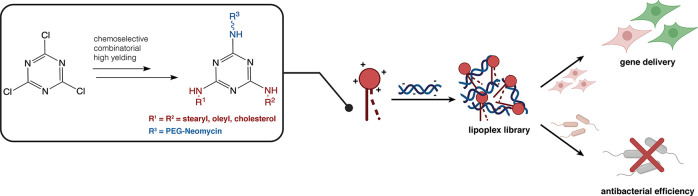
Abstract
Cationic lipids (CLs) have gained significant attention among nonviral gene delivery vectors due to their ease of synthesis and functionalization with multivalent moieties. In particular, there is an increasing request for multifunctional CLs having gene delivery capacity and antibacterial activity. Herein, we describe the design and synthesis of a novel class of aminoglycoside (AG)-based multifunctional vectors with high transfection efficiency and noticeable antibacterial properties. Specifically, cationic amphiphiles were built on a triazine scaffold, allowing for an easy derivatization with up to three potentially different substituents, such as neomycin (Neo) that serves as the polar head and one or two lipophilic tails, namely stearyl (ST) and oleyl (OL) alkyl chains and cholesteryl (Chol) tail. With the aim to shed more light on the effect of different types and numbers of lipophilic moieties on the ability of CLs to condense and transfect cells, the performance of Neo–triazine-based derivatives as gene delivery vectors was evaluated and compared. The ability of Neo–triazine-based derivatives to act as antimicrobial agents was evaluated as well. Neo–triazine-based CLs invariably exhibited excellent DNA condensation ability, even at a low charge ratio (CR, +/−). Besides, each derivative showed very good transfection performance at its optimal CR on two different cell lines, along with negligible cytotoxicity. CLs bearing symmetric two-tailed OL proved to be the most effective in transfection. Interestingly, Neo–triazine-based derivatives, used as either free lipids or lipoplexes, exhibited strong antibacterial activity against Gram-negative bacteria, especially in the case of CLs bearing one or two aliphatic chains. Altogether, these results highlight the potential of Neo–triazine-based derivatives as effective multifunctional nonviral gene delivery vectors.
Introduction
In recent years, the concept of introducing foreign genetic material into cells has gained increasing attention within the clinical and scientific communities. Despite the first success of viral vectors as gene delivery carriers, the need for safer and more effective carriers has boosted current research toward the development of nonviral, synthetic gene delivery systems. In this context, the biggest challenge is to find nonviral options as performant as viral counterparts along with overcoming virus-related drawbacks (high immunogenicity and limited carrying capacity, as well as high production costs).1−6 In this context, cationic lipids (CLs) are the flagship of nonviral gene delivery vectors due to the ease of synthesis and tailoring.7,8
Generally speaking, CLs are amphiphiles prepared by linking a cationic headgroup to lipophilic tail(s) through a suitable linker moiety. Cationic headgroups, encompassing all kinds of amines and quaternary ammonium salts, guanidiniums, heterocycles, peptides, and other unconventional moieties, have been found to play a role in the binding and condensation of the genetic material through electrostatic interactions (i.e., lipoplex formation), in the interaction of the lipoplex with the cell membrane and its internalization, and in the overall toxicity of the vectors.9−11 Depending on the structure, lipophilic tails are classified as (linear or branched saturated/unsaturated) alkyl chains or cyclic (steroid-based) domains.12,13 They play an important role in manifold aspects of the gene delivery process, such as the overall stability and cytotoxicity of the resulting lipoplexes, the protection and release of the genetic cargo, the endosomal escape, and the nuclear penetration. On the other hand, the linker moiety has a stake in the behavior of the CL as a whole, that is, the stability, biodegradability, cytotoxicity, and transfection efficiency of the resulting particles.14,15 Moreover, even if often neglected, some features of the linker, including the overall charge, length, relative orientation, and steric hindrance, are responsible for the conformational flexibility of the amphiphile and, in turn, for the ultimate gene transfer efficiency and cytotoxicity.15,16 In keeping with these findings, a number of structure–activity relationship (SAR) studies have been focused to engineering the ideal CL through the rational design of each and every building block.17−19 However, the complexity and poor understanding of the exact transfection mechanism, that is, the turn of events involving cellular internalization, endosomal escape, plasmid DNA (pDNA) translocation from the cytosol to the nucleus, and the eventual nucleic acids (NAs) release, still greatly impact the development of more and more effective CLs.20
Among different cationic head groups, aminoglycosides (AGs) have gained significant attention mainly due to their natural affinity with NAs, structural and functional variety, and inherent antibacterial activity.21−24 In this context, neamine, neomycin (Neo), paromomycin, tobramycin, and kanamycin, have extensively been used for the synthesis of multifunctional CLs25−29 and polymers.26,30−33 Generally speaking, AG-based CLs have been synthesized keeping the balance 1:1 between the headgroup and the lipophilic tail using 6-aminocaproic acid, succinic acid, and lysine linkers and different lipophilic tails. Such conjugates proved to be very promising in terms of transfection performance in both in vitro and in vivo studies, especially when formulated with the helper lipid DOPE.
Relying on these works, we developed a new class of Neo-based cationic amphiphiles using a triazine scaffold as the linker between the cationic head and the lipophilic tail(s). The triazine scaffold allows for an easy derivatization with up to three potentially different substituents, such as lipophilic tails and polar heads. Following thorough chemical characterization, Neo–triazine derivatives were evaluated for their ability to bind and condense DNA and transfect cells in vitro. Besides, an important feature sometimes undervalued by fundamental scientists but receiving considerable attention from clinical investigators is the desired side antibacterial effect of chemotherapeutics. Indeed, chemotherapy can induce damage to the patient’s immune system, which may elicit bacterial infections. Consequently, there is an urgent need to develop multifunctional materials with high gene delivery and antimicrobial efficiencies. Accordingly, the antibacterial activity against Gram-negative bacteria of the new Neo-based cationic amphiphiles synthesized in this work was assessed as well.
Results and Discussion
Synthesis of Neo–Triazine Cationic Amphiphiles
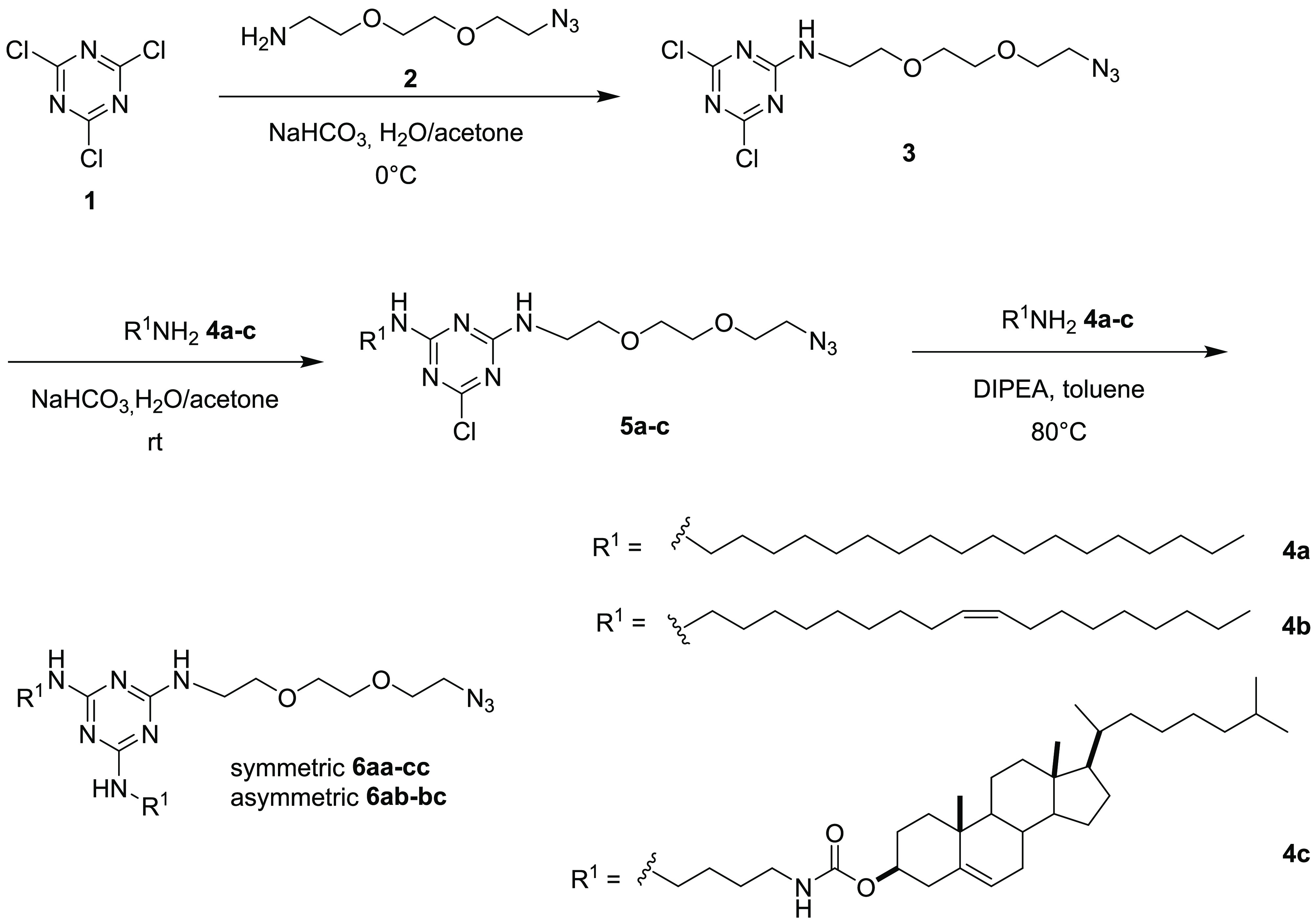
CLs are commonly built by linking a cationic headgroup to a lipophilic tail through a suitable linker or spacer. In the present study, we used 2,4,6-trichlorotriazine (cyanuric chloride) as a tripodal linker in order to link Neo to one or two lipophilic tails. We did choose the triazine scaffold because trichlorotriazine can be chemoselectively functionalized with amines by simply controlling the reaction conditions, in particular the temperature, in multigram scale, being suitable for combinatorial synthesis/SAR purposes.34 Indeed, such scaffold has been widely used so far for the synthesis of libraries of small molecules and multifunctional warheads for a number of applications.35,36 Besides, the triazine core has been exploited in the field of gene/drug delivery mainly to build dendrimers of diverse generations displaying orthogonal outer functional surfaces in order to facilitate further functionalization.2 Despite their synthetic versatility, triazines have been poorly exploited for the synthesis of CLs.37−41 In the frame of a project dealing with the use of AG-based lipids and polymers as multifunctional gene delivery vehicles,26,31,42 three types of Neo–triazine conjugates were synthesized through combinatorial chemistry, including monotailed, symmetric two-tailed, and asymmetric two-tailed Neo–triazine conjugates. Due to the harsh conditions needed for an effective third substitution (80 °C, 12 h) and the relatively low stability of AGs at high temperature, we introduced Neo at the end of the synthetic pathway by alkyne–azide “click” reaction with mono- or two-tailed triazine intermediates bearing an N3–PEG2 arm. Accordingly, 2,4,6-trichlorotriazine 1 was first reacted with NH2–PEG2-N3 linker 2 at 0 °C in order to obtain monosubstituted dichlorotriazine 3 which was reacted at room temperature (rt) with commercially available stearyl amine 4a, oleyl amine 4b, and synthetically accessible cholesterol-amine 4c, leading to selective formation of monotailed intermediates 5a, 5b, and 5c, respectively, in very good yield (Scheme 1). A third substitution with the same amines 4a–c was obtained by performing the reaction overnight, at 80 °C, using DIPEA as the base and toluene as the solvent in a sealed tube. Symmetric two-tailed azides 6aa–cc and asymmetric two-tailed azides 6ab–bc were obtained with good yields. We selected amines 4a–c considering the results obtained by Prof. Lehn and co-workers in studies dealing with the impact of the lipophilic chain on the transfection efficiency of AG-monotailed CLs coformulated with DOPE, showing higher values of transfection efficiency with cholesterol and saturated or unsaturated C18 alkyl chains.25,27−29
Scheme 1. Synthesis of Monotailed 5a–c, Symmetric Two-Tailed 6aa–cc, and Asymmetric Two-Tailed 6ab–bc Triazine-PEG2-N3.
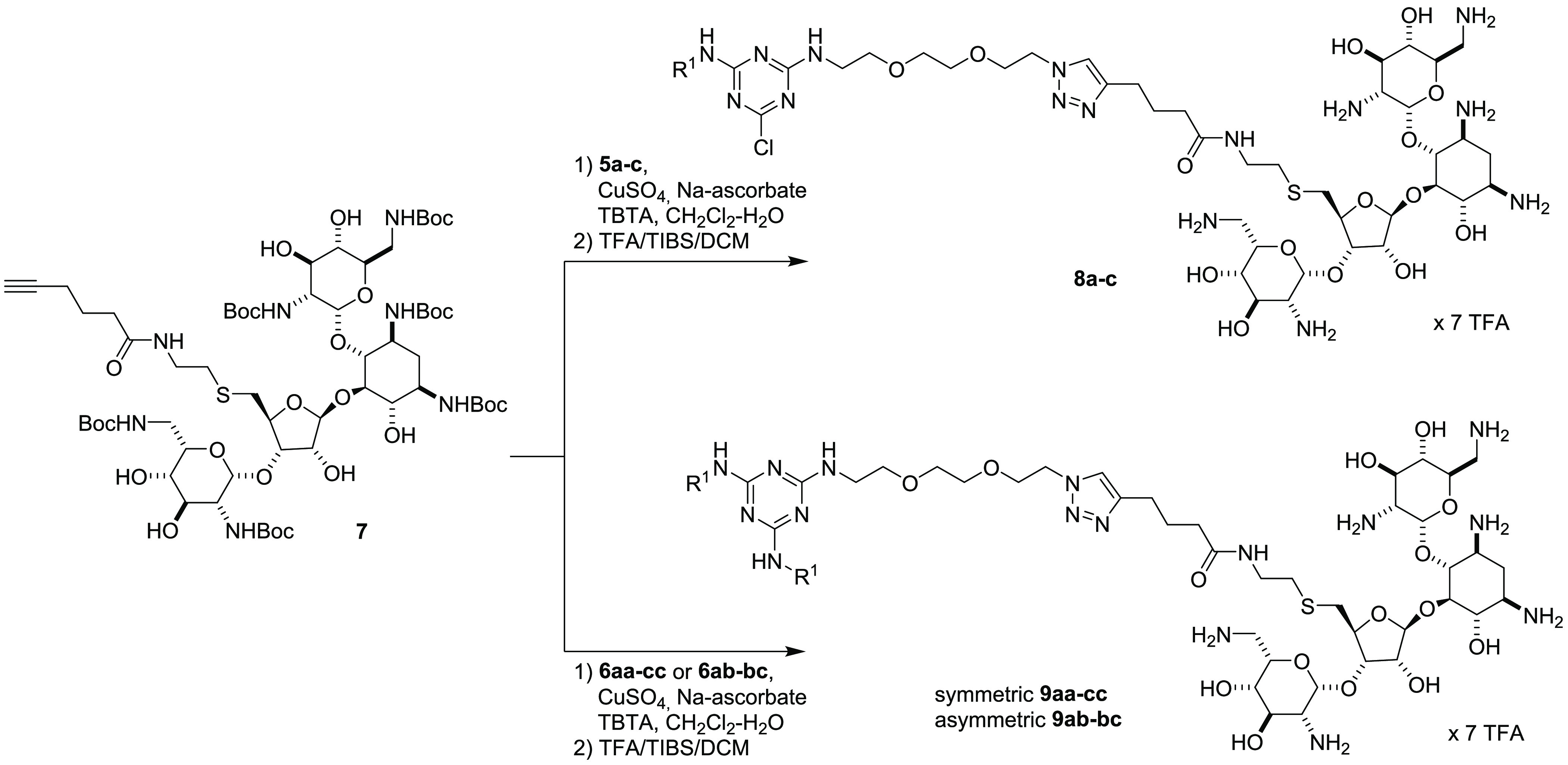
It is worth noting that monotailed triazine-PEG2-N35a–c and two-tailed triazine-PEG2-N36aa–bc are universal intermediates because, in principle, they can be functionalized by “click” reaction with different cationic head groups bearing a linker with a terminal alkyne group. Among the array of AGs, we decided to use Neo because of its ease of functionalization. Going into more detail, we prepared N-Boc-neomycin-alkyne 7 in four steps according to the literature procedure.26 Alkyne 7 was “clicked” with monotailed and two-tailed triazine-PEG2-N35 and 6, respectively, in the presence of the catalytic triad CuSO4/Na-ascorbate/TBTA in a mixture DCM/water affording, after TFA-promoted N-Boc deprotection, final conjugates monotailed 8a–c, symmetric two-tailed 9aa-cc, and asymmetric two-tailed 9ac–bc (Scheme 2).
Scheme 2. Synthesis of Monotailed Neo–Triazine 8a–c and Two-Tailed Neo–Triazine 9aa–cc and 9ac–bc.
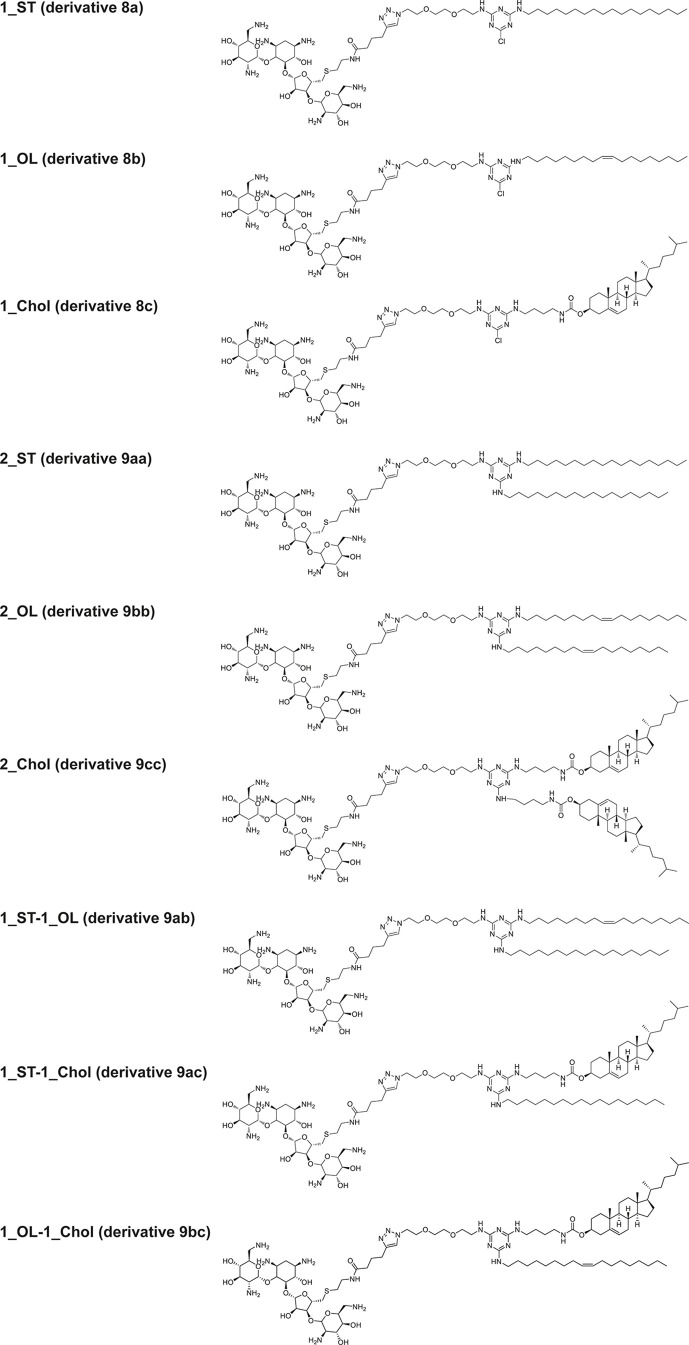
The structures of monotailed Neo–triazine conjugates 8a–c, symmetric two-tailed Neo–triazine conjugates 9aa–cc, and asymmetric two-tailed Neo–triazine conjugates 9ab–bc (Figure 1) were confirmed by 1H and 13C NMR results and mass spectrometry (please refer to Supporting Information Figures S11–S19). 19F NMR titration experiments (Supporting Information Figures S20–S21) showed that each conjugate incorporates 7 equiv of trifluoroacetic acid (TFA), confirming that only one nitrogen of the melamine scaffold is protonated, as already reported in the literature.37
Figure 1.
Structures of monotailed Neo–triazine conjugates 8a–c (1_ST, 1_OL, 1_Chol), symmetric two-tailed Neo–triazine conjugates 9aa–cc (2_ST, 2_OL, 2_Chol), and asymmetric two-tailed Neo–triazine conjugates 9ab, 9ac, and 9bc (1_ST-1_OL, 1_ST-1_Chol, 1_OL-1_Chol).
With the aim to shed more light on the effect of different lipophilic moieties (in terms of type and number) on the ability of CL to condense and transfect cells, the performance of Neo–triazine-based derivatives as gene delivery vectors was further assessed and compared. For a matter of clarity, Neo–triazine conjugates are hereinafter referred to as follows: derivative 8a, 1_ST; derivative 8b, 1_OL; derivative 8c, 1_Chol; derivative 9aa, 2_ST; derivative 9bb, 2_OL; derivative 9cc, 2_Chol; derivative 9ab, 1_ST-1_OL; derivative 9ac, 1_ST-1_Chol; derivative 9bc, 1_OL-1_Chol (Table 1).
Table 1. Details about Compounds and Concentrations of Positive Charges ([+]) for Every Neo–Triazine Lipid Stock Solution.
| compound | [compound] (mg/mL) | [+] (mM) |
|---|---|---|
| 1_ST (derivative 8a) | 10.58 | 34.9 |
| 1_OL (derivative 8b) | 10.57 | 34.9 |
| 1_Chol (derivative 8c) | 11.80 | 35.1 |
| 2_ST (derivative 9aa) | 11.81 | 35.1 |
| 2_OL (derivative 9bb) | 11.79 | 35.1 |
| 2_Chol (derivative 9cc) | 14.24 | 35.4 |
| 1_ST-1_OL (derivative 9ab) | 11.80 | 35.1 |
| 1_ST-1_Chol (derivative 9ac) | 13.03 | 35.3 |
| 1_OL-1Chol (derivative 9bc) | 13.01 | 35.3 |
DNA Condensation Ability of Monotailed, Symmetric Two-Tailed, and Asymmetric Two-Tailed Neo–Triazine-Based Transfectants
Formation of lipoplexes results from charge neutralization of the anionic NAs through the electrostatic interactions with the cationic headgroup(s) of the transfectant.1,43,44 Due to the well-renowned great charge density of Neo,27,45 in this work this AG was used as the polar head. Since a slight-to-moderate excess of positive charge is needed to bury the NA within lipoplexes,46 we evaluated the ability of every Neo–triazine derivative to complex pDNA at progressively increasing charge ratios (CR, +/−). Of note, this index is defined as the ratio between amine moles (N, cationic moiety) of the transfectant with respect to the phosphate moles (P, anionic moiety) borne by a given pDNA amount.1 Quantification of the NAs condensation was performed with a fluorophore-exclusion titration assay using SYBR Green I as the fluorescent probe, which reveals the percentage of uncomplexed DNA that is accessible for dye intercalation at a given CR. As reported in Figure 2, Neo–triazine derivatives invariably exhibited a maximal complexation ability at CR ≥ 1.5. This means that they have greater affinity for NAs if compared to the gold standard 25 kDa branched PEI (bPEI), which exhibited a maximal complexation at CR ≥ 3.1 A reason for that relies on the basicity of the Neo and the melamine scaffold that contributed to the electrostatic interactions with DNA. As all of the transfectants displayed very similar behavior, the lipophilic tails were shown to have no effects on NAs condensation ability. In fact, these results are not surprising if considering that nonpolar tails are responsible for the lipid behavior in water, including phase transition, thus having an effect on the overall stability and finally on the cytotoxicity and transfection efficiency of the resulting lipoplexes.8
Figure 2.
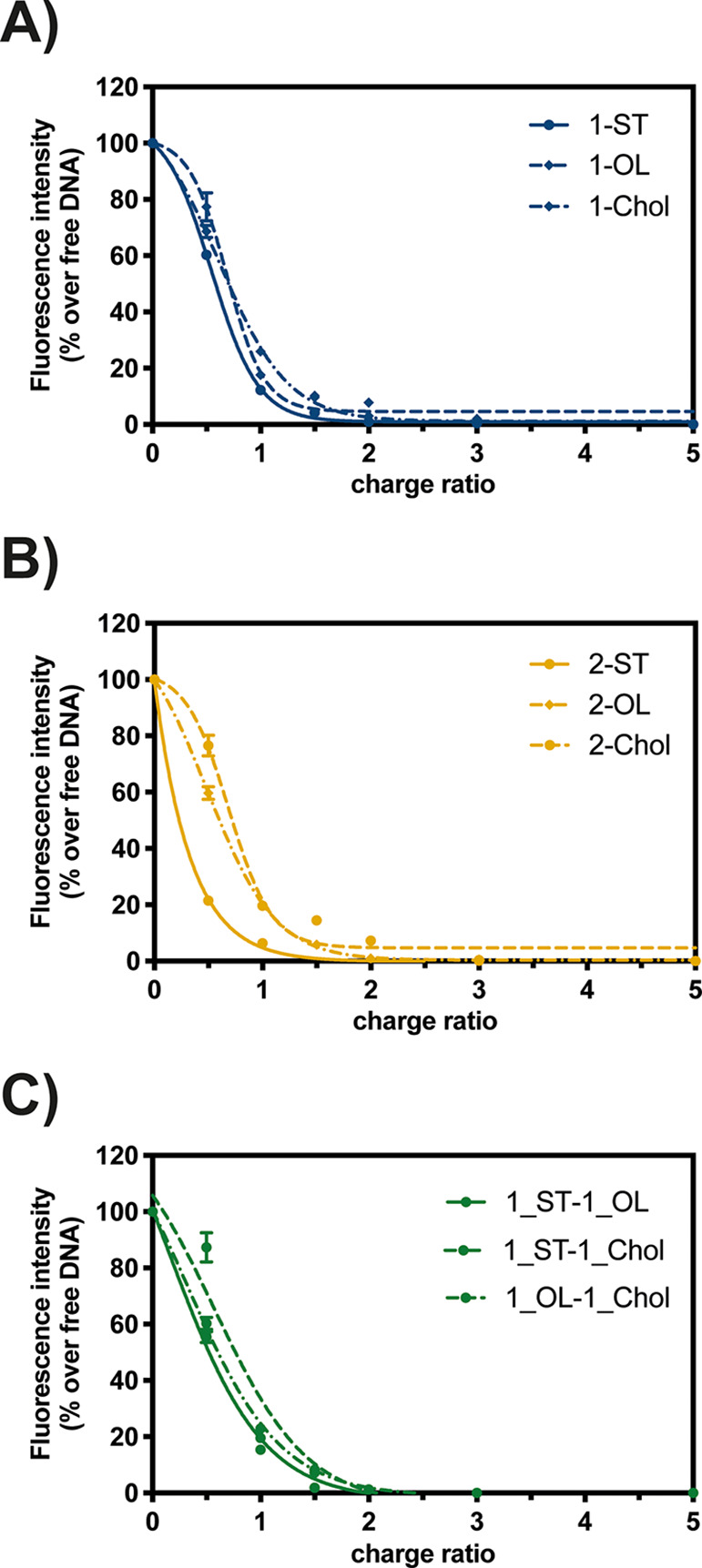
Comparative DNA complexation ability: (A) monotailed Neo–triazine conjugates 1_ST, 1_OL, and 1_Chol; (B) symmetric two-tailed Neo–triazine conjugates 2_ST, 2_OL, and 2_Chol; (C) asymmetric two-tailed Neo–triazine conjugates 1_ST-1_OL, 1_ST-1_Chol, and 1_OL1_Chol. Complexation was evaluated by monitoring the fluorochrome (SYBR Green I) exclusion from lipoplexes as a function of the charge ratio (CR, +/−).
In Vitro Biological Performance of Neo–Triazine Conjugates
The ability shown by Neo–triazine derivatives to effectively bind the DNA prompted us to challenge cells in vitro with such vectors. The main goal was to assess the role played by the number and type (chemical structure) of hydrophobic tails on the overall biological behavior of AG-based lipids.
In Vitro Transfection Performance of Neo–Triazine-Based Lipoplexes
It is a matter of fact that among the three domains of a CL, hydrophobic tails do influence mostly the transfection efficiency and cytotoxicity of the resulting lipoplexes.8,47 Generally, nonpolar tails are grouped as aliphatic- and steroid-based domains, and both types have shown intriguing results in transfection.12 In this context, there is no general consensus as to which the optimal tail design should be. In this study we thus evaluated the effectiveness of an array of Neo–triazine derivatives bearing tails of various type and number. Specifically, two different aliphatic chains, either saturated (i.e., stearyl, ST) or unsaturated (i.e., oleyl, OL), and a cyclic (i.e., Chol) domain were used as hydrophobic motifs to synthesize CLs. Neo–triazine derivatives were therefore evaluated and compared for their ability to transfect two types of mammalian cells, namely L929 and HeLa cell lines.
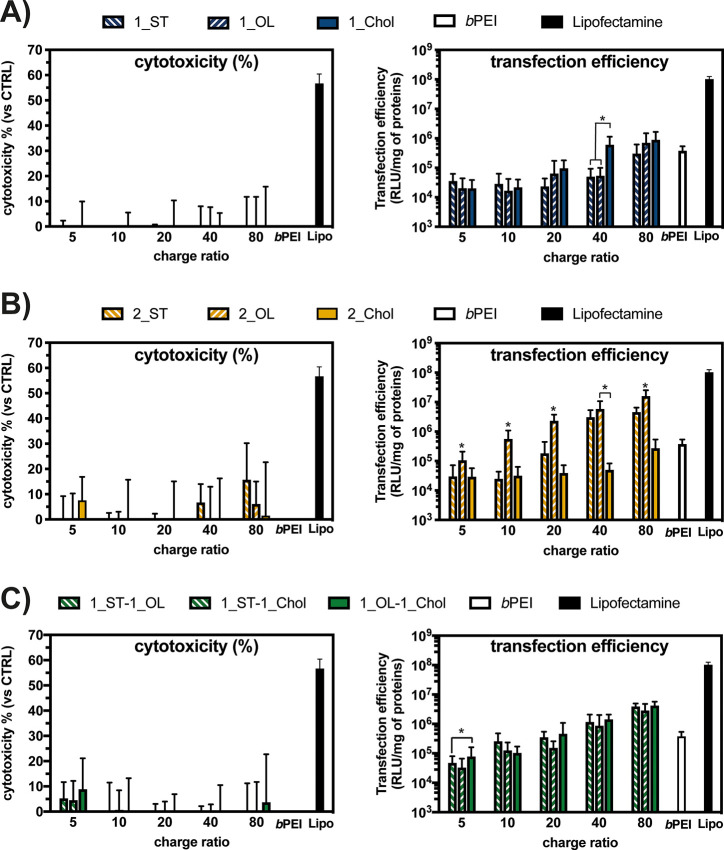
As shown in Figures 3 and S22, Neo–triazine-based CLs exhibited low-to-negligible cytotoxicities, very high transfection efficiencies, and overall performance similar to or even greater than the gold standard bPEI and the commercially sourced Lipofectamine 2000. As a rule of thumb, although Neo–triazine derivatives were generally slightly less effective in transfection than the benchmark lipid transfectant, they were far less toxic to cells. This implies that their overall performance was greater. Besides, as expected, the transfection profiles of Neo–triazine-based lipoplexes were strongly dependent on the CR used to carry out the experiments, such that the higher the CR, the greater the transfection efficiency.25
Figure 3.
Comparative cytotoxicity and transfection efficiency, evaluated on L929 cells, of complexes prepared by mixing pGL3 with Neo–triazine derivatives at different charge ratios (CR, +/−), 25 kDa bPEI and Lipofectamine 2000 (Lipo). Transfection efficiency is expressed as luminescence signal (RLU) normalized to the total protein content in each cell lysate: (A) monotailed Neo–triazine conjugates 1_ST, 1_OL, and 1_Chol; (B) symmetric two-tailed Neo–triazine conjugates 2_ST, 2_OL, and 2_Chol; (C) asymmetric two-tailed Neo–triazine conjugates 1_ST-1_OL, 1_ST-1_Chol, and 1_OL-1_Chol. Results are expressed as mean ± SD (n ≥ 3) (*, p < 0.05 vs other lipids for any given CR).
When comparing monotailed CLs, 1_ST and 1_OL (8a and 8b, respectively) displayed similar trends as a function of CR and an optimum at CR 80. On the other hand, 1_Chol (derivative 8c), which is the CL bearing one Chol molecule, was found to be the slightly more effective in both cell lines (Figures 3A and S22A) already at CR 40.
On the other hand, it is generally accepted that lipids bearing more than one lipophilic moiety are more effective than their monotailed analogues.47 With this in mind, we sought to evaluate whether the combinations of different aliphatic- and steroid-based tails had an effect on the ultimate transfection performance of Neo–triazine-based CLs. An opposite transfection trend was found for symmetric two-tailed derivatives 2_ST, 2_OL, and 2_Chol (9aa, 9bb, and 9cc, respectively) with respect to monotailed counterparts. In more detail, two-tailed lipids bearing Chol were found to be less efficient than the other two kinds of lipids with aliphatic chains (Figures 3B and S22B). Of note, lipoplexes based on 2_OL at CR ≥ 40 were even more effective in transfection than the bPEI-based complexes. Such results are in good agreement with previously published findings on AG-based CLs.28,29 The superior transfection performance of the dioleyl derivatives (2_OL) may be ascribed to the presence of unsaturated hydrophobic chains, which are known to increase the membrane fluidity and fusogenicity of the resulting lipoplexes.48,49 Our results are also in good agreement with Loizeau et al., who showed that C18-long, monounsaturated amphiphiles were the most effective in terms of DNA complexation and transfection efficiency.50
Besides, it is accepted that lipids having asymmetric tails are generally very effective in transfection because of the different arrangement of the resulting supramolecular structures.51 Again, each and every asymmetric two-tailed derivative, namely 1_ST-1_OL, 1_ST-1_Chol, and 1_OL-1_Chol (9ab, 9ac, and 9bc, respectively) showed a good transfection behavior at CR > 40 (Figures 3C and S22C), even greater than the gold standard bPEI.
It is worthy of note that the newly synthesized Neo–triazine-based derivatives proved to be effective gene delivery vehicles due to the negligible cytotoxicities and very high transfection efficiencies, the latter being even superior to those of commercially sourced gold standard transfectants. It is even more important that Neo–triazine-based lipids were not formulated with any helper lipids, such as DOPE, which are often used in order to increase the ultimate transfection efficiency of the CLs.
Overall, we found that two-tailed derivatives were far more effective than one-tailed counterparts. A possible explanation as to why the transfection efficiency of two-tailed CLs was the highest may rely on their tendency to aggregate into stable assemblies once in aqueous solutions.52,53 By contrast, single-chained CLs are considered more prone to form unstable lipoplexes, which are less effective in transfection.54,55
Antibacterial Activity of Neo–Triazine-Based Conjugates
When dealing with AGs as building blocks for the development of gene delivery vectors, one must remind that such class of molecules are relevant in clinical practice as antibiotics for treatment of Gram-negative bacterial infections22,24 and were even used as antiviral agents.56 Therefore, we hypothesized that Neo-based CLs may have a noteworthy antimicrobial activity.
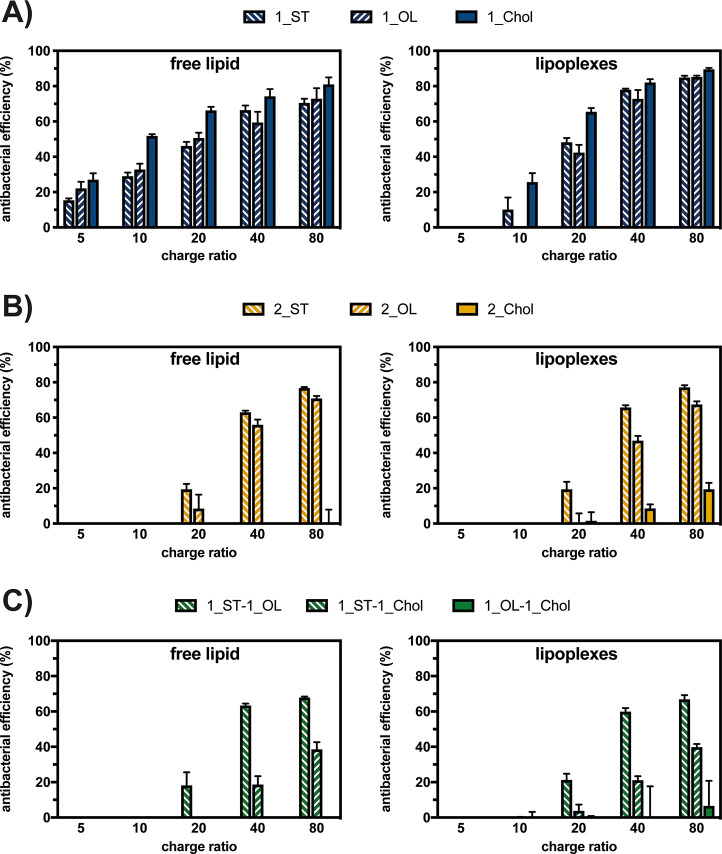
With this in mind, we investigated the antibacterial efficiency of Neo–triazine-based CLs used as free lipids and complexed with pDNA to give lipoplexes (Figure 4). We tested the antibacterial efficacy of the two preparations against the Gram-negative bacteria Escherichia coli (E. coli). As depicted in Figure 4, all derivatives displayed a dose-dependent antibacterial activity. Monotailed CLs 1_ST (8a), 1_OL (8b), and 1_Chol (8c) were effective in inhibiting bacterial growth, reaching up to 90% of bacterial inhibition when prepared as lipoplex suspensions at the optimal CR ≥ 40 (Figure 4A). Overall, the mono-Chol-bearing lipid (1_Chol) exhibited the greatest antibacterial activity. Conversely, two-tailed CLs bearing at least one Chol moiety, namely 2_Chol (derivative 9cc), 1_ST-1_Chol (9ac), and 1_OL-1_Chol (9bc) (Figure 4B and 4C), showed poor, if not negligible, antibacterial efficiency. On the other hand, two-tailed aliphatic and symmetric derivatives 2_ST (9aa) and 2_OL (9bb) as well as the asymmetric 1_ST-1_OL (9ab) conjugate were quite effective as antibacterial agents and showed antibacterial efficiencies > 50% at CR ≥ 40. Such percentage values were defined according to the literature.57 Interestingly, we found a slight difference between the antibacterial performance of the AG derivatives when used as free lipids or mixed with pDNA to give lipoplexes (Figure 4), according to what we previously found in a study on calixarene-based antimicrobial CLs.26 In this regard, single-tailed aliphatic CLs (1_ST, 1_OL, and 1_Chol) were found to be significantly more effective against bacteria when delivered in the form of lipoplexes (p < 0.05).
Figure 4.
Comparative antibacterial activity on E. coli of DNA-free lipids and lipoplexes prepared by mixing pGL3 with Neo–triazine derivatives at different charge ratios (CRs, +/−), corresponding to different [Neo]: (A) monotailed Neo–triazine conjugates 1_ST, 1_OL and 1_Chol; (B) symmetric two-tailed Neo–triazine conjugates 2_ST, 2_OL, and 2_Chol; (C) asymmetric two-tailed Neo–triazine conjugates 1_ST-1_OL, 1_ST-1_Chol, and 1_OL-1_Chol. Results are expressed as mean ± SD (n ≥ 3) (*, p < 0.05 vs each respective lipid counterpart for any given CR).
We can speculate that some of the differences in the antibacterial activity found among mono- and bisubstituted CLs and their extent may rely on the presence of different hydrophobic moieties invariably linked to Neo. To support further this hypothesis and to shed some light on the effect of the lipophilic tail on the antibacterial activity of Neo-bearing CLs, we tested the antibacterial efficiency of the antibiotic Neo per se. As we found that the minimum inhibitory concentration (MIC) of Neo against E. coli was 2 μg/mL (∼2.2 μM) (Figure S23), one may speculate that the steric hindrance and the lipophilicity caused by the tail(s) may account for the slightly lower antibacterial efficiency of Neo–triazine conjugates, as reported elsewhere.26 Nevertheless, while their antibacterial mode of action is still poorly understood, lipids have been found to destabilize bacterial membranes and cause a wide variety of other outcomes, such as inhibition of enzyme activity, impairment of nutrient uptake, generation of peroxidation and auto-oxidation degradation products, or direct bacteriolysis as well as inhibition of fatty acid synthesis.58−60
Taken together, these data disclose the multifunctionality of Neo–triazine-based CLs as effective gene delivery vectors with noteworthy antimicrobial activity.
Conclusions
Herein, we reported the synthesis and characterization of an array of multifunctional Neo–triazine-based CLs differing by the presence of specific lipid moieties, which possess combined virtues such as gene transfection capability, negligible cytotoxicity, and antibacterial activity. The synthetic strategy envisioned to produce these vectors is very straightforward, and thus, it is particularly suitable for the synthesis of large libraries of triazine-based cationic amphiphiles. Indeed, intermediates 5 and 6, bearing one and two lipophilic tails, respectively, are obtained in multigram scale thanks to the easy chemoselective functionalization of the triazine core and can be further functionalized with different cationic moieties through azide–alkyne click reaction.
Biophysical studies indicated that Neo can profitably be used as a cationic headgroup to synthesize CLs very effective in transfection. Among the whole array of synthesized AG-triazine-based CLs, monotailed, Chol-bearing lipid (1_Chol = 8c derivative) and symmetric two-tailed ST and OL derivatives (2_ST = 9aa and 2_OL = 9bb, respectively) were found to be the most promising lipid transfectants because they displayed negligible cytotoxicity and great transfection efficiency. Notably, Neo–triazine-based CLs exerted a marked and dose-dependent activity against Gram-negative bacteria.
The versatility of the synthetic pathway along with knowledge about the basic structure–function relationships obtained in this work can give some hints on the design of next-generation biomedical materials able to elicit pharmacological effects and fight bacterial infections at once.
Experimental Procedures
Materials
L929 (murine fibroblast from subcutaneous connective tissue; ATCC-CCL-1) and HeLa (human cervix carcinoma, CCL-2) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). AlamarBlue Cell Viability Assay was from Life Technologies Italia (Monza, Italy), while the BCA Protein Assay Kit was from ThermoFisher (Monza, Italy). pDNA encoding the modified firefly luciferase (pGL3-Control Vector, 5.2 kbp) and Luciferase Assay System were obtained from Promega (Milan, Italy). pDNA was amplified, isolated, purified, and diluted in 0.1× TE buffer (1 mM Tris, pH 8; 0.1 mM EDTA) as previously described.61
Gram-negative E. coli JM109 bacterial strain (BioSafety Level 1) was purchased from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).
All of the other chemicals were from Merck Life Science S.r.l. (Rome, Italy), if not differently specified.
Synthesis of Neo–Triazine Conjugates
Synthesis of N-(2-(2-(2-Azidoethoxy)ethoxy)ethyl)-4,6-dichloro-1,3,5-triazine-2-amine 3
Cyanuric chloride 1 (500 mg, 2.7 mmol) was dissolved in 20 mL of a 1:1 (v/v) mixture of H2O/acetone and cooled in an ice bath. Compound 2 (472 mg, 2.7 mmol) was dissolved in the minimum amount of acetone by dropwise addition. NaHCO3 (228 mg, 2.7 mmol) was added, then the reaction mixture was stirred for 3 h at 0 °C. The solvents were evaporated under reduced pressure, then the crude product was dissolved in 20 mL of ethyl acetate and washed with a 1 M HCl (2 × 10 mL), a saturated NaHCO3 aqueous solution (2 × 10 mL), and brine (2 × 10 mL), dried over Na2SO4, and concentrated. Flash column chromatography (FCC) of the residue (hexane–ethyl acetate 8:2) afforded the desired product 3 as a colorless oil in 80% yield.
Synthesis of One-Tailed Intermediates 5a–c
3 (600 mg, 1.9 mmol) was dissolved in 15 mL of a 1:1 (v/v) mixture of H2O/acetone. R-NH2 (2.8 mmol) and NaHCO3 (235 mg, 2.8 mmol) were added, then the reaction mixture was stirred at room temperature (rt) overnight. The volatiles were removed under reduced pressure. The residue was dissolved in CH2Cl2 and washed with 1 M HCl (2 × 10 mL), saturated NaHCO3 aqueous solution (2 × 10 mL), and brine (2 × 10 mL), dried over Na2SO4, and concentrated. FCC of the residue (hexane–ethyl acetate 7:3) afforded the desired products.
Synthesis of Two-Tailed Intermediates 6aa–cc and 6ab–bc
Compound 5 (1 equiv) was dissolved in 3 mL of toluene; then DIPEA (1.5 equiv) and R’NH2 (1.5 equiv) were added. The reaction was put under shaking in a sealed vial at 80 °C for 2 days, then the solvent was removed under reduced pressure. The residue was dissolved in CH3Cl and washed with 1 M HCl, a saturated NaHCO3 aqueous solution, and brine. FCC (hexane:ethyl acetate 1:1) afforded the desired products as powders.
Synthesis of One-Tailed Neo Derivatives 8a–c
Neo derivative 7 (1 equiv) was dissolved in CH2Cl2, then compound 5 (1.5 equiv) was added to the reaction mixture. CuSO4 (0.1 equiv), Na–ascorbate (0.1 equiv), and TBTA (0.01 equiv) dissolved in water were added to the reaction mixture. The final mixture was stirred for 15 h at rt. Following solvent evaporation, the crude product was dissolved in EtOAc and washed with a 5% EDTA solution and brine. FCC (MeOH in CH2Cl2 0–5%) afforded compounds Boc-8a–c. The derivatives Boc-8a–c were treated with a mixture of TFA:CH2Cl2 (1:1) for 2 h. After a freeze-drying cycle, 8a–c were obtained as fluffy solids.
Synthesis of Two-Tailed Neo Derivatives 9aa–cc and 9ab–bc
Neo derivative 7 (1 equiv) was dissolved in CH2Cl2; then compound 6 (1.5 equiv) was added to the reaction mixture. CuSO4 (0.1 equiv), Na–ascorbate (0.1 equiv), and TBTA (0.01 equiv) dissolved in water were added, then the final reaction mixture was stirred for 15 h at rt. Following solvent evaporation, the crude product was dissolved in EtOAc and washed with a 5% EDTA solution and brine. FCC (MeOH in CH2Cl2 0–5%) afforded compounds Boc-9aa–cc and Boc-9ab–bc. The derivatives Boc-9aa–cc and Boc-9ab–bc were treated with a mixture of TFA:CH2Cl2 (1:1) for 2 h. After freeze drying, 9aa–cc and 9ab–bc were obtained as fluffy solids.
Chemical Characterization of Neo–Triazine Conjugates
Thin-layer chromatography (TLC) was carried out on sheets of silica gel 60 F254 on aluminum (layer thickness 0.25 mm, Merck). Visualization of the developed chromatogram was achieved with UV light and ceric ammonium molybdate (CAM) or ninhydrin stains. Chromatographic purification of products was accomplished by column chromatography on silica gels (mesh 100–200). 1H NMR, 13C NMR, and 19F NMR spectra were recorded on Bruker spectrometers (400 and 500 MHz). Chemical shifts are expressed in ppm (δ) using tetramethylsilane (TMS) as internal standard for 1H and 13C nuclei (δH and δC = 0.00).
Preparation of the Transfectant Stock Solutions
Neo–triazine-based compounds were diluted in deionized water to the final compound and positive charges concentrations ([+]) reported in Table 1, while 25 kDa bPEI was diluted in 10 mM HEPES to a final concentration of 0.86 mg/mL and a [N] = [+] = 20 mM.1 Neo–triazine-based lipid solutions and bPEI solutions were stored at 4 °C until use.
Preparation of Neo–Triazine/pDNA Complexes
Before complexation, transfectant and pDNA stock solutions were warmed to rt. Complexes were prepared by mixing (through vigorous pipetting) the pDNA and transfectant solutions, prepared at the desired concentration to yield different CRs (i.e., 5, 10, 20, 40 and 80), followed by a 20 min incubation at rt.
Evaluation of DNA Binding Ability
The DNA binding ability of every Neo–triazine conjugate was monitored by a fluorophore-displacement assay, as reported elsewhere.1,26,42 Briefly, complexes were invariably prepared by mixing 0.25 μg of pDNA in 1.0 μL of 200× SYBR Green I (λex = 497 nm, λem = 520 nm) with 11.8 μL of lipid solutions at different concentrations, yielding a final DNA concentration of 20 ng/μL and different CRs. Afterward, complexes were incubated for 20 min at rt in the dark and then diluted to a final volume of 200 μL in 10 mM HEPES. Fluorescence measurements (n = 3 per condition) were performed with a Synergy H1 spectrophotometer (BioTek, Italy) in 384-multiwell black plates (λex = 487 nm, λem = 528 nm). Data are given as relative fluorescence values normalized to the fluorescence of uncomplexed pDNA.
In Vitro Cells Transfection Experiments
Mycoplasma-free L929 and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 2 mM glutamine and supplemented with 10% (v/v) fetal bovine serum (FBS) (i.e., culture medium) in a humidified atmosphere under constant supply of 5% CO2 and at 37 °C.
Twenty-four hours before transfection assays, cells were seeded onto 96-well plates at a density of 2 × 104 cells/cm2 and maintained in standard culture conditions. Next, 160 ng/well of pGL3 was complexed in 10 mM HEPES with Neo–triazine solutions to yield different CRs, as described above, and cells were incubated with complexes in 100 μL/well of culture medium under standard culture conditions for 24 h. Cells transfected with 25 kDa bPEI/DNA (i.e., complexes prepared in 10 mM HEPES at N/P 10) and Lipofectamine 2000/DNA complexes (prepared at a 2.5:1 (v/w) lipid/DNA ratio in Opti-MEM, according to manufacturer’s instructions) were used as internal references.26,42
Following a 24 h transfection, cytotoxicity was evaluated by means of AlamarBlue assay according to the manufacturer’s instructions. Briefly, medium was removed, and each well was loaded with 100 μL of 1× resazurin dye-containing culture medium. Cells were next incubated in standard culture conditions for 2 h; then the fluorescence of the medium was read with a Synergy H1 spectrophotometer (BioTek, Italy) (λex = 540 nm; λem = 595 nm). Viability of untransfected cells (CTRL) was assigned to 100%, and cytotoxicity was calculated as follows
Transfection efficiency was evaluated measuring the luciferase activity by means of the Luciferase Assay System according to the manufacturer’s instructions. Briefly, cells were washed with phosphate-buffered saline (PBS) and lysed with 110 μL/well of Cell Culture Lysis Reagent (Promega, Italy); then 20 μL of cell lysates was added to 50 μL of Luciferase Assay Reagent, and luminescence was measured by means of a Synergy H1 spectrophotometer. The luminescence signal (expressed as relative light units, RLU) of each sample was normalized to its protein content determined by BCA assay. Transfection efficiencies were thus expressed as RLU/mg of proteins.
Antibacterial Tests
The antibacterial effectiveness of Neo–triazine derivatives was tested against Gram-negative E. coli JM109 strain as previously described.26,42 Briefly, bacteria were cultured in 5 mL of Luria–Bertani (LB) broth at 37 °C under shaking at 130 rpm, overnight, until reaching an optical density at λ = 600 nm (OD600 nm) ≈ 1, corresponding to ∼109 bacteria/mL. Bacterial suspensions were next diluted in LB broth to a final concentration of ∼106 bacteria/mL (referred to as bacterial test inoculum for the experiments). For antibacterial tests, 50 μL/well of the bacterial test inoculum was transferred to separate wells of 96-well plates, then 50 μL/well of LB broth containing Neo–triazine/pDNA complexes prepared at varying CRs, their corresponding (uncomplexed, DNA-free) Neo–triazine solutions, or Neo at different concentrations were added. Positive controls of bacterial growth (CTRL+) were 50 μL/well of bacteria added to 50 μL/well of LB. Afterward, bacteria were incubated at 37 °C for 24 h, then the antibacterial efficacy of every derivative was evaluated by means of the turbidity method (i.e., OD600 nm measurements).26,42 Briefly, 24 h post inoculum, the OD600 nm of each well (n = 3 per compound) was read by means of a GENios Plus reader (λ = 600 nm). The antibacterial activity was calculated according to the following equation:
MIC is the lowest [Neo] that gave rise to 100% antibacterial activity.
Statistical Analysis
Statistical analysis was carried out by GraphPad version 8 (GraphPad software, La Jolla, CA, USA). All data were initially analyzed using D’Agostino and Pearson omnibus normality test. Comparisons among groups were performed by one-way ANOVA (Tukey’s multiple comparisons test) and multiple t test (Holm-Sidak method). Significance was retained when p < 0.05. Data are expressed as mean ± standard deviation (SD). Experiments were performed at least in triplicate.
Acknowledgments
Politecnico di Milano and CNR are gratefully acknowledged for economic support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.bioconjchem.0c00616.
Chemical procedures and characterization (1H,13C NMR, and MS spectra for all newly synthesized compounds) (PDF)
Author Contributions
‡ C.P. and N.B.: These authors equally contributed to this work.
The authors declare no competing financial interest.
Dedication
This paper is dedicated to Mr. Massimo Frigerio on the occasion of his retirement from Politecnico di Milano.
This article was initially published with an incorrect copyright statement and was corrected on or around May 5, 2021.
Supplementary Material
References
- Bono N.; Ponti F.; Mantovani D.; Candiani G. (2020) Non-viral in vitro gene delivery: it is now time to set the bar!. Pharmaceutics 12, 183. 10.3390/pharmaceutics12020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.; Simanek E. E. (2012) Triazine dendrimers as drug delivery systems: from synthesis to therapy. Adv. Drug Delivery Rev. 64, 826–835. 10.1016/j.addr.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Steinhauff D.; Ghandehari H. (2019) Matrix mediated viral gene delivery: a review. Bioconjugate Chem. 30, 384–399. 10.1021/acs.bioconjchem.8b00853. [DOI] [PubMed] [Google Scholar]
- Wahane A.; Waghmode A.; Kapphahn A.; Dhuri K.; Gupta A.; Bahal R. (2020) Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules 25, 2866. 10.3390/molecules25122866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricobaraza A.; Gonzalez-Aparicio M.; Mora-Jimenez L.; Lumbreras S.; Hernandez-Alcoceba R. (2020) High-capacity adenoviral vectors: expanding the scope of gene therapy. Int. J. Mol. Sci. 21, 3643. 10.3390/ijms21103643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haasteren J.; Li J.; Scheideler O. J.; Murthy N.; Schaffer D. V. (2020) The delivery challenge: fulfilling the promise of therapeutic genome editing. Nat. Biotechnol. 38, 845–855. 10.1038/s41587-020-0565-5. [DOI] [PubMed] [Google Scholar]
- Yin H.; Kanasty R. L.; Eltoukhy A. A.; Vegas A. J.; Dorkin J. R.; Anderson D. G. (2014) Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555. 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- Buck J.; Grossen P.; Cullis P. R.; Huwyler J.; Witzigmann D. (2019) Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano 13, 3754–3782. 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- Zhi D.; Zhang S.; Cui S.; Zhao Y.; Wang Y.; Zhao D. (2013) The headgroup evolution of cationic lipids for gene delivery. Bioconjugate Chem. 24, 487–519. 10.1021/bc300381s. [DOI] [PubMed] [Google Scholar]
- Nie X.; Zhang Z.; Wang C. H.; Fan Y. S.; Meng Q. Y.; You Y. Z. (2019) Interactions in DNA condensation: an important factor for improving the efficacy of gene transfection. Bioconjugate Chem. 30, 284–292. 10.1021/acs.bioconjchem.8b00805. [DOI] [PubMed] [Google Scholar]
- Chen J.; Wang K.; Wu J.; Tian H.; Chen X. (2019) Polycations for gene delivery: dilemmas and solutions. Bioconjugate Chem. 30, 338–349. 10.1021/acs.bioconjchem.8b00688. [DOI] [PubMed] [Google Scholar]
- Zhi D.; Zhang S.; Wang B.; Zhao Y.; Yang B.; Yu S. (2010) Transfection efficiency of cationic lipids with different hydrophobic domains in gene delivery. Bioconjugate Chem. 21, 563–577. 10.1021/bc900393r. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-Y. (2017) Fluorinated polymers in gene delivery. Acta Polym. Sin. 8, 1215–1219. 10.11777/j.issn1000-3304.2017.17095. [DOI] [Google Scholar]
- Pezzoli D.; Chiesa R.; De Nardo L.; Candiani G. (2012) We still have a long way to go to effectively deliver genes!. J. Appl. Biomater. Funct. Mater. 2, 82–91. 10.5301/JABFM.2012.9707. [DOI] [PubMed] [Google Scholar]
- Srinivas R.; Samanta S.; Chaudhuri A. (2009) Cationic amphiphiles: promising carriers of genetic materials in gene therapy. Chem. Soc. Rev. 38, 3326–3338. 10.1039/b813869a. [DOI] [PubMed] [Google Scholar]
- Zhi D.; Bai Y.; Yang J.; Cui S.; Zhao Y.; Chen H.; Zhang S. (2018) A Review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 253, 117–140. 10.1016/j.cis.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Niculescu-Duvaz D.; Heyes J.; Springer C. (2003) Structure-activity relationship in cationic lipid mediated gene transfection. Curr. Med. Chem. 10, 1233–1261. 10.2174/0929867033457476. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Mahato R. I. (2010) Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opin. Drug Delivery 7, 1209–1226. 10.1517/17425247.2010.513969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinoglu S.; Wang M.; Xu Q. (2015) Combinatorial library strategies for synthesis of cationic lipid-like nanoparticles and their potential medical applications. Nanomedicine 10, 643–657. 10.2217/nnm.14.192. [DOI] [PubMed] [Google Scholar]
- Karmali P. P.; Chaudhuri A. (2007) Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med. Res. Rev. 27, 696–722. 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- Tor Y. (2003) Targeting RNA with small molecules. ChemBioChem 4, 998–1007. 10.1002/cbic.200300680. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Dix A. V.; Tor Y. (2010) Antibiotic selectivity for prokaryotic vs. eukaryotic decoding sites. Chem. Commun. 46, 5542–5544. 10.1039/c0cc00423e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosso M. Y.; Li Y.; Garneau-Tsodikova S. (2014) New trends in the use of aminoglycosides. MedChemComm 5, 1075–1091. 10.1039/C4MD00163J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci M. C.; Volonterio A. (2020) Aminoglycosides: from antibiotics to building blocks for the synthesis and development of gene delivery vehicles. Antibiotics 9, 504. 10.3390/antibiotics9080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desigaux L.; Sainlos M.; Lambert O.; Chevre R.; Letrou-Bonneval E.; Vigneron J. P.; Lehn P.; Lehn J. M.; Pitard B. (2007) Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proc. Natl. Acad. Sci. U. S. A. 104, 16534–16539. 10.1073/pnas.0707431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono N.; Pennetta C.; Sganappa A.; Giupponi E.; Sansone F.; Volonterio A.; Candiani G. (2018) Design and synthesis of biologically active cationic amphiphiles built on the calix[4]Arene scaffold. Int. J. Pharm. 549, 436–445. 10.1016/j.ijpharm.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Le Gall T.; Baussanne I.; Halder S.; Carmoy N.; Montier T.; Lehn P.; Décout J. L. (2009) Synthesis and transfection properties of a series of lipidic neamine derivatives. Bioconjugate Chem. 20, 2032–2046. 10.1021/bc900062z. [DOI] [PubMed] [Google Scholar]
- Mével M.; Sainlos M.; Chatin B.; Oudrhiri N.; Hauchecorne M.; Lambert O.; Vigneron J. P.; Lehn P.; Pitard B.; Lehn J. M. (2012) Paromomycin and neomycin B derived cationic lipids: synthesis and transfection studies. J. Controlled Release 158, 461–469. 10.1016/j.jconrel.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Habrant D.; Peuziat P.; Colombani T.; Dallet L.; Gehin J.; Goudeau E.; Evrard B.; Lambert O.; Haudebourg T.; Pitard B. (2016) Design of ionizable lipids to overcome the limiting step of endosomal escape: application in the intracellular delivery of mRNA, DNA, and siRNA. J. Med. Chem. 59, 3046–3062. 10.1021/acs.jmedchem.5b01679. [DOI] [PubMed] [Google Scholar]
- Chen M.; Hu M.; Wang D.; Wang G.; Zhu X.; Yan D.; Sun J. (2012) Multifunctional hyperbranched glycoconjugated polymers based on natural aminoglycosides. Bioconjugate Chem. 23, 1189–1199. 10.1021/bc300016b. [DOI] [PubMed] [Google Scholar]
- Ghilardi A.; Pezzoli D.; Bellucci M. C.; Malloggi C.; Negri A.; Sganappa A.; Tedeschi G.; Candiani G.; Volonterio A. (2013) Synthesis of multifunctional PAMAM-aminoglycoside conjugates with enhanced transfection efficiency. Bioconjugate Chem. 24, 1928–1936. 10.1021/bc4003635. [DOI] [PubMed] [Google Scholar]
- Miryala B.; Godeshala S.; Grandhi T. S. P.; Christensen M. D.; Tian Y.; Rege K. (2016) Aminoglycoside-derived amphiphilic nanoparticles for molecular delivery. Colloids Surf., B 146, 924–937. 10.1016/j.colsurfb.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Sganappa A.; Wexselblatt E.; Bellucci M. C.; Esko J. D.; Tedeschi G.; Tor Y.; Volonterio A. (2017) Dendrimeric guanidinoneomycin for cellular delivery of bio-macromolecules. ChemBioChem 18, 119–125. 10.1002/cbic.201600422. [DOI] [PubMed] [Google Scholar]
- Blotny G. (2006) Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 62, 9507–9522. 10.1016/j.tet.2006.07.039. [DOI] [Google Scholar]
- Zacharie B.; Abbott S. D.; Bienvenu J. F.; Cameron A. D.; Cloutier J.; Duceppe J. S.; Ezzitouni A.; Fortin D.; Houde K.; Lauzon C.; Moreau N.; Perron V.; Wilb N.; Asselin M.; Doucet A.; Fafard M.-E.; Gaudreau D.; Grouix B.; Sarra-Bournet F.; St-Amant N.; Gagnon L.; Penney C. L. (2010) 2,4,6-Trisubstituted triazines as protein A mimetics for the treatment of autoimmune diseases. J. Med. Chem. 53, 1138–1145. 10.1021/jm901403r. [DOI] [PubMed] [Google Scholar]
- Vineberg J. G.; Zuniga E. S.; Kamath A.; Chen Y. J.; Seitz J. D.; Ojima I. (2014) Design, synthesis, and biological evaluations of tumor-targeting dual-warhead conjugates for a taxoid-camptothecin combination chemotherapy. J. Med. Chem. 57, 5777–5791. 10.1021/jm500631u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiani G.; Frigerio M.; Viani F.; Verpelli C.; Sala C.; Chiamenti L.; Zaffaroni N.; Folini M.; Sani M.; Panzeri W.; Zanda M. (2007) Dimerizable redox-sensitive triazine-based cationic lipids for in vitro gene delivery. ChemMedChem 2, 292–296. 10.1002/cmdc.200600267. [DOI] [PubMed] [Google Scholar]
- Merkel O. M.; Mintzer M. A.; Sitterberg J.; Bakowsky U.; Simanek E. E.; Kissel T. (2009) Triazine dendrimers as nonviral gene delivery systems: effects of molecular structure on biological activity. Bioconjugate Chem. 20, 1799–1806. 10.1021/bc900243r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel O. M.; Mintzer M. A.; Librizzi D.; Samsonova O.; Dicke T.; Sproat B.; Garn H.; Barth P. J.; Simanek E. E.; Kissel T. (2010) Triazine dendrimers as nonviral vectors for in vitro and in vivo RNAi: the effects of peripheral groups and core structure on biological activity. Mol. Pharmaceutics 7, 969–983. 10.1021/mp100101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel O. M.; Zheng M.; Mintzer M. A.; Pavan G. M.; Librizzi D.; Maly M.; Höffken H.; Danani A.; Simanek E. E.; Kissel T. (2011) Molecular modeling and in vivo imaging can identify successful flexible triazine dendrimer-based siRNA delivery systems. J. Controlled Release 153, 23–33. 10.1016/j.jconrel.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiani G.; Pezzoli D.; Cabras M.; Ristori S.; Pellegrini C.; Kajaste-Rudnitski A.; Vicenzi E.; Sala C.; Zanda M. A. (2008) Dimerizable cationic lipid with potential for gene delivery. J. Gene Med. 10, 637–645. 10.1002/jgm.1186. [DOI] [PubMed] [Google Scholar]
- Bono N.; Pennetta C.; Bellucci M. C.; Sganappa A.; Malloggi C.; Tedeschi G.; Candiani G.; Volonterio A. (2019) Role of generation on successful DNA delivery of PAMAM-(guanidino)neomycin conjugates. ACS Omega 4, 6796–6807. 10.1021/acsomega.8b02757. [DOI] [Google Scholar]
- Lucotti A.; Tommasini M.; Pezzoli D.; Candiani G. (2014) Molecular interactions of DNA with transfectants: a study based on infrared spectroscopy and quantum chemistry as aids to fluorescence spectroscopy and dynamic light scattering analyses. RSC Adv. 4, 49620–49627. 10.1039/C4RA08845J. [DOI] [Google Scholar]
- Mintzer M. A.; Simanek E. E. (2009) Nonviral vectors for gene delivery. Chem. Rev. 109, 259–302. 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- Wang H.; Tor Y. (1997) Dimeric aminoglycosides: design, synthesis and RNA binding. Bioorg. Med. Chem. Lett. 7, 1951–1956. 10.1016/S0960-894X(97)00339-9. [DOI] [Google Scholar]
- Elouahabi A.; Ruysschaert J. M. (2005) Formation and intracellular trafficking of lipoplexes and polyplexes. Mol. Ther. 11, 336–347. 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Jones C. H.; Chen C. K.; Ravikrishnan A.; Rane S.; Pfeifer B. A. (2013) Overcoming nonviral gene delivery barriers: perspective and future. Mol. Pharmaceutics 10, 4082–4098. 10.1021/mp400467x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpicco S.; Canevari S.; Ceruti M.; Galmozzi E.; Rocco F.; Cattel L. (2004) Synthesis, characterization and transfection activity of new saturated and unsaturated cationic lipids. Farmaco 59, 869–878. 10.1016/j.farmac.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Heyes J.; Palmer L.; Bremner K.; MacLachlan I. (2005) Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Controlled Release 107, 276–287. 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Loizeau D.; Le Gall T.; Mahfoudhi S.; Berchel M.; Maroto A.; Yaouanc J. J.; Jaffrès P. A.; Lehn P.; Deschamps L.; Montier T.; Giamarchi F. (2013) Physicochemical properties of cationic lipophosphoramidates with an arsonium head group and various lipid chains: a atructure-activity approach. Biophys. Chem. 171, 46–53. 10.1016/j.bpc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Meka R. R.; Godeshala S.; Marepally S.; Thorat K.; Reddy Rachamalla H. K.; Dhayani A.; Hiwale A.; Banerjee R.; Chaudhuri A.; Vemula P. K. (2016) Asymmetric cationic lipid based non-viral vectors for an efficient nucleic acid delivery. RSC Adv. 6, 77841–77848. 10.1039/C6RA07256A. [DOI] [Google Scholar]
- Wang H.; Liu Y.; Zhang J.; Zhang Y.; Xia Y.; Yu X. (2014) Cyclen-based cationic lipids with double hydrophobic tails for efficient gene delivery. Biomater. Sci. 2, 1460–1470. 10.1039/C4BM00174E. [DOI] [PubMed] [Google Scholar]
- Li L.; Wang F.; Wu Y.; Davidson G.; Levkin P. A. (2013) Combinatorial synthesis and high-throughput screening of alkyl amines for nonviral gene delivery. Bioconjugate Chem. 24, 1543–1551. 10.1021/bc400158w. [DOI] [PubMed] [Google Scholar]
- Lv H.; Zhang S.; Wang B.; Cui S.; Yan J. (2006) Toxicity of cationic lipids and cationic polymers in gene delivery. J. Controlled Release 114, 100–109. 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Pinnaduwage P.; Schmitt L.; Huang L. (1989) Use of a quaternary ammonium detergent in liposome mediated DNA transfection of mouse L-cells. Biochim. Biophys. Acta, Biomembr. 985, 33–37. 10.1016/0005-2736(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Wang G.; Zhang L. H.; Ye X. S. (2007) Modifications of aminoglycoside antibiotics targeting RNA. Med. Res. Rev. 27, 279–316. 10.1002/med.20085. [DOI] [PubMed] [Google Scholar]
- Soothill J. S.; Ward R.; Girling A. J. (1992) The IC50: an exactly defined measure of antibiotic sensitivity. J. Antimicrob. Chemother. 29, 137–139. 10.1093/jac/29.2.137. [DOI] [PubMed] [Google Scholar]
- Zheng C. J.; Yoo J. S.; Lee T. G.; Cho H. Y.; Kim Y. H.; Kim W. G. (2005) Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 579, 5157–5162. 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Desbois A. P.; Smith V. J. (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 85, 1629–1642. 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- Yoon B. K.; Jackman J. A.; Valle-González E. R.; Cho N. J. (2018) Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 19, 1114. 10.3390/ijms19041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloggi C.; Pezzoli D.; Magagnin L.; De Nardo L.; Mantovani D.; Tallarita E.; Candiani G. (2015) Comparative evaluation and optimization of off-the-shelf cationic polymers for gene delivery purposes. Polym. Chem. 6 (35), 6325–6339. 10.1039/C5PY00915D. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.