Abstract

Based on the increasing importance of intermetallic compounds and alloys in heterogeneous catalysis, we explore the possibilities of using selected intermetallic compounds and alloy structures and phases as catalyst precursors to prepare highly active and CO2-selective methanol steam reforming (MSR) as well as dry reforming of methane (DRM) catalyst entities by controlled in situ decomposition and self-activation. The exemplary discussed examples (Cu51Zr14, CuZn, Pd2Zr, GaPd2, Cu2In, ZnPd, and InPd) show both the advantages and pitfalls of this approach and how the concept can be generalized to encompass a wider set of intermetallic compounds and alloy structures. Despite the common feature of all systems being the more or less pronounced decomposition of the intermetallic compound surface and bulk structure and the in situ formation of much more complex catalyst entities, differences arise due to the oxidation propensity and general thermodynamic stability of the chosen intermetallic compound/alloy and their constituents. The metastability and intrinsic reactivity of the evolving oxide polymorph introduced upon decomposition and the surface and bulk reactivity of carbon, governed by the nature of the metal/intermetallic compound-oxide interfacial sites, are of equal importance. Structural and chemical rearrangements, dictating the catalytic performance of the resulting entity, are present in the form of a complete destruction of the intermetallic compound bulk structure (Cu51Zr14) and the formation of an metal/oxide (Cu51Zr14, InPd) or intermetallic compound/oxide (ZnPd, Cu2In, CuZn) interface or the intertranformation of intermetallic compounds with varying composition (Pd2Zr) before the formation of Pd/ZrO2. In this Perspective, the prerequisites to obtain a leading theme for pronounced CO2 selectivity and high activity will be reviewed. Special focus will be put on raising awareness of the intrinsic properties of the discussed catalyst systems that need to be controlled to obtain catalytically prospective materials. The use of model systems to bridge the material’s gap in catalysis will also be highlighted for selected examples.
Keywords: methanol steam reforming, methane dry reforming, dynamics, thermodynamic stability, phase boundary, carbon reactivity, water activation
1. Introduction into the Scientific Concept
Intermetallic compounds represent an important and very fast growing group of materials in heterogeneous catalysis.1−6 Significant progress has been made over the past two decades with respect to synthesis, adsorption behavior, and the general understanding of bonding properties and structures. Several reviews covering almost all aspects of intermetallic compounds and alloys are currently available.1−6 With respect to catalytic applications, intermetallic compounds and alloys have continued to contribute significant progress to the understanding of a range of reactions, with the semihydrogenation of acetylene and methanol steam reforming at the forefront.2 Despite the large number of intermetallic compounds and alloys that are principally known (e.g., 6000 different only binary intermetallic compounds were known in 2014,2 a total number of 2500 publications with respect to the use of intermetallic compounds in catalysis have been published up to 20201) and their widespread use in catalytic research, one key obstacle in their use clearly remains: even in the simplest reactions (and more valid for complex reactions, such as methanol steam reforming), the bulk and surface structure of the intermetallic compounds are generally not static, but increasingly dynamic.1,2 This renders the establishment of structure–property/activity/selectivity relationships not straightforward. The exemplary ZnPd intermetallic compound, which has particularly stirred up catalytic research in the past two decades, serves as a highly illustrative example in this respect. ZnPd is one of the most CO2-selective methanol steam reforming catalysts, and many aspects of its properties are already known.7−11 This structurally quite simple intermetallic compound is a prime example to show the highly dynamic nature of such materials in catalysis. ZnPd features structural alterations even if exposed to CO. Its catalytic performance in methanol steam reforming can be directly related to its structural instability and highly dynamic surface and bulk structure upon contact with the methanol and water reaction mixture.2 It is now widely accepted that the active phase is not the self-supported isolated intermetallic compound, but in fact an intermetallic compound-oxide ZnPd–ZnO interface, that is in situ formed during catalytic operation.8 A bifunctional operating mechanism is prevalent: ZnPd ensures methanol, and ZnO, water activation. Whether the interfacial region itself holds the active centers, or if spillover effects of activated species occur, is still subject to discussion. The features of ZnPd could be generalized to similar intermetallic compounds, where the structural dynamics appear unfortunate at first sight. However, this apparent disadvantage can be overturned if the structural alterations are controlled and the subsequent partial or complete decomposition of the intermetallic compound/alloy is steered in a catalytically meaningful way. As a consequence, intermetallic compounds and alloys would therefore be used only as highly defined precursor structures that are transformed—either by a selected pretreatment or in the reaction mixture itself—into the active/selective structure or phase.
This concept itself is not new: already in 1976, it was recognized that synthetic ammonia catalysts on the basis of intermetallic compounds consisting of a transition metal and a rare earth element, upon contact with the ammonia synthesis reaction mixture, give rise to decomposition and the formation of a metal–nitride interface.12 An array of such intermetallic compounds incorporating Co and Fe, e.g., HoFe3 (resulting in HoN and Fe) or CeCo3 (resulting in CeN and Co), yielded such interfaces. Similar observations were made for PrCo2, CeCo2, or PrCo3.12 Most remarkably, the authors particularly stated that only the in situ decomposed composite is active. The concept of using intermetallic compounds as precursor structures to generate more active materials has been extended to other reactions such as CO and CO2 methanation and hydrocarbon synthesis. For CO methanation, Coon et al. studied combinations of Ni, Co, Fe, and rare earth elements and found similar results (e.g., for LaNi5, ErNi5 or ErFe3, among others).13−15 More input has been provided by Craig et al. using actinide–transition metal intermetallic compounds for hydrocarbon synthesis. Despite their obvious niche application, the observations on ThNi5, ZrNi5, and UNi5 are of prime importance in the understanding of the operational principles of more recent and applicable intermetallic compounds.13In situ formation of Ni/ThO2, Ni/ZrO2, and Ni/UO2 upon exposure to the CO + H2 mixture was observed, and it was specifically stated that “...specific interfaces or specific interactions between metal and support [are observed]...”15 and that “...conventional Ni on ThO2 [prepared by impregnation] is less active than the Ni/ThO2 system obtained by ThNi5 decomposition...”13,16 The so-obtained mixture was identified as the active phase.13,17 For the Ni/ThO2 system, the increased H2S poisoning tolerance was attributed to a “bifunctional synergism,” resulting from the specifics of the element with which Ni was combined in the intermetallic compound precursor state.15 This already points to some kind of “memory effect,” indicating potential use for steering the catalytic properties of the resulting decomposition mixture. For ThNi5, it was stated that “nickel, formed as a decomposition product by the nature of the MNi5 compound, is probably the active species, but its properties are influenced by the nature of M in the MNi5 precursor state.”15
Decomposition of intermetallic compound catalysts for ammonia synthesis, CO oxidation, and selective hydrogenation, of Fe91Zr918 and Pd8Si19,19 into (surface) Fe + ZrO2–x, as well as Pd + SiO2 was also observed. For the latter, the activity is due to a “very special surface distribution [of Pd and SiO2].”20 Recently, the concept of decomposing intermetallic compounds into an active state has also been extended to the methanol steam reforming performance of single-phase quasicrystals on especially an Al–Fe–Cu basis. The leaching behavior and the resulting formation of small copper particles has been determined to be strongly dependent on its individual composition.21
In recent years, the mostly unwanted, or at least not deliberately induced, decomposition of Pd- and Cu-based intermetallic compounds has given rise to especially CO2-selective methanol steam reforming catalysts.7−11,22−35 ZnPd, GaPd2, InPd, InPt, GaPt2, Cu51Zr14, or ZnNi, to name just a few, have one common structural denominator: resulting from partial or full in situ decomposition of an intermetallic compound precursor, the CO2-selective state is exclusively composed of an intermetallic compound (or metal)–oxide interface with shared activation and catalytic duties between the two constituting entities.36 Strong differences among the individual precursor materials with respect to adsorption, stability, or oxidation propensity have been observed, emphasizing the need for an approach less reliant on trial and error in order to induce and understand decomposition. Another reaction, where the concept of controlled intermetallic compound/alloy precursor decomposition is increasingly exploited, is the dry reforming of methane. Here, an additional level of complexity related to the carbon reactivity on mostly Pd–Zr systems is introduced,37,38 although the underlying principles of the concept are similar. A recent study on Ni–Y alloys also revealed in situ decomposition into Ni/Y2O3 composites with superior dry reforming activity.39 In a similar fashion, the stability of different Hf-based intermetallic compounds (e.g., NiHf or CoHf2) during dry reforming has been assessed.40
The same concept of creating supported-metal catalysts via decomposition of precursor structures was previously discussed for amorphous metal alloys (i.e., “metal glasses”). Several examples in the literature exist, which have demonstrated the potential to use such materials as promising catalyst precursors.41,42 In the present Perspective, we deliberately do not discuss such metal glasses but rather focus on prospective intermetallic compound precursors, which have the advantage of providing a highly defined starting structure. The corresponding alloy-related studies are essentially used to highlight the use of model systems to elucidate underlying mechanistic details of in situ decomposition, such as the reactivity of intermediary hydroxyl species resulting from water activation or reaction-induced carbon from methane activation.
As a consequence, the high structural dynamics giving in many cases rise to an at least partial decomposition is a matter of fact. However, destability of an intermetallic compound or alloy need not be a disadvantage per se. If a knowledge-based concept is established that allows the use of such materials to reproducibly and in a controlled way act as precursor structures for decomposition, access to more active and selective entities is granted.43 In the best way, nanocrystalline, highly stable supported intermetallic compound (or metal)–oxide composites of defined geometry, morphology, and electronic and thus, superior catalytic properties result. To accomplish this task successfully, the in-depth knowledge of factors and parameters influencing the decomposition is of the utmost importance. As will be clear from sections 2 and 3, for most of the examples these parameters (e.g., oxidation behavior, thermodynamic stability, modification, and catalytic performance of the resulting oxide or carbon reactivity) usually appear entangled. The term “knowledge-based concept or decomposition” is in the following used for an approach that takes advantage of the intrinsic properties of structurally, chemically, and electronically comparable intermetallic compounds/alloys to steer the decomposition without a widescale trial-and-error approach. Extending e.g., the ZnPd studies to GaPd2, CdPd, InPd, or ZnPt is such an example, which is documented by the similar valence band structure causing similar catalytic patterns in MSR.1
This Perspective introduces the widespread possibilities of using intermetallic compounds and alloys as precursor materials to prepare highly active and selective entities by controlled in situ decomposition and self-activation. We exemplify the advantages and possible pitfalls in using this approach by reviewing illustrative examples from our own expertise in section 2. Alongside the common feature of partial and/or full decomposition, the individual aspects of each discussed system will be assessed. Wherever possible, the discussed examples will be used to extrapolate the features to similar structures, thus, generalizing the concept. For each case study and material, a very short introduction into the state-of-the-art of the particular material in the chosen reaction will be given. The selection of the presented case studies is, on the one hand, driven by their use in two important reactions in the hydrogen economy and environmental science, methanol steam reforming and methane dry reforming. On the other hand, the selected materials are especially well-suited to show the scientific concept of this Perspective. The leading theme of the case studies with respect to methanol steam reforming is the importance of water activation and how this activation can be influenced by controlled decomposition. We selected two groups of intermetallic compounds/alloys: In section 2.1, two Cu-based materials, Cu51Zr14 and CuZn, are compared in their structural stability and methanol steam reforming performance, as the corresponding Cu/ZnO and/or Cu/ZrO2 systems have already displayed superior MSR properties. Section 2.2 is devoted to a direct comparison of the two Pd-based intermetallic compounds ZnPd and Ga2Pd. We link the (missing) structural instability of the respective intermetallic compounds directly to the MSR performance of the respective Pd/ZnO and Pd/Ga2O3 catalysts and the catalytic contribution of the reaction-induced oxide phase. The reactivity of reaction-induced carbon at Pd/ZrO2 interfaces resulting from in situ decomposition of Pd2Zr intermetallic compounds and Pd–Zr alloys is discussed in section 2.3. Section 3 deals with a set of key parameters that directly controls the catalytic performance outlined in section 2. The resulting metal-oxide phase boundary as the single most important parameter is discussed, alongside the consequences that arise in terms of oxide and carbon reactivity (sections 3.1 and 3.2). The combined knowledge of the intrinsic properties of both intermetallic compound/alloy structure and resulting decomposition products will then yield prerequisites to control the decomposition and obtain a leading theme to pronounced selectivity and activity. Control and steering of the decomposition is essentially possible by adjustment of the reaction environment (e.g., by changing the stoichiometry of the dry reforming reaction mixture to yield different interfacial carbon reactivities) and, therefore, its reduction/oxidation chemical potential. Another pathway of steering is related to varying the initial stoichiometry of the intermetallic compounds and alloys. We will show that, e.g., the water activation properties of stoichiometrically different Cu–Zn or Zn–Pd alloy samples is very much dependent on the initial stoichiometry.
2. Use of Controlled in Situ Decomposition of Intermetallic Compound and Alloy Precursor Structures to Create Highly Active and Selective Methanol Steam and Methane Dry Reforming Catalysts
2.1. Enhancing the Water Activation by in Situ Activation and Decomposition of Cu51Zr14 and CuZn Intermetallic Compounds and Alloys: Pathways to Metal–Oxide Systems with Superior Methanol Steam Reforming Performance
A first example to exploit the in situ decomposition of well-defined intermetallic compounds is the oxidative decomposition of Cu51Zr14 in a methanol steam reforming mixture into a very CO2-selective Cu/t-ZrO2 composite mixture.23,28,29 The addition (or substitution) of ZrO2 to already well-established Cu/ZnO catalysts allows an overcoming of the Cu sintering by structural stabilization of Cu by ZrO2. Direct interaction of Cu and the participating Zr species, including the formation of Cu–O–Zr bonds, has been suggested.44−47 The resulting Cu–ZrO2 interface has been suspected to host the active and selective sites. The contact of Cu metal to the tetragonal ZrO2 modification yields a particularly CO2-selective material.42 Structure-wise, the Zr–O phase diagram is a complex issue, as the thermodynamically more stable monoclinic polymorph has been previously reported to be of minor catalytic relevance for high CO2 selectivity.45 Tetragonal (or cubic) ZrO2 needs to be either externally stabilized by dopants (e.g., Y) or intrinsically stabilized by oxygen vacancies and/or particle size effects. Taking these features into account in the search for alternative preparation pathways to optimize the metal–oxide interfacial sites of CO2-selective Cu/ZrO2 methanol steam reforming catalysts, we end up with a difficult task.
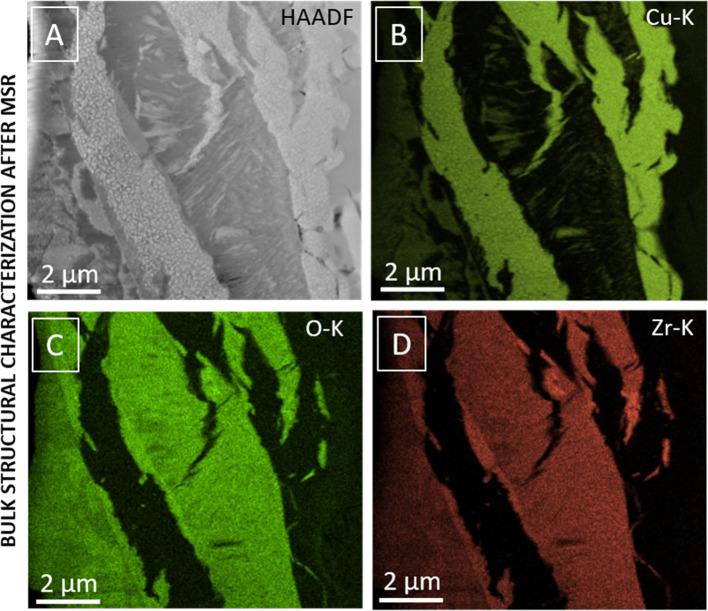
The use of Cu–Zr alloys or intermetallic compounds as precursor structures to access active and selective Cu/ZrO2 catalysts has been up to now limited to the exploitation of Pd- and Au-doped Cu–Zr metallic glasses.48−51 However, we experience limitations with respect to the ill-defined initial glassy state and the necessity for oxidative pretreatments to decompose the alloy before the methanol steam reforming experiment. The selection of a more defined Cu–Zr precursor intermetallic compound structure is a logical extension of the concept. Figures 1 and 2 highlight our results using a defined Cu51Zr14 intermetallic compound structure for oxidative decomposition in a methanol steam reforming mixture. We synthesized the intermetallic compound by reactive co-melting of metallic Cu and Zr, resulting in a quite uniform distribution of Cu and Zr within surface and bulk regions (Figure 1, panel A). Subjecting the Cu51Zr14 material to a methanol steam reforming reaction up to 623 K (Figure 1, panel B) yields decomposition into a Cu/ZrO2 composite consisting of surface-bound small Cu particles embedded in a ZrO2 matrix. Both X-ray and electron diffraction confirm the almost exclusive presence of tetragonal ZrO2. In essence, the in situ decomposition during methanol steam reforming yields the anticipated Cu/t-ZrO2 sample with an extended—due to the intimate contact between Cu in the ZrO2 matrix—interface between Cu and tetragonal ZrO2. Destruction of Cu51Zr14 affects the surface and bulk in a similar way (Figure 2).23,28,29
Figure 1.
(Surface) structural, chemical, and catalytic characterization of the Cu51Zr14 intermetallic compound structure during methanol steam reforming up to 623 K. Panels A and B: STEM/EDX analysis of the surface-near regions of decomposed Cu51Zr14 before (A) and after one catalytic MSR cycle up to 623 K (B). The individual panels highlight the HAADF image and the Cu–K and Zr–K intensities. The overlay shows also the O–K intensity and to the right the Pt signal from the FIB sample preparation. Panel C: XPS surface chemical characterization before and after several MSR cycles. Panel D: Four consecutive catalytic methanol steam reforming profiles starting from the Cu51Zr14 intermetallic compound. Reproduced with permission from ref (28). Copyright 2021 Wiley-VCH.
Figure 2.
STEM/EDX characterization of the Cu51Zr14 intermetallic compound structure after a methanol steam reforming reaction up to 623 K. The individual panels highlight the HAADF image (panel A), the Cu–K (panel B), O–K (panel C), and Zr–K intensities (panel D). Reproduced with permission from refs (28) and (29).32 Copyright 2021 Wiley-VCH and American Chemical Society.
Revisiting the prerequisites for CO2-selective MSR, we note that the key criterion is efficient methanol and water at dedicated (interfacial) sites. The role of Cu is still controversially discussed, with both purely geometric strain and ionic effects (Cu vs. Cu+ species) put forward.28 However, water activation remains crucial. Numerous studies on different intermetallic compounds reveal the role of the “support” formed by decomposition of the catalytic precursor7−11,22−35 (ZrO2 in this case), going well beyond simple stabilization of the distribution of reactive Cu particles. Reversible hydroxylation of ZrO2 (or special interfacial sites), invoking a bifunctional synergism with shared duties between Cu (methanol activation) and ZrO2 (water activation), is very important. Tetragonal ZrO2 arising from in situ decomposition is indeed capable of efficient reversible formation of surface Zr–OH species, as documented by the Zr–OH component in Zr 3d XP spectra after each of the four consecutive MSR runs (Figure 1, panel C). This directly translates into a very CO2-selective Cu/tetragonal ZrO2 material (Figure 1, panel D). Ongoing in situ activation of Cu51Zr14 during the four displayed MSR cycles occurs, as judged by the shift of the CO2 light off temperatures to lower values after each consecutive run. The activity of the observed material is 102 times higher than similar Cu/ZrO2 systems described in the literature and still exceeds a conventional Cu/ZnO catalyst by a factor of 3 (studied under identical conditions).28
We identify essentially two factors steering the decomposition of Cu51Zr14 and the exclusive formation of Cu/tetragonal ZrO2. First, we note the high oxidation propensity of Zr and the associated high formation enthalpy of ZrO252 formed by Cu51Zr14 decomposition. Also, our own model system studies on differently preprared Cu-ZrO2 materials starting from different alloy precursors have shown that keeping Zr in its metallic state during preparation is extremely difficult. However, the inevitable oxidation of Zr and the formation of Zr0/Zr4+ entities in fact provide an efficient approach to Cu–Zr0/Zr4+ materials that can be deliberately switched between a CO-, HCHO-, and CO2-selective state by the preparation process.53,54 The key criterion is the different hydroxylation ability of the different Zr species formed during synthesis. A second (geometric) steering factor is the particular epitaxial stabilization of the Cu metal–tetragonal ZrO2 interface.29 The observed almost perfect epitaxial match is particularly aided by the well-defined precursor Cu51Zr14 structure.
Summarizing, the Cu51Zr14 case study provides a perfect example of how the decomposition of a highly defined precursor state yields an outstandingly CO2-selective methanol steam reforming catalyst, with a bifunctional operating mechanism being directly deducible. The discussed concept is also valid for other nominal initial Cu/Zr stoichiometries. Variation of the Cu/Zr ratio from 9:2 over 2:1 to 1:2 (essentially starting from Cu5Zr or CuZr2 structures with Cu metal and Zr metal by-components) and in situ decomposition during MSR essentially yields similar catalytic patterns with respect to CO2 selectivity and hydroxylation propensity of the participating Zr4+ species.23
The results from the Cu51Zr14 case study can be perfectly generalized to investigations of the hydrogen production following CO2-selective methanol steam reforming on CuZn alloy precursor states. Mainly driven by the in-depth understanding of technical Cu/ZnO methanol synthesis catalysts, the importance of the Cu0/ZnO interface in both preparation and activation has been repeatedly stressed.55 The addition of Zn has been previously suspected to lead to a clear improvement of catalytic properties, despite the fact that the exact role of Zn had not been clarified until recently. In particular, a plethora of essentially contradictory interpretations of the role of the Cu/ZnO interface have been put forward, including a “Cu–Zn” alloy model, with the suspected formation of a Cu–Zn(OH) species during MSR.55
The model concept using a UHV-based methodological approach is particularly feasible here, as the eventual segregation behavior of Zn and the associated redox chemistry of both Cu and ZnO (and their interface) are more easily followed. To optimize the CO2 selectivity, we scrutinized a series of brass samples with different nominal stoichiometries (CuZn37, CuZn10, and CuZn15) and a near-surface Cu–Zn alloy state, accessed through thermal Zn deposition and subsequent annealing treatments. Evaporation of between 5 and 12 monolayers of Zn onto a Cu foil at 300 K, followed by a short thermal annealing step (10 min) at 523 K, induces the formation of a CuZn ∼10:1 near-surface alloy state with superior MSR properties.
The MSR profile (Figure 3A) of this alloy state in relation to pure Cu reveals that methanol is fast converted with water at almost 100% CO2 selectivity between 530 and 623 K. The in situ near-ambient pressure XP spectra collected during catalytic MSR operation (Figure 2B) point out that this CO2-selectivity goes along with a transition from a purely bimetallic Zn 3d component at 300 K to an almost 1:1 mixture of oxidic and bimetallic Zn at 543 K. The start of the Zn segregation to the surface can be pinpointed to ∼450 K, as indicated by a binding energy shift of the Zn 3d peak and a rise in the Zn 3d/Cu 3d peak ratio. Summarized in the inset in Figure 3A, the CuZn ∼10:1 near-surface alloy precursor state provides the optimum Zn loading and distribution for an Cu/Znox interface with a high number of active sites providing high CO2 selectivity. A bifunctional synergism prevails, with Cu providing fast methanol dehydrogenation to formaldehyde, while Znox sites are essentially responsible for water activation. Missing Znox sites, as well as the presence of a fully Cu blocking passivating Znox layer lead to catalyst deactivation.
Figure 3.
Panel A: Catalytic methanol steam reforming profiles on clean Cu (lower panel) and a Cu:Zn = 10:1 near surface alloy (top panel). A schematic of the formation of the active centers during MSR is shown as inset. Panel B: In situ X-ray photoelectron spectra (top, Cu 3d; bottom, Zn 3d region) collected on an initial Cu/Zn = 10:1 near surface alloy at 130 eV during methanol steam reforming (0.12 mbar methanol + 0.24 mbar water). The Zn 3d region is deconvoluted into bimetallic CuZn (blue, 10.25 eV) and oxidic Zn (red, 11.2 eV) components. Reproduced with permission from ref (55). Copyright 2021 Wiley-VCH.
The obtained results already point out an inherent problem of a delicate stoichiometric balance of Cu and Zn in the precursor state to obtain a CO2 selective state during MSR. To underline this importance, in the present case study all brass samples and all too Zn-rich near-surface alloys exhibited formation of such a Znox passivating layer. Exact tuning of the initial level of Zn doping is imperative to, e.g., promoting formate reactivity at an optimized Cu/Znox interface. Both CO2 selectivity and MSR activity directly scale with the extent of the Cu/Znox interface, which is a result of the optimum precursor stoichiometry and the subsequent Zn segregation (and oxidation) to the surface during in situ activation.
2.2. Teamwork or Not? Enhancing the Methanol Steam Reforming Performance by Bifunctionally Operating in Situ Activated Intermetallic Compound–Oxide Interfaces: ZnPd vs GaPd2
The group of intermetallic compounds based on 8–10 group metals was initially introduced by Iwasa et al. in the mid 1990s, mostly to overcome the poor sintering stability and associated deactivation of conventional Cu/Zn/Al2O3 catalysts.10 Starting with Pd and Pt particles on selected oxides, reduction in hydrogen at temperatures up to 773 K (depending on the specific oxide) yielded small intermetallic compound particles supported on a more or less reduced oxide support. Depending on the oxide, very different catalytic patterns in methanol steam reforming resulted. Depositing Pd or Pt on, e.g., SiO2, an oxide usually considered hard to reduce, preserves the metallic state upon reduction and only methanol dehydrogenation is observed (intermetallic compound formation starting from Pd or Pt on SiO2 (or Al2O3) can be triggered upon reduction in hydrogen, but needs much higher temperatures (T ≥ 873 K)56). In contrast, deposition of small Pd or Pt particles on ZnO, Ga2O3, or In2O3 and subsequent hydrogen reduction yields the highly CO2-selective intermetallic compounds ZnPd, GaPd2, and InPd.7−11,24−27,30−35 These early observations have triggered extensive studies to unravel the full mechanistic details of MSR operation. Extension to structurally similar compounds, such as CdPd, revealed the common electronic valence band structure of metallic Cu, ZnPd, GaPd2, InPd, and CdPd (among others) to be the crucial catalytic steering parameter.43 In due course, first attempts were made to separate the structural and catalytic contributions of intermetallic compounds and oxides. Several key observations, in turn fitting to a larger picture of in situ activation of intermetallic compounds and the establishment of structure-selectivity correlations, were made: (i) As discussed for Cu–Zn,55 the stoichiometric balance between both constituents of the intermetallic compounds was found to be a generally important parameter to establish a highly CO2-selective material. This was by far best studied on the archetypical ZnPd compound.7−11 ZnPd exhibits a rather large compositional range, where a large deviation from the ideal stoichiometry can be structurally tolerated.9 However, these compositional deviations go at the cost of a different electronic structure, formation of passivating oxide layers, and composition-dependent catalytic patterns.43 Largely neglected for a long time were in fact the catalytic and structural properties of the supporting oxide.10,43 Different catalytic profiles were obtained using either ZnO, Ga2O3, or In2O3 as supports.7−11,24,25,27,33 ZnO and In2O3 are both highly CO2-selective methanol steam reforming catalysts,11,57 but Ga2O3 itself features a vital formate- and oxygen vacancy-mediated (reverse) water–gas shift reactivity, spoiling the CO2 selectivity.58,59 In comparison to In2O3, ZnO and Ga2O3 are hard-to-reduce oxides. In2O3 readily loses lattice upon annealing in either pure hydrogen or during MSR, and as such, most catalytically relevant properties are oxygen vacancy-dominated.60,61 As a consequence of both points discussed above, the in situ stability and eventual decomposition into an (inter)metallic compound/metal-oxide system and its dependence on composition has drawn particular attention. Naturally, this again raises the question of the influence of the metal-oxide phase boundary on the catalytic properties.
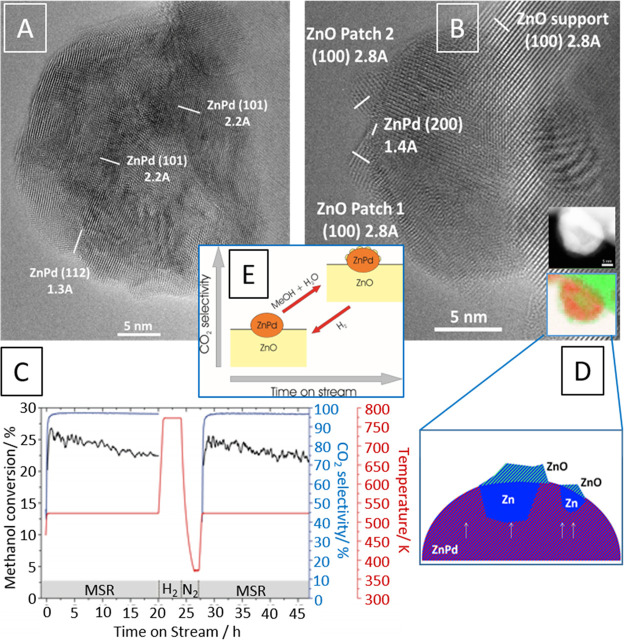
The importance of this (in situ formed) boundary is best appreciated if the MSR performance of isolated ZnPd8 and GaPd230 is compared. Figure 4, panels A and B showcase a direct high-resolution TEM comparison of ZnPd after initial contact with the reaction mixture at 573 K (panel A) and in the most CO2-selective state (panel B). Following the initial explanation of assigning the full catalytic action to ZnPd alone,10,43 the catalyst state displayed in panel A would be essentially CO2-selective, as it apparently consists of small ZnPd particles supported on reduced ZnO. The MSR profile (panel C), however, features an induction period of 30–60 min before the CO2 selectivity strongly increases. Thus, the catalyst in situ self-activates and transforms itself into the state of panel B. The critical structural difference between the two states is the appearance of ZnO patches on the surface of the ZnPd particle. This ZnO arises from oxidative in situ decomposition of Zn-rich areas within the chemically extremely inhomogeneous ZnPd particles (inset in panel B and panel D) and is structurally and electronically very different from the ZnO support.62 It is a direct consequence of in situ activation. The sequence of transforming the initial impregnated Pd/ZnO catalyst into the CO2-selective state involves (i) a reduction in hydrogen to form the ZnPd/ZnO state (which is per se not CO2-selective) and in situ formation of ZnPd(ZnO)/ZnO, which represents the CO2-selective state. The formation of the ZnO patches does not occur via a classic “strong metal-support interaction” effect but is purely a result of a chemical reaction of the Zn-rich areas with the MSR reaction mixture.62
Figure 4.
High-resolution electron microscopy images of a ZnPd/ZnO catalyst before CO2 selectivity is observed (panel A) and in the CO2-selective state (panel B). The catalytic methanol steam reforming experiment is highlighted in panel C. Panel D schematically depicts the CO2-selective ZnPd/ZnO interface in situ formed during catalytic MSR operation as derived from high-resolution and EELS imaging (inset in panel B). Panel E shows an overview of the CO2 selectivity as a function of structural transformation of the catalyst. Reproduced with permission from ref (8). Copyright 2021 Wiley-VCH.
The importance of the in situ activation of intermetallic compounds during MSR to deliver CO2-selective materials is further strengthened by similar experiments starting from isolated oxide-free GaPd2. In theory, this particular material is expected to behave similarly to ZnPd on the basis of reports on the MSR performance of Pd/Ga2O3 catalysts.22,24,33,63 As for Pd/Ga2O3, reduction yields a CO2-selective Ga2O3-supported GaPd2 intermetallic compound. To answer the question whether the isolated GaPd2 is equally prone to self-activation, a self-supporting bulk-like GaPd2 film was prepared by alternating deposition of Pd and Ga layers and subsequent thermal annealing (Figure 5, panel A). The catalytic MSR profile, however, indicates no CO2-selective state (Figure 5, panel B).22 CO is the main product due to the dominating methanol dehydrogenation with both CO2 and formaldehyde only formed as minor byproducts. The reason for this behavior is clear from the in situ collected XP spectra during MSR operation (Figure 5, panel C): no oxidic Ga2O3 component arises during MSR operation, pointing toward missing self-activation. Only if the Ga2O3 support is present from the beginning can a selectively functioning entity with shared duties between GaPd2 (methanol activation) and Ga2O3 (water activation) arise.22,24,63
Figure 5.

Panel A: SEM/EDX analysis of the isolated self-supported bulk GaPd2 intermetallic compound with the elemental Pd (Pd-M, green) and Ga (Ga-L, red) distribution as determined by EDX. Panel B: Catalytic methanol steam reforming profiles (12 mbar methanol + 24 mbar water). Experimental details given in ref (22). Panel C: In situ collected Pd 3d5/2(left), Ga 3d (middle), and valence band (right) XP spectra collected during methanol steam reforming (12 mbar methanol + 24 mbar water) on GaPd2. For maximum surface sensitivity, the Pd 3d5/2 signal was measured at 470 eV photon energy and the Ga 3d and valence band signals at 170 eV. The arrow in panel C indicates the increasing temperature from 332 to 573 K. Reproduced with permission from ref (24). Copyright 2021 Elsevier.
The outstanding role of ZnPd with respect to self-activation is confirmed by dedicated model catalyst studies utilizing differently prepared ZnPd materials (Figure 6) and also provides the link to the CuZn experiments discussed in section 2.1.35 The electronic structure of a thin ZnPd monolayer alloy very much resembles the one of pure metallic Pd. A missing oxidized Zn component in the respective in situ collected XP spectra (panel A) explains the suppressed water activation and full methanol dehydrogenation to CO (panel B, lower panel). In contrast, a bulk-like ZnPd alloy features both the electronic valence band structure of Cu and an oxidized Zn component. The latter arises from in situ self-activation—similarly as discussed for the powder ZnPd/ZnO material—and gives rise to a CO2-selective material in MSR (panel B, upper panel). The bifunctional operating mechanism enabling methanol and water activation on different sites of the bulk ZnPd sample is schematically depicted in panel C.
Figure 6.
Panel A: In situ XP spectra (Pd 3d5/2, Zn 3d and valence band regions) collected during MSR on a 1:1 ZnPd multilayer (red spectra) and on a respective ZnPd monolayer (blue spectra). Black spectra: metallic Pd reference. The in situ formed, oxidized ZnOH component is shown as a broken red line in the middle panel. For maximum surface sensitivity, the Pd 3d5/2 signal has been measured at 650 eV photon energy, the Zn 3d and valence band signals at 120 eV. Reaction conditions: 0.12 mbar methanol + 24 mbar water at 553 K. Panel B: MSR profiles on the multilayer PdZn 1:1 alloy (upper panel) vs MSR reaction on a monolayer PdZn surface and MSR reaction on clean Pd foil (lower panel). Reaction conditions: 12 mbar methanol + 24 mbar water. Experimental details given in ref (35). Panel C: Side view of the multilayer PdZn alloy with possible surface intermediates en route toward CO2. Reproduced with permission from ref (35). Copyright 2021 Wiley-VCH.
2.3. Steering the Methane Dry Reforming Activity of Pd–Zr Intermetallic Compounds and Alloys by Controlled in Situ Decomposition Yielding Pd-ZrO2 Interfaces with Beneficial Carbon Reactivity
The dry reforming of methane (DRM) reaction is considered a promising method to convert two harmful climate-harming gases, CO2 and CH4, into useful syngas, which can be further used to access a range of useful synthetic fuels. It is possible to steer the follow-up reactions by adjusting the H2/CO ratio in the produced syngas mixture. 1:1 ratios allow carbonylation or hydroformylation processes, while the synthesis of renewable fuels requires H2/CO ratios higher than 2.64,65 Application-wise, the coking issues, especially on the widely used Ni-based materials, represent the most serious obstacle.66−69 Early attempts to improve the Ni coking resistance yielded promising bimetallic NiPd DRM catalysts supported on ZrO2.70 On a mechanistic level, both ensemble and ligand effects at the bimetallic surface can account for the methane-activating role of the intermetallic components, but the duty of the intermetallic compound (or alloy)–oxide interface is less clear. For inert supports, an eventual cocatalytic role of the metal–oxide interface is apparently less pronounced in the presence of a material that may activate both CO2 and CH4, such as pure Ni. Steering the level of bifunctional operation is possible by mixing Ni with an oustanding CH4 activator with at the same time inferior CO2 activation properties in its pure state, such as Pd. Consequently, the associated promotion of CO2 activation on Pd requires a comparatively higher number of Pd–oxide interfacial sites.37,38
This lays out the general strategy to employ intermetallic compounds and alloys in the knowledge-driven development of active methane dry reforming catalysts: we should focus on the preparation of the most extended (inter)metal(lic)–oxide interface providing superior methane activation on the in situ activated intermetallic compound or metal component and enhanced CO2 activation properties of the oxide component. Both oxygen vacancy-mediated (as a consequence of surface reducibility) and surface-chemistry mediated (as a consequence of basic surface sites enabling CO2 activation as reactive carbonate intermediates) parameters are considered central for high DRM activity. With respect to the use of intermetallic or alloy precursor structures, bulk intermetallic samples are particularly suited to trigger partial or quantitative decomposition into metal–oxide systems with a large contact area. This can in principle be achieved by precatalytic treatments, such as reductive activation or following special leaching techniques, or achieved through direct in situ activation in the reaction mixture. We have shown in the preceding sections that this is a particularly worthwhile approach for Pd–Zr and Cu–Zr systems in methanol steam reforming to access a large amount of phase boundary sites.23,28,29,37,38
The importance of the quality of the evolving Pd-ZrO2 phase boundary sites with respect to activation properties and the associated carbon reactivity evolving from in situ decomposition of different Pd–Zr intermetallic compounds and defined alloys is summarized in Figure 7. A comparative catalytic DRM characterization of a subsurface Zr0-doped Pd sample (representing a near-surface model alloy catalyst), a bulk Pd2Zr intermetallic compound, and a Pd/ZrO2 reference catalyst already reveals different active states (panel A). The structural denominator of the intermetallic compound/alloy sample is the decomposition into Pd/ZrO2 during a DRM treatment (monitored by in situ X-ray diffraction during DRM operation up to 1073 K, panel B) exceeding the activity of the impregnated Pd/ZrO2 catalyst by a factor of 100. The high activity is directly linked to the fast reaction of highly reactive Pd carbide species (i.e., dissolved carbon species inside the Pd0 bulk) toward CO at the Pd-ZrO2 phase boundary, providing the necessary efficient CO2 activation sites. This carbide species is visible in the corresponding in situ collected XP spectra of both the C 1s and the Pd 3d region (panel C). This obviously crucial component is missing for the subsurface Pd–Zr alloy, which forms extended ZrO2 islands on top of a quasi-infinite Pd bulk, serving as a sink for carbon.37,38 Consequently, the transport of reactive carbon to the interface is suppressed, deactivating the associated Pd–ZrO2 interface in comparison to the bulk Pd2Zr sample.37,38 This observation is similar to the ones made for the ThNi5 materials discussed in the context of hydrocarbon synthesis.15 The crucial role of the carbon reactivity will focused upon in section 3.2 in more detail.
Figure 7.
Panel A: DRM profiles on the CVD-prepared subsurface Zr0–Pd foil precatalyst vs a single-ZrO2 film (upper panel), on the Pd2Zr bulk-intermetallic precatalyst (middle panel), and on the supported Pd–ZrO2 powder reference catalyst. Detailed reaction conditions given in refs (37) and (38). Panel B: Synchrotron-based in situ X-ray diffractograms of the bulk-intermetallic Pd2Zr catalyst collected in a CH4/CO2 (ratio 1:1) reaction mixture between 293 and 1073 K. Gas flow: 2 mL min–1 at ambient pressure with a heating rate of 20 K min–1. The colored bars mark the positions of the respective reference reflections. Panel C: High-resolution in situ XP spectra of the C 1s, Zr 3d, and Pd 3d5/2 recorded at 973 K on the Pd2Zr precatalyst (excitation energies chosen for 400 eV photoelectron kinetic energy). Left spectra, 0.3 mbar pure CH4; middle spectra, 0.3 mbar pure CO2; right spectra, 0.15 mbar CH4 + 0.15 mbar CO2. TOF values obtained by normalization of the molar rates to the geometrically estimated total number of surface Pd atoms. Details of calculations given in refs (37) and (38). Reproduced with permission from refs (37) and (38). Copyright 2021 Wiley-VCH and MDPI.
3. Phase Boundary Effects to Prepare Selective and Active Materials Following in Situ Decomposition of Intermetallic Compound/Alloy Precursors
The present section seeks to identify key factors determining the pathway of structural decomposition en route to active and selective catalysis for the materials outlined in the case studies. We restrict ourselves here to the methanol steam reforming and methane dry reforming performance, but the concepts can be extended to similar systems at will. An obvious prerequisite is the existence of an intermetallic compound or at least an alloy, which is directly linked to the formation of metal–metal bonds or the (partial) solubility of at least two metals. Subsequently, the thermodynamic stability limits of the intermetallic compounds/alloys need to be approached under the chosen reaction conditions. As such, these conditions are not static in the course of the reaction and may switch between reductive and oxidative. For methanol steam reforming, the reaction conditions change from oxidative in the beginning to increasingly reductive as the reaction progresses and more hydrogen is formed. To obtain a highly dispersed metal–oxide system via intermetallic compound/alloy in situ decomposition, a high oxidation propensity of one part (in case of a binary intermetallic compound) of the catalyst material is imperative. Hence, the combination of a noble metal with an easily passivating metal is usually a promising starting point. As a conclusion, we will use the knowledge derived from the identified key factors to propose promising candidates of intermetallic compounds, whose testing might result in catalytically prospective materials
The stability of the in situ formed active and selective metal-oxide phase boundary is the single most important parameter, determining the catalytic properties of the entire catalyst material. It is connected not only to the stability of the intermetallic compound or alloy precursor structure, steering the structure, morphology, and electronic properties of the resulting metal-oxide phase boundary, but directly influences the physicochemical properties of the phase boundary itself. Two of these properties are discussed in the next section: the reactivity of the resulting oxide polymorph and the reactivity of reaction-induced carbon.
In this section, we focus on one key parameter, featuring two sides of the same coin and serving as a prime example to show its entangled nature. As discussed for the CO2-selective state of Cu/ZrO2 catalysts, the interface of Cu to tetragonal ZrO2 particularly stands out in high CO2 selectivity. Apart from beneficial surface chemical issues of tetragonal ZrO2, we have shown that the interface between Cu and tetragonal ZrO2 is particularly stabilized by epitaxial effects.29 The reported lattic mismatch between the tetragonal ZrO2 (012) and cubic Cu (111), as well as between tetragonal ZrO2 (112) and cubic Cu (311), is less than 4% (Figure 8). This facilitates the formation of a well-defined, extended Cu/tetragonal ZrO2 interface with superior CO2 selectivity in methanol steam reforming. The prevailing epitaxial relation is Cu(001)//tetragonal ZrO2 (112). Note that the discussed epitaxial Cu-tetragonal ZrO2 effects are very similar to those reported, e.g., for Au/rutile TiO2 in CO oxidation.71 For Cu/tetragonal ZrO2, the role of the initial hexagonal intermetallic compound Cu51Zr14 is central, as structural similarities between Cu51Zr14 and tetragnal ZrO2 additionally prevail. The dominating epitaxial relation between Cu51Zr14 and tetragonal ZrO2 is Cu51Zr14(0001)//tetragonal ZrO2 (112). The in situ decomposition of Cu51Zr14 is then directly steered by the energy gain of massively segregating and enriching metallic Cu at the surface, also facilitating the formation of well-ordered tetragonal ZrO2.
Figure 8.
Ball models of the epitaxial Cu/t-ZrO2 (panel A) and Cu51Zr14/t-ZrO2 (panel B) relationships. Side view of Cu(001)//tetragonal ZrO2 (112) and Cu51Zr14(0001)//tetragonal ZrO2 (112). Color code: Zr, green; O, red; Cu, blue. Reproduced with permission from ref (29). Copyright 2021 American Chemical Society.
The role of tetragonal ZrO2 in CO2-selective methanol steam reforming on Cu catalysts45 reveals another very important feature: despite the prominent role of ZrO2, its use is severly hampered by the complex Zr–O phase diagram. At least three different crystalline ZrO2 polymorphs are known: the ambient-stable monoclinic modification and two high-temperature stable cubic and tetragonal structures.72 The latter two can be stabilized under ambient conditions by deliberate doping or particle size effects.73 Structural effects leading to high CO2 selectivity are only known for Cu in contact with tetragonal ZrO2, hence from the structural point of view, knowledge-based catalyst development should aim at providing synthesis pathways leading to maximum Cu-tetragonal ZrO2 phase boundary sites. The controlled in situ decomposition of Cu51Zr14 is one of the key preparation approaches to accomplish this, facilitated by the epitaxial relationships.
A similar metal-oxide phase boundary effect has been observed for a Pd2Zr intermetallic compound under methane dry reforming operation already discussed in the context of Figure 7.23 The exclusive activity-steering role of the Pd/ZrO2 interface can be directly appreciated by the comparison of the dry reforming reactivities of a surface Pd–Zr alloy, a Pd2Zr bulk intermetallic compound, and an impregnated Pd/ZrO2 catalyst.38 Mechanistic-wise, the extended Pd–ZrO2 interface present for the Pd2Zr intermetallic compound (and to a lesser extent for the impregnated Pd/ZrO2 material) allows efficient supply of the interface with reactive carbon arising from methane activation on metallic Pd (cf. Figure 7; Figure 9, middle and right panel).
Figure 9.
Schematic representation of the initial and reactive states of the three Pd–Zr materials. Reproduced with permission from ref (38). Copyright 2021 MDPI.
Epitaxial relationships, as discussed in Figure 8, seem also to play a major role in the stabilization of the Pd/tetragonal ZrO2 interface during in situ activation of Pd2Zr in the methane dry reforming mixture, as the deviation in the lattice constant between Cu and Pd is only 7%.
3.1. Reactivity of the Resulting Oxide Polymorph
Elaborating on the importance of the metal–oxide phase boundary effects raises the question about the explicit structural and catalytic role of the oxide component as an integral part of the metal–oxide entity formed by in situ decomposition of the intermetallic compound/alloy. Apart from the general relevance of the oxide component for water activation in methanol steam reforming or carbon dioxide activation in methane dry reforming, the catalytic properties of the oxide can beneficially or detrimentally impact the total catalytic performance of the metal–oxide composite. To illustrate the general principles, we again turn to the group of Pd-based intermetallic compounds, specificially to the comparison of Zn–Pd, Ga–Pd, and In–Pd. The overall qualitative CO2 selectivity in the state after entering the intermetallic compound state following hydrogen reduction is comparable at >90%,7,10,25 but the minute differences can be also directly related to the intrinsic catalytic differences of ZnO, Ga2O3, and In2O3. The formation of ZnO and In2O3 patches on the (partially) decomposed ZnPd and InPd intermetallic compound has been directly proven by electron microscopy and directly correlated to an improved methanol steam reforming performance.8,27,31 Whether full decomposition of the intermetallic compound into a metal–oxide system or only a partial decomposition and the creation of an intermetallic compound/oxide interface occurs, the only important factor is that the nonoxidic component must be capable of efficient methanol activation. The CO2 selectivity of the resulting ZnPd/ZnO and InPd/In2O3 interfaces is more pronounced compared to Ga2Pd/Ga2O3.8,22,24,26,27,31 Especially for the InPd bimetallic catalysts, the synergy of the InPd bimetallic phase in contact with In2O3 has been documented for methanol steam reforming and methanol synthesis likewise.31,74,75
In contrast to ZnPd and InPd, the corresponding isolated Ga2Pd intermetallic compound is not susceptible to decomposition into Ga2Pd/Ga2O3.30 The only way to use Ga2Pd as an efficient methanol steam reforming catalyst is the preparation routine via reactive metal–support interaction, i.e., the reductive formation of GaPd2 particles by hydrogen reduction of a Pd/Ga2O3 catalyst at 773 K.32 The so-formed GaPd2/Ga2O3 interface is 95% CO2 selective in methanol steam reforming, which is less than that for ZnPd/ZnO (>99%) and InPd/In2O3 (>98%).8,22,24,26,27,31,63 The reason for this discrepancy is the pronounced water–gas shift reactivity of Ga2O3, which is spoiling the CO2 selectivity of the entire Ga2Pd/Ga2O3 catalyst at the methanol steam reforming reaction temperatures.58,59 Mechanistic-wise, the water–gas shift reaction on Ga2O3 can be purely surface-bound (formate-mediated) or involve oxygen vacancies (vacancy-mediated). ZnO and In2O3 are, however, very CO2-selective methanol steam reforming catalysts themselves, and the CO2 spoiling effect is efficiently suppressed.11,57,60,61 Especially on In2O3 as a highly reducible oxide, the water–gas shift route is purely oxygen vacancy-dominated (i.e., CO is very efficiently transformed into CO2), but the reverse reaction is effectively blocked due to the missing replenishment of oxygen vacancies by CO2.57,60,61
Exceeding the importance of the “simple” intrinsic catalytic properties of the oxide, the situation is significantly complicated by the fact that the adsorption and catalytic properties of a given oxide can be deliberately influenced by the synthesis protocol and steering the distribution of Brønsted and Lewis acidic and basic surface sites. Especially for intermetallic compounds involving Zr, eventually giving rise to ZrO2 during decomposition, this is a delicate issue. Although in situ decomposition of Cu-containing intermetallic compounds (Cu51Zr14 and CuZr2) yields a composite of metallic Cu and tetragonal ZrO2 due to the already discussed epitaxial stabilization, the previously anticipated exclusive role of tetragonal ZrO2 in CO2 selective methanol steam reforming cannot be upheld anymore. On the contrary, both ZrO2 modifications (monoclinic and tetragonal) can be switched between CO- and CO2-selective in contact with metallic Cu, depending on the surface acidity or basicity of ZrO2 as a consequence of the synthesis protocol.76
3.2. Carbon Reactivity
Carbon reactivity and clean-off as a key parameter for methane dry reforming activity is a direct consequence of the quality and quantity of the phase boundary sites (i.e., their activation capability and associated amount) arising from in situ decomposition of the intermetallic compound/alloy. As we have shown in comparative methane dry reforming studies using near-surface Pd–Zr alloys and bulk Pd2Zr intermetallic compounds,23,38 efficient carbon chemistry and loading can only be obtained on an extended Pd/tetragonal ZrO2 interface accessed through decomposition of the Pd2Zr intermetallic compound. Transfer of interfacial carbon as a consequence of methane activation on metallic Pd to redox-active ZrOx sites assisting in CO2 activation is very efficient, and diffusive carbon loss into deeper Pd bulk regions is suppressed. Only starting from Pd2Zr yields the necessary small Pd particle dimensions for an increased amount of reactive carbide-like and/or dissolved carbon at the Pd-tetragonal ZrO2 phase boundary. The discussed carbon management, especially on Ni-containing intermetallic compounds, is very much related to the attempts to understand and accordingly suppress the coking on conventional Ni catalysts by active supports on a Zr- or La-oxide basis.77,78 “Active” support refers to the ability to decrease the carbon amount on the metal by usage of the phase boundary and the suppression of nucleation and formation of graphitic carbon layers also on the metal. Recent studies on Ni/MnO catalysts indicated that surface carbon can also act as a reactive intermediate under methane dry reforming operation but piles up as a significant amount of bulk carbon upon recooling to room temperature.79In situ characterization therefore is imperative to understand the carbon reactivity during catalytic operation, especially if intermetallic compounds are used as precursor structures to access the active metal-oxide phase. Decomposition of intermetallic compounds/alloys allows direct steerig of the dry reforming performance by optimization of the metal-oxide phase boundary and the associated metal particle size. In due course, the carbon dioxide activation properties, nucleation, and growth kinetics of graphite species or the role of reactive interfacial carbon can be influenced. As such, the requirements on the use and decomposition of intermetallic compounds in methane dry reforming are much higher compared to in methanol steam reforming, essentially due the carbon reactivity issue.
4. Conclusions and Outlook
We have shown the capabilities of using defined and ordered intermetallic compounds and alloys to prepare highly active and selective metal–oxide composite materials by in situ decomposition in the respective reaction mixtures. Exemplified for the methanol steam reforming and methane dry reforming reaction, we are able to identify a number of key factors that need to be carefully controlled to steer the decomposition pathway to catalytically prospective materials. The resulting quality (opening the desired reaction channels by selective activation) and associated large amounts of metal–oxide phase boundary sites is the single most important parameter that controls epitaxial relationships, the contribution of the intrinsic physicochemical/catalytic properties of the resulting oxide polymorph or the carbon reactivity. Thus, it determines the catalytic performance of the entire catalytic composite resulting from the in situ decomposition of intermetallic compound/alloy precursor structures. Appreciating the importance of the discussed key factors now allows projection of the performance of relevant catalytic materials beyond the exemplified case studies and eventual identification of similar materials on a knowledge-based basis. For methanol steam reforming, the prerequisite for an active and selective material is efficient methanol and water activation; therefore, it appears feasible to test the in situ decomposition of the respective group of intermetallic compounds on a copper basis, Cu–Ga,80,81 Cu–Sn,82,83 and Cu–Y;79 a palladium basis, Pd–Sn84 and Pd–Y;85 a platinum basis, Pt–Sn86 and Pt–Y;87 or an iridium basis, Ir–Ga,88,89 Ir–Sn,88 Ir–In,90 or Ir–Y.91 Intermetallic compounds exist in all binary phase diagrams. The selection of the “metal” part as Cu, Pd, Pt, or Ir is derived from the already documented methanol activation capabilities,10,89 the one for the “oxide” part from the known water activation capabilities of the oxide formed by the decomposition of the precursor structures.11,57−59,92,93 We expect the formation of Ga2O3, SnO2, and Y2O3 during decomposition—especially the latter two are proven to be highly CO2 selective methanol steam reforming catalysts themselves.92−94 Whether full decomposition to the metal–oxide systems or partial decomposition into oxide-supported intermetallic compounds, eventually through compositional intertransformations of different structures, occurs remains to be tested. In the best scenario, steering the decomposition process as a function of reaction temperature allows access to different structural stages of decomposition. The already tested Cu–In phase diagram is such a system, where through the combination of in situ decomposition studies of intermetallic compound precursor structures with different Cu/In ratios and impregnated Cu/In2O3 catalysts, the highly CO2 selective nature of the Cu–In2O3 interface was assessed.95
To extrapolate the use of in situ decomposition of Pd–Zr intermetallic compounds to access active methane dry reforming Pd-ZrO2 metal–oxide interfaces, its carbon management is crucial. For efficient bifunctional operation, it is necessary that the metal formed upon decomposition either forms a reactive carbide or actually dissolves carbon to yield a distinct carbon reactivity and allows for efficient carbon dioxide activation. The true nature of the activated carbon dioxide species, e.g., as intermediate (oxy)carbonate species, remains to be determined. The minimum requirement is that a full carbon dioxide activation–carbon monoxide release cycle must be enabled. This is particularly aided by basic surface sites, which have been documented to be crucial for CO2 activation and improvement of catalyst deactivation. For methane dry reforming, the addition of La2O3 to Co/SiO2 catalysts was reported to positively affect the surface basicity and catalytic properties.96 In due course, La-, Zr-, or Sm-containing intermetallic compounds represent a promising group as test structures, as—in situ decomposition of the intermetallic compounds provided—the resulting oxide parts La2O3, ZrO2, and Sm2O3 are already known from complementary studies on metal exsolution from perovskite-type oxides and intermetallic compounds during in situ dry reforming treatment to enable such a CO2 activation cycle.38,77,97−100 The decomposition of intermetallic compounds is a similar process insofar as an in situ formed metal-oxide interface is the active catalytic center. Intermetallic compounds such as binary Ni–La,101,102 Pd–La,101 or the corresponding Zr-103,104 or Sm-containing systems100 provide a reasonable starting point for in situ decomposition studies. The common reactivity denominator of Ni and Pd is the rich and vital carbon chemistry that has already been proven crucial for Pd–Zr systems, where only the in situ decomposition of Pd2Zr yielded the necessary active nanoparticulate Pd-ZrO2 composite.
Reaction-wise, we note that the concept, which was outlined for two examples, can be projected to related reactions. For CO and/or CO2 methanation and ammonia synthesis, such a concept was already introduced. A necessary prerequisite is that the oxidation/reduction chemical potential of the respective reaction mixture allows an approach to the stability limits of the intermetallic compound/alloy structures under the chosen reaction conditions.
As an important feature for full appreciation of the used concept, which has unfortunately not been touched so far, is related to the regeneration of the final metal-oxide composite mixture. This is of obvious importance for repeated use in catalytic cycles. If a full regeneration cycle can be repeatedly accessed and the final metal-oxide mixture can be obtained as a “steady state” of reversible decomposition and regeneration remains to be tested for each individual case. Attempts for such oxidative regeneration of In–Pd intermetallic compounds (which have been decomposed to Pd//In2O3 during activation) yielded unsatisfactory results. Although the reduced InxOy could be restored, it forms a passivating layer around the Pd particles, preventing full regeneration of In–Pd.27 For UHV-based alloy model catalysts such as Cu–Zn or Zn–Pd discussed in this work, oxidative regeneration was possible by resegregation of Zn to the surface and associated removal.35,55
Acknowledgments
This work was financially supported by the Austrian Science Fund (FWF) through projects F 45 and DACH project I 2877-N34 and performed within the framework of the special research platform “Advanced Materials” and the special Ph.D. program “Reactivity and Catalysis” at the University of Innsbruck. P.D.K.N. thanks the ÖAD for financial support through an Ernst Mach scholarship.
The authors declare no competing financial interest.
References
- Armbrüster M. Intermetallic Compounds In Catalysis - A Versatile Class of Materials Meets Interesting Challenges. Sci. Technol. Adv. Mater. 2020, 21, 303–322. 10.1080/14686996.2020.1758544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbrüster M.; Schlögl R.; Grin Y. Intermetallic Compounds In Heterogeneous Catalysis—A Quickly Developing Field. Sci. Technol. Adv. Mater. 2014, 15, 034803. 10.1088/1468-6996/15/3/034803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A.; Rioux R. M. Intermetallics In Catalysis: An Exciting Subset of Multimetallic Catalysts. Catal. Today 2019, 330, 2–15. 10.1016/j.cattod.2018.05.048. [DOI] [Google Scholar]
- Furukawa S.; Komatsu T. Intermetallic Compounds: Promising Inorganic Materials for Well-Structured and Electronically Modified Reaction Environments for Efficient Catalysis. ACS Catal. 2017, 7, 735–765. 10.1021/acscatal.6b02603. [DOI] [Google Scholar]
- Marakatti V. S.; Peter S. C. Synthetically Tuned Electronic and Geometrical Properties of Intermetallic Compounds As Effective Heterogeneous Catalysts. Prog. Solid State Chem. 2018, 52, 1–30. 10.1016/j.progsolidstchem.2018.09.001. [DOI] [Google Scholar]
- Rößner L.; Armbrüster M. Electrochemical Energy Conversion on Intermetallic Compounds: A Review. ACS Catal. 2019, 9, 2018–2062. 10.1021/acscatal.8b04566. [DOI] [Google Scholar]
- Armbrüster M.; Behrens M.; Föttinger K.; Friedrich M.; Gaudry É.; Matam S. K.; Sharma H. R. The Intermetallic Compound ZnPd and Its Role in Methanol Steam Reforming. Catal. Rev.: Sci. Eng. 2013, 55, 289–367. 10.1080/01614940.2013.796192. [DOI] [Google Scholar]
- Friedrich M.; Penner S.; Heggen M.; Armbrüster M. High CO2 Selectivity in Methanol Steam Reforming through ZnPd/ZnO Teamwork. Angew. Chem., Int. Ed. 2013, 52, 4389–4392. 10.1002/anie.201209587. [DOI] [PubMed] [Google Scholar]
- Friedrich M.; Teschner D.; Knop-Gericke A.; Armbrüster M. Influence of Bulk Composition of The Intermetallic Compound ZnPd on Surface Composition and Methanol Steam Reforming Properties. J. Catal. 2012, 285, 41–47. 10.1016/j.jcat.2011.09.013. [DOI] [Google Scholar]
- Iwasa N.; Takezawa N. New Supported Pd and Pt Alloy Catalysts for Steam Reforming and Dehydrogenation of Methanol. Top. Catal. 2003, 22, 215–224. 10.1023/A:1023571819211. [DOI] [Google Scholar]
- Lorenz H.; Friedrich M.; Armbrüster M.; Klötzer B.; Penner S. ZnO Is a CO2-Selective Steam Reforming Catalyst. J. Catal. 2013, 297, 151–154. 10.1016/j.jcat.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T.; Wallace W. E.; Craig R. S. Rare Earth Intermetallics As Synthetic Ammonia Catalysts. J. Catal. 1976, 44, 236–243. 10.1016/0021-9517(76)90394-8. [DOI] [Google Scholar]
- Elattar A.; Wallace W. E.; Craig R. S.. Hydrocarbon Synthesis Using Catalysts Formed by Intermetallic Compound Decomposition. In Hydrocarbon Synthesis from Carbon Monoxide and Hydrogen; American Chemical Society, 1979; Vol. 178; pp 7–14. 10.1021/ba-1979-0178.ch002. [DOI] [Google Scholar]
- Coon V. T.; Takeshita T.; Wallace W. E.; Craig R. S. Rare Earth Intermetallics as Catalysts For The Production of Hydrocarbons From Carbon Monoxide and Hydrogen. J. Phys. Chem. 1976, 80, 1878–1879. 10.1021/j100558a013. [DOI] [Google Scholar]
- Elattar A.; Takeshita T.; Wallace W.; Craig R. Intermetallic Compounds of The Type MNi5 as Methanation Catalysts. Science 1977, 196, 1093–1094. 10.1126/science.196.4294.1093. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Verykios X. E.; MacDonald S. M.; Affrossman S. Comparative Study of Carbon Dioxide Reforming of Methane to Synthesis Gas over Ni/La2O3 and Conventional Nickel-Based Catalysts. J. Phys. Chem. 1996, 100, 744–754. 10.1021/jp951809e. [DOI] [Google Scholar]
- Moldovan A.; Elattar A.; Wallace W. Studies of the Methanation Catalysts ThNi5 and ZrNi5 by Auger and Characteristic Energy Loss Spectra. J. Solid State Chem. 1978, 25, 23–29. 10.1016/0022-4596(78)90039-7. [DOI] [Google Scholar]
- Baiker A.; Schlog̈l R.; Armbruster E.; Güntherodt H. J. Ammonia Synthesis over Supported Iron Catalyst Prepared From Amorphous Iron-Zirconium Precursor: I. Bulk Structural and Surface Chemical Changes of Precursor During Its Transition to The Active Catalyst. J. Catal. 1987, 107, 221–231. 10.1016/0021-9517(87)90287-9. [DOI] [Google Scholar]
- Noack K.; Rehren C.; Zbinden H.; Schloegl R. Modification of the Catalytic Hydrogenation Activity of Glassy Pd81Si19. Surface Analysis by ISS and XPS. Langmuir 1995, 11, 2018–2030. 10.1021/la00006a031. [DOI] [Google Scholar]
- Baiker A.; Gasser D.; Lenzner J.; Reller A.; Schlogl R. Oxidation of Carbon Monoxide over Palladium on Zirconia Prepared from Amorphous Pd—Zr Alloy I. Bulk Structural, Morphological, and Catalytic Properties of Catalyst. J. Catal. 1990, 126, 555–571. 10.1016/0021-9517(90)90020-K. [DOI] [Google Scholar]
- Tsai A. P.; Yoshimura M. Highly Active Quasicrystalline Al-Cu-Fe Catalyst For Steam Reforming of Methanol. Appl. Catal., A 2001, 214, 237–241. 10.1016/S0926-860X(01)00500-2. [DOI] [Google Scholar]
- Haghofer A.; Föttinger K.; Girgsdies F.; Teschner D.; Knop-Gericke A.; Schlögl R.; Rupprechter G. In Situ Study of The Formation And Stability of Supported Pd2Ga Methanol Steam Reforming Catalysts. J. Catal. 2012, 286, 13–21. 10.1016/j.jcat.2011.10.007. [DOI] [Google Scholar]
- Köpfle N.; Mayr L.; Schmidmair D.; Bernardi J.; Knop-Gericke A.; Hävecker M.; Klötzer B.; Penner S. A Comparative Discussion of the Catalytic Activity and CO2-Selectivity of Cu-Zr and Pd-Zr (Intermetallic) Compounds in Methanol Steam Reforming. Catalysts 2017, 7, 53. 10.3390/catal7020053. [DOI] [Google Scholar]
- Lorenz H.; Penner S.; Jochum W.; Rameshan C.; Klötzer B. Pd/Ga2O3 Methanol Steam Reforming Catalysts: Part II. Catalytic Selectivity. Appl. Catal., A 2009, 358, 203–210. 10.1016/j.apcata.2009.02.027. [DOI] [Google Scholar]
- Lorenz H.; Rameshan C.; Bielz T.; Memmel N.; Stadlmayr W.; Mayr L.; Zhao Q.; Soisuwan S.; Klötzer B.; Penner S. From Oxide-Supported Palladium to Intermetallic Palladium Phases: Consequences for Methanol Steam Reforming. ChemCatChem 2013, 5, 1273–1285. 10.1002/cctc.201200712. [DOI] [Google Scholar]
- Lorenz H.; Thalinger R.; Köck E.-M.; Kogler M.; Mayr L.; Schmidmair D.; Bielz T.; Pfaller K.; Klötzer B.; Penner S. Methanol Steam Reforming: CO2-Selective Pd2Ga Phases Supported on α- and γ-Ga2O3. Appl. Catal., A 2013, 453, 34–44. 10.1016/j.apcata.2012.11.010. [DOI] [Google Scholar]
- Lorenz H.; Turner S.; Lebedev O. I.; Van Tendeloo G.; Klötzer B.; Rameshan C.; Pfaller K.; Penner S. Pd-In2O3 Interaction Due to Reduction In Hydrogen: Consequences for Methanol Steam Reforming. Appl. Catal., A 2010, 374, 180–188. 10.1016/j.apcata.2009.12.007. [DOI] [Google Scholar]
- Mayr L.; Klötzer B.; Schmidmair D.; Köpfle N.; Bernardi J.; Schwarz S.; Armbrüster M.; Penner S. Boosting Hydrogen Production from Methanol and Water by In Situ Activation of Bimetallic Cu-Zr Species. ChemCatChem 2016, 8, 1778–1781. 10.1002/cctc.201600361. [DOI] [Google Scholar]
- Mayr L.; Köpfle N.; Klötzer B.; Götsch T.; Bernardi J.; Schwarz S.; Keilhauer T.; Armbrüster M.; Penner S. Microstructural and Chemical Evolution and Analysis of a Self-Activating CO2-Selective Cu-Zr Bimetallic Methanol Steam Reforming Catalyst. J. Phys. Chem. C 2016, 120, 25395–25404. 10.1021/acs.jpcc.6b07824. [DOI] [Google Scholar]
- Mayr L.; Lorenz H.; Armbruster M.; Villaseca S. A.; Luo Y.; Cardoso R.; Burkhardt U.; Zemlyanov D.; Haevecker M.; Blume R.; Knop-Gericke A.; Klotzer B.; Penner S. The Catalytic Properties of Thin Film Pd-Rich GaPd2 In Methanol Steam Reforming. J. Catal. 2014, 309, 231–240. 10.1016/j.jcat.2013.10.002. [DOI] [Google Scholar]
- Neumann M.; Teschner D.; Knop-Gericke A.; Reschetilowski W.; Armbrüster M. Controlled Synthesis and Catalytic Properties of Supported In-Pd Intermetallic Compounds. J. Catal. 2016, 340, 49–59. 10.1016/j.jcat.2016.05.006. [DOI] [Google Scholar]
- Penner S.; Armbrüster M. Formation of Intermetallic Compounds by Reactive Metal-Support Interaction: A Frequently Encountered Phenomenon In Catalysis. ChemCatChem 2015, 7, 374–392. 10.1002/cctc.201402635. [DOI] [Google Scholar]
- Penner S.; Lorenz H.; Jochum W.; Stöger-Pollach M.; Wang D.; Rameshan C.; Klötzer B. Pd/Ga2O3 Methanol Steam Reforming Catalysts: Part I. Morphology, Composition and Structural Aspects. Appl. Catal., A 2009, 358, 193–202. 10.1016/j.apcata.2009.02.026. [DOI] [Google Scholar]
- Rameshan C.; Stadlmayr W.; Penner S.; Lorenz H.; Mayr L.; Hävecker M.; Blume R.; Rocha T.; Teschner D.; Knop-Gericke A.; Schlögl R.; Zemlyanov D.; Memmel N.; Klötzer B. In Situ XPS Study of Methanol Reforming on PdGa Near-Surface Intermetallic Phases. J. Catal. 2012, 290, 126–137. 10.1016/j.jcat.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshan C.; Stadlmayr W.; Weilach C.; Penner S.; Lorenz H.; Hävecker M.; Blume R.; Rocha T.; Teschner D.; Knop-Gericke A.; Schlögl R.; Memmel N.; Zemlyanov D.; Rupprechter G.; Klötzer B. Subsurface-Controlled CO2 Selectivity of PdZn Near-Surface Alloys in H2 Generation by Methanol Steam Reforming. Angew. Chem., Int. Ed. 2010, 49, 3224–3227. 10.1002/anie.200905815. [DOI] [PubMed] [Google Scholar]
- Furukawa S.; Endo M.; Komatsu T. Bifunctional Catalytic System Effective for Oxidative Dehydrogenation of 1-Butene and n-Butane Using Pd-Based Intermetallic Compounds. ACS Catal. 2014, 4, 3533–3542. 10.1021/cs500920p. [DOI] [Google Scholar]
- Köpfle N.; Götsch T.; Grünbacher M.; Carbonio E. A.; Hävecker M.; Knop-Gericke A.; Schlicker L.; Doran A.; Kober D.; Gurlo A.; Penner S.; Klötzer B. Zirconium-Assisted Activation of Palladium To Boost Syngas Production by Methane Dry Reforming. Angew. Chem., Int. Ed. 2018, 57, 14613–14618. 10.1002/anie.201807463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpfle N.; Ploner K.; Lackner P.; Götsch T.; Thurner C.; Carbonio E.; Hävecker M.; Knop-Gericke A.; Schlicker L.; Doran A.; Kober D.; Gurlo A.; Willinger M.; Penner S.; Schmid M.; Klötzer B. Carbide-Modified Pd on ZrO2 as Active Phase for CO2-Reforming of Methane—A Model Phase Boundary Approach. Catalysts 2020, 10, 1000. 10.3390/catal10091000. [DOI] [Google Scholar]
- Shoji S.; Peng X.; Imai T.; Murphin Kumar P. S.; Higuchi K.; Yamamoto Y.; Tokunaga T.; Arai S.; Ueda S.; Hashimoto A.; Tsubaki N.; Miyauchi M.; Fujita T.; Abe H. Topologically immobilized catalysis centre for long-term stable carbon dioxide reforming of methane. Chem. Sci. 2019, 10, 3701–3705. 10.1039/C8SC04965C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T.; Uezono T. CO2 Reforming of Methane on Ni- and Co-based Intermetallic Compound Catalysts. J. Jpn. Pet. Inst. 2005, 48, 76–83. 10.1627/jpi.48.76. [DOI] [Google Scholar]
- Baiker A. Metallic Glasses In Heterogeneous Catalysis. Faraday Discuss. Chem. Soc. 1989, 87, 239–251. 10.1039/dc9898700239. [DOI] [Google Scholar]
- Takahashi T.; Inoue M.; Kai T. Effect of Metal Composition on Hydrogen Selectivity In Steam Reforming of Methanol over Catalysts Prepared From Amorphous Alloys. Appl. Catal., A 2001, 218, 189–195. 10.1016/S0926-860X(01)00641-X. [DOI] [Google Scholar]
- Pang Tsai A.; Kameoka S.; Ishii Y. PdZn = Cu: Can an Intermetallic Compound Replace an Element?. J. Phys. Soc. Jpn. 2004, 73, 3270–3273. 10.1143/JPSJ.73.3270. [DOI] [Google Scholar]
- Breen J. P.; Ross J. R. H. Methanol Reforming For Fuel-Cell Applications: Development of Zirconia-Containing Cu-Zn-Al Catalysts. Catal. Today 1999, 51, 521–533. 10.1016/S0920-5861(99)00038-3. [DOI] [Google Scholar]
- Purnama H.; Girgsdies F.; Ressler T.; Schattka J. H.; Caruso R. A.; Schomäcker R.; Schlögl R. Activity and Selectivity of a Nanostructured CuO/ZrO2 Catalyst in the Steam Reforming of Methanol. Catal. Lett. 2004, 94, 61–68. 10.1023/B:CATL.0000019332.80287.6b. [DOI] [Google Scholar]
- Velu S.; Suzuki K.; Gopinath C. S.; Yoshida H.; Hattori T. XPS, XANES and EXAFS Investigations of CuO/ZnO/Al2O3/ZrO2 Mixed Oxide Catalysts. Phys. Chem. Chem. Phys. 2002, 4, 1990–1999. 10.1039/b109766k. [DOI] [Google Scholar]
- Velu S.; Suzuki K.; Kapoor M. P.; Ohashi F.; Osaki T. Selective Production of Hydrogen For Fuel Cells Via Oxidative Steam Reforming of Methanol over CuZnAl(Zr)-Oxide Catalysts. Appl. Catal., A 2001, 213, 47–63. 10.1016/S0926-860X(00)00879-6. [DOI] [Google Scholar]
- Gasser D.; Baiker A. Hydrogenation of Carbon Dioxide Over Copper—Zirconia Catalysts Prepared by In-Situ Activation of Amorphous Copper—Zirconium Alloy. Appl. Catal. 1989, 48, 279–294. 10.1016/S0166-9834(00)82799-2. [DOI] [Google Scholar]
- Domokos L.; Katona T.; Molnár Á.; Lovas A. Amorphous Alloy Catalysis VIII. A New Activation of An Amorphous Cu41Zr59 Alloy In The Transformation of Methyl Alcohol To Methyl Formate. Appl. Catal., A 1996, 142, 151–158. 10.1016/0926-860X(96)00051-8. [DOI] [Google Scholar]
- Jennings J. R.; Owen G.; Nix R. M.; Lambert R. M. Methanol Synthesis Catalysts Derived From Copperintermetallic Precursors: Transient Response to Pulses of Carbon Dioxide, Oxygen And Nitrous Oxide. Appl. Catal., A 1992, 82, 65–75. 10.1016/0926-860X(92)80006-X. [DOI] [Google Scholar]
- Owen G.; Hawkes C. M.; Lloyd D.; Jennings J. R.; Lambert R. M.; Nix R. M. Methanol Synthesis Catalysts Derived From Ternary Rare Earth, Copper, Zirconium And Rare Earth, Copper, Titanium Intermetallic Alloys. Appl. Catal. 1990, 58, 69–81. 10.1016/S0166-9834(00)82279-4. [DOI] [Google Scholar]
- Chase M. W. Jr. NIST-JANAF Themochemical Tables, Fourth Edition. J. Phys. Chem. Ref. Data 1998, 9, 1–1951. Monograph. [Google Scholar]
- Mayr L.; Klötzer B.; Zemlyanov D.; Penner S. Steering of Methanol Reforming Selectivity By Zirconia-Copper Interaction. J. Catal. 2015, 321, 123–132. 10.1016/j.jcat.2014.10.012. [DOI] [Google Scholar]
- Mayr L.; Shi X.; Köpfle N.; Klötzer B.; Zemlyanov D. Y.; Penner S. Tuning of The Copper-Zirconia Phase Boundary For Selectivity Control of Methanol Conversion. J. Catal. 2016, 339, 111–122. 10.1016/j.jcat.2016.03.029. [DOI] [Google Scholar]
- Rameshan C.; Stadlmayr W.; Penner S.; Lorenz H.; Memmel N.; Hävecker M.; Blume R.; Teschner D.; Rocha T.; Zemlyanov D.; Knop-Gericke A.; Schlögl R.; Klötzer B. Hydrogen Production by Methanol Steam Reforming on Copper Boosted by Zinc-Assisted Water Activation. Angew. Chem., Int. Ed. 2012, 51, 3002–3006. 10.1002/anie.201106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner S.; Wang D.; Su D. S.; Rupprechter G.; Podloucky R.; Schlögl R.; Hayek K. Platinum Nanocrystals Supported by Silica, Alumina And Ceria: Metal-Support Interaction Due to High-Temperature Reduction In Hydrogen. Surf. Sci. 2003, 532, 276–280. 10.1016/S0039-6028(03)00198-5. [DOI] [Google Scholar]
- Lorenz H.; Jochum W.; Klötzer B.; Stöger-Pollach M.; Schwarz S.; Pfaller K.; Penner S. Novel Methanol Steam Reforming Activity and Selectivity of Pure In2O3. Appl. Catal., A 2008, 347, 34–42. 10.1016/j.apcata.2008.05.028. [DOI] [Google Scholar]
- Jochum W.; Penner S.; Föttinger K.; Kramer R.; Rupprechter G.; Klötzer B. Hydrogen on Polycrystalline β-Ga2O3: Surface Chemisorption, Defect Formation, And Reactivity. J. Catal. 2008, 256, 268–277. 10.1016/j.jcat.2008.03.019. [DOI] [Google Scholar]
- Jochum W.; Penner S.; Kramer R.; Föttinger K.; Rupprechter G.; Klötzer B. Defect Formation and The Water-Gas Shift Reaction on B-Ga2O3. J. Catal. 2008, 256, 278–286. 10.1016/j.jcat.2008.03.018. [DOI] [Google Scholar]
- Bielz T.; Lorenz H.; Amann P.; Klötzer B.; Penner S. Water-Gas Shift and Formaldehyde Reforming Activity Determined by Defect Chemistry of Polycrystalline In2O3. J. Phys. Chem. C 2011, 115, 6622–6628. 10.1021/jp111739m. [DOI] [Google Scholar]
- Bielz T.; Lorenz H.; Jochum W.; Kaindl R.; Klauser F.; Klötzer B.; Penner S. Hydrogen on In2O3: Reducibility, Bonding, Defect Formation, and Reactivity. J. Phys. Chem. C 2010, 114, 9022–9029. 10.1021/jp1017423. [DOI] [Google Scholar]
- Heggen M.; Penner S.; Friedrich M.; Dunin-Borkowski R. E.; Armbrüster M. Formation of ZnO Patches on ZnPd/ZnO during Methanol Steam Reforming: A Strong Metal-Support Interaction Effect?. J. Phys. Chem. C 2016, 120, 10460–10465. 10.1021/acs.jpcc.6b02562. [DOI] [Google Scholar]
- Haghofer A.; Ferri D.; Föttinger K.; Rupprechter G. Who Is Doing the Job? Unraveling the Role of Ga2O3 in Methanol Steam Reforming on Pd2Ga/Ga2O3. ACS Catal. 2012, 2, 2305–2315. 10.1021/cs300480c. [DOI] [Google Scholar]
- Ghoneim S. A.; El-Salamony R. A.; El-Temtamy S. A. Review on Innovative Catalytic Reforming of Natural Gas to Syngas. World J. Eng. Technol. 2016, 4, 116. 10.4236/wjet.2016.41011. [DOI] [Google Scholar]
- Wang S.; Lu G. Q.; Millar G. J. Carbon Dioxide Reforming of Methane To Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. 10.1021/ef950227t. [DOI] [Google Scholar]
- Ashcroft A. T.; Cheetham A. K.; Green M. L. H.; Vernon P. D. F. Partial Oxidation of Methane to Synthesis Gas Using Carbon Dioxide. Nature 1991, 352, 225–226. 10.1038/352225a0. [DOI] [Google Scholar]
- Bradford M. C. J.; Vannice M. A. Catalytic Reforming of Methane With Carbon Dioxide over Nickel Catalysts I. Catalyst Characterization and Activity. Appl. Catal., A 1996, 142, 73–96. 10.1016/0926-860X(96)00065-8. [DOI] [Google Scholar]
- Rostrupnielsen J. R.; Hansen J. H. B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. 10.1006/jcat.1993.1312. [DOI] [Google Scholar]
- Wolfbeisser A.; Sophiphun O.; Bernardi J.; Wittayakun J.; Föttinger K.; Rupprechter G. Methane Dry Reforming over Ceria-Zirconia Supported Ni Catalysts. Catal. Today 2016, 277, 234–245. 10.1016/j.cattod.2016.04.025. [DOI] [Google Scholar]
- Steinhauer B.; Kasireddy M. R.; Radnik J.; Martin A. Development of Ni-Pd Bimetallic Catalysts For The Utilization of Carbon Dioxide and Methane by Dry Reforming. Appl. Catal., A 2009, 366, 333–341. 10.1016/j.apcata.2009.07.021. [DOI] [Google Scholar]
- Haruta M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. 10.1002/tcr.10053. [DOI] [PubMed] [Google Scholar]
- Abriata J. P.; Garcés J.; Versaci R. The O-Zr (Oxygen-Zirconium) System. Bull. Alloy Phase Diagrams 1986, 7, 116–124. 10.1007/BF02881546. [DOI] [Google Scholar]
- Shukla S.; Seal S. Mechanisms of Room Temperature Metastable Tetragonal Phase Stabilisation In Zirconia. Int. Mater. Rev. 2005, 50, 45–64. 10.1179/174328005X14267. [DOI] [Google Scholar]
- Köwitsch N.; Thoni L.; Klemmed B.; Benad A.; Paciok P.; Heggen M.; Köwitsch I.; Mehring M.; Eychmüller A.; Armbrüster M. Proving a Paradigm in Methanol Steam Reforming: Catalytically Highly Selective InxPdy/In2O3 Interfaces. ACS Catal. 2021, 11, 304–312. 10.1021/acscatal.0c04073. [DOI] [Google Scholar]
- Snider J. L.; Streibel V.; Hubert M. A.; Choksi T. S.; Valle E.; Upham D. C.; Schumann J.; Duyar M. S.; Gallo A.; Abild-Pedersen F.; Jaramillo T. F. Revealing the Synergy between Oxide and Alloy Phases on the Performance of Bimetallic In-Pd Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 3399–3412. 10.1021/acscatal.8b04848. [DOI] [Google Scholar]
- Ploner K.; Watschinger M.; Kheyrollahi Nezhad P. D.; Götsch T.; Schlicker L.; Köck E.-M.; Gurlo A.; Gili A.; Doran A.; Zhang L.; Köwitsch N.; Armbrüster M.; Vanicek S.; Wallisch W.; Thurner C.; Klötzer B.; Penner S. Mechanistic Insights Into The Catalytic Methanol Steam Reforming Performance of Cu/ZrO2 Catalysts By In Situ and Operando Studies. J. Catal. 2020, 391, 497–512. 10.1016/j.jcat.2020.09.018. [DOI] [Google Scholar]
- Bekheet M. F.; Delir Kheyrollahi Nezhad P.; Bonmassar N.; Schlicker L.; Gili A.; Praetz S.; Gurlo A.; Doran A.; Gao Y.; Heggen M.; Niaei A.; Farzi A.; Schwarz S.; Bernardi J.; Klötzer B.; Penner S. Steering the Methane Dry Reforming Reactivity of Ni/La2O3 Catalysts by Controlled In Situ Decomposition of Doped La2NiO4 Precursor Structures. ACS Catal. 2021, 11, 43–59. 10.1021/acscatal.0c04290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Li L.; Zhang X.; Wang Z.; Wang X.; Peng H. Design of Ni-ZrO2@SiO2 Catalyst with Ultra-High Sintering and Coking Resistance For Dry Reforming of Methane to Prepare Syngas. J. CO2 Util. 2018, 27, 297–307. 10.1016/j.jcou.2018.08.003. [DOI] [Google Scholar]
- Gili A.; Schlicker L.; Bekheet M. F.; Görke O.; Penner S.; Grünbacher M.; Götsch T.; Littlewood P.; Marks T. J.; Stair P. C.; Schomäcker R.; Doran A.; Selve S.; Simon U.; Gurlo A. Surface Carbon as a Reactive Intermediate in Dry Reforming of Methane to Syngas on a 5% Ni/MnO Catalyst. ACS Catal. 2018, 8, 8739–8750. 10.1021/acscatal.8b01820. [DOI] [Google Scholar]
- Liu S.; McDonald S.; Gu Q.; Matsumura S.; Qu D.; Sweatman K.; Nishimura T.; Nogita K. Properties of CuGa2 Formed Between Liquid Ga and Cu Substrates at Room Temperature. J. Electron. Mater. 2020, 49, 128–139. 10.1007/s11664-019-07688-4. [DOI] [Google Scholar]
- Li J.-B.; Ji L.N.; Liang J.K.; Zhang Y.; Luo J.; Li C.R.; Rao G.H. A Thermodynamic Assessment of the Copper-Gallium System. CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 2008, 32, 447–453. 10.1016/j.calphad.2008.03.006. [DOI] [Google Scholar]
- Li D.; Franke P.; Fürtauer S.; Cupid D.; Flandorfer H. The Cu-Sn Phase Diagram Part II: New Thermodynamic Assessment. Intermetallics 2013, 34, 148–158. 10.1016/j.intermet.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürtauer S.; Li D.; Cupid D.; Flandorfer H. The Cu-Sn Phase Diagram, Part I: New Experimental Results. Intermetallics 2013, 34, 142–147. 10.1016/j.intermet.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H. Pd-Sn (Palladium-Tin). J. Phase Equilib. Diffus. 2012, 33, 253–254. 10.1007/s11669-012-0025-0. [DOI] [Google Scholar]
- Du Z.; Yang H.; Li C. Thermodynamic Modeling of the Pd-Y System. J. Alloys Compd. 2000, 297, 185–191. 10.1016/S0925-8388(99)00582-4. [DOI] [Google Scholar]
- Okamoto H. The Pt-Sn (Platinum-Tin) System. J. Phase Equilib. 2003, 24, 198–198. 10.1361/105497103770330938. [DOI] [Google Scholar]
- Palenzona A.; Cirafici S. The Pt-Y (Platinum-Yttrium) System. J. Phase Equilib. 1990, 11, 493–497. 10.1007/BF02898267. [DOI] [Google Scholar]
- Ir (Iridium) Binary Alloy Phase Diagrams. In Alloy Phase Diagrams; Okamoto H., Schlesinger M. E., Mueller E. M., Eds.; ASM International, 2016; Vol. 3, p 1, 10.31399/asm.hb.v03.a0006172. [DOI] [Google Scholar]
- Weststrate C. J.; Ludwig W.; Bakker J. W.; Gluhoi A. C.; Nieuwenhuys B. E. Methanol Decomposition and Oxidation on Ir(111). J. Phys. Chem. C 2007, 111, 7741–7747. 10.1021/jp070539k. [DOI] [PubMed] [Google Scholar]
- Anres P.; Fossati P.; Richter K.; Gambino M.; Gaune-Escard M.; Bros J. P. Thermodynamics of the [Ir-In] System. J. Alloys Compd. 2000, 296, 119–127. 10.1016/S0925-8388(99)00507-1. [DOI] [Google Scholar]
- Okamoto H. The Ir-Y (Iridium-Yttrium) System. J. Phase Equilib. 1992, 13, 651–653. 10.1007/BF02667218. [DOI] [Google Scholar]
- Kuo H.-T.; Chen H.-W. Effect of Y2O3 and Nd2O3 on the Steam Reforming of Methanol over Cu/ZnO Catalysts. Sci. Adv. Mater. 2013, 5, 1895–1906. 10.1166/sam.2013.1655. [DOI] [Google Scholar]
- Zhao Q.; Lorenz H.; Turner S.; Lebedev O. I.; Van Tendeloo G.; Rameshan C.; Klötzer B.; Konzett J.; Penner S. Catalytic Characterization of Pure SnO2 and GeO2 In Methanol Steam Reforming. Appl. Catal., A 2010, 375, 188–195. 10.1016/j.apcata.2009.12.027. [DOI] [Google Scholar]
- Lorenz H.; Zhao Q.; Turner S.; Lebedev O. I.; Van Tendeloo G.; Klötzer B.; Rameshan C.; Pfaller K.; Konzett J.; Penner S. Origin of Different Deactivation of Pd/SnO2 and Pd/GeO2 Catalysts In Methanol Dehydrogenation and Reforming: A Comparative Study. Appl. Catal., A 2010, 381, 242–252. 10.1016/j.apcata.2010.04.015. [DOI] [Google Scholar]
- Ploner K.; Schlicker L.; Gili A.; Gurlo A.; Doran A.; Zhang L.; Armbrüster M.; Obendorf D.; Bernardi J.; Klötzer B.; Penner S. Reactive Metal-Support Interaction In The Cu-In2O3 System: Intermetallic Compound Formation and Its Consequences For CO2-Selective Methanol Steam Reforming. Sci. Technol. Adv. Mater. 2019, 20, 356–366. 10.1080/14686996.2019.1590127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouarab R.; Cherifi O.; Auroux A. Effect of The Basicity Created By La2O3 Addition on The Catalytic Properties of Co(O)/SiO2 In CH4+CO2 Reaction. Thermochim. Acta 2005, 434, 69–73. 10.1016/j.tca.2005.01.019. [DOI] [Google Scholar]
- Osazuwa O. U.; Cheng C. K. Catalytic Conversion of Methane And Carbon Dioxide (Greenhouse Gases) Into Syngas over Samarium-Cobalt-Trioxides Perovskite Catalyst. J. Cleaner Prod. 2017, 148, 202–211. 10.1016/j.jclepro.2017.01.177. [DOI] [Google Scholar]
- Osazuwa O. U.; Setiabudi H. D.; Abdullah S.; Cheng C. K. RETRACTED: Syngas Production From Methane Dry Reforming over SmCoO3 Perovskite Catalyst: Kinetics and Mechanistic Studies. Int. J. Hydrogen Energy 2017, 42, 9707–9721. 10.1016/j.ijhydene.2017.03.061. [DOI] [Google Scholar]
- Zhang W. D.; Liu B. S.; Zhan Y. P.; Tian Y. L. Syngas Production via CO2 Reforming of Methane over Sm2O3-La2O3-Supported Ni Catalyst. Ind. Eng. Chem. Res. 2009, 48, 7498–7504. 10.1021/ie9001298. [DOI] [Google Scholar]
- Taherian Z.; Yousefpour M.; Tajally M.; Khoshandam B. A Comparative Study of ZrO2, Y2O3 and Sm2O3 Promoted Ni/SBA-15 Catalysts For Evaluation of CO2/Methane Reforming Performance. Int. J. Hydrogen Energy 2017, 42, 16408–16420. 10.1016/j.ijhydene.2017.05.095. [DOI] [Google Scholar]
- La (Lanthanum) Binary Alloy Phase Diagrams. Alloy Phase Diagrams; Okamoto H., Schlesinger M. E., Mueller E. M., Eds.; ASM International, 2016; Vol. 3, p 1. 10.31399/asm.hb.v03.a0006173. [DOI] [Google Scholar]
- Deyuan Z.; Tang J.; Gschneidner K. A. A Redetermination of The La-Ni Phase Diagram From LaNi To LaNi5 (50–83.3 at.% Ni). J. Less-Common Met. 1991, 169, 45–53. 10.1016/0022-5088(91)90234-U. [DOI] [Google Scholar]
- Pd (Palladium) Binary Alloy Phase Diagrams. Alloy Phase Diagrams; Okamoto H., Schlesinger M. E., Mueller E. M., Eds.; ASM International, 2016; Vol. 3, p 1. 10.31399/asm.hb.v03.a0006193. [DOI] [Google Scholar]
- Su X.; Zhang W.; Du Z. A Thermodynamic Assessment of the Ni-Sm System. J. Alloys Compd. 1998, 278, 182–184. 10.1016/S0925-8388(98)00560-X. [DOI] [Google Scholar]