Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is most closely related to severe respiratory syndrome; however, recent reports showed that it is capable of causing neurological disease. Here we report a case-series of 4 critically ill COVID-19 patients who recovered from pneumonia but showed serious neurological symptoms and eventually died.
Keywords: Brain, Disfunction, Brain damage, COVID-19
Introduction
The COVID-19 disease has emerged as a pandemic and the current data reveals that adults and children are affected in different ways [1]. Clinical manifestations represent a wide spectrum of diseases ranging from mild to severe respiratory syndrome influenza-like illness with lower respiratory tract symptoms, loss of taste and smell, and severe gastrointestinal symptoms [2,3]. COVID-19 can be even a life-threatening disease due to the development of severe pneumonia, acute respiratory distress syndrome (ARDS), multi-organ dysfunction and neurological damages [3,4]. Indeed, coronaviruses are known as pathogens with neuroinvasive potential. There is increasing evidence that coronavirus infections are not always confined to the respiratory tract but involved even the central nervous system (CNS). When this happens, it may also occur and contribute to overall morbidity and mortality in acute care setting [5]. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is most closely related to severe respiratory syndrome, however recent reports showed that it is capable to cause neurological disease [4]. Here we report a case-series of 4 critically ill COVID-19 patients who recovered from pneumonia but showed serious neurological symptoms and eventually died.
Case descriptions
From the 10th of March our ICU was completely dedicated to COVID-19 patients and has been one of the largest cohorted ICU in Southern Italy, admitting 12 positive critically ill patients [3]. Four out of 12 critically ill COVID-19 patients, recovered from pneumonia and not sedated from 6 days, showed a sudden onset of serious neurological symptoms. Table 1 reported the main characteristics and the neurological symptoms.
Table 1.
Neurologic features of included patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Main characteristics | Male, 66 y, Hypertension, Diabetes | Female, 47y, Obesity | Female, 67 y, Obesity | Male, 63y, Hypertension, Diabetes |
| Time from the first positive swab to neurological assessment (days) | 18 | 22 | 13 | 16 |
| Mental status | Awareness markedly reduced, able to perform only simple commands with to verbal stimulus like close the eyes and protrude the tongue | Awareness markedly reduced, able to perform only simple commands with to verbal stimulus like close the eyes and protrude the tongue | Awareness markedly reduced, open the yes but not able to perform any simple command | Awareness markedly reduced, open the yes but not able to perform any simple command |
| Response to painful stimulus | Only facial grimace | Only facial grimace | Only facial grimace | Only facial grimace |
| GCS score | ||||
| • Eye response | 4 | 4 | 4 | 4 |
| • Motor response | 1 | 1 | 1 | 1 |
| • Verbal response | 1 | 1 | 1 | 1 |
| Ocular response | ||||
| • Pupillary size | Equal Present |
Equal Present |
Equal Present |
Equal Present |
| • Pupillary light reflex | Absent | Absent | Present | Present |
| • Eye movements | Fixed | Fixed | Not fixed | Not fixed |
| • Eye position | Absent | Absent | Present | Present |
| • Oculovestibular response | Absent | Absent | Present | Present |
| • Oculocephalic response | Absent | Absent | Present | Present |
| Corneal reflex | Absent | Absent | Present | Present |
| Grimace | Present | Present | Present | Present |
| Cough reflex | Diminished | Diminished | Absent | Absent |
| Respiratory pattern | No trigger for spontaneous breathing | No trigger for spontaneous breathing | No trigger for spontaneous breathing | No trigger for spontaneous breathing |
| Upper limbs movement | Absent | Absent | Absent | Absent |
| Lower limbs movement | Absent | Absent | Absent | Absent |
| Upper limbs tone | Markedly diminished (hypotonia) | Markedly diminished (hypotonia) | Markedly diminished (hypotonia) | Markedly diminished (hypotonia) |
| Lower limbs tone | Markedly diminished (hypotonia) | Markedly diminished (hypotonia) | Markedly diminished (hypotonia) | Markedly diminished (hypotonia) |
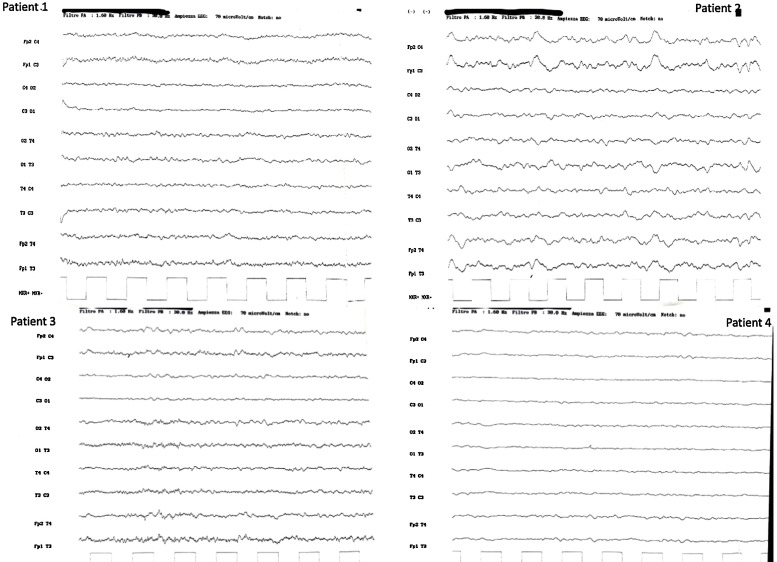
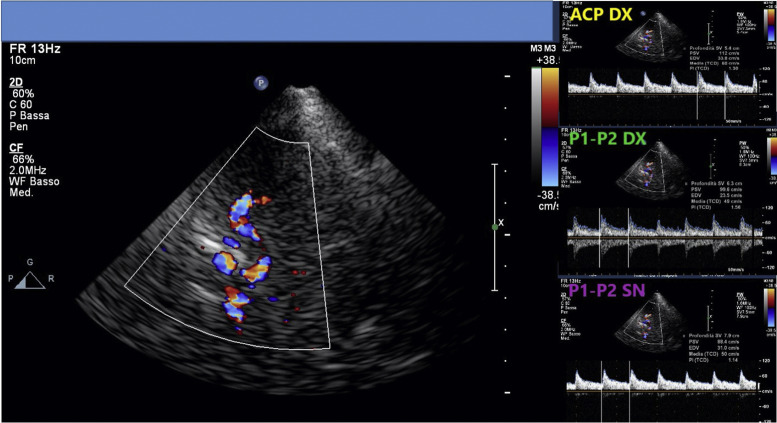
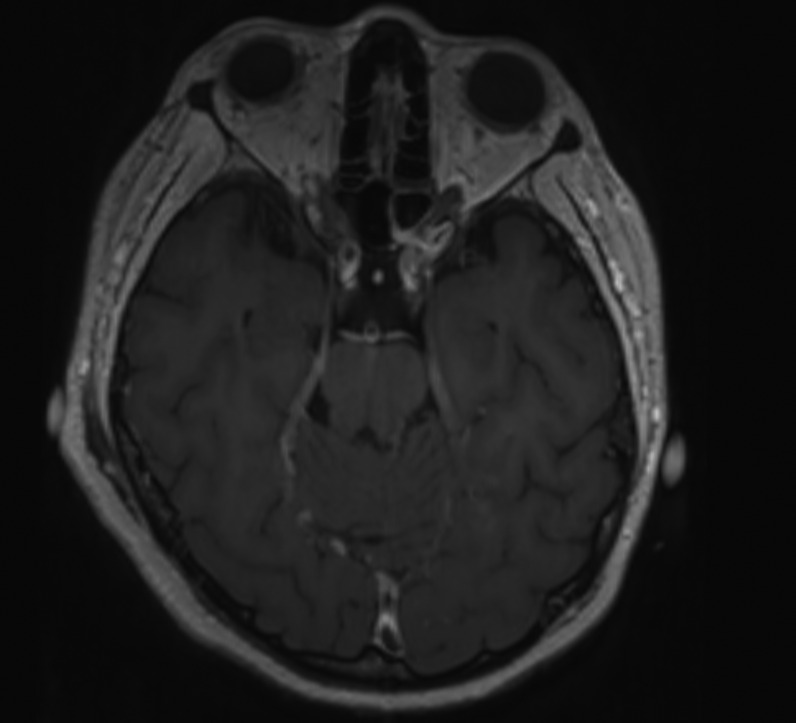
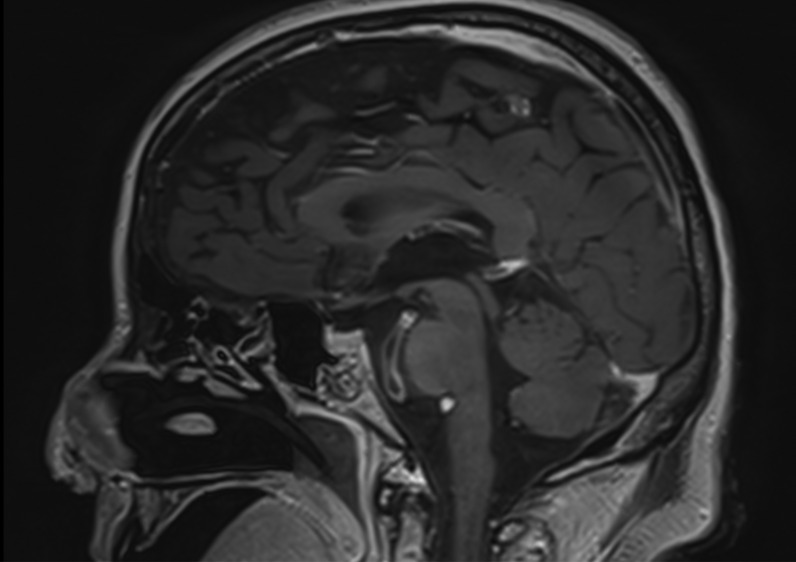
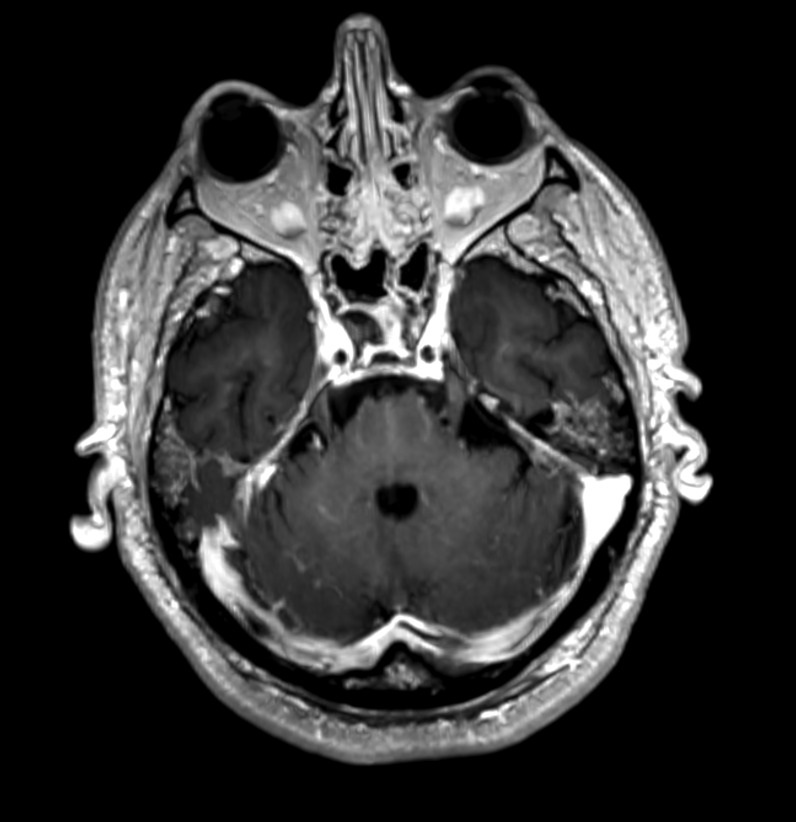
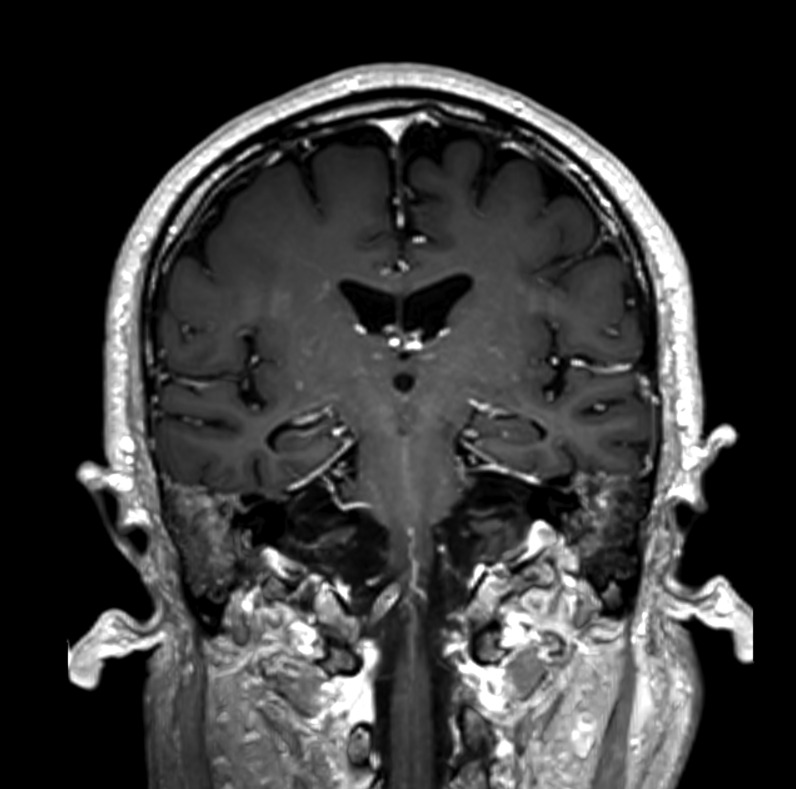
Their mental status was markedly reduced, even if 2/4 patients were able to follow simple commands with eyes or tongue. All of them reacted to body painful stimuli with facial grimace and had a Glasgow coma scale (GCS) of 6 points. Oculovestibular and oculocephalic response were absent in 2/4 patients. All patients had no trigger for spontaneous breathing and were tetraplegic. Within 3 days from symptom onset, we performed electroencephalogram (EEG) and a brain CT scan in all of them, while a brain magnetic resonance imaging (MRI) was obtained in 2 patients within 24 h from CT. All patients showed a generalized slow activity at the EEG (Fig. 1 ). The 16-slice brain CT scans were negative in all patients (supplementary material). No significant parenchymal alterations were evident on pre-contrast MRI sequences (including DWI, SWI, FLAIR-T2 and TSE T2w) in the two patients studied. Conversely, CE-MRI showed some abnormal enhancement along the arterial walls, mainly in the vertebro-basilar system in one case (supplementary materials), and scattered punctuate enhancing foci in the deep white matter, presumably due to vessel engorgement in the other patient (supplementary material). No abnormal findings were evident on post-contrast FLAIR images neither in the parenchyma nor in the leptomeningeal spaces. We further performed a transcranial doppler that showed an increased flow velocity in 2 cases (Fig. 2 ). All patients died.
Fig. 1.
EEG activities of included patients. Each patient shows a continuous generalized slow activity at different waves.
Fig. 2.
The transcranial doppler of the patients n°4 catch at the mesencephalic plane. The main box shows the transcranial doppler of the Circle of Willis. The right upper box shows the transcranial doppler of right cerebral posterior artery (named ACP DX in the figure). The right middle box shows the transcranial doppler of the first and second segments (named P1 and P2 DX in the figure) of the of right cerebral posterior artery. The right lower box shows the transcranial doppler of the first and second segments (named P1 and P2 SN in the figure) of the left cerebral posterior artery.
Discussion
We describe the fatal outcome of 4 critically ill COVID-19 patients who recovered from SARS pneumonia but developed severe neurologic symptoms. All patients had no respiratory drive but still minimal level of consciousness. Furthermore, all of them showed a generalized slow activity at the EEG but normal brain CT scan. Interestingly, we found a slightly increase of flow velocity at transcranial doppler in 2 of them. This data was further confirmed by the vessel engorgement found at the brain MRI of one patient.
The respiratory control system consists of three components: the central neural respiratory generator, the sensory input system, and the muscular effector system. The rate and strength at which the diaphragm contracts, hence the frequency and volume of respiration, depend heavily on the firing pattern of pacemaker cells in the brainstem [6]. Critically ill patients with serious cerebral damages and brainstem injuries often require airway protection and mechanical ventilation [7,8]. According to this, the absence of respiratory drive in critically ill COVID-19 patients may be consistent with an involvement of the brainstem respiratory center even if a clear evidence of brain structural radiological changes is lacking. Indeed, while the pathophysiology is not completely understood, it seems that COVID-19 could directly invade the CNS [9].
Generalized slow activity at the EEG, negative brain CT/MRI scan and the presence of severe neurological clinical symptoms may suggest a CNS dysfunction [10]. Description of the MR/CT findings in patients with end-stage COVID-19 are still scarce at the time of this writing [5]. Most of them describe some type of vascular (ischemic) or inflammatory lesions. Our cases showed abnormal enhancement of the vessel walls, suggesting endothelial inflammation without signs of parenchymal/leptomeningeal involvement, although PWI was not performed. The question whether these abnormal findings are causative of the impairment clinical state and/or the EEG pattern observed remains unsolved. We are led to believe that a diffuse functional brain disconnection syndrome may occur in these patients, and the use of resting-state functional MRI techniques is warranted.
Conclusions
The present case series thus highlights that in critically ill patients with COVID-19, the impairment of blood flow showed by MRI and TCD may have a role in the loss of brain function.
Funding
No funding sources.
Competing interests
None declared.
Ethics approval and consent to participate
Obtained. Ethical approval 155/20.
Availability of data and material
NA.
Authors contributions
MV, CI, ET, ADS, AB, GS collected the data, evaluated the data, draft the paper and approved the final version.
Acknowledgements
NA.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.05.011.
Appendix A. Supplementary data
The following are Supplementary data to this article:
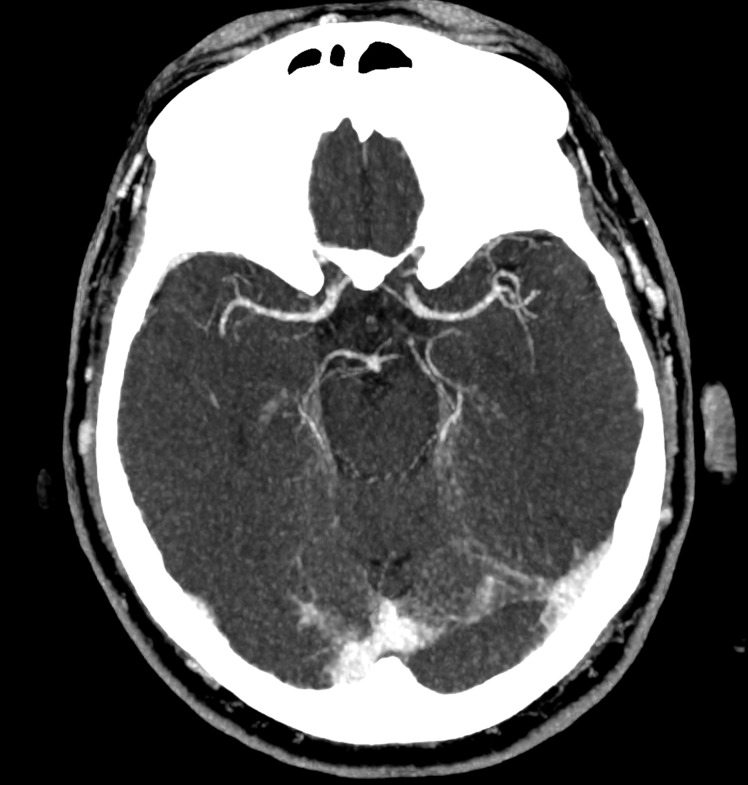
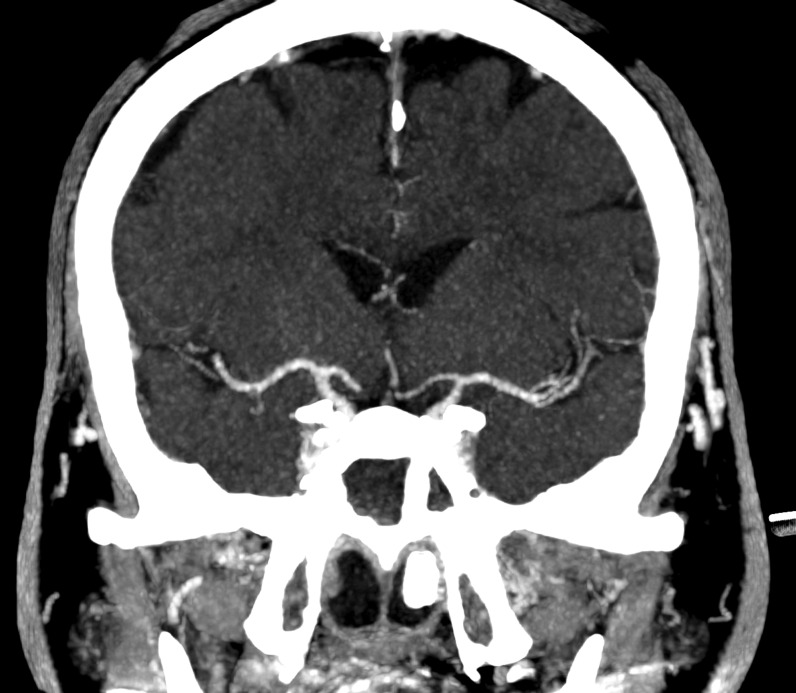
Axial and coronal reformatted contrast-enhanced CT scans showing absence of significant stenosis of the main arterial branches of the Willis circle and normal appearance of the brain parenchyma.
Reformatted axial (a) and sagittal (b) 1mm-thick contrast-enhanced 3D-T1 images in a 46-year old woman (patient 2). Thickening and abnormal wall enhancement of the basilar artery is evident. Reformatted axial (a) and coronal (c) 1mm-thick contrast-enhanced 3D-T1 images in a 67-year old man (patient 3). Punctuate foci of contrast enhancement are evident in the cerebellar hemispheres (a) and at the nucleo-capsular level (b)
References
- 1.Rehman S., Majeed T., Azam Ansari M., Ali U., Sabit H., et al. Current scenario of COVID-19 in pediatric age group and physiology of immune and thymus response. Saudi J Biol Sci. 2020;27(10):2567–2573. doi: 10.1016/j.sjbs.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S., Tombuloglu H., Hassanein S.E., et al. Coronavirus diseases 2019: current biological situation and potential therapeutic perspective. Eur J Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman S., Majeed T., Ansari M.A., Al-Suhaimi E.A. Syndrome resembling Kawasaki disease in COVID-19 asymptomatic children. J Infect Public Health. 2020;13(12):1830–1832. doi: 10.1016/j.jiph.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manganelli F., Vargas M., Iovino A., Iacovazzo C., Santoro L., Servillo G. Brainstem involvement and respiratory failure in COVID-19. Neurol Sci. 2020;41(7):1663–1665. doi: 10.1007/s10072-020-04487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez C.A. Looking ahead: the risk of neurologic complications due to COVID-19. Neurol Clin Pract. 2020;(April) doi: 10.1212/CPJ.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas M., Servillo G., Tessitore G., Aloj F., Brunetti I., Arditi E., et al. Double lumen endotracheal tube for percutaneous tracheostomy. Respir Care. 2014;59(11):1652–1659. doi: 10.4187/respcare.03161. [DOI] [PubMed] [Google Scholar]
- 7.Vargas M., Servillo G., Striano P. Serum lactate dehydrogenase as early marker of posterior reversible encephalopathy syndrome: keep your eyes open. Anaesth Intensive Care. 2012;40(3):570–571. [PubMed] [Google Scholar]
- 8.Striano P., Bifulco F., Servillo G. The saga of Eluana Englaro: another tragedy feeding the media. Intensive Care Med. 2009;35(6):1129–1131. doi: 10.1007/s00134-009-1484-6. [DOI] [PubMed] [Google Scholar]
- 9.Deana C., Verriello L., Pauletto G., et al. Insights into neurological dysfunction of critically ill COVID-19 patients. Trends Anaesthesia Crit Care. 2021;36:30–38. doi: 10.1016/j.tacc.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Axial and coronal reformatted contrast-enhanced CT scans showing absence of significant stenosis of the main arterial branches of the Willis circle and normal appearance of the brain parenchyma.
Reformatted axial (a) and sagittal (b) 1mm-thick contrast-enhanced 3D-T1 images in a 46-year old woman (patient 2). Thickening and abnormal wall enhancement of the basilar artery is evident. Reformatted axial (a) and coronal (c) 1mm-thick contrast-enhanced 3D-T1 images in a 67-year old man (patient 3). Punctuate foci of contrast enhancement are evident in the cerebellar hemispheres (a) and at the nucleo-capsular level (b)
Data Availability Statement
NA.