Abstract
Reproducible and in situ microbial detection, particularly of microbes significant in urinary tract infections (UTIs) such as Candida albicans, provides a unique opportunity to bring equity in the healthcare outcomes of disenfranchised groups like women in low-resource settings. Here, we demonstrate a system to potentially detect vulvovaginal candidiasis by leveraging the properties of multifilament cotton threads in the form of microfluidic-thread-based analytical devices (μTADs) to develop a frugal microbial identification assay. A facile mercerization method using heptane wash to boost reagent absorption and penetration is also performed and is shown to be robust compared to other existing conventional mercerization methods. Furthermore, the twisted mercerized fibers are drop-cast with media consisting of l-proline β-naphthylamide, which undergoes hydrolysis by the enzyme l-proline aminopeptidase secreted by C. albicans, hence signaling the presence of the pathogen via simple color change with a limit of detection of 0.58 × 106 cfu/mL. The flexible and easily disposable thread-based detection device when integrated with menstrual hygiene products showed a detection time of 10 min using spiked vaginal discharge. The developed method boasts a long shelf life and high stability, making it a discreet detection device for testing, which provides new vistas for self-testing multiple diseases that are considered taboo in certain societies.
Introduction
Presenting as the most common outpatient disease, the incidence of urinary tract infections (UTIs) is reported at least once in 50–60% of adult women worldwide.1 The regular occurrence and recurrent nature of certain UTIs manifest themselves as increased burdens on primary healthcare systems, as their incidence as a complication has been linked to multiple additional diseases such as diabetes and postpregnancy infections.1−6 Along with a significant economic burden,7 UTIs have a prominent global social burden as well, leading to a significant drop in the quality of life in the form of sexual, physical, and emotional deterioration particularly in the lives of women.2,8,9 The development of UTIs exacerbates the existing inequalities present in societal structures particularly affecting economically and socially disenfranchised women, which tend to have lower access to healthcare facilities.2,9−11 The disparity is further cemented by the lack of resources present for the reliable diagnosis of UTIs like bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC), denying high-risk groups information vital for informed decision making.4,7 Particularly in the case of VVC, the gold standard of diagnosis consists of a combined approach integrating microscopic examination from a vaginal or vulval swab with relevant symptoms presenting on the patient.3,6
Around 75% of women belonging to childbearing age experience VVC once in their life. Up to 9% of women in different populations experience >3 episodes per year, considered as recurrent vulvovaginal candidiasis (RVVC).12 Denning et al. estimated that by 2030, the population of women with RVCC each year will increase to almost 158 million. This alone leads to the economic burden from lost productivity of up to US$14·39 billion annually.4 These facilities are limited to testing labs frequently absent or overburdened in low-resource areas and do not facilitate reliable in situ self-diagnosis of the infection, making cost a limiting factor in the diagnosis of UTIs in high-risk low-income groups. This inequality is most pronounced in developing areas where the lack of substantial public outreach and existing societal taboos results in instances of shame and guilt associated with contracting UTIs, lowering the overall number of cases reported and tracked.13,14 The lack of reliable, rapid, and inexpensive tools to self-diagnose a UTI in a discreet manner presents a sizable problem affecting a large section of vulnerable people leading to underdiagnosis3,7 of the problem and a severe gap of information due to the inability to test a large representative part of the population.
The loss of equity due to the erosion of autonomy disallows personal choice in health significant decisions that furthers the burden of the disease and loss of productivity, undermining the social upliftment of many disenfranchised people.15 Sustainable and more equitable growth on the lines of the sustainable development goals put forth by the United Nations, particularly goal 3 and goal 5, calls for the promotion of good health and well-being supplemented by gender equality.16 These targets heavily rely on the availability of relevant healthcare data to individuals, particularly women. Empowering women by providing an avenue for them to personally contribute to their own healthcare needs requires developing testing techniques and modalities, which allow for self-diagnosis of diseases of global interest, particularly UTIs.1,2
To benefit the primary stakeholders, nontraditional solutions striking a balance between cost and performance as a means to solve contextual challenges must be developed. A viable approach toward this end can be pursued by deploying microfluidic devices. Microfluidics possesses multiple favorable properties such as low-volume operation, greater fluid manipulation, facile operation, and modular construction facilitating miniaturization and high portability, which make them highly relevant in multiple interdisciplinary research applications particularly in the field of diagnostics and biosensing.17−22 Initially being fabricated by labor- and cost-intensive processes like photolithography23 and micromachining out of materials like poly(dimethyl siloxane) and poly(ethylene terephthalate),24 the current generation of devices tend to employ materials that favor cost-effectiveness and high scalability.25 They achieve these targets by implementing cheaper and more readily available materials such as cellulose-based substrates like paper with more “Do it yourself” approaches to fabrication, which can further be automated.26−32 Paper-based analytical devices or μPADs have seen applications in fields such as diagnostics,29,33,34 environmental monitoring,28 manufacturing, and food safety control.35
A further simplified version of analytical devices leverages threads as the base medium has garnered significant attention recently.36−45 These thread-based analytical devices or μTADs provide further advantages of not needing the creation of barriers and wells on the substrate itself. μTAD manufacturing does not need multiple complicated steps and is usually done via household items such as sewing needles or machines.41,45,46 These devices also easily integrate into objects such as sensors and can provide additional tensile strength to the added object.37 The base processes of operation in the μTAD are driven by capillary action between the threads that drive the sample to the sites where reagents are immobilized so as to promote a reaction.38 These reagents can be designed to leverage detection techniques such as colorimetry,47−49 which provides a qualitative signal to the eye or to a smartphone-based detection, which are further highlighted by the thread's white color.49−51 Owing to their utility, μTADs have been used to perform a myriad of assays including glucose assays,52 enzyme-linked immune-sorbent assays (ELISA),53 immunochromatographic assays.54−61
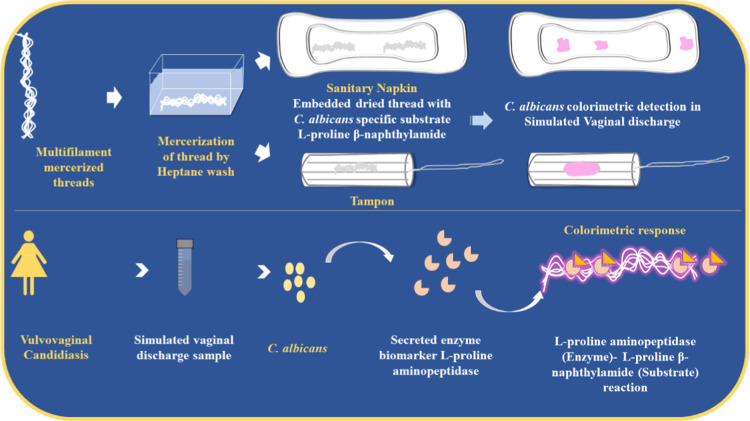
Using the tenants of frugal science that promote the development of cost-effective and highly scalable solutions,62 we have combined multifilament threads with a simple colorimetric test, which uses an enzyme–substrate reaction as a means of detecting the target biomarker. Generally, the wicking property is essential in thread devices and is generally impeded by the presence of hydrophobic substances like wax and binders added to cotton threads in the manufacturing process.42,63 This issue has been combatted by mercerizing the thread to improve tensile and absorbing properties.62 Conventionally, many groups have used hydrochloric acid (HCl)64,65 or sodium hydroxide (NaOH)41 and integrated sonication66 as a means of mercerization. Using the aforementioned tedious approaches for the removal of leftover residues in threads may affect the pH of the substrate, thereby hindering the outcome of the colorimetric test.67 To answer this burgeoning problem, our group chose to explore and develop a noncaustic and easy-to-use mercerization option using heptane wash in our experiments. Then, we leveraged the mercerized μTADs as a tool for microbial assay, which employs a colorimetric substrate specific to a secretory enzyme of Candida albicans. The relevant colorimetric substrate was drop-cast onto the thread device, which wicked it up and created a device, which could signal the presence of microbe qualitatively with a clear and distinct color change. We have also integrated μTADs into menstrual hygiene products, allowing for the detection of C. albicans in simulated vaginal discharge samples, signaling an infection (Figure 1). To the best of our knowledge, this is the first study involving μTADs and menstrual hygiene products like napkins and tampons for in situ detection of vulvovaginal candidiasis in a robust and discreet manner, which can be deployable in resource-constrained settings.
Figure 1.
Schematic illustration of mercerized microfluidic-thread-based analytical devices (μTADs) and their utility in menstrual hygiene products.
Results and Discussion
Characterization of Mercerization Methods
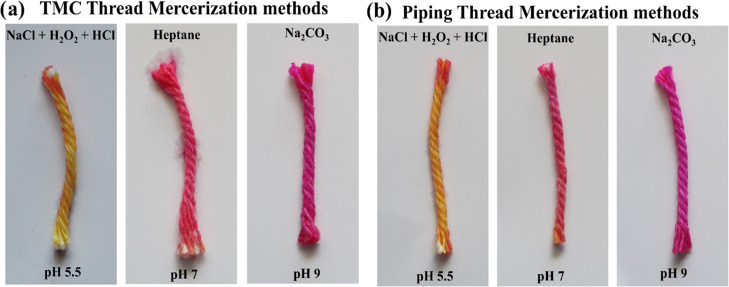
Commercial mercerization methods tend to use harsh chemicals with high pH, which if left as a residue can be hard to remove and may influence the results of colorimetric, immuno, and pH-based assays performed on it. To test the effect of the mercerization method on a pH assay, we used a thread substrate to perform a pH-based colorimetric test in which phenol red dye was drop-cast on the thread matrix. Then, the color change of the dye was noted to assess the pH of the thread. We observed that the in-lab developed heptane wash technique, compared to other mercerization methods (Figure 2), was not caustic and easy to clean, therefore not impeding the color change in the thread by possible residues. The developed method allows for the addition of a new method to the arsenal of mercerization methods, potentially increasing sensitivity in assays in which pH is the principle indicating property being pursued. The thread’s wicking properties are the most essential part of a μTAD, and since the mercerization methods are applied to boost the same, it is essential to compare different methods available in their mercerizing merit. We performed experiments to ascertain the water absorbency and water penetration rates of two commercially available threads that were used as the matrix for developing μTADs. The threads used were twisted multifilament cotton (TMC) thread and piping white glazed cotton (PWGC) thread. These two particular threads are representative of the majority of threads found in the market. The mercerization methods tested include a sodium carbonate (Na2CO3) treatment, a sodium chloride (NaCl)–hydrogen peroxide (H2O2)–hydrochloric acid (HCl) treatment, and our lab’s proposed heptane treatment.
Figure 2.
pH study and comparison of mercerization methods: (a) TMC thread and (b) piping thread.
We assessed the penetration rate of water through 10 cm of thread after adding 150 μL of the sample to both the threads and the distance the water wicked to was noted with respect to time and plotted for all of the methods and untreated thread (Figure 3). We found that mercerized threads possessed significantly more rapid penetration rates, possibly due to the removal of hydrophobic substances and binders. On the other hand, untreated threads displayed poor or nil penetration of dye due to the presence of hydrophobic and waxy residues. In-between the mercerization methods, varying results were found for both threads. In the TMC thread (Figure 3a), heptane wash performed better than the NaCl–H2O2–HCl treated thread, but the best results were given by Na2CO3 treatment. In the PWGC thread (Figure 3b), the heptane wash had comparable results to the Na2CO3 treatment, but the NaCl treatment afforded the best results. In both results, the heptane wash performed best as a general method of mercerization with reliably robust results in both threads.
Figure 3.
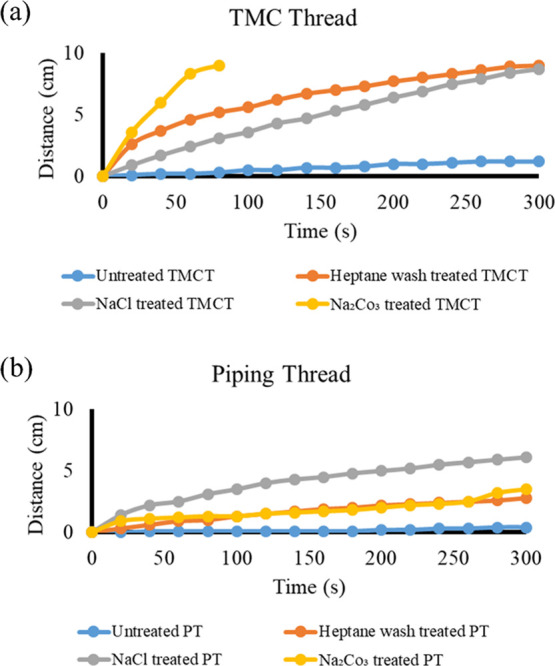
Water penetration rate (comparison) of (a) TMC thread and (b) piping thread.
A water absorbency assay was also performed on the TMC and PWGC threads in which the water absorbency was found out of 100% for all of the methods and plotted (Figure 4). The heptane wash performed best in the TMC thread (Figure 4a) and was comparably effective to the other methods in the PWGC thread (Figure 4b), where the Na2CO3 showed the best performance. We found that heptane wash was the best general-purpose mercerization method compared to other commercial methods.
Figure 4.
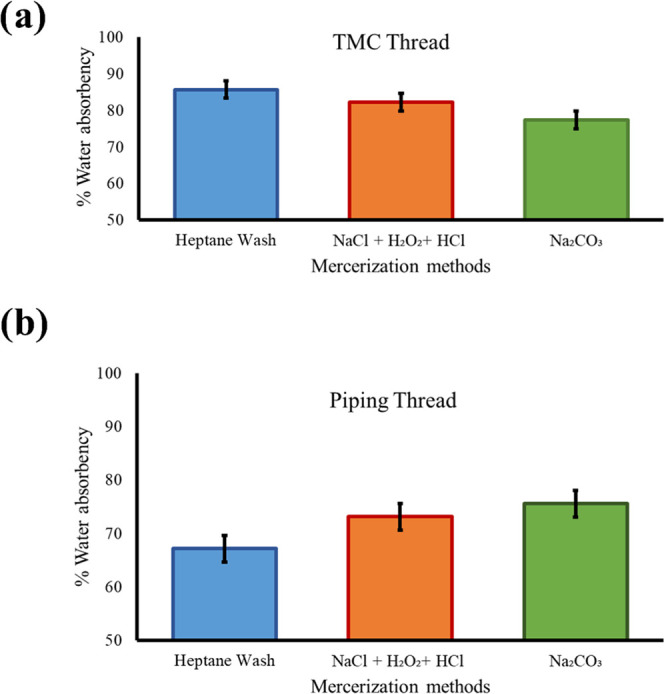
Water absorbency assay (comparison) of (a) TMC thread and (b) piping thread. Each experiment was performed three times. Average value ± standard deviation was measured.
A typical stress–strain curve is shown in Figure 5. We observed that treatment of TMC and PWGC with heptane, NaCl-H202-HCl, and Na2CO3 has a considerable effect on the tensile strength. TMC thread showed maximum tensile stress around 53.25 MPa, which is significantly higher than their treated counterparts, which has a breaking point around 29 MPa. However, in the case of PWGC thread, both untreated and heptane-treated threads showed similar tensile stress values. On the other hand, NaCl- and Na2CO3-treated threads showed slightly lesser tensile stress.
Figure 5.
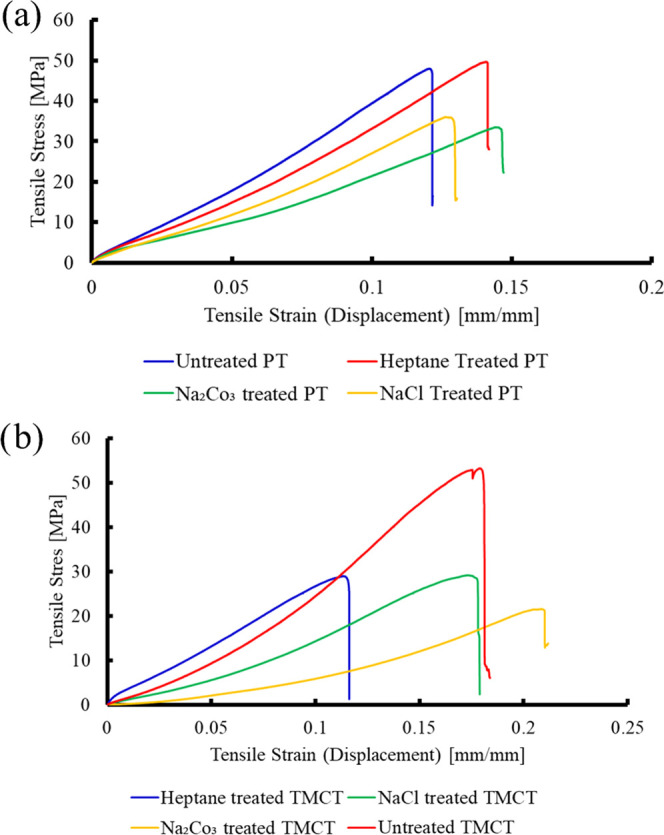
Stress–strain curve for different mercerization methods: (a) piping thread and (b) TMC thread.
Thread-Based Colorimetric Microbial Assay
As a way to test the efficacy of the medium, we used thread as the matrix for a detection assay for C. albicans, a major opportunistic fungal pathogen causing invasive and debilitating female genital diseases.2,3,6,8 The gold standard methods for diagnosing C. albicans infections include culture and nucleic acid-based testing, which are labor- and technically intensive. Instead, we implemented a method using l-proline β-naphthylamide (PRO) substrate for targeting the enzyme l-proline aminopeptidase, secreted by C. albicans to indicate its presence in solution68 within 10 min colorimetrically with a pink hue using cinnamaldehyde as an indicator. The assay demonstrated competence quantitatively, indicating the presence of C. albicans, as we observed that the intensity of the pink color increased as the concentration of C. albicans was increased (Figure 6).
Figure 6.
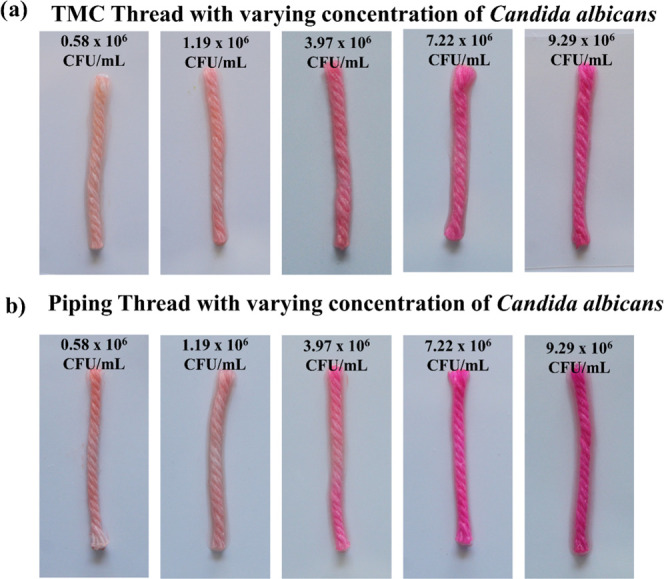
Thread-based colorimetric assay using heptane-mercerized (a) TMC thread and (b) piping thread.
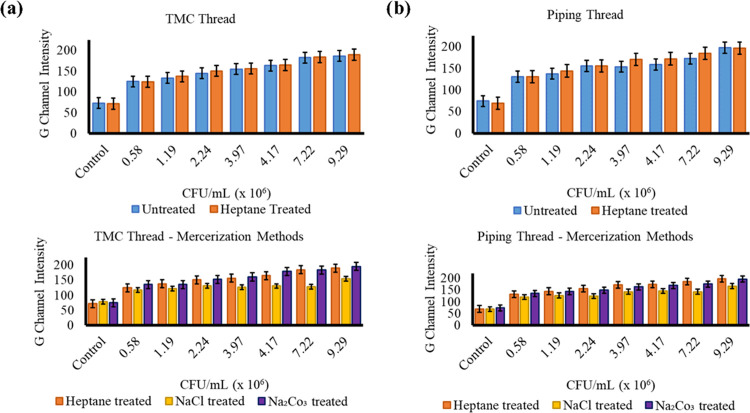
Leveraging the quantitative change of color is a way to visually signal the presence of C. albicans. We developed a frugal and robust μTAD and assessed implementing the thread as an alternative substrate to the culture-based time-intensive methods used in diagnostic labs. We also assessed the effect of mercerization on the intensity of the color change by plotting the G-channel intensities of all of the color changes in the treated and untreated thread with respect to the concentration of C. albicans present (Figure 7), particularly by heptane wash. Compared to the untreated thread, heptane-treated thread showed better results for lower concentrations of C. albicans and comparable results for higher concentrations, a phenomenon more pronounced in the PWGC thread (Figure 7b) than the TMC thread (Figure 7a). Compared to other methods of mercerization, heptane wash showed comparably robust results to the Na2CO3-treated thread. In the PWGC thread, we observed relatively better performance of heptane wash compared to the other mercerization methods and to the mercerization in the TMC thread. These results further prove the utility of heptane wash as a viable mercerization alternative to traditional methods.
Figure 7.
G-channel intensity comparison of mercerization methods in (a) TMC thread and (b) piping thread. Each experiment was performed three times. Average value ± standard deviation was measured.
Vulvovaginal Candidiasis Detection Device
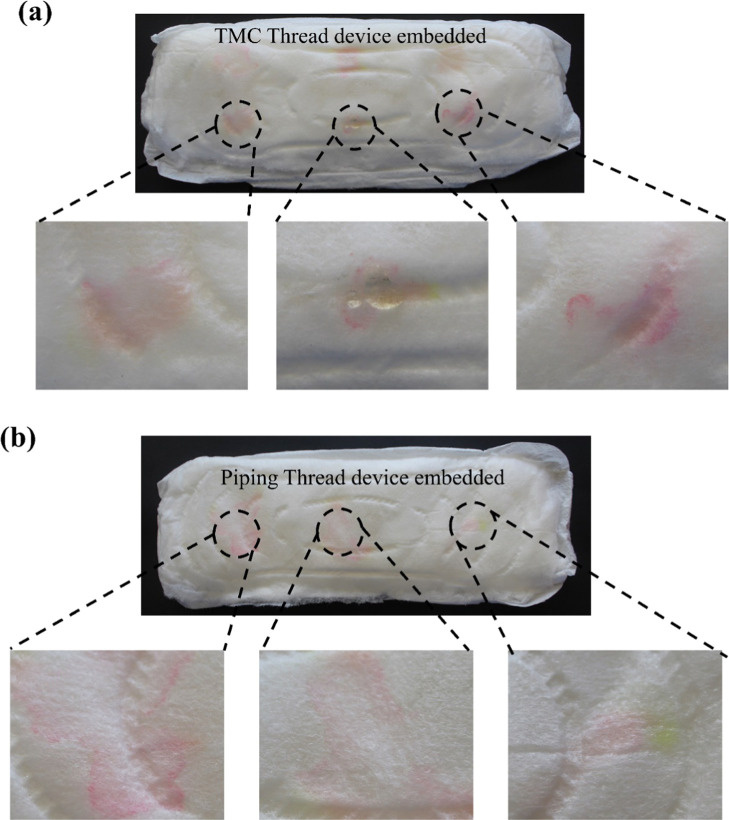
The true utility of the μTAD developed is explored as a wearable, robust, and in situ detection device for vaginal infections. The flexible and discreet nature of the device allows for its seamless integration into menstrual hygiene products, particularly sanitary napkins and tampons (Figures 8 and 9). When the μTAD was combined with menstrual hygiene products, the signaling ability was tested using simulated vaginal discharge samples. The results showed robust signaling and color change, which could be used to detect infection. Biochemical indication of infection tends to be a long and arduous process involving diagnostic labs and culture times of up to 24–72 h requiring significant prior expertise to perform. The high volumes of biohazardous waste produced can pose great disposal and storage issues as well. As a result, low-resource settings tend to lack access to robust testing facilities. Our proposed method is frugal, robust, and simple enough to be performed at home discreetly with little to no technical expertise. When integrated with menstrual hygiene products, the device does not need additional disposal methods and can be disposed of safely, mainly through incineration, like other menstrual hygiene products. These devices have been shown to have a significant shelf life and can be used immediately, circumventing the need for fresh reagent preparation.
Figure 8.
C. albicans detection in spiked vaginal discharge using thread-device embedded in sanitary napkins: (a) TMC thread and (b) piping thread.
Figure 9.
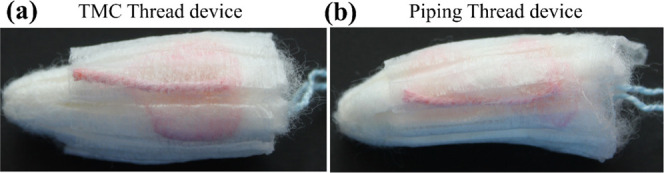
C. albicans detection in spiked vaginal discharge using thread-device embedded in tampons: (a) TMC thread and (b) piping thread.
Realistically, the number of pathogens that lead to the development of UTIs span a wider range. Compared to the number of infections caused by Candida species, Enterobacteriaceae cause the majority of UTIs detected.69,70 As a means to widen the net cast by the device when testing for UTIs, multiplexing multiple detection modalities such as nitrite tests and leukocyte esterase activity tests onto a singular platform can help span the whole gamut of potential pathogenic infections.71 We identify the utility of the medium and device we have proposed to provide fabricating guidelines for devices that potentially leverage the robust properties of the thread-based assay toward detecting UTIs with sufficient accuracy. Multiple threads spanning the surface of detection can be used to simultaneously perform multiple assays, a method that has been demonstrated before in other applications.72 This method can boost the number of data points that can be offered to a tester without sacrificing the device’s flexibility when integrated into a menstruation product.
Conclusions
Our research evidently establishes the utility of thread as a viable substrate for microbial detection assays showing multiple desirable properties such as low cost and ease of disposal. We developed a completely novel device for the detection of a globally significant disease, vulvovaginal candidiasis, which relies on enzyme (l-proline aminopeptidase) and substrate (l-proline β-naphthylamide) reaction to the presence of pathogen or microbe. Furthermore, our research proposes a new and viable mercerization method of using heptane wash (≈0.21 US$) to boost absorption and penetration rates in threads and shows how this method is highly robust compared to other methods of mercerization. The efficacy of the method demonstrated provides another option for mercerizing threads, which can benefit from the advantageous properties of heptane, mainly its nonpolar and noncaustic nature, availability, and ease of separation. We also achieved a low cost of 0.22–0.28 US$ for the thread-integrated sanitary napkin or tampon test for C. albicans. Additionally, the ease of manufacture and high scalability of its production due to easily automated production and less added parts make it perfect for use in resource-scarce settings. This approach also pave the way for other wearable devices used to detect diseases that disenfranchise women, especially those that are sexually transmitted. We envisage to improve and further explore better manufacturing methods aimed toward reducing evaporation-linked degradation and integrating image processing techniques through smartphones to boost detection time and objectivity in results.
Experimental Section
Materials
Twisted multifilament cotton (TMC) thread (Simco) and piping white glazed cotton (PWGC) thread (Simco) were purchased from a local craft store. Sodium chloride (NaCl), hydrogen peroxide (H2O2), sodium carbonate (Na2CO3), acetic acid, glycerol, urea, and glucose were procured from Merck, India. Hydrochloric acid (HCl), n-heptane, and potassium hydroxide were obtained from Loba Chemie and SRL, India. l-proline β-naphthylamide (PRO) and p-dimethylaminocinnamaldehyde (DCA) were purchased from Sigma-Aldrich, India, and Loba Chemie, India. Calcium hydroxide, bovine serum albumin (BSA), and lactic acid were procured from Titan Biotech, HiMedia, and Finar Limited, India. Sanitary napkins and tampons (Bella) were procured from a local pharmacy. C. albicans (ATCC 24433) was taken from the culture collection at Mycology Laboratory, Department of Microbiology, Kasturba Medical College, Manipal.
Thread Mercerization
Method 1: NaCl-, H2O2-, and HCl-Based Method
Twisted multifilament cotton (TMC) and piping white glazed cotton (PWGC) thread pieces (3 cm) were taken and boiled in 2 M NaCl solution at 100 °C for 30 min on a heating mantle. Then, the thread pieces were transferred to 0.01% H2O2 and soaked for 5 min, followed by soaking in 0.01 M HCl for 5 min. Further, the thread pieces were soaked and washed in Milli-Q water three times for 10 min each and then dried in a hot-air oven at 100 °C for 1.5 h.
Method 2: Na2CO3-Based Method
TMC and PWGC thread pieces (3 cm) were boiled in 10 mg/mL sodium carbonate (Na2CO3) solution at 100 °C for 5 min on a heating mantle, followed by soaking and washing in Milli-Q water three times for 10 min each. The thread pieces were dried in a hot-air oven at 100 °C for 1.5 h.
Method 3: Heptane Wash-Based Method
TMC and PWGC thread pieces (3 cm) were dipped in appropriate volume of n-heptane solvent and washed thoroughly three times (for about 30 s each), replacing the solvent before every wash. The thread pieces were then washed in Milli-Q water three times and dried in a hot-air oven at 100 °C for 1.5 h.
pH Study of Threads Treated with Different Mercerization Techniques
To assess the effect of the mercerization techniques on the pH of the thread, different mercerized TMC and PWGC thread pieces (3 cm) were taken and 100 μL of phenol red pH indicator dye solution was added to it. The change in the color of the indicator on the thread based on the pH of the thread was captured using Canon Eos 3000D DSLR camera, and the pH values of the thread pieces corresponding to the different mercerization treatments were measured using pH strips.
Penetration Rate of Threads
To analyze the water penetration rate in different mercerized TMC and PWGC threads (10 cm), 150 μL of phenol red indicator solution in water was added and the distance moved by the dye in thread with time was noted using a ruler and stopwatch.
Percentage Water Absorbency of Threads
TMC and PWGC thread pieces (3 cm) treated through different mercerization techniques were taken and their dry weight was measured using a weighing balance. Further, they were dipped and soaked for 5 min in 1 mL of water taken in vials separately. The water-soaked thread pieces were then weighed again, and this weight was recorded as the wet weight of the threads. The percentage water absorbency was measured using the formula [((wet weight – dry weight)/ wet weight) × 100].
Tensile Testing
The experiment of tensile testing was done in the universal testing machine (Instron 3366). In general, tensile test is used to measure the tensile strength of the specimen by applying load on two ends. In this study, tensile testing was done for the TMC and PWGC threads treated with heptane, NaCl-H202-HCl, and Na2CO3 and untreated thread (control) using universal testing machine. Threads measuring 10 cm were clamped onto the grip mechanism connected to the machine, and the load was provided.
Thread-Based Colorimetric Detection of C. albicans
PRO substrate solution (50 μL, 2 mg/mL) was added to the three sets of mercerized TMC and PWGC threads (3 cm) and dried for 15 min after the substrate solution wicked properly through the threads. Next, 100 μL of C. albicans cell suspensions (different cell concentrations or CFU/mL spiked in water) was added to the thread pieces and incubated at 37 °C for 15 min. DCA indicator solution (80 μL, 5.1 mg/mL) was added, and the color change in the thread device was captured after 10 min using a Canon Eos 3000D DSLR camera. The green channel intensity values of the color formed in the different threads were measured from the images using Fiji software and normalized suitably.
Further, we embedded the PRO substrate-imbibed 3 cm TMC and PWGC thread pieces (heptane wash mercerized) in the inner layer of sanitary napkins and tampons. Simulated vaginal discharge samples (300 μL/thread; 3.510 g/L sodium chloride, 1.4 g/L potassium hydroxide, 0.222 g/L calcium hydroxide, 0.018 g/L bovine serum albumin, 2 g/L lactic acid, 1 g/L acetic acid, 0.160 g/L glycerol, 0.4 g/L urea, 5 g/L glucose, pH 4.6) spiked with C. albicans (2.24 × 106 CFU/mL) were added to the sanitary napkins and tampons and incubated at 37 °C for 15 min. DCA indicator (80 μL/thread) was added to the thread-embedded spots of sanitary napkins and tampons, and the color change in the regions was captured after 10 min using a Canon Eos 3000D DSLR camera.
Acknowledgments
This work was partially funded by Manipal-McGill Centre for Infectious Diseases [Seed Grant Award No. MAC ID/SGA/2017/21]. N.K.M. & A.P. acknowledge the financial support from Vision Group on Science and Technology, Government of Karnataka, under SMYSR and RGS/F Scheme [Sanction Letter No. KSTePS/VGST/SMYSR-2016–17/GRD-595/2017–18, KSTePS/ VGSTRGS/F/GRD No. 711/2017–18]. A.P. acknowledges Indian Council of Medical Research (ICMR) for providing Senior Research Fellowship [File No. 5/3/8/91/ITR-F/2020]. N.K.M. acknowledges the financial support received from Science and Engineering Research Board (SERB), Department of Science and Technology, Govt of India, under Core Research Grant (CRG) Scheme (File No. CRG/2020/003060). The authors extend their special thanks to Department of Biotechnology, Manipal Institute of Technology. M.S.G.N. acknowledges Science and Engineering Research Board (SERB), Department of Science and Technology, Govt of India, for National Post-Doctoral Fellowship [Sanction No. -PDF/2018/001486]. N.K.M. thanks Dr. Praveen Kumar and Dr. Vijendra Prabhu for their fruitful discussions. N.K.M. specially thanks Dr. Roopa PS, Department of Obstetrics and Gynaecology, Kasturba Medical College, Manipal for providing insights on Vulvovaginal Candidiasis.
Author Contributions
# A.P. and H.S. contributed equally.
The authors declare no competing financial interest.
References
- Medina M.; Castillo-Pino E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 3–7. 10.1177/1756287219832172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilardi J. E.; Walker S.; Temple-Smith M.; McNair R.; Mooney-Somers J.; Bellhouse C.; Fairley C. K.; Chen M. Y.; Bradshaw C. The Burden of Bacterial Vaginosis: Women’s Experience of the Physical, Emotional, Sexual and Social Impact of Living with Recurrent Bacterial Vaginosis. PLoS One 2013, 8, e74378 10.1371/journal.pone.0074378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D. Vulvovaginal Candidosis. Lancet 2007, 369, 1961–1971. 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- Denning D. W.; Kneale M.; Sobel J. D.; Rautemaa-Richardson R. Global Burden of Recurrent Vulvovaginal Candidiasis: A Systematic Review. Lancet Infect. Dis. 2018, 18, e339–e347. 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- Eckert L. O.; Hawes S. E.; Stevens C. E.; Koutsky L. A.; Eschenbach D. A.; Holmes K. K. Vulvovaginal Candidiasis: Clinical Manifestations, Risk Factors, Management Algorithm. Obstet. Gynecol. 1998, 92, 757–765. 10.1097/00006250-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Gonçalves B.; Ferreira C.; Alves C. T.; Henriques M.; Azeredo J.; Silva S. Vulvovaginal Candidiasis: Epidemiology, Microbiology and Risk Factors. Crit. Rev. Microbiol. 2016, 42, 905–927. 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- Peebles K.; Velloza J.; Balkus J. E.; McClelland R. S.; Barnabas R. V. High Global Burden and Costs of Bacterial Vaginosis. Sex. Transm. Dis. 2019, 46, 304–311. 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- Johnson S. R.; Griffiths H.; Humberstone F. J. Attitudes and Experience of Women to Common Vaginal Infections. J. Lower Genital Tract Dis. 2010, 14, 287–294. 10.1097/LGT.0b013e3181d85bb7. [DOI] [PubMed] [Google Scholar]
- Payne S. C.; Cromer P. R.; Stanek M. K.; Palmer A. A. Evidence of African-American Women’s Frustrations with Chronic Recurrent Bacterial Vaginosis. J. Am. Acad. Nurse Pract. 2010, 22, 101–108. 10.1111/j.1745-7599.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- Paul K.; Boutain D.; Manhart L.; Hitti J. Racial Disparity in Bacterial Vaginosis: The Role of Socioeconomic Status, Psychosocial Stress, and Neighborhood Characteristics, and Possible Implications for Preterm Birth. Soc. Sci. Med. 2008, 67, 824–833. 10.1016/j.socscimed.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Jones F. R.; Miller G.; Gadea N.; Meza R.; Leon S.; Perez J.; Lescano A. G.; Pajuelo J.; Caceres C. F.; Klausner J. D.; Coates T. J. Prevalence of Bacterial Vaginosis among Young Women in Low-Income Populations of Coastal Peru. Int. J. STD AIDS 2007, 18, 188–192. 10.1258/095646207780132505. [DOI] [PubMed] [Google Scholar]
- Rosati D.; Bruno M.; Jaeger M.; Ten Oever J.; Netea M. G. Recurrent Vulvovaginal Candidiasis: An Immunological Perspective. Microorganisms 2020, 8, 144 10.3390/microorganisms8020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail S.; Shajahan A.; Sathyanarayana Rao T. S.; Wylie K. Adolescent Sex Education in India: Current Perspectives. Indian J. Psychiatry 2015, 57, 333–337. 10.4103/0019-5545.171843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk R.; Murt A. Epidemiology of urological infections: a global burden. World J. Urol. 2020, 38, 2669–2679. 10.1007/s00345-019-03071-4. [DOI] [PubMed] [Google Scholar]
- Osamor P. E.; Grady C. Women’s Autonomy in Health Care Decision-Making in Developing Countries: A Synthesis of the Literature. Int. J. Women’s Health 2016, 8, 191–202. 10.2147/IJWH.S105483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odera J. A.; Mulusa J.. SDGs, Gender Equality and Women’s Empowerment: What Prospects for Delivery?. In Sustainable Development Goals and Human Rights; Springer, 2019; pp 95–118. [Google Scholar]
- Gong M. M.; Sinton D. Turning the Page: Advancing Paper-Based Microfluidics for Broad Diagnostic Application. Chem. Rev. 2017, 117, 8447–8480. 10.1021/acs.chemrev.7b00024. [DOI] [PubMed] [Google Scholar]
- Marx V. Putting Microfluidics in Other People’s Hands. Nat. Methods 2018, 15, 167–170. 10.1038/nmeth.4609. [DOI] [PubMed] [Google Scholar]
- Whitesides G. M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Nguyen H.-T.; Thach H.; Roy E.; Huynh K.; Perrault C. Low-Cost, Accessible Fabrication Methods for Microfluidics Research in Low-Resource Settings. Micromachines 2018, 9, 461 10.3390/mi9090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H. Y.; Sousa F. B.; Carlo H. L.; Maciel P. P.; Macena M. S.; Han J. Enhanced Transport of Materials into Enamel Nanopores via Electrokinetic Flow. J. Dent. Res. 2015, 94, 615–621. 10.1177/0022034515572189. [DOI] [PubMed] [Google Scholar]
- Mani N. K.; Rudiuk S.; Baigl D. Spatially Controlled DNA Unzipping by Microfluidic Interface Positioning on a Molecule Perpendicular to a Multicomponent Flow. Chem. Commun. 2013, 49, 6858–6860. 10.1039/c3cc44016h. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B.; Hanson R. L.; Almughamsi H. M.; Pang C.; Fish T. R.; Woolley A. T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. 10.1021/acs.analchem.9b04986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstmann-Olsen C.; Hanczyc M. M.; Hoyland J.; Rasmussen S.; Rubahn H. G. Uniform Droplet Splitting and Detection Using Lab-on-Chip Flow Cytometry on a Microfluidic PDMS Device. Sens. Actuators, B 2016, 229, 7–13. 10.1016/j.snb.2016.01.120. [DOI] [Google Scholar]
- Carrell C.; Kava A.; Nguyen M.; Menger R.; Munshi Z.; Call Z.; Nussbaum M.; Henry C. Beyond the Lateral Flow Assay: A Review of Paper-Based Microfluidics. Microelectron. Eng. 2019, 206, 45–54. 10.1016/j.mee.2018.12.002. [DOI] [Google Scholar]
- Martinez A. W.; Phillips S. T.; Butte M. J.; Whitesides G. M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem., Int. Ed. 2007, 46, 1318–1320. 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbioli G. G.; Mazzu-Nascimento T.; Stockton A. M.; Carrilho E. Technical Aspects and Challenges of Colorimetric Detection with Microfluidic Paper-Based Analytical Devices (MPADs) - A Review. Anal. Chim. Acta 2017, 970, 1–22. 10.1016/j.aca.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Kung C. T.; Hou C. Y.; Wang Y. N.; Fu L. M. Microfluidic Paper-Based Analytical Devices for Environmental Analysis of Soil, Air, Ecology and River Water. Sens. Actuators, B 2019, 301, 126855 10.1016/j.snb.2019.126855. [DOI] [Google Scholar]
- Yetisen A. K.; Akram M. S.; Lowe C. R. Paper-Based Microfluidic Point-of-Care Diagnostic Devices. Lab Chip 2013, 13, 2210–2251. 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- Martinez A. W.; Phillips S. T.; Whitesides G. M.; Carrilho E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- Mani N. K.; Prabhu A.; Biswas S. K.; Chakraborty S. Fabricating Paper Based Devices Using Correction Pens. Sci. Rep. 2019, 9, 1752 10.1038/s41598-018-38308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh Singhal H.; Prabhu A.; Giri Nandagopal M. S.; Deivasigamani T.; Mani N. K. One-Dollar Microfluidic Paper-Based Analytical Devices: Do-It-Yourself Approaches. Microchem. J. 2021, 165, 106126 10.1016/j.microc.2021.106126. [DOI] [Google Scholar]
- Prabhu A.; Giri Nandagopal M. S.; Peralam Yegneswaran P.; Singhal H. R.; Mani N. K. Inkjet Printing of Paraffin on Paper Allows Low-Cost Point-of-Care Diagnostics for Pathogenic Fungi. Cellulose. 2020, 27, 7691–7701. 10.1007/s10570-020-03314-3. [DOI] [Google Scholar]
- Mani N. K.; Das S. S.; Dawn S.; Chakraborty S. Electro-kinetically Driven Route for Highly Sensitive Blood Pathology on a Paper-based Device. Electrophoresis 2020, 41, 615–620. 10.1002/elps.201900356. [DOI] [PubMed] [Google Scholar]
- Hristov D. R.; Rodriguez-Quijada C.; Gomez-Marquez J.; Hamad-Schifferli K. Designing Paper-Based Immunoassays for Biomedical Applications. Sensors (Switzerland). MDPI AG February 2019. Sensors 2019, 19, 554 10.3390/s19030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A.; Shang J.; Cheng F.; Paik B. A.; Kaplan J. M.; Andrade R. B.; Ratner D. M. Biofunctional Paper via the Covalent Modification of Cellulose. Langmuir 2012, 28, 11265–11273. 10.1021/la301661x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reches M.; Mirica K. A.; Dasgupta R.; Dickey M. D.; Butte M. J.; Whitesides G. M. Thread as a Matrix for Biomedical Assays. ACS Appl. Mater. Interfaces 2010, 2, 1722–1728. 10.1021/am1002266. [DOI] [PubMed] [Google Scholar]
- Lin S. C.; Hsu M. Y.; Kuan C. M.; Wang H. K.; Chang C. L.; Tseng F. G.; Cheng C. M. Cotton-Based Diagnostic Devices. Sci. Rep. 2015, 4, 6976 10.1038/srep06976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier J.; Brakke K. A.; Gosselin D.; Berthier E.; Navarro F. Thread-Based Microfluidics: Flow Patterns in Homogeneous and Heterogeneous Microfiber Bundles. Med. Eng. Phys. 2017, 48, 55–61. 10.1016/j.medengphy.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Weng X.; Kang Y.; Guo Q.; Peng B.; Jiang H. Recent Advances in Thread-Based Microfluidics for Diagnostic Applications. Biosens. Bioelectron. 2019, 132, 171–185. 10.1016/j.bios.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilghaz A.; Ballerini D. R.; Shen W. Exploration of Microfluidic Devices Based on Multi-Filament Threads and Textiles: A Review. Biomicrofluidics 2013, 7, 051501 10.1063/1.4820413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajikhah S.; Cabot J. M.; Innis P. C.; Paull B.; Wallace G. Life-Saving Threads: Advances in Textile-Based Analytical Devices. ACS Comb. Sci. 2019, 21, 229–240. 10.1021/acscombsci.8b00126. [DOI] [PubMed] [Google Scholar]
- Li X.; Tian J.; Shen W. Thread as a Versatile Material for Low-Cost Microfluidic Diagnostics. ACS Appl. Mater. Interfaces 2010, 2, 1–6. 10.1021/am9006148. [DOI] [PubMed] [Google Scholar]
- Owens T. L.; Leisen J.; Beckham H. W.; Breedveld V. Control of Microfluidic Flow in Amphiphilic Fabrics. ACS Appl. Mater. Interfaces 2011, 3, 3796–3803. 10.1021/am201003b. [DOI] [PubMed] [Google Scholar]
- Ballerini D. R.; Li X.; Shen W. Flow Control Concepts for Thread-Based Microfluidic Devices. Biomicrofluidics 2011, 5, 014105 10.1063/1.3567094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini D. R.; Li X.; Shen W. An Inexpensive Thread-Based System for Simple and Rapid Blood Grouping. Anal. Bioanal. Chem. 2011, 399, 1869–1875. 10.1007/s00216-010-4588-5. [DOI] [PubMed] [Google Scholar]
- Tomimuro K.; Tenda K.; Ni Y.; Hiruta Y.; Merkx M.; Citterio D. Thread-Based Bioluminescent Sensor for Detecting Multiple Antibodies in a Single Drop of Whole Blood. ACS Sensors 2020, 5, 1786–1794. 10.1021/acssensors.0c00564. [DOI] [PubMed] [Google Scholar]
- Jarujamrus P.; Prakobkij A.; Puchum S.; Chaisamdaeng S.; Meelapsom R.; Anutrasakda W.; Amatatongchai M.; Chairam S.; Citterio D. Acid-Base Titration Using a Microfluidic Thread-Based Analytical Device (MTAD). Analyst 2020, 145, 4457–4466. 10.1039/D0AN00522C. [DOI] [PubMed] [Google Scholar]
- Xiao G.; He J.; Chen X.; Qiao Y.; Wang F.; Xia Q.; Yu L.; Lu Z. A Wearable, Cotton Thread/Paper-Based Microfluidic Device Coupled with Smartphone for Sweat Glucose Sensing. Cellulose 2019, 26, 4553–4562. 10.1007/s10570-019-02396-y. [DOI] [Google Scholar]
- Prabhu A.; Nandagopal M. S. G.; Peralam Yegneswaran P.; Prabhu V.; Verma U.; Mani N. K. Thread Integrated Smart-Phone Imaging Facilitates Early Turning Point Colorimetric Assay for Microbes. RSC Adv. 2020, 10, 26853–26861. 10.1039/D0RA05190J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sateanchok S.; Wangkarn S.; Saenjum C.; Grudpan K. A Cost-Effective Assay for Antioxidant Using Simple Cotton Thread Combining Paper Based Device with Mobile Phone Detection. Talanta. 2018, 177, 171–175. 10.1016/j.talanta.2017.08.073. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.; Estala L.; Gaines M.; Gomez F. A. Mixed Thread/Paper-Based Microfluidic Chips as a Platform for Glucose Assays. Electrophoresis 2016, 37, 1685–1690. 10.1002/elps.201600029. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.; Gaines M.; Gallegos L. Y.; Guevara R.; Gomez F. A. Enzyme-Linked Immunosorbent Assays (ELISA) Based on Thread, Paper, and Fabric. Electrophoresis 2018, 39, 476–484. 10.1002/elps.201700354. [DOI] [PubMed] [Google Scholar]
- Seth M.; Mdetele D.; Buza J. Immunochromatographic Thread-Based Test Platform for Diagnosis of Infectious Diseases. Microfluid. Nanofluid. 2018, 22, 45 10.1007/s10404-018-2065-1. [DOI] [Google Scholar]
- Vatansever F.; Burtovyy R.; Zdyrko B.; Ramaratnam K.; Andrukh T.; Minko S.; Owens J. R.; Kornev K. G.; Luzinov I. Toward Fabric-Based Flexible Microfluidic Devices: Pointed Surface Modification for PH Sensitive Liquid Transport. ACS Appl. Mater. Interfaces 2012, 4, 4541–4548. 10.1021/am3008664. [DOI] [PubMed] [Google Scholar]
- Cabot J. M.; Breadmore M. C.; Paull B. Thread Based Electrofluidic Platform for Direct Metabolite Analysis in Complex Samples. Anal. Chim. Acta. 2018, 1000, 283–292. 10.1016/j.aca.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.; Gaines M.; Gomez F. A. Thread-Based Microfluidic Chips as a Platform to Assess Acetylcholinesterase Activity. Electrophoresis 2017, 38, 996–1001. 10.1002/elps.201600476. [DOI] [PubMed] [Google Scholar]
- Erenas M. M.; De Orbe-Payá I.; Capitan-Vallvey L. F. Surface Modified Thread-Based Microfluidic Analytical Device for Selective Potassium Analysis. Anal. Chem. 2016, 88, 5331–5337. 10.1021/acs.analchem.6b00633. [DOI] [PubMed] [Google Scholar]
- Agustini D.; Fedalto L.; Bergamini M. F.; Marcolino-Junior L. H. Microfluidic Thread Based Electroanalytical System for Green Chromatographic Separations. Lab Chip 2018, 18, 670–678. 10.1039/C7LC01267E. [DOI] [PubMed] [Google Scholar]
- Mao X.; Du T. E.; Wang Y.; Meng L. Disposable Dry-Reagent Cotton Thread-Based Point-of-Care Diagnosis Devices for Protein and Nucleic Acid Test. Biosens. Bioelectron. 2015, 65, 390–396. 10.1016/j.bios.2014.10.053. [DOI] [PubMed] [Google Scholar]
- Choi J. R.; Nilghaz A.; Chen L.; Chou K. C.; Lu X. Modification of Thread-Based Microfluidic Device with Polysiloxanes for the Development of a Sensitive and Selective Immunoassay. Sens. Actuators, B 2018, 260, 1043–1051. 10.1016/j.snb.2018.01.102. [DOI] [Google Scholar]
- Gemci R. Examining the effects of mercerization process applied under different conditions to dimensional stability. Sci. Res. Essays 2010, 5, 560–571. [Google Scholar]
- Agustini D.; Bergamini M. F.; Marcolino-Junior L. H. Characterization and Optimization of Low Cost Microfluidic Thread Based Electroanalytical Device for Micro Flow Injection Analysis. Anal. Chim. Acta 2017, 951, 108–115. 10.1016/j.aca.2016.11.046. [DOI] [PubMed] [Google Scholar]
- Du T. E.; Wang Y.; Zhang Y.; Zhang T.; Mao X. A Novel Adenosine-Based Molecular Beacon Probe for Room Temperature Nucleic Acid Rapid Detection in Cotton Thread Device. Anal. Chim. Acta 2015, 861, 69–73. 10.1016/j.aca.2014.12.044. [DOI] [PubMed] [Google Scholar]
- Mao X.; Du T. E.; Meng L.; Song T. Novel Gold Nanoparticle Trimer Reporter Probe Combined with Dry-Reagent Cotton Thread Immunoassay Device for Rapid Human Ferritin Test. Anal. Chim. Acta 2015, 889, 172–178. 10.1016/j.aca.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Arroyo M. J.; Erenas M. M.; Orbe-Payá I.; de Cantrell K.; Dobado J. A.; Ballester P.; Blondeau P.; Salinas-Castillo A.; Capitán-Vallvey L. F. Thread Based Microfluidic Platform for Urinary Creatinine Analysis. Sens. Actuators, B 2020, 305, 127407 10.1016/j.snb.2019.127407. [DOI] [Google Scholar]
- Suarez W. T.; Franco M. O. K.; Capitán-Vallvey L. F.; Erenas M. M. Chitosan-Modified Cotton Thread for the Preconcentration and Colorimetric Trace Determination of Co(II). Microchem. J. 2020, 158, 105137 10.1016/j.microc.2020.105137. [DOI] [Google Scholar]
- Perry J. L.; Miller G. R.; Carr D. L. Rapid, Colorimetric Identification of Candida Albicans. J. Clin. Microbiol. 1990, 28, 614–615. 10.1128/JCM.28.3.614-615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers R. D. New Directions in the Diagnosis and Therapy of Urinary Tract Infections. Am. J. Obstet. Gynecol. 1991, 164, 1387–1389. 10.1016/0002-9378(91)91478-F. [DOI] [PubMed] [Google Scholar]
- Andriole V. T. Perspective: Urinary Tract Infections: Recent Developments. J. Infect. Dis. 1987, 156, 865–869. 10.1093/infdis/156.6.865. [DOI] [PubMed] [Google Scholar]
- Oneson R.; Groschel D. H. M. Leukocyte Esterase Activity and Nitrite Test as a Rapid Screen for Significant Bacteriuria. Am. J. Clin. Pathol. 1985, 83, 84–87. 10.1093/ajcp/83.1.84. [DOI] [PubMed] [Google Scholar]
- Terse-Thakoor T.; Punjiya M.; Matharu Z.; Lyu B.; Ahmad M.; Giles G. E.; Owyeung R.; Alaimo F.; Shojaei Baghini M.; Brunyé T. T.; Sonkusale S. Thread-Based Multiplexed Sensor Patch for Real-Time Sweat Monitoring. npj Flexible Electron. 2020, 4, 18 10.1038/s41528-020-00081-w. [DOI] [Google Scholar]