Abstract
Introduction
As researchers and academics around the world scramble ahead to dissect and analyse every aspect of the SARS-CoV2 virus, one such study explored the various underlying electrolyte abnormalities that were precipitated in patients suffering from a confirmed COVID-19 infection. A significant proportion of such patients were noted to be hypokalemic. Hypokalemia can be life threatening as it is known to cause cardiac arrhythmia.
Case presentation
Our encounter with said presentation was incidental. The on-call medical team was involved in a cardiac arrest call for a 74-year -old gentleman who developed torsades de pointes, Retrospective analysis of the clinical picture pointed out the fact that he was found to be persistently hypokalemic and bradycardic solely triggered as a result of COVID-19.
Conclusion
Although initially thought to be a pulmonary disease but along with time extra-pulmonary manifestations of Covid-19 has demonstrated significant consequences. Electrolyte abnormalities and cardiac dysfunction are examples of such extra-pulmonary pathologies. Therefore, it is important to keep close monitoring for such abnormalities otherwise could lead into life threatening arrythmias.
Keywords: Covid-19, SARS-CoV2 related bradycardia, SARS-CoV2 related hypokalemia, Torsades de pointes
Abbreviations: SARS-CoV2, Severe acute respiratory syndrome coronavirus 2; Lab, laboratory; NA, sodium; K, potassium
HIGHLIGHTS
-
•
Covid-19 is associated with cardiac conduction defects.
-
•
Covid-19 is associated with hypokalemia.
-
•
Hypokalemia and bradycardia can result in Torsades de pointes.
1. Introduction
As 2019 drew to a close, little did humans assume that they are about to embark on a journey so perilous that it could very well be drafted to history books as a once in a lifetime phenomenon. Just as the spanish flu wreaked havoc in the early 20th century, another virus was about to leave its permanent mark in the annals of human life. Novel coronavirus or SARS-CoV 2 as branded by the WHO, started in a province in china. Its ability to sustain its spread changed its designation from an epidemic to a global pandemic. A plethora of research is being conducted worldwide to have a better understanding of the patho-physiological consequences of COVID-19. As researchers and academics around the world scramble ahead to dissect and analyse every aspect of the SARS-CoV2 virus, one such study explored the various underlying electrolyte abnormalities that were precipitated in patients suffering from a confirmed COVID-19 infection. A significant proportion of such patients were noted to be hypokalemic. Hypokalemia can be life threatening as it is known to cause cardiac arrhythmia.
2. Case presentation
A 74-year-old gentleman presented to the emergency triage with worsening shortness of breath and desaturation (80% on room air). Symptoms improved with 15L non-rebreathing mask. Upon stabilizing the patient, history revealed that he had been experiencing viral prodrome (fever, cough with clear expectoration) for the past 7 days. He is known hypertensive on losartan 50mg once daily.
General examination revealed he was tachypneic, tachycardic, dyspneic with saturation remaining within 92–95% on 15L non-rebreathing mask. Chest auscultation revealed bi-basal crackles without any wheeze.
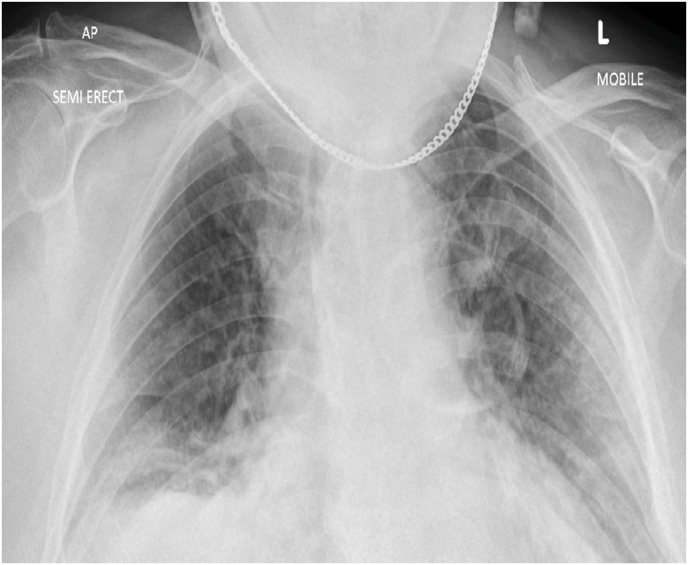
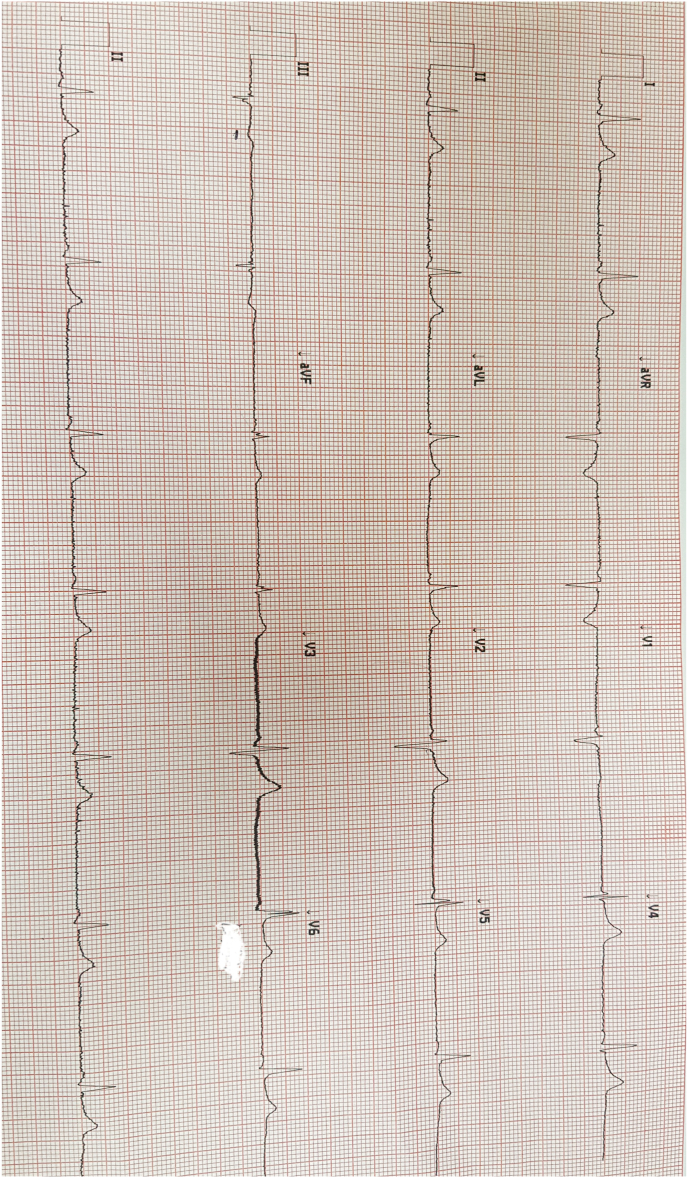
Lab results revealed euvolemic hypo-osmolar hypokalemia with a serum potassium of 2.5 mmol/L and osmolality 262 mOsm/L (280–296 mOsm/L), urine potassium 48 mmol/L and osmolality 510 mmol/L (300–900 m.mol/L). He had a normal sodium level of 138 m.mol/L. Inflammatory markers were elevated i.e. C-reactive protein was 157 mg/dL with a WCC of 16.5 and d-dimer 1.75. CK was elevated to 566 whereas LDH level was 488. Urine potassium to creatinine ratio was above 1.5. He had normal urine magnesium, calcium, and chloride levels. His clotting screen, renal function, liver function, thyroid profile, calcium, and phosphate remained unremarkable. Magnesium level was 0.58 m.mol/l. Mg levels and urine potassium were retrieved after the peri cardiac arrest event. X-ray chest showed bi-lateral opacities (Fig. 1). Arterial blood gas showed hypoxic respiratory failure. Ecg on admission showed sinus bradycardia with long standing LBBB (Fig. 2). His venous blood gas did not show metabolic alkalosis.
Fig. 1.
X-ray chest showing bi-lateral opacities.
Fig. 2.
ECG revealing bradycardia with long standing LBBB.
His oxygen therapy was therefore escalated to CPAP to support his oxygen requirement. He was started on intravenous potassium replacement, antibiotics.He was given intravenous dexamethasone 6mg as a stat dose and was later continued on daily basis for 10days. He also received intravenous remdesivir. Early review from critical care outreach team was completed keeping in mind of the severity of illness. Later-on the day of admission to the hospital a medical emergency call was put on as he went into peri-arrest. Cardiac monitor showed rhythm suggestive of torsade's de pointes.He was therefore immediately treated with intravenous magnesium 2 gm which was repeated in 5 minutes. A plan was discussed with the on-call cardiology team for pacing if patient didn't respond to intravenous magnesium. As hypokalemia and bradycardia were thought to be the predisposing factors a central line was inserted to give him high dose of intravenous potassium (40 m.mol/hr). Fortunately, his heart rate and rhythm settled without requiring any further interventional cardiac procedures. He was then transferred to intensive care unit for close monitoring. He made good recovery within following 48 hours and eventually able to step down out of intensive care unit. Later on all other possible causes for bradycardia and hypokalemia were explored and were found to be negative. His heart rate and potassium level both improved with time as the effect of the virus weaned off after a 12-days hospital stay. Therefore, we came into a conclusion of COVID-19 induced hypokalemia and bradycardia resulting in torsades de pointes for the gentleman.
3. Clinical discussion
Following a global outbreak of SARS-COV-2 world is continuing to witness various spectrum of COVID-19. A high prevalence of hypokalemia have been noted amongst SARS-COV-2 infected patients. Stand out theory to explain such presentation is disturbed renin-angeotensin system activity which increases as a result of losing counteractivity of angiotensin converting enzyme-2 [1]. SARS-COV-2 is known to enter into the human cells by binding with ACE2 receptor located on the cell membrane [2]. In a healthy individual ACE1 increases RAS activity whereas ACE2 opposes it. Number of ACE2 receptor degradation increases secondary to binding of SARS- COV-2 with the receptor. This leads into elevated RAS activity to an extent where it acts like secondary hyper aldesteronism [1]. End result is increase retention of sodium and water along with more excretion of potassium from the kidney. Studies have noted to find increase urinary potassium in hypokalemic COVID-19 patients compared to those with normokalemia [1]. Other suggested explanation is GI loss which is often associated with SARS-COV-2 infected patients. Potassium levels therefore should be closely monitored as it regulates cell polarity and action potential. A period of hypokalemia can lead into prolong resting membrane potential and cellular hyperpolarity which can potentially trigger ventricular arrythmia once it effects the cardiac myocyte. Appropriate plasma levels of potassium is therefore required in preventing myocardial failure through weakening cellular hyperpolarity and depolarization. It is ideal to keep a range of potassium about 4–5.5 m mol/l to prevent myocardial dysfunction [3].
Hypokalemia is popularly known as a risk factor for arrythmogenic deaths. Suggested theories are slow conduction (due to membrane hyper-polarization), prolong ventricular repolarization (due to inhibition of potassium efflux), and abnormal pacemaker activity largely resulting from increase diastolic repolarization in parkinje fibers [4,5]. High calcium is known to cause pacemaker abnormalities. Elevated calcium levels are found due to failure of NA-K pump which results in raised NA-CA exchange and thusly calcium overload [4]. Other suggested mechanism for high calcium is inhibition of NKA activity [4]. NKA is an ATP and voltage dependent ion transporter that exchanges 3 NA from cytosol and 2 K from the extracellular compartment leading to a net abundant current. It is the major NA efflux mechanism in cardiac myocytes that regulates intracellular NA by balancing NA efflux against NA influx. NKA activity is maintained by extracellular K, intracellular NA, and membrane action potential. NKA is composed of alfa-beta dimers. Reduce activity of NKA alfa 2 increases cellular calcium by limiting forward NCX activity (increase calcium efflux) and raises reverse NCX activity (increase calcium influx). NCX is NA- CA exchange that uses electrochemical gradient of NA and Calcium to exchange 3NA for 1 CA. Hypokalemia reduces outward repolarizing currents as well as both potassium and NKA current. This increases ADP, allowing more CA influx through L-type CA channels during plateau phase. Secondary elevated high calcium caused by NKA inhibitor and reduce inward NCX currents combined with APD prolongation, initiates a positive feedback loop. According to this model, high calcium activates CaMK2 which phosphorylates and activates late NA current (INAL) and increase Ica. This causes activation of INAL and Ica which amplifies intracellular NA-CA resulting in detrimental and downward activity of CaMK2 leading to after depolarization and ventricular arrythmia. All such mechanism can trigger abnormal automaticity and generate cardiac arrythmia.
In addition to hypokalemia COVID-19 infection itself has been reported to cause bradycardia. Aetiopathological consequences seems to be multifactorial which includes viral myocardial damage, hypoxia, raised pro-inflammatory cytokines [6]. Possible explanation for bradycardia associated with COVID-19 is release of pro-inflammatory cytokines (IL 6, IL 10, IL 12 & TNF-alfa) which targets the SA node resulting in change of heart rate and rhythm [6]. Bradycardia and hypokalemia both have been known to be triggering factors for ventricular tachycardia, ventricular fibrillation, torsades de pointes. Inverse relationship between heart rate and repolarization time primarily accounts for bradycardia induced QT prolongation.
We to the best of our knowledge are presenting a case for the first time about COVID-19 associated bradycardia and hypokalemia resulting in torsades de pointes. We ruled out all other possible causes for hypokalemia and bradycardia making COVID-19 to remain as the sole risk factor. Emergency treatment is removal of predisposing factors and suppression of EADS (Early after depolarizations) [7]. Mg suppresses TDP (Torsades de pointes) by lowering CA influx therefore eventually reducing EAD amplitude. 2 gm (20ml of 10% solution) intravenous over 1–2 minutes, repeated every 5–15 minutes interval to a maximum of 6 gm can be given. Aside from correcting any reversible causes for long qt other potential therapies to treat such a catastrophic clinical condition includes use of beta blocker if not bradycardic. In case of bradycardia or where beta blocker is contra-indicated left cardiac denervation, transvenous/transcutaneous pacing could be lifesaving whereas ICD remains the last resort if already mentioned therapies fail.
4. Conclusion
Along with the course of time we have been introduced to various spectrum of Covid-19. This case report depicts electrolyte and cardiac manifestations related to SARS-CoV2. It raises the view to give importance to monitoring electrolytes and cardiac electrical activities in patients suffering from Covid-19 to avoid life threatening complications.
Please state any conflicts of interest
No conflict of interest
Please state any sources of funding for your research
This case report did not require any funding
Sources of funding
This case report did not receive any funding
Ethical approval
The case report is exempt from ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr. Saquib Navid Sidiqui: Lead and corresponding author. Dr. Muhammad Memon, Dr. Sonam Tshering, Dr. Tanveer Hasan, Dr. Asheer Jawed, Dr. Pranav Kumar Jha, Dr. Roland Jayasekhar: Co-authors.
Trial registry number
-
1)
Name of the registry: Not applicable to case report
-
2)
Unique Identifying number or registration ID: Not applicable to case report
-
3)
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable to case report.
Guarantor
Dr. Saquib Navid Siddiqui.
Declaration of competing interest
No conflicts of interest.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
We thank our patient to give us consent to elaborate his case for the benefit of medical education and medical science in general.
References
- 1.Dong Chen, Xiaokun Li, Qifa Song, Chenchan Hu, Fefei SU, Jianyi Dai et al. Assessment of Hypokalemia and Clinical Characteristics in Patients with Coronavirus Disease 2019 in Wenzhou, China. PMID: 32525548; doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed]
- 2.Lu Roujian, Zhou Xiang, Li Juan, Niu Peihua, Yang Bo, Wu Honglong. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald J.E., STruthers A.D. What is the optimal serum potassium level in cardiovascular patients? J. Am. Coll. Cardiol. 2004;43(2):155–161. doi: 10.1016/j.jack.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Jonas Skogestad, Jan M. A. Hypo Kale Mia-Induced Arrhythmia and Heart Failure: New Insights and Implications for Therapy. PMID: PMC6234658; PMID:30464746; doi:10.3389/fphys.2018.01500. [DOI] [PMC free article] [PubMed]
- 5.James N. Weiss, Zhilin Qu, Kalyanam Shivkumar. The Electrophysiology of Hypo- and Hyperkalemia. PMID:28314851; doi:10.1161/CIREP.116.004667. [DOI] [PMC free article] [PubMed]
- 6.Eluwana A. A., Douglas S. C., Lynn Moran, Richard Synder. Bradycardia in Patients with Covid-19: A Calm before the Storm? PMID: 32550090; doi:10.7759/cereus.8599. [DOI] [PMC free article] [PubMed]
- 7.P. T. Munro, C. A. Graham. Torsade de Pointes. Doi:10.1136/emu.19.5.485. [DOI] [PMC free article] [PubMed]