Abstract

This communication describes the development of a metal-free catalytic decarboxylation of silyl alkynoates to alkynylsilanes. Treatment of a silyl alkynoate with a catalytic amount of tetrabutylammonium difluorotriphenylsilicate (TBAT) in N,N-dimethylformamide at 150 °C resulted in decarboxylation to give the corresponding alkynylsilane in good to excellent yield (75 → 95%). The TBAT system was applicable to the decarboxylation of sterically demanding silyl alkynoates such as tert-butyldiphenylsilyl 3-phenylpropiolate. Mechanistic studies revealed that the tetrabutylammonium alkynoate derived from TBAT and the silyl alkynoate act as a catalyst for the decarboxylation.
Introduction
Decarboxylative transformations are recognized as useful reactions in organic synthesis, because carboxylic acids and their ester derivatives are readily available starting materials.1 Among them, the class of decarboxylation reactions that connect two moieties in a molecule separated by a carboxyl group, such as the protodecarboxylation of carboxylic acids and decarboxylative allylic alkylation of β-keto allyl esters, are operationally advantageous: the only stoichiometric coproduct is carbon dioxide, which is nontoxic, nonflammable, and readily removable from the reaction medium.2
Alkynylsilanes are useful building blocks in organic synthesis.3 While the nucleophilic substitution of a halosilane with a metal acetylide prepared by deprotonating a terminal alkyne with an organometallic reagent is one of the most common methods for the synthesis of alkynylsilanes (Scheme 1a),4 the high nucleophilicity of metal acetylides limits their functional group tolerance, and the coproducts derived from the organometallic reagents are sometimes problematic from the viewpoints of practicality and atom economy. Therefore, the catalytic Si–C cross-coupling of a terminal alkyne with a silicon electrophile has attracted increasing attention, and various silicon electrophiles such as halosilanes,5,6 hydrosilanes,7 and vinylsilanes8 are now available for this purpose (Scheme 1b). On the other hand, we recently reported a catalytic decarboxylation approach for the synthesis of alkynylsilanes (Scheme 1c).9−11 In the presence of a copper-based catalyst, a silyl alkynoate is decarboxylated to afford the corresponding alkynylsilane. Although the copper-catalyzed system has some advantages such as a low catalyst loading, mild reaction conditions, and easy-to-remove carbon dioxide as the coproduct, the steric hindrance of the silyl substituents strongly affected the reaction progress and the decarboxylation of silyl alkynoates with bulky silyl groups such as the triisopropylsilyl group was significantly impeded.
Scheme 1. Approaches to the Synthesis of Alkynylsilanes.
Owing to the high affinity of the fluoride ion to silicon atoms, the activation of organosilicon compounds by fluoride ion is a common procedure for the transformations of organosilicon compounds.12 Indeed, alkaline metal fluorides have been applied as catalysts in the decarboxylation of trimethylsilyl perfluorobenzoates.13 Although the applicable substrates are limited to trimethylsilyl penta- and tetrafluorobenzoates, and the applicability of the approach to the decarboxylation of other silyl esters was unclear, we expected that the small, hard fluoride ion would activate silyl alkynoates to induce decarboxylation, irrespective of the steric bulk of the silyl substituents (Scheme 1d).
Results and Discussion
The decarboxylation of triisopropylsilyl 3-phenylpropiolate (1a) was initially investigated in the presence of 5 mol % KF in N,N-dimethylformamide (DMF) at 150 °C, and the desired alkynylsilane 2a was obtained in 42% yield after 1 h (Table 1, entry 1). Other potassium salts (KCl, KBr, and KI) showed remarkably diminished catalytic performance, demonstrating the importance of fluoride ion (entries 2–4). While NaF gave an inferior result (entry 5), a higher yield of 55% was observed in the decarboxylation with CsF (entry 6). An alkaline earth metal fluoride, MgF2, was also examined, but only a trace amount of alkynylsilane 2a was produced (entry 7). Next, we investigated organic fluorides instead of metal fluorides. When tetrabutylammonium fluoride (TBAF) was used as a catalyst, the yield of 2a was 50%, even though 1a was completely consumed (entry 8). The major byproduct was the corresponding alkynoic acid, which was generated by hydrolysis of 1a with the water contained in TBAF.14 Therefore, we focused on anhydrous organic fluoride sources, and found that tetrabutylammonium difluorotriphenylsilicate (TBAT) gave 2a in a higher yield of 75%, albeit with 19% of the hydrolyzed byproduct, the corresponding alkynoic acid (entry 9).15 Because the participation of water from the glassware was suspected in the hydrolysis, the decarboxylation was conducted in a poly(tetrafluoroethylene) (PTFE) vessel, which afforded the product in 65% with a negligible amount of the hydrolyzed byproduct (entry 10). Finally, when the reaction was carried out for 3 h, silyl alkynoate 1a was completely consumed, yielding the desired alkynylsilane 2a in 93% (entry 11).
Table 1. Optimization of the Reaction Conditions.
| entry | catalyst | conv. (%) | yield (%)a |
|---|---|---|---|
| 1 | KF | 46 | 42 |
| 2 | KCl | 23 | 19 |
| 3 | KBr | 11 | 2 |
| 4 | KI | <5 | trace |
| 5 | NaF | 27 | 22 |
| 6 | CsF | 65 | 55 |
| 7 | MgF2 | <5 | trace |
| 8 | TBAF | >95 | 50 |
| 9 | TBAT | 94 | 75 |
| 10b | TBAT | 71 | 65 |
| 11b,c | TBAT | >95 | 93 (89)d |
Determined by 1H NMR analysis using mesitylene as internal standard.
PTFE vessel was used instead of glassware.
The reaction was carried out for 3 h.
Isolated yield.
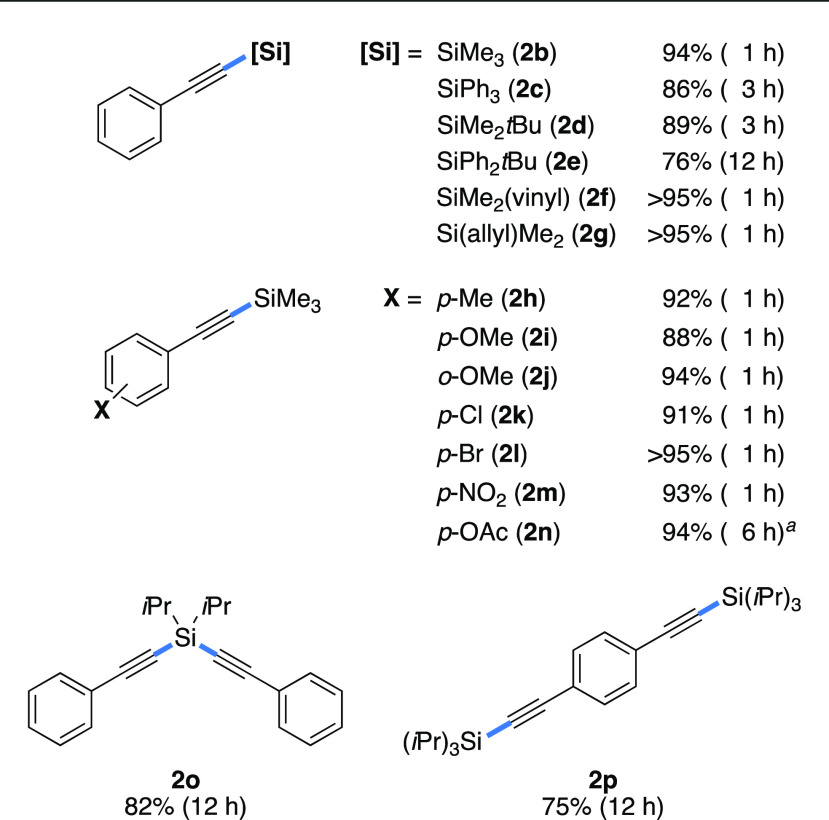
Under the optimal reaction conditions, the scope of silyl alkynoates was investigated (Table 2). The decarboxylation of trimethylsilyl 3-phenylpropiolate (1b) was completed in 1 h, affording desired alkynylsilane 2b in 94% yield. Triphenylsilyl 3-phenylpropiolate (1c) and tert-butyldimethylsilyl 3-phenylpropiolate (1d) were completely consumed in 3 h, furnishing products 2c (86%) and 2d (89%), respectively. Even more sterically demanding tert-butyldiphenylsilyl 3-phenylpropiolate (1e) was efficiently decarboxylated to give alkynylsilane 2e in 76% yield, albeit with a longer reaction time of 12 h. Silyl alkynoates with vinyl (1f) and allyl (1g) groups on the silicon atom were also decarboxylated in excellent yields without any side reactions. The reactions of trimethylsilyl 3-arylpropiolates with either electron-donating (−Me and −OMe) or electron-withdrawing (−Cl, −Br, and −NO2) groups at the para- and ortho-position on the benzene ring afforded the corresponding alkynylsilanes (2h–2m) in high yields (88 → 95%). These results show that the electronic nature of the aryl groups has little effect on the product yield. It is noteworthy that, because this system contains no transition metals, substrates with C–Cl and C–Br functional groups are tolerated under the reaction conditions. Silyl alkynoate 1n with an acetoxy group was found to decompose to some extent at 150 °C; thus, the reaction was carried out at 80 °C for 6 h to give alkynylsilane 2n in 94% yield. Diisopropylsilanediyl bis(3-phenylpropiolate) (1o) smoothly underwent two-fold decarboxylation to give the corresponding dialkynylsilane (2o) in 82% yield. Bis((triisopropylsilyl)ethynyl)benzene (2p) was afforded in 75% yield by the two-fold decarboxylation of silyl alkynoate 1p.
Table 2. Scope and Limitations of Silyl Alkynoates.
Reaction was carried out at 80 °C.
To shed light on the reaction mechanism, the decarboxylation of 1a was followed by 19F NMR spectroscopy (Figure S1). When 1a was reacted with 5 mol % TBAT in DMF-d7 at room temperature, the complete consumption of TBAT and the formation of iPr3SiF and Ph3SiF were observed; no other compounds containing fluorine atoms were detected. The production of iPr3SiF and Ph3SiF was also confirmed by 29Si{1H} NMR spectroscopy (Figure S2). These observations suggest that TBAT is not the true catalyst for the decarboxylation, but rather, the tetrabutylammonium alkynoate that is produced by the reaction of the silyl alkynoate with TBAT functions catalytically. Indeed, tetrabutylammonium 3-phenylpropiolate, which was prepared from 3-phenylpropiolic acid, NaH, and tetrabutylammonium chloride, smoothly catalyzed the decarboxylation of silyl alkynoate 1a to give alkynylsilane 2a in 95% NMR yield under fluoride-free conditions (Scheme 2a). The clean decarboxylation of 1a with 5 mol % nBu4NOAc also indicated that the fluoride ion is not an essential component of the catalyst, but that the carboxylate ion plays a critical role in the decarboxylation (Scheme 2b). While the formation of alkynylsilane 2a was not observed in the stoichiometric reaction of tetrabutylammonium 3-phenylpropiolate and iPr3SiF (Scheme 2c and Figure S3), not only alkynytriisopropylsilane 2a (11%) but also alkynyltriphenylsilane 2c (13%) were produced in the stoichiometric reaction of silyl alkynoate 1a and TBAT (Scheme 2d and Figure S4), implying that the formation of the tetrabutylammonium alkynoate is reversible.
Scheme 2. (a) Catalytic Reaction of Silyl Alkynoate 1a with Tetrabutylammonium 3-Phenylpropiolate, (b) Catalytic Reaction of 1a with nBu4NOAc, (c) Stoichiometric Reaction of Tetrabutylammonium 3-Phenylpropiolate and iPr3SiF, and (d) Stoichiometric Reaction of 1a and TBAT.
Based on these findings, a possible reaction mechanism is proposed, as illustrated in Scheme 3. First, TBAT reacts with the silyl alkynoate to produce a tetrabutylammonium alkynoate as well as two fluorosilanes (R3SiF and Ph3SiF) derived from the silyl alkynoate and TBAT. The thus-obtained tetrabutylammonium alkynoate reacts with the silyl alkynoate to form a pentacoordinate silicon intermediate, which undergoes decarboxylation to give the desired alkynylsilane and carbon dioxide with regeneration of the tetrabutylammonium alkynoate.
Scheme 3. Proposed Reaction Mechanism for the Catalytic Decarboxylation of Silyl Alkynoates with TBAT.
In summary, we have developed a facile method for the synthesis of alkynylsilanes by decarboxylating silyl alkynoates in the presence of a catalytic amount of commercially available TBAT. A wide variety of substrates, including those with bulky silyl groups such as the tert-butyldiphenylsilyl group or functional groups such as chloro, bromo, and acetoxy groups, were efficiently decarboxylated in good to excellent yields. Mechanistic studies revealed that the tetrabutylammonium alkynoate is the catalyst for the decarboxylation and TBAT acts as a precatalyst. Our group is now investigating catalytic systems for the decarboxylation of silyl esters other than silyl alkynoates.
Experimental Section
General Procedure for Decarboxylation of Silyl Alkynoates 1
In a PTFE vessel, a solution of silyl alkynoate 1 (0.50 mmol) and tetrabuthylammonium difluorotriphenylsilicate (5 mol %, 0.025 mmol) in DMF (1.0 mL) was stirred at 150 °C. After 1–12 h, the reaction mixture was diluted in CH2Cl2 (0.5 mL) and passed through a silica gel column. After evaporation, the desired alkynylsilane 2 was obtained in 75 → 95% yield.
Acknowledgments
This work was supported by the “Development of Innovative Catalytic Processes for Organosilicon Functional Materials” project (Project Leader: K.S.) from the New Energy and Industrial Technology Development Organization (NEDO).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01256.
Experimental procedures, characterization data, and copies of NMR spectra (1H, 13C{1H}, and 29Si{1H}) of the alkynylsilane products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Rodríguez N.; Goossen L. J. Decarboxylative Coupling Reactions: a Modern Strategy for C–C-Bond Formation. Chem. Soc. Rev. 2011, 40, 5030–5048. 10.1039/c1cs15093f. [DOI] [PubMed] [Google Scholar]; b Wei Y.; Hu P.; Zhang M.; Su W. Metal-Catalyzed Decarboxylative C–H Functionalization. Chem. Rev. 2017, 117, 8864–8907. 10.1021/acs.chemrev.6b00516. [DOI] [PubMed] [Google Scholar]; c Patra T.; Maiti D. Decarboxylation as the Key Step in C–C Bond–Forming Reactions. Chem.—Eur. J. 2017, 23, 7382–7401. 10.1002/chem.201604496. [DOI] [PubMed] [Google Scholar]; d Moon P. J.; Lundgren R. J. Metal-Catalyzed Ionic Decarboxylative Cross-Coupling Reactions of C(sp3) Acids: Reaction Development, Mechanisms, and Application. ACS Catal. 2020, 10, 1742–1753. 10.1021/acscatal.9b04956. [DOI] [Google Scholar]; e Daley R. A.; Topczewski J. J. Aryl-Decarboxylation Reactions Catalyzed by Palladium: Scope and Mechanism. Synthesis 2020, 52, 365–377. 10.1055/s-0039-1690769. [DOI] [Google Scholar]
- Weaver J. D.; Recio A.; Grenning A. J.; Tunge J. A. Transition Metal-Catalyzed Decarboxylative Allylation and Benzylation Reactions. Chem. Rev. 2011, 111, 1846–1913. 10.1021/cr1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Larson G. Some Aspects of the Chemistry of Alkynylsilane. Synthesis 2018, 50, 2433–2462. 10.1055/s-0036-1591979. [DOI] [Google Scholar]; b Yamamoto Y. Silver-Catalyzed C–H and C–Si Bond Transformations and Related Processes. Chem. Rev. 2008, 108, 3199–3222. 10.1021/cr078359u. [DOI] [PubMed] [Google Scholar]; c Blumenkopf T. A.; Overman L. E. Vinylsilane- and Alkynylsilane-Terminated Cyclization Reactions. Chem. Rev. 1986, 86, 857–873. 10.1021/cr00075a009. [DOI] [Google Scholar]
- a Greene T. W.; Wutts P. G.. Protective Groups in Organic Synthesis, 5th ed.; John Wiley & Sons: New York, 2006. [Google Scholar]; b Davidsohn W. E.; Henry M. C. Organometallic Acetylenes of the Main Groups III-V. Chem. Rev. 1967, 67, 73–106. 10.1021/cr60245a003. [DOI] [Google Scholar]; c Huang P.; Xu D.; Reich R. M.; Kaiser F.; Liu B.; Kühn F. E. Et2Zn-Mediated Stoichiometric C(sp)-H Silylation of 1-Alkynes and Chlorosilanes. Tetrahedron Lett. 2019, 60, 1574–1577. 10.1016/j.tetlet.2019.05.017. [DOI] [Google Scholar]
- a Lapouyade P.; Deleris G.; Dunogues J.; Calas R. Synthese nouvelle d’alcynes α-silicies. J. Organomet. Chem. 1974, 80, C45–C46. 10.1016/s0022-328x(00)80020-0. [DOI] [Google Scholar]; b Taniguchi Y.; Inanaga J.; Yamaguchi M. Use of 1,8-Diazabicyclo[5.4.0]undec-7-ene in Preparation of Trimethylsilyl Enol Ethers and Trimethylsilylacetylenes. Bull. Chem. Soc. Jpn. 1981, 54, 3229–3230. 10.1246/bcsj.54.3229. [DOI] [Google Scholar]; c Kownacki I.; Marciniec B.; Dudziec B.; Kubicki M. Silylative Coupling of Terminal Alkynes with Iodosilanes: New Catalytic Activation of sp-Hybridized Carbon–Hydrogen Bonds. Organometallics 2011, 30, 2539–2545. 10.1021/om200038r. [DOI] [Google Scholar]; d Kownacki I.; Orwat B.; Marciniec B.; Kownacka A. A New and Efficient Route for the Synthesis of Alkynyl Functionalized Silicon Derivatives. Tetrahedron Lett. 2014, 55, 548–550. 10.1016/j.tetlet.2013.11.103. [DOI] [Google Scholar]; e Kownacki I.; Orwat B.; Marciniec B. Iridium-Promoted Conversion of Chlorosilanes to Alkynyl Derivatives in a One-Pot Reaction Sequence. Organometallics 2014, 33, 3051–3059. 10.1021/om500320t. [DOI] [Google Scholar]; f Spesivaya E. S.; Lupanova I. A.; Konshina D. N.; Konshin V. V. Zn(OTf)2/i-Pr2NEt Promoted Synthesis of Tetraalkynylsilanes. Tetrahedron Lett. 2021, 63, 152713. 10.1016/j.tetlet.2020.152713. [DOI] [Google Scholar]
- Rahaim R. J. Jr.; Shaw J. T. Zinc-Catalyzed Silylation of Terminal Alkynes. J. Org. Chem. 2008, 73, 2912–2915. 10.1021/jo702557d. [DOI] [PubMed] [Google Scholar]
- a Jun C.-H.; Crabtree R. H. Dehydrogenative Silation, Isomerization and the Control of Syn- vs. Anti-Addition in the Hydrosilation of Alkynes. J. Organomet. Chem. 1993, 447, 177–187. 10.1016/0022-328x(93)80236-5. [DOI] [Google Scholar]; b Itoh M.; Mitsuzuka M.; Utsumi T.; Iwata K.; Inoue K. Dehydrogenative Coupling Reactions between Hydrosilanes and Monosubstituted Alkynes Catalyzed by Solid Bases. J. Organomet. Chem. 1994, 476, c30–c31. 10.1016/0022-328x(94)87091-8. [DOI] [Google Scholar]; c Tsuchimoto T.; Fujii M.; Iketani Y.; Sekine M. Dehydrogenative Silylation of Terminal Alkynes with Hydrosilanes under Zinc–Pyridine Catalysis. Adv. Synth. Catal. 2012, 354, 2959–2964. 10.1002/adsc.201200310. [DOI] [Google Scholar]; d Toutov A. A.; Betz K. N.; Schuman D. P.; Liu W.-B.; Fedorov A.; Stoltz B. M.; Grubbs R. H. Alkali Metal-Hydroxide-Catalyzed C(sp)–H Bond Silylation. J. Am. Chem. Soc. 2017, 139, 1668–1674. 10.1021/jacs.6b12114. [DOI] [PubMed] [Google Scholar]; e Ma Y.; Lou S. J.; Luo G.; Luo Y.; Zhan G.; Nishiura M.; Luo Y. B(C6F5)3/Amine-Catalyzed C(sp)–H Silylation of Terminal Alkynes with Hydrosilanes: Experimental and Theoretical Studies. Angew. Chem., Int. Ed. 2018, 57, 15222–15226. 10.1002/anie.201809533. [DOI] [PubMed] [Google Scholar]
- Marciniec B.; Dudziec B.; Kownacki I. A New Catalytic Route for the Activation of sp-Hybridized Carbon–Hydrogen Bonds. Angew. Chem., Int. Ed. 2006, 45, 8180–8184. 10.1002/anie.200603582. [DOI] [PubMed] [Google Scholar]
- Kawatsu T.; Aoyagi K.; Nakajima Y.; Choi J.-C.; Sato K.; Matsumoto K. Catalytic Decarboxylation of Silyl Alkynoates to Alkynylsilanes. Organometallics 2020, 39, 2947–2950. 10.1021/acs.organomet.0c00433. [DOI] [Google Scholar]
- a Hergott H. H.; Simchen G. Eine Einfache Synthese von Trichloromethyltrimethylsilan und Carbonsäure-Trimethylsilylestern. Synthesis 1980, 1980, 626–627. 10.1055/s-1980-29144. [DOI] [Google Scholar]; b Kornev A. N.; Donnikova O. S.; Semenov V. V.; Kurskii Y. A. Synthesis of (Trichloromethyl)organosilanes by Catalytic Decarboxylation of (Trichloroacetoxy)organosilanes. Russ. Chem. Bull. 1995, 44, 145–148. 10.1007/bf00696977. [DOI] [Google Scholar]
- Idris M. A.; Lee S. Recent Advances in Decarboxylative Reactions of Alkynoic Acids. Synthesis 2020, 52, 2277–2298. 10.1055/s-0040-1707600. [DOI] [Google Scholar]
- a Abele E.; Abele R. Recent Advances in Activation of Silicon Bonds by Fluoride Ion. Main Group Met. Chem. 2009, 32, 165–194. 10.1515/mgmc.2009.32.4.165. [DOI] [Google Scholar]; b Abele E. Activation of Silicon Bonds by Fluoride Ion in the Organic Synthesis in The New Millennium: A Review. Main Group Met. Chem. 2005, 28, 45–69. 10.1515/mgmc.2005.28.2.45. [DOI] [Google Scholar]; c Abele E.; Lukevics E. Recent Advances in Fluoride Ion Activation of Silicon Bonds in Organic Synthesis. Main Group Met. Chem. 2001, 24, 315–350. 10.1515/mgmc.2001.24.6.315. [DOI] [Google Scholar]; d Chuit C.; Corriu R. J. P.; Reye C.; Young J. C. Reactivity of Penta- and Hexacoordinate Silicon Compounds and Their Role as Reaction Intermediates. Chem. Rev. 1993, 93, 1371–1448. 10.1021/cr00020a003. [DOI] [Google Scholar]; e Furin G. G.; Vyazankina O. A.; Gostevsky B. A.; Vyazankin N. S. Synthetic Aspects of the Use of Organosilicon Compounds under Nucleophilic Catalysis Conditions. Tetrahedron 1988, 44, 2675–2749. 10.1016/s0040-4020(88)90008-7. [DOI] [Google Scholar]
- Igumnov S. M.; Boyko V. E.; Don V. L.. Method of Producing Fluorinated Aryl(trimethyl)silane. Russia Patent RU 2521168 C1, 2014.
- Sharma R. K.; Fry J. L. Instability of Anhydrous Tetra-n-alkylammonium Fluorides. J. Org. Chem. 1983, 48, 2112–2114. 10.1021/jo00160a041. [DOI] [Google Scholar]
- Pilcher A. S.; Ammon H. L.; DeShong P. Utilization of Tetrabutylammonium Triphenylsilyldifluoride as a Fluoride Source for Nucleophilic Fluorination. J. Am. Chem. Soc. 1995, 117, 5166–5167. 10.1021/ja00123a025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.