle, and Tanner and Whitehouse (TW2) methods. Arch Dis Child. 1999; 81:172–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner JM, Healy MJR, Goldstein H, Cameron N. Assessment of Skeletal Maturity and Prediction of Adult Height (TW3 Method). WB Saunders, London. 2001. [Google Scholar]
- 21.Luk KD, Saw LB, Grozman S, Cheung KM, Samartzis D. Assessment of skeletal maturity in scoliosis patients to determine clinical management: a new classification scheme using distal radius and ulna radiographs. Spine J. 2014; 14:315–325. [DOI] [PubMed] [Google Scholar]
- 22.Sitoula P, Verma K, Holmes L, Jr, Gabos PG, Sanders JO, Yorgova P, et al. Prediction of curve progression in idiopathic scoliosis: validation of the sanders skeletal maturity staging system. Spine (Phila Pa 1976). 2015; 40:1006–1013. [DOI] [PubMed] [Google Scholar]
- 23.Charles YP, Diméglio A, Canavese F, Daures JP. Skeletal age assessment from the olecranon for idiopathic scoliosis at risser grade 0. J Bone Joint Surg Am. 2007; 89:2737–2744. [DOI] [PubMed] [Google Scholar]
- 24.Cheung JP, Cheung PW, Samartzis D, Cheung KM, Luk KD. The use of the distal radius and ulna classification for the prediction of growth: peak growth spurt and growth cessation. Bone Joint J. 2016; 98-B:1689–1696. [DOI] [PubMed] [Google Scholar]
- 25.Cheung JP, Samartzis D, Cheung PW, Leung KH, Cheung KM, Luk KD. The distal radius and ulna classification in assessing skeletal maturity: a simplified scheme and reliability analysis. J Pediatr Orthop B. 2015; 24:546–551. [DOI] [PubMed] [Google Scholar]
- 26.Goodman LA, Kruskal WH. Measures of association for cross classifications*. J Am Statis Assoc. 1954; 49:732–764. [Google Scholar]
- 27.Adeyemi O. Measures of association for research in educational planning and administration. Res J Math Statis. 2011; 3:82–90. [Google Scholar]
- 28.Kviz FJ. Interpreting proportional reduction in error measures as percentage of variation explained. Sociological Quarter. 1981; 22:413–420. [Google Scholar]
- 29.Pitlović V, Sarić G, Pitlović H, Jovanović S, Jurisić D. A correlation of peak height velocity and olecranon apophysis ossification assessed by ultrasound. Coll Antropol. 2013; 37:1285–1289. [PubMed] [Google Scholar]
- 30.Sanders JO, Qiu X, Lu X, Duren DL, Liu RW, Dang D, et al. The uniform pattern of growth and skeletal maturation during the human adolescent growth spurt. Sci Rep. 2017; 7:16705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimeglio A. Growth in pediatric orthopaedics. J Pediatr Orthop. 2001; 21:549–555. [PubMed] [Google Scholar]
- 32.Kim HJ, Yoon JR, Modi C, Modi H, Song HR, Song SY. Interrelationship of the Risser sign, knee epiphysis, and bone age in determining skeletal maturity: a case-control study. J Pediatr Orthop B. 2011; 20:173–177. [DOI] [PubMed] [Google Scholar]
- 33.Canavese F, Charles YP, Dimeglio A. Skeletal age assessment from elbow radiographs. Review of the literature. Chir Organi Mov. 2008; 92:1–6. [DOI] [PubMed] [Google Scholar]
- 34.Canavese F, Charles YP, Dimeglio A, Schuller S, Rousset M, Samba A, et al. A comparison of the simplified olecranon and digital methods of assessment of skeletal maturity during the pubertal growth spurt. Bone Joint J. 2014; 96-B:1556–1560. [DOI] [PubMed] [Google Scholar]
- 35.Soliman A, De Sanctis V, Elalaily R, Bedair S. Advances in pubertal growth and factors influencing it: can we increase pubertal growth? Indian J Endocrinol Metab. 2014; 18:S53–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson AD, Sanders JO, Liu RW, Cooperman DR. The relationship of calcaneal apophyseal ossification and sanders hand scores to the timing of peak height velocity in adolescents. Bone Joint J. 2015; 97-B:1710–1717. [DOI] [PubMed] [Google Scholar]


