Abstract

Dissolution of metals in organic solvents is relevant to various application fields, such as metal extraction from ores or secondary resources, surface etching or polishing of metals, direct synthesis of organometallic compounds, and separation of metals from other compounds. Organic solvents for dissolution of metals can offer a solution when aqueous systems fail, such as separation of metals from metal oxides, because both the metal and metal oxide could codissolve in aqueous acidic solutions. This review critically discusses organic media (conventional molecular organic solvents, ionic liquids, deep-eutectic solvents and supercritical carbon dioxide) for oxidative dissolution of metals in different application areas. The reaction mechanisms of dissolution processes are discussed for various lixiviant systems which generally consist of oxidizing agents, chelating agents, and solvents. Different oxidizing agents for dissolution of metals are reviewed such as halogens, halogenated organics, donor–acceptor electron-transfer systems, polyhalide ionic liquids, and others. Both chemical and electrochemical processes are included. The review can guide researchers to develop more efficient, economic, and environmentally friendly processes for dissolution of metals in their elemental state.
1. Introduction
In contrast to many metals salts, metals in their elemental state cannot be dissolved in conventional solvents. Their dissolution must necessarily involve simultaneously an oxidation step, so that the dissolution of metals can be described as oxidative dissolution. In case only part of the solid is dissolved, oxidative dissolution of metals can be described as a leaching process. The solution used for leaching is called a leaching system, leaching agent, or lixiviant. For oxidative dissolution of metals, the lixiviant must contain at least one oxidizing agent (to oxidize the metal), often a solvent (water or a nonaqueous solvent) to dissolve the oxidizing agent, and sometimes a complexing agent to adjust the redox potential of the metals and/or the solubility of the produced metal compounds. Oxidative dissolution of metallic elements happens in various applications such as extraction of gold from gold-bearing ores, separation of metals from other compounds, chemical etching in the microelectronics industry, surface polishing of metals or alloys, and direct synthesis of organometallic compounds from metals.1−4 Moreover, oxidative dissolution of metals could be relevant for recycling of valuable metals from secondary resources such as preconsumer production scrap and end-of-life products.5,6 Many metals and alloys are essential for the production of components in high-tech devices, such as neodymium–iron–boron (NdFeB) permanent magnets used in electric motors and wind turbines and platinum-group metals (PGMs) used in car exhaust catalysts. Rare-earth elements (REEs), together with PGMs, are on the lists of critical raw materials of the European Commission and the United States of America, because of their high economic importance and high supply risk.7,8 Recycling of metals is essential in a circular economy.
Conventional methods for dissolution of metals are water-based and the metals are oxidized in aqueous solutions by, for example, strong mineral acids such as hydrochloric acid and sulfuric acid with evolution of hydrogen gas. However, these nonoxidizing mineral acids are not able to dissolve noble metals such as gold, silver, and platinum. Current technologies for dissolution of noble metals involve the use of aqua regia or sodium cyanide (in the presence of oxygen gas), which cause serious safety and environmental concerns.9,10 Their use will probably be restricted in the near future. Therefore, the development of new efficient, economic, and environmentally benign leaching systems for metal dissolutions is a very relevant research activity.
Dissolution of metals by oxidizing agents in organic solvents is an alternative approach. The organic solvents can be conventional molecular organic solvents, ionic liquids (ILs), deep-eutectic solvents (DESs), or supercritical carbon dioxide (scCO2). Metal processing in organic solvents or mixed with small amounts of water (<50 vol %) is termed “solvometallurgy”, which is an emerging branch of extractive metallurgy and complementary to hydrometallurgy and pyrometallurgy.11 Examples of solvometallurgy are solvo-leaching, nonaqueous solvent extraction, nonaqueous precipitation and electrodeposition.12−16 When ionic solvents such as ionic liquids (ILs) or deep-eutectic solvents (DESs) are involved, the term “ionometallurgy” could be used.17 Dissolution of metals in organic solvents offer several advantages compared to aqueous solvents. Organic solvent systems usually generate smaller volumes of aqueous waste streams and are often more selective than aqueous systems. One of the earliest studies on the dissolution of metals in organic solvents dates from more than 70 years ago and is about the dissolution of uranium using elemental halogens as oxidizing agents.18 The number of papers published on the topic of oxidative dissolutions of metals in organic solvents grew very slowly in the beginning, but increased rapidly after the year 2000 (Figure 1), due to the search for new, efficient, and environmentally friendly processes for the extraction of metals from metal ores and secondary resources. At present, the total number of publications on oxidative dissolution of metals in their elemental state in organic solvents is still less than 200.
Figure 1.
Number of publications on dissolution of metals in organic solvents.
This review describes the different strategies for the oxidative dissolution of metals in organic solvents and possible applications of this approach. The review covers the literature from the 1950s until December 2020. Only research of oxidative dissolution of metals in their metallic state (oxidation state zero) is presented in this review, so that research on dissolution of other metal compounds such as metal oxides and sulfides has been omitted. Special attention has been paid to ionic liquids and deep-eutectic solvents, because their use for oxidative dissolution of metals is emerging. The various oxidizing systems show different mechanisms for the oxidative dissolution. To better understand the reaction mechanism in the different reaction media, this review is organized according to the different types of oxidizing agents. ILs and DESs are all combined in one section, irrespective of whether they have been used as oxidizing agent or as solvents, in order to highlight their application in oxidative dissolution of metals. The different types of organic leaching systems, together with the studied oxidative dissolution of metals in the corresponding systems, are summarized in Figure 2. The aim of this review is to give an overview of the emerging research field of oxidative dissolution of metals in organic solvents. By highlighting the oxidation reaction mechanisms, this review can be helpful for the development of new efficient, environmentally benign, and economic processes for dissolution of metals in their elemental state for various applications.
Figure 2.
Overview of the organic leaching systems and the metals that have been studied for oxidative dissolution in the corresponding leaching systems.
2. Solutions of Halogens in Polar Organic Solvents
Many studies on the oxidizing dissolution of metals in organic solvents use halogens, because they are strong oxidizing agents. Various metals can be dissolved directly in solutions of halogens in polar organic solvents, such as bromine in ethyl acetate (EtOAc) or methanol (MeOH) or chlorine in N,N-dimethylformamide (DMF). Addition of suitable ligands can enhance the dissolution of noble metals such as gold and platinum. These ligands can be simply halide compounds (e.g., tetraethylammonium iodide) or organic compounds such as dithio-oxamide. The subsections in section 2 are defined on the basis of the types of ligands in the leaching system. Subsection 2.1 describes halogen leaching systems without added ligands, and subsection 2.2 covers halogen leaching systems with halide anions as ligands, whereas subsection 2.3 is about organic compounds that can form adducts with halogens.
2.1. Halogens in Organic Solvents
The simplest organic lixiviants for the dissolution of elemental metals are solutions of halogens in an organic solvent. The halogens act as oxidizing agents to oxidize the metal from oxidation state zero to a higher oxidation state, which can be dissolved in the organic solvent. Examples of commonly used organic solvents are ethanol (EtOH), methyl acetate (MeOAc), butyl acetate (BuOAc), ethylene glycol (EG), acetonitrile (ACN), and tri-n-butylphosphate (TBP). A major application of organic solvent–halogen mixtures is the quantitative analysis of nonmetallic phase impurities in metals and alloys, because the metals in their elemental state are oxidatively dissolved, but metallic compounds (e.g., oxides and borides) and nonmetallic components (e.g., boron) are not soluble.19−23 Other applications are the dissolution of metals or metal alloys, the chlorination of metals, and the chemical etching and polishing of semiconductors.3,24−30 Leaching systems containing halogens and organic solvents used for dissolution of metals in various application fields are summarized in Table 1. Due to the large variety of combinations of halogens with organic solvents, this subsection is further divided into three subsections based on the type of halogens.
Table 1. Leaching Systems Using Halogens as Oxidizing Agents in Organic Solvents for Dissolution of Metals in Various Application Fields.
| Solvent system | Metals studied | Application | References |
|---|---|---|---|
| Br2-EtOAc | U, Nd, Sm, Ce, Pr, La, Y, Ga, Zr | Separation of metals and metal oxides | (2, 18, 31) |
| Br2-MeOH | Be, Zr, Th, U | Phase analysis (B content in metals) | (19) |
| GaAs, GaP | Polishing or etching of semiconductors | (3, 24, 25) | |
| Fe | Determination of iron content in rusted iron sponge | (32) | |
| Ni | Phase analysis (TiC-Ni cermets) | (33) | |
| X2 (Cl2, Br2, I2, ICl, Cl3–) -organic solvents (MeOH, MeOAc, BuOAc, ACN) | Al, Cr, Co, Cu, Fe, Pb, Mn, FeMo, Ni, FeNi, P, FeSi, S, Sn, Ti, FeW, V, | Phase analysis | (20−23) |
| Cl2-DMF | Mo | Chlorination | (26) |
| Cl2-DMF-H2O | Re, W, Mo | Chlorination | (27) |
| Cl2-DMF-HCl | Re, Pt | Chlorination | (28) |
| Cl2-DMF-FeCl3 | Zr | Chlorination | (29) |
| Cl2-tetrachloroethylene (TCE)-TBP | U | Chlorination | (30) |
| Br2-organic solvent (EtOH, DMF, EG, EtOAc, MeOAc) | Ni, Cu | Remove coatings from SmCo magnets | (34) |
| Br2-EtOH | Au, Ag, Pt | extracting noble metals from solid residues after mineral acid leaching of raw materials | (35) |
2.1.1. Bromine in Organic Solvents
Bromine is the most used halogen in organic solvents for oxidative dissolution of elemental metals, because of its moderate oxidizing power, which allows bromine to oxidize metals but not aggressively react with organic solvents. Various organic solvents (e.g., EtOAc, MeOAc, ACN, MeOH, EtOH) mixed with bromine have been studied for dissolution of metals in various applications. The reaction of bromine with metals can be described in eq 1:
| 1 |
where M represents a metallic element and x is the oxidation state of the metal. Solutions of bromine in ethyl acetate (Br2-EtOAc) have been investigated mostly for dissolution of uranium metal for nuclear applications. Elemental uranium is very reactive and can be dissolved rapidly in common mineral acids, such as nitric acid or hydrochloric acid. However, for the dissolution of uranium alloys with refractory alloying metals such as zirconium, aggressive reagents such as hydrofluoric acid, with or without extra added fluoride salts, have to be used to ensure complete dissolution of the uranium–zirconium alloy.18 Dissolution of such alloys in organic solvents is useful when fluoride ions must be absent for the subsequent analysis or for avoiding aggressive reagents. Bromine in ethyl acetate (Br2-EtOAc) reacts rapidly with both uranium and zirconium metal to form UBr4 and ZrBr4.18 Organic solvents other than ethyl acetate have been tested as solvents for bromine to dissolve uranium metal, but these solvents did not perform as well as ethyl acetate.18 Br2-EtOAc was successfully used for separation of metals from their oxides, including metallic uranium, magnesium, aluminum, and zinc. By using a solution of Br2-EtOAc to selectively separate uranium and rare-earth metals from their oxides, the yield of electrochemical reduction of the simulated oxide spent nuclear fuel in the molten salt mixture LiCl-Li2O was estimated.31 However, Br2-EtOAc is not reactive enough for dissolution of noble metals and their alloys.
Solutions of bromine in alcohols are one of the most studied leaching systems in the category of bromine in organic solvents. Bromine in EtOH can be used for extracting noble metals such as gold, silver, and platinum from raw materials.35 The raw materials containing noble metals were first leached in a mineral acid at temperatures 95–100 °C to leave the noble metals in a solid residue that was then treated with bromine in organic solvents twice. The recovery efficiency of noble metals could reach >95%. Recently, bromine in organic solvents has shown potential for removal of the metallic nickel–copper–nickel protective coatings of rare-earth permanent magnets (Nd–Fe–B and Sm–Co magnets), prior to recycling of the rare-earth elements.34 Among five solvents tested, namely 1 vol % of bromine in EtOH, DMF, EG, EtOAc, and MeOAc, the first two solutions could selectively dissolve nickel and copper without leaching the magnet alloy (Figure 3). Dissolution of metals in Br2-EG was slow due to the high viscosity of EG which is 1 order of magnitude higher than that of ethanol or DMF. The slow dissolution rate of metals in the MeOAc and EtOAc mixtures was attributed to the low solubility of the formed metal salts in these solvents. This solvometallurgical process performed better than an aqueous 2 M HNO3 solution, which could dissolve nickel but not copper.
Figure 3.

Screening dissolution test for the SmCo5 (top) and Sm2Co17 (bottom) permanent magnets at 20 min in 1 vol % Br2 in EG, DMF, EtOH, MeOAc, and EtOAc. The recovery yields, η (%), were the ratio of the concentration of a metal in solution after leaching for 20 min to the concentration of the same metal if all the content of the coating would be dissolved in solution. Reprinted from ref (34). Copyright 2019 Royal Society of Chemistry under [CC BY-NC 3.0] [https://creativecommons.org/licenses/by-nc/3.0/].
Bromine in methanol (Br2-MeOH) is another example of a bromine-alcohol mixture. It has been tested for the determination of boron in metals such as beryllium, zirconium, thorium, and uranium, by selective dissolution of the metal, followed by distillation of the formed boron methyl ester.19 With the anhydrous reaction mixture, traces of boron could be completely recovered by distillation. Another application of Br2-MeOH is for phase analysis, to quantify the composition of the titanium carbide-nickel cermets, more specifically to determine the amount of titanium present in a metallic nickel binder. Br2-MeOH was used as solvent at a low temperature (−20 °C) to dissolve the metallic phase without affecting the titanium carbide.33 Br2-MeOH has also been used for processing of semiconductors, for instance for chemical polishing of gallium arsenide and for etching of gallium phosphide.3,24,25 The etching behaviors of GaP in Br2-MeOH and in aqueous solutions of Br2 were similar, so that it seems preferable to use the aqueous solution for etching, considering the toxicity of Br2-MeOH. However, the advantages of Br2-MeOH solutions must be taken into account as well. For example, the reproducibility of the measurements in Br2-MeOH solutions was higher than that in the aqueous Br2 solutions. Moreover, Br2 has a higher solubility in methanol than in water. The metallic iron content in a rusted iron sponge was determined by using a solution of Br2-MeOH to selectively dissolve iron metal, leaving behind the iron oxides.32
Although Br2-MeOH can oxidatively dissolve metals, this system must be used with much caution. First, it must be stressed that it can be dangerous to prepare solutions of bromine in alcohol solvents, especially when the concentration of bromine is above 10 vol %. It was reported that by mixing a 50 vol % Br2-MeOH solution in a 250 mL volumetric flask, within seconds the solution was violently expelled from the flask.36 This is due to a strongly exothermic reaction between bromine and alcohol. Moreover, the vigorous reaction of bromine with methanol may generate gaseous products which could also eject the solution from the flask. Therefore, leaching of the metal with Br2-MeOH should be performed at room temperature, to avoid the rapid reaction between Br2 and MeOH at elevated temperatures. Second, the reaction of Br2 with MeOH could produce HBr and water. These products dissolve oxides so that the selectivity of metal dissolution decreases when metal oxides are present. Therefore, magnesium oxide is sometimes used to neutralize the formed acids to prevent the dissolution of oxides. Third, the reaction should be performed under anhydrous conditions, since water can react with Br2 forming HBr as well. For example, the use of a nonaqueous solution is mandatory in the application of quantification of titanium in titanium carbide-nickel cermets, because the HBr formed by hydrolysis of bromine can react with the carbide, resulting in errors in the phase analysis.33
2.1.2. Chlorine in Organic Solvents
Chlorination of refractive metals such as molybdenum, tungsten, uranium, zirconium, rhenium, and platinum has been studied by using chlorine in organic solvents, among which chlorine in DMF was the often used leaching system.26−29 It is known that halogens can react with organic solvents,18,36 but the influence of these side reactions on the dissolution of metals has been studied only occasionally. Drobot et al. studied the oxidative dissolution of molybdenum by chlorine gas dissolved in DMF.26 The IR and EPR spectra of the solutions obtained after chlorination of molybdenum suggested that the formed complexes are the diamagnetic Mo(VI) and paramagnetic Mo(V) complexes of composition R2[MoOCl5], where R is [(CH3)2NCOH2]+ and [(CH3)2NH2]+. Moreover, the complex [(CH3)2NH2][MoOCl5] is not stable upon prolonged storage and decomposes to form [(CH3)2NH2]Cl, indicating that the DMF solvent participates in the chlorination reaction. Therefore, DMF not only acts as solvent for chlorine but also enhances the chlorination reaction due to the formation and destruction of the formed molybdenum complex. The mechanism of chlorination of molybdenum could be described by the reactions given in Scheme 1.26
Scheme 1. Chlorination of Molybdenum by Chlorine in DMF.
Addition of water can enhance the oxidative dissolution of metals in chlorine-DMF.27 For example, when chlorine in DMF was used as a leaching system, only small amounts of rhenium and tungsten (<5%) could be oxidatively dissolved. However, an increase in the water content in the leaching system resulted in a higher amount of metals dissolved in the solution. Further increase in the water content resulted in a decrease of the dissolution of molybdenum and tungsten (Figure 4). Quantitative dissolution of rhenium was attained by adding 30 to 40% water with respect to the volume of DMF.
Figure 4.
Effect of water on dissolution of metals in solutions of chlorine in DMF. (Data were extracted from ref (27).)
Chlorination of refractory metals in DMF–H2O mixtures is much more efficient than that in the neat organic solvent. This is due to the fact that mixing of water and DMF can rupture some hydrogen bonds between water molecules and form new hydrogen bonds between the carbonyl oxygen atoms of DMF and water molecules. This new arrangement facilitates the molecular interaction between DMF and other molecules in the system. On the other hand, in the presence of chlorine gas, addition of water induces the formation of HCl and HClO. The new complex [(CH3)2N–CH=OH]+Cl– is formed with a hydrogen bond between the carbonyl oxygen atom of DMF and HCl (see Scheme 2). This protonated DMF molecule can further react with the chlorine-containing anion, e.g. [MOCl5]2–, to form an adduct, and this enhances the dissolution of metals.
Scheme 2. Complex Formed between DMF and HCl.

Similarly, addition of HCl in Cl2-DMF can enhance the oxidative dissolution of metals as well. Chlorine in DMF with addition of water or HCl solutions was applied to recover rhenium and platinum from end-of-life platinum–rhenium catalyst on an aluminum support.28 The influence of HCl on the dissolution efficiency was similar to that of water. One leaching step at 25–60 °C could dissolve 80 to 90% of rhenium, while less than 50% of platinum was dissolved, due to the modified (coked) state of platinum in the spent catalyst. After a decarbonization process (to burn off carbon at about 500 °C), a second chlorination leaching step was conducted, resulting in 70–94% of platinum recovery and 91–96% of rhenium recovery.28 No heating was required for the leaching step, and this is a cheap leaching system.
The presence of the Lewis acid FeCl3 in Cl2-DMF can influence the kinetics of dissolution of zirconium as well.29 The dissolution rate of zirconium metal increased appreciably relative to the FeCl3-free system. A maximum in the dissolution rate was observed at 1.5 g/L of FeCl3, and further increase of the FeCl3 content resulted in a rapid decrease of the dissolution rate. The dissolution of zirconium and the catalytic effect of FeCl3 could be described by eqs 2–5:
| 2 |
| 3 |
| 4 |
| 5 |
Besides DMF, tetrachloroethylene (TCE) was used as solvent, in combination with TBP (complexing agent) and chlorine as oxidizing agent for low-temperature chlorination of uranium metal.30 Uranium reacted with chlorine to form UCl4, which further complexed with TBP to form UCl4·2TBP (eq 6):
| 6 |
The dissolution rate increased with increasing concentrations of chlorine and TBP. Similar to the DMF-Cl2 leaching system, addition of water (0.2–0.6 vol%) did increase the dissolution rate significantly. The dissolution rate decreased with a further increase in water content. A maximum in dissolution rate was observed for a water content of 0.2–0.6 vol%. The function of TCE was not specifically mentioned in the paper, but it can be supposed to act as a diluent. The reason for the positive effect of water addition is probably the formation of HCl that can complex with TBP and enhance the metal dissolution.11
Chlorine can be used as a strong oxidizing agent for metals, but the fact that it can react with organic solvents as well makes the recycling of the organic solvent difficult. In some cases the strong reactivity of chlorine toward organic solvents with formation of chlorinated organic compounds has a negative effect on the dissolution of metals. For example, bromine in EtOAc was preferred over chlorine in EtOAc for dissolution of uranium metal.18
2.1.3. Other Halogens in Organic Solvents
One of the main applications of solutions of halogens in organic solvents is phase analysis of metals or alloys, to determine the concentration of impurities such as metallic compounds (e.g., oxides, sulfides) and nonmetallic compounds (e.g., boron, carbon) in metal alloys by selectively dissolution of metals. Pioneering work was performed by Beeghly, who used bromine-methyl acetate (Br2-MeOAc) solvent for the quantitative isolation of aluminum nitride from steel alloys.20 All nitrogen was recovered as insoluble aluminum nitride after dissolution of steel samples in the lixiviant. Inspired by this work, Headridge and co-workers investigated the feasibility of quantitative separation of carbide, nitride, oxide, and sulfide inclusions from metals by using solutions of halogens in organic solvents.21−23 For useful phase analysis, it is necessary that only the metals in their elemental state oxidatively dissolve in the leaching system, and other compounds such as oxides and nitrides that are present in metal alloys do not. Therefore, the reactivity of various metals in the elemental state and of metal compounds (oxides, nitrides, sulfides, carbides) with bromine in organic solvents MeOAc, BuOAc, and ACN was investigated. Additionally, the solubilities of the corresponding metal bromides formed by the reactions and of the metal compounds (oxides, nitrides, sulfides, carbides) in the leaching systems were reported. All the studied elements or alloys were found to react with bromine: aluminum, chromium, cobalt, copper, iron, lead, manganese, ferromolybdenum, nickel, ferroniobium, phosphorus, ferrosilicon, sulfur, tin, titanium, ferrotungsten, and vanadium. The study of the solubility showed that most of the metal bromides can dissolve in the organic solvent–bromine mixtures, but some metal bromides were poorly soluble, such as the bromides of lead, molybdenum, silicon, and tungsten.21 The poor solubility of metal bromides could limit the oxidative dissolution of these metals in the leaching systems. Most studied carbides, nitrides, and oxides of metals have low reactivity and solubility in Br2-ACN and Br2-MeOAc, whereas most sulfides have a significant solubility and some sulfides can react with the leaching agents violently. Therefore, organic solvent–bromine mixtures can be used for isolation of most carbides, nitrides, and oxides but not sulfides from metals.23
Subsequently, more organic solvent–halogen mixtures were used to study the solubilities of manganese silicon nitride, metals and their nitrides, carbides, oxides, and sulfides. The studied organic solvents were MeOH and MeOAc, and the halogens were Cl2, Br2, I2, ICl, and ICl3.22 Based on the reactivity and solubility of the metals and metal compounds in the leaching systems, a method was proposed to determine in steel the contents of total nitrogen, of mobile nitrogen, of nitrogen as manganese silicon nitride, and of nitrogen as aluminum nitride. A mixture of I2-MeOAc can be used in the first step to isolate manganese silicon nitride and aluminum nitrides while dissolving metals; subsequently, ICl3-MeOAc can be used to isolate aluminum nitride, while dissolving manganese silicon nitride.22 Therefore, organic solvent–halogen mixtures can be used to quantify nonmetallic phases in metals. Moreover, the organic solvent has a slight influence on the solubility of the metal bromide. For example, acetonitrile with its higher dielectric constant (ε = 36.5 at 25 °C) is a better coordinating solvent for ions and is thus a better solvent for divalent metal bromides than methyl acetate and butyl acetate with dielectric constants of ε = 6.7 (25 °C) and ε = 5.0 (20 °C), respectively.21
2.2. Halogens + Halide Ligands + Organic Solvents
The dissolution efficiency of metals largely depends on the solubility of the reaction products (metal halide salts) in the organic solvents. Increasing the solubility of the salts formed could suppress formation of a passivating layer and hence increase the dissolution efficiency. Moreover, addition of complexing agents to the organic solvent could change the redox potential of metals and hence the dissolution behavior of the metals. In the 1990s, Nakao and co-workers carried out a series of studies on the dissolution of metals with an organic lixiviant consisting of an elemental halogen, a halide salt, and a common organic solvent.4,37−43 They used the halogens chlorine, bromine, and iodine. Most of the used halide salts were quaternary ammonium halide, but metal halides such as KI, KBr, and NaI were tested as well. A variety of organic solvents including hydrocarbons, organic halides, alcohols, ketones, esters, and nitro compounds was investigated. Bromine–cetylpyridinium bromide (CPB)–benzene and iodine–cetylpyridinium iodide (CPI)–benzene were found to be effective for the dissolution of various metals, such as Fe, Ni, Cu, Zn, Au, Ag, Pd, Cr, Mn, Co, Ga, Ge, Se, Cd, In, Sb, Hg, and Pb.37 The metals were dissolved in the form of metal complex anions, such as [AgBr2]−, [AuBr4]−, [NiBr4]2–, [ZnBr4]2–, and [PdBr4]2–. In the dissolution process, the halogens acted as oxidizing agents and the halide compounds as complexing agents to allow the products to be soluble in the leaching systems. Besides tests on pure metal wires, gold and silver were efficiently extracted from quartzite ore (containing 13.1 ppm of gold and 389.2 ppm of silver) with the same lixiviant.41 Organic solvents can react with halogens, but in the presence of CPB or CPI, the reactivity of halogens is largely reduced, due to the formation of tribromide or triiodide anions by reaction between the halogens and the halide salts. These trihalide anions are still active to metals, but less active toward the organic solvents than elemental bromine and iodine. However, in most cases, elemental chlorine could not be used, because the trichloride anion is quite reactive toward organic solvents. On the other hand, it was observed that acetonitrile is very resistant toward chlorine at low temperatures. Thus a mixture of chlorine with chloride salts in acetonitrile can be used for oxidative dissolution of metals. Several lixiviants in acetonitrile comprising chlorine and different organic halide salts such as tetraethylammonium chloride, trimethylamine hydrogen chloride (Me3NHCl), and tetraethylammonium bromide were compared with lixiviants using inorganic halide salts such as I2–NaI–acetone, Br2–KBr–methanol, and I2–KI–methanol for dissolution of noble metals.38 All these systems could dissolve palladium, gold, and silver, except that silver could not be dissolved in the methanol systems, because of the formation of a passivating layer of silver halide on the silver metal surface. It was observed that chlorine reacted faster than bromine and iodine. Moreover, it was found that the system Cl2–Me3NHCl–acetonitrile did dissolve gold more rapidly than aqua regia at 30 °C.
An interesting phenomenon was observed in the I2–I– organic solvent system: when the molar ratio I2/I– was larger than 0.5, gold dissolved on heating as [AuI2]− and, upon cooling, part of the gold was deposited from the pregnant solution via formation of [AuI4]− and Au by a disproportionation reaction. When a new piece of gold was added to the resulting solution under reflux, an amount of gold equal to that deposited before was redissolved and upon cooling was redeposited. This process could be repeated several times. Such reversible dissolution/deposition behavior was not observed for any other metals, nor in Cl2–Cl–-organic solvent and Br2–Br–-organic solvent systems, in which gold dissolved exclusively in the form of very stable [AuX4]− complexes.40 However, upon addition of methanol, the reversible dissolution/deposition processes can be induced in all systems of X2-X––organic solvent (X = Cl, Br, and I).39
Organometallic complexes can be synthesized in one step by dissolving the corresponding metals in the above-mentioned organic solutions.4 This direct synthesis method can avoid the handling of hygroscopic metal halides as reagents. Lower valence metal compounds (for instance ferrous or cuprous compounds) can be obtained by selecting an appropriate molar ratio of X2/metal.
2.3. Dihalogen or Interhalogen Adducts
Halogen adducts are formed by the interaction between a σ-donor with a relatively high-lying HOMO and a dihalogen as an acceptor with a relatively low-energy LUMO (σ*). It has been found that these dihalogen adducts are capable of oxidizing elemental metals, including noble metals. The reactions between metals and diiodine and dibromine adducts with phosphine, arsine, and antimony donors have been studied.44−49 Examples of such adducts are diiodotrimethylarsine (Me3AsI2), diiodotriphenylantimony (Ph3SbI2), and dibromotrimethylphosphorus (Me3PBr2). These adducts can react with metals (e.g., Mn, Zn, Co, Ni, and Au) to form complexes with various structures. The nature of the formed metal complexes is strongly dependent on both the organic group and the halogen. Gold reacts with Me3AsI2 to form the square-planar complex [AuI3(AsMe3)], whereas the same metal reacts with Me3PI2 to form the trigonal-bipyramidal complex [AuI3(PMe3)2].49 Besides new perspectives in the field of inorganic chemistry, these results open the door to new applications of dihalogen adducts for recovery of noble metals from secondary resources or for metal refining. Unfortunately, the adducts with phosphine and arsine donors are very toxic and they require strictly anhydrous, anaerobic conditions and long reaction times.
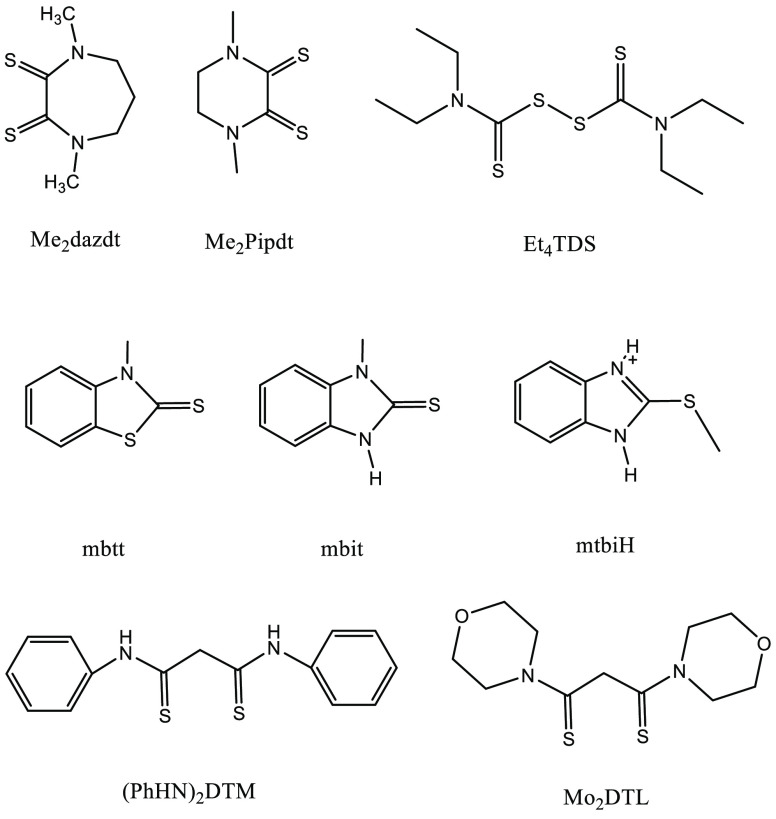
A series of new oxidation reagents of dihalogen adducts bearing S,S-donors or adducts on the basis of thioamide have been reported for dissolution of metals such as noble metals, cadmium, and mercury (Table 2 and Scheme 3).50−61 These adducts were prepared by simply mixing dihalogen or interhalogen with the donors in organic solvents such as CHCl3 or THF at room temperature. The resulting compounds have been claimed to be safe, inexpensive, easy to handle, and noncytotoxic. Proper choice of polyfunctional donors that favors the preferred geometry required by the metal undergoing oxidation is believed to enhance the oxidation reaction. Most of the synthesized adducts were applied for dissolution of noble metals such as gold, palladium, and platinum. The softness of the donor atoms, as well as the chelating properties of the two vicinal thiones stabilize the oxidized noble metals in the d8 configuration.
Table 2. Names and Abbreviations of Organic Ligands to Form Halogen Adducts.
| Name | Abbreviation |
|---|---|
| N,N’-dimethylperhydrodiazepine-2,3-dithione | Me2dazdt |
| N,N’-dimethylpiperazinium-2,3-dithione | Me2Pipdt |
| tetraethylthiuram disulfide | Et4TDS |
| 3-methyl-benzothiazole-2-thione | mbtt |
| 1-methyl-1H-benzimidazole-2(3H)-thione | mbit |
| N,N’-diphenyl dithiomalonamide | (PhHN)2DTM |
| dimorpholyldithiomalonamide | Mo2DTM |
Scheme 3. Structures and Abbreviations of the Reported Organic Ligands to Form Halogen Adducts.
The most extensively studied compound is the bis-diiodine adduct of N,N’-dimethylperhydrodiazepine-2,3-dithione (Me2dazdt·2I2) which can oxidatively dissolve gold and palladium under mild conditions in organic solvents.50,61 The oxidation reactions are shown in Scheme 4. The reaction product after dissolution of gold was identified as the square-planar [AuI2(Me2dazdt)]I3, where gold(III) is coordinated with two iodide ligands and one S,S-chelating ligand Me2dazdt. However, the reaction product after palladium dissolution was identified as [Pd(Me2dazdt)2](I3)2, wherein the first coordination sphere of palladium(II) comprises two bidentate Me2dazdt ligands. The reagent Me2dazdt·2I2 was tested for practical applications, for example the recovery of gold from waste inkjet printer cartridges and SIM cards.57 It was used for failure analysis on end-of-life microelectronic devices by selectively removing the upper layer of gold from the multilayer system such as Ti/Pt/Au in GaAs-based devices, without destroying the electrical contacts.52 The reagent allowed for quantitative recovery of palladium from a spent car exhaust catalyst (0.5–3.0 wt % Pd supported on Al2O3 and CeO2–ZrO2/Al2O3).61 The adduct Me2dazdt·2I2 cannot dissolve platinum or rhodium metal, either at room temperature or in refluxing THF or CH3CN. Therefore, palladium can be selectively recovered when platinum or rhodium is also present in the spent car catalyst. Moreover, it was found to be much more efficient than the aqueous lixiviant I2/I–, which could recover only 11% of the palladium from the same sample of spent car exhaust catalyst under the same conditions. Metallic palladium could easily be obtained by heating the formed complex [Pd(Me2dazdt)2](I3)2 at 600 °C for a few minutes. However, the ligand is destroyed during the thermal treatment of the complex to recover metallic palladium. This lowers the economic, environmental, and elemental sustainability of the recovery process. Therefore, the obtained complexes [Pd(Me2dazdt)2](I3)2 and [PdI2(Me2dazdt)] have been valorized themselves rather than recovering palladium metal from them, for instance as catalysts in the regio- and chemoselective C–H functionalization of benzo[h]quinoline to 10-alkoxybenzo[h]quinoline and 8-methylquinoline to 8-(methoxymethyl)quinoline in the presence of the oxidant PhI(OAc)2.59 Palladium powder can also be oxidized by a diiodine tellurium adduct (2-PyTe)2···I2 at room temperature with formation of a square planar geometry complex [PdI(TePy-2)(I2TePy-2)2] (Scheme 5).62
Scheme 4. Oxidative Dissolution of Gold and Palladium with Me2dazdt·2I2 in Organic Solvent at Room Temperature.
Scheme 5. Synthesis of a Palladium Complex from a Diiodine Tellurium Adduct.
Various dihalogens or interhalogens can form adducts with dithio-oxamide donors such as IBr, ICl, Br2, or I2.54 The oxidation power of the adducts can be tuned by varying the halogens. The adducts of Me2dazdt·2IBr have been tested for dissolution of gold. The square-planar complex [Au(Me2dazdt)Br2]IBr2, with a structure similar to that of the complex [AuI2(Me2dazdt)]I3, was formed by reaction between gold and Me2dazdt·2I2. The ligands act as an S,S-chelating ligand, and two halide ligands complete the first coordination sphere of gold(III). The effectiveness of these adducts in THF solutions for gold dissolution was compared with conventional I2/KI aqueous lixiviants, and the following trend was found: Me2dazdt·2I2 > Me2dazdt·2IBr > I2/KI. Besides a faster dissolution rate, the adducts also showed better etching behavior than I2/KI solutions. It was observed that gold etching by adducts was uniform, while the I2/KI-etched gold surface appeared porous and its color changed to red. This color was attributed to gold nanoparticles. A comparison of the cyclic voltammograms of the Me2dazdt·2IBr and Me2dazdt·I2 adducts with that of IBr and I2 in anhydrous CH2Cl2 suggests that the halogens are the only electroactive species in the oxidative dissolution process.
The adduct N,N’-dimethylpiperazinium-2,3-dithione triiodide, [Me2Pipdt]I3, is of interest because it can act as a powerful oxidation agent toward metallic platinum, with formation of the square-planar complex [Pt(Me2pipdt)2](I3)2 (Scheme 6).51 In contrast to the neutral adduct Me2dazdt·2I2, the adduct [Me2Pipdt]I3 is a salt, in which the cation is formed by the protonated ligand and the anion is a triiodide. Similarly to Me2dazdt·2I2, the adduct [Me2Pipdt]I3 can also react with other metals such as copper, silver, gold, and platinum. However, only [Me2Pipdt]I3 reacts with platinum, whereas Me2dazdt·2I2 does not. This difference in reactivity is most likely due to differences in the geometry of the ligand: Me2dazdt has a seven-ring structure, while Me2Pipdt has a six-ring structure. The six-ring structure probably favors the required geometry of the formed platinum complex. The adduct [Me2Pipdt]I3 can also quantitatively dissolve the hazardous elements cadmium and mercury in a one-step reaction.53 The formed complex [CdI(Me2pipdt)2]I3 has a coordination geometry intermediate between square-pyramidal and trigonal-bipyramidal, while the complex [HgI2(Me2pipdt)] shows a distorted tetrahedral geometry (Figure 5).
Scheme 6. Oxidative Dissolution Reaction of Platinum by [Me2Pipdt]I3 in Acetonitrile (Reflux for 4 days).
Figure 5.

Molecular structure of (a) [CdI(Me2pipdt)2]I3 and (b) [HgI2(Me2pipdt)], with thermal ellipsoids depicted at the 30% probability level. Symmetry code = −x; y; 1/2 – z. Reprinted with permission from ref (53). Copyright 2014 Elsevier B.V.
The donors of dihalogen adducts have been extended to other types, such as tetraethylthiuram disulfide (Et4TDS), 3-methyl-benzothiazole-2-thione (mbtt), and 1-methyl-1H-benzimidazole-2(3H)-thione (mbit).56,58 Their structures are shown in Scheme 3. The mixture of Et4TDS and I2 in acetone solutions can dissolve metallic gold powders and can etch a thin layer of gold from photonic devices with Si/SiO2/Ti/Au multilayer structures. By changing the molar ratio of Et4TDS to I2, a variety of gold complexes can be formed, such as [Au(Et2dtc)2][AuI2], [Au(Et2dtc)I2], [Au(Et2dtc)2]I, and [Au(Et2dtc)2]I3 (Et2dtc = N,N-diethyldithiocarbamate). The examples of formation of different complexes are shown in Scheme 7. The mixture of Et4TDS and I2 was also applied for gold recovery from real Waste Electrical and Electronic Equipment (WEEE) materials.60
Scheme 7. Oxidative Dissolution of Gold by Et4TDS/I2 in Acetone at Room Temperature with the Formation of Four Complexes by Varying the Molar Ratio of Et4TDS to I2.
Reprinted with permission from ref (58). Copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA.
Two thioamide donors 3-methyl-benzothiazole-2-thione (mbtt) and 1-methyl-1H-benzimidazole-2(3H)-thione (mbit) were exploited to form dihalogen adducts.56 Despite the similar structures of mbtt and mbit, their reactivities toward gold are totally different. The adduct mbtt·I2 quantitatively dissolved gold powder at a molar ratio of gold to mbtt·I2 of 1:2 at 25 °C within 3 days in anhydrous diethyl ether, whereas only 45% of gold was dissolved by mbit I2 under the same conditions. Moreover, gold was oxidized to the +1 oxidation state by mbtt I2 but to the +3 oxidation state by mbit I2. The cationic gold complex [Au(mbtt)2]I3 was formed with mbtt, whereas with mbit, the anionic gold complex [(mtbiH)2](AuI4)I3 was formed, which was not bound to any cationic 2-methylthiobenzimidazolium (mtbiH) unit but rather to iodides to form the square-planar [AuI4]− anion. The structures of mbtt, mbit, and mtbiH are shown in Scheme 3. The difference in reactivity of mbtt and mbit toward gold was attributed to the presence of the imido group that can form an intramolecular hydrogen bond with the S-bonded iodine atom (Ib) in the form of N–H···Ib. This new moiety might modify its reactivity with the gold surface.
The organic molecules dithiomalonamides (PhHN)2DTM and Mo2DTM (see Scheme 3) can react with iodine in organic solvent to form organic triiodides (OrgI3), namely 3,5-bis(phenylamino)-1,2-dithiolylium triiodide ([(PhHN)2DTL]I3) and 3,5-bis(morpholino)-1,2-dithiolylium triiodide [Mo2DTL]I3.55 These were applied for leaching of palladium from metal powders and model spent car exhaust catalysts at room temperature. Two more triiodides with tetrabutylammonium (N4444) and tetraphenylphosphonium (Ph4P) cations were formed directly from the corresponding iodide salt with iodine, and they were studied for leaching of palladium as well. Actually, these two triiodides are trihalide ionic liquids, but due to their high melting point, they are solid at room temperature, so that they can only be used when dissolved in organic solvents. Therefore, we include them in this section rather than in section 5. The crystals obtained after dissolution of palladium metal were characterized by single-crystal X-ray diffraction and showed that the formed complex had the formula Org2[Pd2I6] (Figure 6). The palladium recovery yields from spent car exhaust catalyst showed the trend following: [(PhHN)2)DTL]I3 > [Ph4P]I3 ≥ [N4444]I3 ≫ KI3. The aqueous KI3 solution gave the lowest recovery yield, due to the formation of a passivation layer of PdI2 on the palladium metal surface. The higher leaching yields of OrgI3 compounds than KI3 were ascribed to the formation of the Org2[Pd2I6] complex which prevents formation of a passivation layer and facilitates its extraction in organic solvent. Note that [(PhHN)2)DTL]I3 gave a similar leaching yield as the most often studied adduct Me2dazdt·2I2 under the same experimental conditions (98 vs 99%). Gold can be oxidized by [(PhHN)2)DTL]I3 and [Mo2DTL]I3 as well, and the formed complexes have the stoichiometry Org[AuI2]. Unlike for dissolution of palladium, the aqueous solutions of KI/I2 have comparable efficiency as OrgI3 solutions for dissolution of gold, because no occurrence of passivation was observed during the reaction.
Figure 6.

Leaching of palladium from spent catalyst by organic triiodides in methylethylketone (MEK) under reflux for 7 days. Reprinted with permission from ref (55). Copyright 2017 American Chemical Society.
Organic iodide salts which provide both oxidative power and a coordinating ligand could dissolve gold with formation of organo-gold compounds. The iodide salts were iodocarbenium iodides (N,N-dimethyliodomethyleneiminium iodide) and bis-donor-substituted iodocarbenium iodide (bis(N,N’-dimethylamino)iodomethylene iodide and 2-iodo-1,3-dimethylimidazolium iodide) (Scheme 8).63 Gold powder could be oxidized by iodocarbenium iodides in organic solvents (dichloromethane or acetonitrile) to form the gold(III)–carbene complex triiodo[(dimethylamino)methylene]gold(III) which was in equilibrium with gold(I) species iodo(dimethylamino)methylenegold(I) or [AuI2(CHNMe2)2]+[AuI2]− (Scheme 8). The yield of gold(III) complexes was low and could be increased by addition of iodine, whereas bis-donor-substituted iodocarbenium iodide could also react with gold to form complexes with no need of addition of iodine. The mono- and bis-carbene-gold(III) complexes were produced (Figure 7). These reactions provide the routes for preparation of carbene complexes directly from metals.
Scheme 8. Structures of Iodocarbene Iodide and Gold Complexes Formed by Reaction between Compound (a) and Gold.
The names of the compounds are (a) N,N-dimethyliodomethyleneiminium iodide; (b) bis(N,N’-dimethylamino)iodomethylene iodide; (c) 2-iodo-1,3-dimethylimidazolium iodide; (d) triiodo[(dimethylamino)methylene]gold(III); (e) iodo(dimethylamino)methylenegold(I); and (f) [AuI2(CHNMe2)2]+[AuI2]−.
Figure 7.

Crystal structures of carbene complexes: the urea-derived complexes 10-mono (top, left, dAu–C = 2.041(7) Å) and 10-bis (bottom, left, dAu–C = 2.085(4) Å) and the corresponding NHC-complexes 11-mono (top, right, dAu–C = 2.074(15) Å) and 11-bis (bottom, right, dAu–C = 2.021(7) Å). Thermal ellipsoids at 50% probability. Reprinted with permission from ref (63). Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA.
It can be concluded from this subsection that dihalogen adducts bearing S,S-donors or adducts on the basis of thioamide in organic solvents can oxidatively dissolve metals such as noble metals, cadmium, and mercury. The structure and the chelating properties of the organic adducts play an important role in the oxidation reactions. The same adducts can form different crystal structures after oxidation reaction with different metals as shown in Figure 5. In addition, by slightly adjusting the structure of the organic adducts, their oxidizing capability could be changed. For example, [Me2Pipdt]I3 can oxidize platinum, whereas Me2dazdt·2I2 cannot. The dihalogen adducts also provide the possibility to form organo-gold compounds directly from metals as shown in Figure 7. Moreover, the organic lixiviants are sometimes more efficient than the aqueous lixiviant for oxidative dissolution of metals.55 Dihalogen adducts are claimed to be safe, cheap, easy to handle, and noncytotoxic. However, most of the organic compounds are destroyed in order to recover the metals after dissolution, so that these organic compounds cannot be reused. Therefore, economic analysis should be performed prior to large scale applications.
3. Halocarbons as Oxidizing Agent
Organic halides (halocarbons) can act as oxidizing agents in polar organic solvents and have been used for dissolution of metals with the aim of direct synthesis of metal complexes. Tezuka et al. studied the oxidative dissolution of copper by halocarbons in dimethyl sulfoxide (DSMO) with the formation of the complex CuCl2(DMSO).64
Both the halocarbon and the polar solvent played an important role in the dissolution process. The most studied halocarbon was carbon tetrachloride (CCl4), but other halocarbons could be used as oxidizing agents as well, such as diphenyldichloromethane, dichloroacetic acid, or trichloroacetic acid esters and bromoalkanes (e.g., carbon tetrabromide). DMSO could not be replaced by other polar organic solvents, such as methanol, tetrahydrofuran, benzene, chloroform, acetonitrile, or pyridine. The dissolution mechanism is described by eqs 7–9. First, copper is oxidized by CCl4-DMSO with the formation of CuCl2(DMSO)2 and dichlorocarbene (:CCl2). The dichlorocarbene instantaneously reacts with DMSO to produce dimethyl sulfide and phosgene. Phosgene directly reacts further with copper to form CuCl2(DMSO)2 and carbon monoxide.
| 7 |
| 8 |
| 9 |
Dimethylacetamide (DMAA) could replace DMSO for copper dissolution by CCl4 as oxidizing agent. The difference compared to the CCl4-DMSO system is that copper is oxidized to copper(I) by CCl4 in DMAA with the formation of C2Cl6. The formed copper(I) complexes are slowly oxidized by CCl4-DMAA or air to copper(II) complexes.65 Similar as in the CCl4-DMSO system, copper is dissolved in CCl4-DMAA via a radical mechanism, but the formed radical is CCl3 rather than :CCl2. The results showed that the oxidation of copper is a surface chemical reaction, with the interaction of the adsorbed organic compounds with the metal surface as the rate-limiting step.
A similar radical mechanism of oxidative dissolution of titanium was observed by Egorov et al. for the use of benzyl bromide as oxidizing agent in the presence of the polar solvent DMF.66 Analysis of the reaction products in the presence of radical scavengers dicyclohexyldeuterophosphine and in their absence suggested that the reaction occurs via the benzyl radical (·CH2–C6H5). Under oxygen-free conditions, the dissolution of titanium occurs by a one-electron transfer mechanism, with formation of 1,2-diphenylethane and titanium(IV) complexes. The complex [TiBr(DMF)2]Br2 is initially formed but slowly reacts further with benzyl bromide and DMF to yield the complex [TiBr(DMF)5]Br3. Note that titanium is not oxidized by benzyl bromide in DMF in the presence of oxygen, probably because of the formation of a passivating oxide film on the metal surface, which prevents contact between the metal surface and the oxidizing agent.
CCl4 in ammonia solutions can dissolve copper under very mild conditions with formation of [Cu(NH3)m]Cl2·nH2O.67 At the same time, CCl4 is decomposed to CH2Cl2. The reaction is believed to have the same radical mechanism as in DMSO, with the formation of intermediate :CCl2. Eight additional halogenated compounds were tested for copper dissolution in the presence of ammonia solution: CHCl3, CHBr2CHBr2, CH2Br2, CH3CCl3, CHCl2CHCl2, CH2ClCHCl2, CCl2CCl2, and CH2ClCH2Cl. More copper could be dissolved in ammonia solutions upon addition of the first four compounds and CCl4, whereas less was dissolved by adding the last four halogenated compounds. Among the nine halogenated compounds and NH3(aq), CCl4 is the best compound to solubilize copper. Although without CCl4, ammonium solution can dissolve copper as well, addition of CCl4 can speed up the reaction by a factor of 150. This system CCl4–NH3(aq) has different oxidizing power toward different metals. For example, zinc can be dissolved with an efficiency of 60% relative to copper. Less than 0.5% of silver and tin can be dissolved, but lead and iron do not react. This difference might be due to the different coordinating behaviors of metals with ammine ligands.
Halocarbons in organic solvents can even dissolve noble metals.68 The studied leaching systems contain halocarbon compounds used as oxidizing agents including CCl4, CBr4, CPh2Cl2, CPhCl3, and C(CN)2Cl2, and coordinating organic solvents including DMSO, DMF, and DMAA. The results for dissolution of various noble metals by different leaching systems are summarized in Table 3. Different combinations of oxidizing agents and solvents gave different behavior for metal dissolutions. It was proposed that the reaction proceeds through a carbene intermediate, similarly to the reaction between copper and CCl4–DMSO. Moreover, dissolution of the oxides, sulfides, tellurides, and selenides of noble metals (e.g., PdO, PtO2, PtS2, Ag2S, Ag2Se, and Ag2Te) was studied as well by this type of leaching system.68
Table 3. Dissolution of Noble Metals in Mixtures of Halocarbons with Organic Solvent68.
| Metal | Lixiviant | Temp (°C) | Reaction product |
|---|---|---|---|
| Ag | CCl4–DMSO | 80 | AgCl2– |
| CBr4–DMSO | 80 | AgBr2– | |
| CPh2Cl2–DMAA | 110 | AgCl2– | |
| Au | CBr4–DMSO | a | AuBr4– |
| Pd | CCl4–DMSO | a | PdCl2·2DMSO |
| CBr4–DMF | a | PdBr2·2DMF | |
| CPh2Cl2–DMAA | 110 | PdCl2·2DMAA | |
| CPh2Cl2–thiophene | a | PdCl2·2(C4H4S) | |
| Pt | CPh2Cl2–DMAA | 110 | PtCl2·2DMAA |
| C(CN)2Cl2–DMF | 100 | PtCl2·2DMF | |
| Rh | CPh2Cl2–DMAA | 110 | RhCl3·3DMAA |
| C(CN)2Cl2–DMF | 100 | RhCl3·3DMF | |
| Ru | No studied system could dissolve Ru | ||
Temperature was not reported.
An aqueous mixture of the halocarbon N-bromosuccinimide (NBS) and the coordinating solvent pyridine (Py) could dissolve gold.1 The leaching yield of gold with NBS/Py was higher than that with the classic cyanide method, with thiourea, and with solutions of KI/I2. NBS/Py also had good leaching selectivity for gold over other metals coexisting in the gold ore such as Fe, Al, Mg, Cu, and Zn (Figure 8). The oxidation by NBS involved mainly the release of a molecular bromine intermediate that oxidizes gold to form the anion complex [AuBr4]−, which subsequently reacted with a pyridine molecule to form PyAuBr3. The metal coordination with a pyridine-like unit decreased the redox potential for oxidation of metallic gold by NBS. Due to the exothermic reaction, the dissolution of gold proceeds better at lower temperatures, as indicated by the fact that the leaching yields of gold at 70 and 50 °C were much lower than the yield at 25 °C.
Figure 8.
Gold leaching from ores by N-bromosuccinimide/pyridine (NBS/Py). (a) Scanning electron microscopy (SEM) image of the gold ore; (b) corresponding metal contents in the raw gold ore; (c) effect of pH on the leaching yield of gold by different methods; (d) effect of pH on the leaching yields for the collective metals contained in the gold ores. The NBS and pyridine concentrations were 10 and 100 mm, respectively. Reprinted with permission from ref (1). Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA.
Stabilized bromine compounds such as 1-chloro-3-bromo-5,5-dimethylhydrantoin (GEOBROM 3114) and 1,3-dibromo-5,5-dimethylhydantoin (GEOBROM 5500) (Scheme 9), which have lower vapor pressures than bromine, can dissolve gold as well.69 During the leaching of gold in GEOBROM 5500 media, gold is oxidized anodically to the auric state, while GEOBROM 5500 is cathodically reduced to the very effective oxidizing agent hypobromous acid. Subsequently, gold goes into solution as the tetrabromoaurate(III) complex, [AuBr4]−, which has a very high formation constant (1032) in aqueous solutions. Different metals and minerals can either decrease or increase the dissolution rate of gold in bromine media by galvanic interactions.69 For example, copper, iron, and galena decrease the dissolution rate of gold, due to the galvanic interaction between the two substances. Sphalerite can enhance the dissolution rate of gold significantly. This can probably be attributed to the action of dissolved Zn(II) ions on the surface of gold and the formation of a film (probably a zinc complex) on a gold surface which can increase its conductivity.
Scheme 9. Chemical Structures of GEOBROM 3114 (left) and GEOBROM 5500 (right).
It is shown in this section that various halocarbons could be used as solvent in leaching systems for dissolution of metals. However, most halocarbons are toxic, especially CCl4. Due to the suspected carcinogenicity of CCl4, the use of this solvent has been banned in many countries. Therefore, alternative green leaching systems should be developed for future use.
4. Donor–Acceptor Electron-Transfer Systems
In a donor–acceptor electron-transfer system, neither the donor nor the acceptor alone directly reacts with metals, but their mixtures do, because new species capable of oxidizing metals are generated by the interaction between the donor and the acceptor. Examples are mixtures of DMSO and ammonium halides (NH4X), DMSO and HX (X = Cl, Br, and I), and DMSO and SO2 and a mixture of thionyl chloride (SOCl2) and pyridine (organic aqua regia).
4.1. DMSO with Halide Acids or Salts
The DMSO–halide acids (HX, X = Cl, Br, I) in both aqueous and organic systems have been studied for oxidative dissolution of metals such as copper, silver, gold, palladium, and platinum.70−73 The final products of the reaction between DMSO and HX are mainly dimethyl sulfide (Me2S), dihalogens, and water, together with some byproducts such as CO and CS2. The intermediate products Me2S(OH)X, Me2S·X2, and X2 are most likely responsible for the oxidation of metals. The sequence of reactions between DMSO and HBr is presented in Scheme 10:
Scheme 10. Sequence of Reactions between DMSO and HBr.
Various metal complexes can be synthesized by dissolution of the corresponding metals in DMSO–HX solutions. For example, after adding palladium to the mixture of DMSO and HBr, the solid product Me2SPdBr2 was isolated. The liquid phase is most likely a mixture of several complexes, depending on the ratio of the components. By dissolving silver in the system of DMSO–HBr, several silver complexes, e.g. AgBr, [Me3S]AgBr2, and [Me3S]Ag2Br3, were formed. Whereas in the system of DMSO–HX–ketone (X = Br, I; ketone = acetone, acetylacetone, or acetophenone), the complexes formed were [Me2S+CH2COR]AgX2– (R = Me, Ph; X = Br, I). The composition of the complexes depended on various factors, including the DMSO-to-HX molar ratio, the nature of HX, and the methods used to isolate the solid products from the solution. It is noteworthy that silver does not dissolve in the DMSO–HF system and that it dissolves only very slowly in the DMSO–HCl system. This is probably due to the stronger reactivity of the formed complex with bounded molecular fluorine or chlorine toward organic molecules than to metals. The reactions between DMSO and HCl are shown in Scheme 11.
Scheme 11. Reactions between DMSO and HCl.
The dissolution of copper was investigated in the system of DMSO–HX (X = Br, Cl). HX was dissolved in water or organic solvents: acetonitrile (MeCN) and nitrobenzene (PhNO2).73 The dissolution rates of copper metal in nonaqueous media are 1 order of magnitude higher than in aqueous media, following the sequence: H2O < MeCN < PhNO2. This difference in reactivity was attributed to a change in the adsorbability of the reagents on the metal surface. The low reactivity of the aqueous system is caused by the strong adsorption of water on the metal surface, which competes with the oxidizing species for contacting with the metal surface. A distinctive feature of metal dissolution in the DMSO-HBraq system is the presence of two or three maxima in the plot of the metal dissolution rate as a function of the molar fraction of HBr, indicating that several species that can oxidize metals are formed upon the interaction of DMSO and HBr. Besides bromine-containing compounds, oxygen-containing compounds (H2O2, HXO) are formed as well, due to the interaction between DMSO and HX in aqueous solutions.72 DMSO-HXaq dissolves both transition metals (Cu) and noble metals (Au, Ag, and Pt), whereas gold is insoluble in the nonaqueous system DMSO–HClsolv. Therefore, the latter system can be used for selective dissolution of copper in the presence of gold.
The alternative system DMSO–NH4Xaq can dissolve copper as well.74 The mechanism for the formation of intermediates active for metal dissolution shown in Scheme 12 is similar to that for the system DMSO–-HX.
Scheme 12. Reactions between DMSO and NH4X.
Moreover, the dissolution rate of copper in air is faster than that in argon, indicating that molecular oxygen probably can react with the intermediate species to form a new species that can oxidize the metal. In the DMSO–HX system, nonaqueous solutions are more reactive toward metals than aqueous solutions. However, copper dissolves faster in NH4Iaq-O2 containing no DMSO than in DMSO–NH4Xaq. This was explained by the formation of the stronger oxidizing agent IO3– in the former system.
4.2. DMSO with SO2
The mixed nonaqueous system DMSO–SO2 has shown to have good potential for dissolution of metals.75−82 The metals Mg, Sr, Ba, V, Mn, Fe, Co, Ni, Cu, Zn, Al, In, Pb, and Yb dissolve in DMSO–SO2 to form metal disulfates (Mx(S2O7)y·zMe2SO), and the metals Sr, Ba, and Pb react as well with formation of metal sulfates which form a passive layer on the metal surface. Chromium is completely inert in the mixed solvent. Other metals, Na, Be, Ca, Ce, Pr, Eu, Dy, Ga, TI, Sn, Sb, Bi, I, and Cd, also react, but the reaction products have not been characterized yet. These metals dissolve neither in DMSO nor in SO2 separately, but they do dissolve in the mixture of DMSO and SO2. Adducts with compositions 2SO2·DMSO, SO2·DMSO, and SO2·2DMSO have been identified by Raman spectroscopy and phase study (i.e., by measuring the eutectic point for mixtures of SO2 with DMSO to obtain the structure of adducts).81,83 These adducts are considered as active intermediates for metal oxidation. Dimethyl sulfide is one of the reaction products, and the existence of the radical ion [SO2]•– and an ion pair containing a metal ion and [SO2]•– have been demonstrated.79 The adduct 2SO2·DMSO provides a starting point for the formation of a radical ion containing the S–O–S group, [DMSO·OS·O·SO2]•–. By stepwise reaction with DMSO, the disulfate anions are formed via a series of successive radical anions.
Besides DMSO, other solvents containing SO2 have been tested for dissolving metals such as Li, Na, Mg, Ca, Fe, and Zn. The solvents DMAA, DMF, formamide, ethanol, water, trimethyl phosphate, and tris(dimethylamino)phosphine oxide react with metals, whereas the solvents tetrahydrothiophen-1,1-dioxide, nitrobenzene, nitromethane, ethyl acetate, acetonitrile, diethyl ether, 1,4-dioxane, tetrahydrofuran, pyridine, and acetone do not. The solvent must have a high dielectric constant (ε > 20), as well as a high Gutmann donor number (DN > 20) for spontaneous dissolution of metals to occur. A solvent with a high dielectric constant is required for the formation of the sulfoxylate radical ion. A solvent with a high donor number is required for the solvation of metal ions with inhibition of recombination of metal ion and [SO2]•– to form metal and SO2. The reaction product after metal dissolution is a metal dithionite rather than a sulfate or disulfate.81 The dithionite anion is formed by direct dimerization of the radical ion [SO2]•–.
4.3. Organic Aqua Regia
“Organic aqua regia” was first reported by Lin and co-workers in 2010, and it can dissolve gold and palladium with a high dissolution rate at room temperature.84 It is a mixture of SOCl2 and a polar organic solvent such as pyridine (Py), DMF, or imidazole. It was named as “organic aqua regia”, because of its similarity to aqua regia: (1) it can dissolve noble metals; (2) noble metals cannot dissolve in either of the components, and only their mixtures are strongly oxidizing. The mixture SOCl2–Py can dissolve gold at a rate of 0.3 mol m–2 h–1 at room temperature, which is faster than the dissolution of gold in conventional cyanide leaching agents (<0.004 mol m–2 h–1) and iodide solutions (<0.16 mol m–2 h–1). The enhanced reactivity of organic aqua regia is ascribed to the formation of the adduct SOCl2·Py, resulting from the charge-transfer interaction between SOCl2 and Py. The sulfur atom in SOCl2 acts as an electron acceptor, and the nitrogen atom in pyridine acts as an electron donor. Gold dissolves in the solution in the form of [AuCl4]− rather than AuCl3 or its complex with pyridine.
Organic aqua regia can be formed not only with pyridine but also with other polar organic solvents.85 The following solvents or organic compounds have been found to be able to effectively dissolve gold in the presence of SOCl2: pyrrole, pyrrolidine, pyrrolidone, isoxazole, isothiazole, pyrazole, imidazole, thiazole, oxazole, pyrazolone, bipyrazole, pyridine, pyridazine, pyrimidine, pyrazine, triazine, indole, quinoline, purine, pteridine, phthalocyanine, N,N’-dicyclohexylcarbodiimide, DMF, N,N’-dimethylbenzylamine, dodecyltrimethylammonium bromide, trip-tolyl-phosphine, etc. However, effective dissolution of gold in a mixture of SOCl2 with any of the following chemicals has not been observed: maleimide, azobis(isobutyronitrile), aniline, polyaniline, phenanthroline, methylbenzyl cyanide, 2-acetyl-1-methylpyrrole, and benzyltriethylammonium tetrafluoroborate.
A qualitative study has shown that copper, iron, nickel, tin, indium, silver, and palladium dissolve in the SOCl2–Py mixture, whereas platinum, titanium, tungsten, tantalum, and chromium do not.85 The fact that some metals cannot be dissolved in SOCl2–Py is probably due to surface passivation.
By varying the composition of organic aqua regia, selective dissolution of noble metals can be achieved. For example, by replacing pyridine with DMF, the mixture SOCl2–DMF can dissolve gold but not palladium or platinum. A mixture of SOCl2–pyridine (3:1) can dissolve gold, silver, and palladium at room temperature, while platinum is completely inert, even at 70 °C (reflux) for 1 week. Therefore, selective dissolution of gold, palladium, and platinum on a silicon substrate could be achieved by first dissolving gold in SOCl2–DMF, followed by dissolution of palladium in the SOCl2–pyridine mixture (Figure 9). It is assumed that selective dissolution is caused by differences in interaction between SOCl2 and the organic solvent.
Figure 9.

Illustration of selective dissolution of gold, palladium, and platinum in organic aqua regia. A silicon substrate was metallized with a Pd/Au/Pt layer (250 nm thick each by electron-beam evaporation or direct current sputtering, with chromium used as the adhesion layer). The top row of images shows the photographs of the Pd/Au/Pt metallization layer on a silicon substrate during the process of selective dissolution. Reprinted with permission from ref (84). Copyright 2010 WILEY-VCH Verlag GmbH & Co. KGaA.
Besides the application in the recovery of noble metals, organic aqua regia can be applied in the microelectronic industry, for instance for “vapor” etching of gold metallization on a circuit board by vaporized organic aqua regia.84 Etching of copper from the fabrication of printed circuit boards has been studied by using the SOCl2–CH3CN solutions as etchant at room temperature. The etching rate was found to be much faster than any currently used etchant for copper.85 Subsequently, this solvent was successfully applied for the separation of platinum from a mixture of gold and platinum nanoparticles and from a Au/Pt core–shell nanoparticle catalyst, with the purity of the recovered platinum above 95%.86 This organic aqua regia was also applied in organic synthesis, for activation of gold/activated-carbon catalysts for hydrochlorination of acetylene.87 Activation of gold/activated-carbon catalyst in organic aqua regia improves the activity and stability of the catalyst compared to the activation procedure in conventional aqua regia.
Organic aqua regia can oxidize noble metals under mild conditions, but the employed reagents are hazardous, which makes this reagent less attractive from an environmental point of view, and this leads to a further search for safer alternatives for aqua regia.
5. Ionic Liquids and Deep Eutectic Solvents
5.1. Ionic Liquids
Ionic liquids (ILs) are solvents that are composed entirely of ions, and some of them can be considered as environmentally friendly solvents. They have been applied as alternatives for molecular organic solvents in various fields, due to their unique properties such as a negligible vapor pressure, a wide liquid range, high thermal and chemical stability, a wide electrochemical window, and poor flammability.88−93 The properties of ILs can be tuned by changing the structure of organic cations and inorganic or organic anions. For example, ILs with oxidizing power can be synthesized by adding molecular halogens to halide ILs so that tri- or polyhalide or interhalide ILs can be formed, such as [Cl3]−, [Br3]−, [I3]−, [ClBr2]−, [BrCl2]−, and [BrI2]−.94−98 The polyhalide ILs are most often used as reagents for halogenation of organic compounds, such as aromatics, alkenes, and alkynes,99−101 but they can be used for oxidative dissolution of metals or alloys as well.15,94,95,102,103 These polyhalide ILs can be used in pure form or as mixtures with halide ILs for dissolution of metals.
5.1.1. ILs as Oxidizing Agents
Trihalide ILs can be prepared by simply mixing equal molar amounts of halogens and halide ionic liquids. They can safely store halogens and in the meantime have oxidizing properties. Various metals can be dissolved in trihalide ionic liquids under mild conditions. The mixtures of trihalide and halide ILs work as well. In this case, halide ILs act as solvent and trihalide ILs as oxidizing agent. A series of trichloride ILs that are liquid form at room temperature was synthesized by Li et al.94 The cationic cores used were phosphonium, ammonium, pyridinium, pyrrolidinium, and imidazolium (Scheme 13), and the full names and abbreviations of the cations are shown in Table 4. It was confirmed by NMR spectroscopy that all the studied cations are stable in the presence of chlorine, except imidazolium cations. The trichloride ILs have a lower viscosity and melting point than the corresponding chloride ILs, due to the more extended charge delocalization in the trichloride anions compared to the chloride anion, and thus weaker electrostatic interactions between cations and anions. A total of 12 metals and two metal alloys have been tested for dissolution in the representative trichloride IL [P444,14][Cl3]: Fe, Cu, In, Zn, Ga, Sb, Au, Pt, Ge, Ta, Sm, Dy, GaAs, and InAs.94 All metals and alloys, except Sm (5 mm pieces), Dy (5 mm pieces), Ta (powder), and Pt (wires) could be dissolved in this trichloride IL at room temperature. Thus, trichloride ILs can selectively dissolve some metals and, for example, allow the separation of the highly valuable gold and platinum. The fact that samarium metal did not dissolve in the trichloride ILs was unexpected, because rare-earth metals are in general very reactive. In follow-up experiments using freshly milled samarium powder instead of samarium metal pieces, different results were obtained: samarium powder could indeed be oxidatively dissolved in trichloride ILs.15 This result showed that samarium metal pieces are difficult to dissolve in trichloride ILs because of the presence of a protective oxide layer on the samarium metal surface which prevents contact between the oxidizing trichloride anion and the metal.
Scheme 13. Structure of Cations of Trichloride ILs.
Reprinted from ref (94). Copyright 2018 Royal Society of Chemistry under [CC BY-NC 3.0] [https://creativecommons.org/licenses/by-nc/3.0/].
Table 4. Full Names and Abbreviations of the Cations of Trichloride ILs.
| Name of IL cation | Abbreviation |
|---|---|
| Trihexyl(tetradecyl)phosphonium | P666,14 |
| Tetrabutylphosphonium | P4444 |
| Tributyl(tetradecyl)phosphonium | P444,14 |
| 1-Butylpyridinium | BPy |
| Methyltrioctylammonium | N1888 |
| 1-Butyl-1-methylpyrrolidinium | BMPyrr |
| 1-Butyl-2-methylpyridinium | 2-MBPy |
| 1-Butyl-1-methylpiperidinium | BMPip |
| 1-Butyl-4-methylpyridinium | 4-MBPy |
| 1-Hexylpyridinium | HPy |
| 1-Butyl-3-methylimidazolium | Bmim |
| 1-Decyl-3-methylimidazolium | Dmim |
Metal recovery from spent samarium–cobalt (Sm–Co) magnets using a trichloride ionic liquid was studied by the same group.11 The results showed that Sm–Co magnets, containing Fe, Co, Cu, and Sm, can be quantitatively dissolved in trichloride ILs. Two processes are involved in oxidative dissolution: oxidation of the metals by trichloride ions and dissolution of the oxidation products in the ionic liquid. The oxidation process converts the metals to the corresponding metal chlorides, and simultaneously each trichloride anion is reduced to three chloride ions. The generated metal chlorides are subsequently dissolved in the IL by complex formation. Thus, the dissolution mechanism of Fe, Co, Cu, and Sm in the trichloride IL [P666,14][Cl3] in the presence of coordinating chloride ions can be expressed by the following equations:
| 10 |
| 11 |
| 12 |
| 13 |
More trihalide or tri-interhalide ionic liquids with a tributyldecylphosphonium cation ([P44410]) and various anions have been prepared, including trichloride ([Cl3]), bromidedichloride ([BrCl2]), chloridedibromide ([ClBr2]), tribromide ([Br3]), iodidedibromide ([IBr2]), bromidediiodide ([BrI2]), and triiodide ([I3]).95 One representative IL, [P44410][Br3], was selected for oxidative dissolution tests for metals. Several base metals and metalloids (iron, zinc, copper, cobalt, bismuth, indium, tin, gallium, antimony, and germanium) can be oxidatively dissolved in [P44410][Br3], as well as the noble metals palladium and gold. However, platinum and rhodium could not be dissolved, similarly to what was observed for the trichloride IL [P666,14][Cl3].94 Copper as a representative metal could be efficiently dissolved in all the synthesized trihalide ILs, suggesting that all these the trihalide ILs can oxidize metals. The same tribromide IL [P44410][Br3] could also efficiently leach gallium, indium, and arsenic from semiconductors (GaIn, GaAs) and light-emitting diodes that contained GaAs.103
May and co-workers studied the reaction of the interhalide IL 1-hexyl-3-methylimidazolium iodide dibromide ([Hmim][Br2I]) with copper, silver, and gold, investigated by using X-ray photoelectron spectroscopy, mass loss measurements, and gas-phase mass spectrometry.104 All the studied metals can be oxidized by the interhalide IL to form molecular iodine and metal ions in the +1 oxidation state, in the form of [Br–M–Br]− (M = Cu, Ag, and Au), which is different from dissolution of metals in trichloride ILs, where the metals are oxidized to their highest oxidation state.94 The reaction can be described as eq 14:
| 14 |
The dissolution behavior of gold in 1-hexyl-3-methylimidazolium dibromoiodide [Hmim][Br2I] and 1-butyl-2,3-dimethylimidazolium dibromoiodide [C4C1C1Im][Br2I] that was methylated at the 2-position of the imidazolium ring was compared, and this study rules out the formation of imidazole-carbene as a cause of the oxidation reaction. Moreover, the oxidation reaction is not sensitive to either water or air. The dissolution efficiency of gold in aqueous solutions of Na[Br2I] is at least ten times less than that in [Hmim][Br2I], indicating that the organic cation [Hmim]+ plays an important role in the dissolution of the formed metal complex.
Recently, ILs with a poly pseudohalide anion such as [Br(BrCN)]− and [Br(BrCN)3]− were synthesized and successfully used for dissolution of gold.105 The vapor pressures of the poly pseudohalogen salts bis(triphenylphosphoranylidene)iminium bromide [PNP]Br are reduced by at least a factor of 10 to 0.1 mbar (1 equiv BrCN) and 15.5 mbar (5 equiv BrCN) at ambient conditions, compared to the vapor pressure of toxic BrCN (160 mbar at 25 °C). Room temperature ILs can be obtained when more than 3 equiv of BrCN is added to bromide salts. Due to the significantly lower vapor pressures, the poly pseudohalide ILs can easily be handled and applied, for example, in metal recycling. A room temperature IL was formed by adding 4 equiv of BrCN to the IL butyldiethylmethylammonium bromide. This IL can dissolve gold with the formation of gold(I), as well as predominantly gold(III) cyanido and gold(III) bromidocyanido species.
Due to the involvement of halogens in ILs, the polyhalide ILs have oxidizing power and hence can be used as both oxidizing agent and solvent. However, ILs without oxidizing power can be applied as solvents for metals dissolutions together with some oxidizing agent, e.g. trihalide ILs in halide ILs as solvents.
5.1.2. ILs as Solvents
Whitehead and co-workers reported a series of studies where ILs were used as solvents for recovery of gold and silver from ores.106−108 Recovery efficiencies of metals were studied by varying solvent ILs and oxidizing and complexing agents. The IL [Bmim][HSO4] was extensively studied for metal extraction due to its acidity. The system of [Bmim][HSO4]/thiourea/Fe2(SO4)3 could effectively leach gold and silver with high selectivity over the base metals Cu, Zn, Pb, and Fe in a gold-bearing ore.106 Here the IL acts as solvent and Fe2(SO4)3 as oxidizing agent. Thiourea was selected as complexing agent because of its high selectivity for gold and silver. This IL system and the aqueous system of H2SO4/thiourea/Fe2(SO4)3 have similar capability for leaching of gold. However, this IL system has higher leaching efficiency of silver than the aqueous system, because in the aqueous system, insoluble silver species precipitate and form a passivation layer on the silver metal surface. An additional benefit was that the IL could be recycled. HSO4-based ILs with different 1-alkyl-3-methyl-imidazolium cations (alkyl = n-butyl, n-pentyl, n-hexyl, n-heptyl, and n-octyl) could also be used as solvents, but as the alkyl chain length was increased, the efficiency of gold recovery dropped, probably due to the higher viscosity of the ILs.108 Bmim-based ILs with varying anions (N(CN)2–, CH3SO3–, HSO4–, BF4–, and Cl–) were used as solvent for recovery of gold and silver, but among all five ILs investigated, only [Bmim][HSO4] showed high extraction efficiency and selectivity (Figure 10). The selectivity differences between different anions result from their differences in coordination ability with metal ions.
Figure 10.

Extraction of gold and silver (top) and base metals (bottom) from a complex sulfidic gold-bearing ore using thiourea leaching with ILs with the [Bmim]+ cation and different anions (50 °C, 48 h leaching). Reprinted with permission from ref (108). Copyright 2007 Elsevier B.V.
Apart from Fe2(SO4)3, also peroxomonosulfate (HSO5–) was tested as oxidizing agent for leaching of gold, silver, and base metals from ores in the ILs [Bmim][HSO4] and [Bmim]Cl, with various complexing agents (thiourea, NaCl, NaBr, and NaI).107 All the components in the lixiviant play important roles, because changing either the solvent, the oxidizing agent, or the complexing agent changes the leaching efficiency, sometimes even dramatically. The best combination for leaching gold and silver is the system [Bmim]Cl/NaI/HSO5–, which can quantitatively leach silver and 85% of gold, with only limited codissolution of base metals. This selectivity was attributed to formation of the stable gold iodo complex and the soluble silver iodo complex in the ionic liquid environment.
By studying the electro-oxidation of iodide on a gold electrode in the room temperature ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, Bentley et al. observed that gold could be oxidized in the presence of iodide.109 Coulometric/electrogravimetric analysis suggested that the oxidation state of the soluble gold species is +1 and that diiodoaurate, [AuI2]−, is the most likely intermediate compound. A proportionally smaller amount of triiodide intermediate was also detected by means of UV–vis absorption spectroscopy. On this experimental evidence, it was proposed that iodide oxidation of gold occurs via two parallel reaction pathways: predominantly via a diiodoaurate intermediate (eqs 15 and 16) and to a lesser extent via a triiodide intermediate (eqs 17 and 18).
| 15 |
| 16 |
| 17 |
| 18 |
ILs were used as electrolytes in the electrochemical process for oxidative dissolution of platinum.110 Bmim-based ILs with various anions were tested for anodic dissolution of platinum, namely, Cl–, Br–, I–, [SCN]−, and [N(CN)2]−. Only IL with chloride anions could dissolve platinum. Platinum dissolution was observed in [Mim]Cl and [Bmim]Cl, but not in [Hmim]Cl at 80 °C, but all these three ILs could dissolve platinum at 150 °C. The platinum dissolution rate in [Bmim]Cl at 80 °C is of the same order of magnitude as in aqua regia at 25 °C. A mixture of Bmim-based ILs with bis(trifluoromethanesulfonyl)imide ([NTf2]) and chloride anions could also dissolve platinum, but the dissolution rate and the amount of dissolved platinum were lower than those in pure [Bmim]Cl. This mixture could leach platinum immobilized on the carbon substrate of the electrodes from fuel-cell membrane electrode assemblies and simultaneously electrodeposit platinum on the counter electrode. The electrochemical dissolution of platinum was achieved in eutectic-based ILs, ZnCl2-1-ethyl-3-methylimidazolium chloride (molar ratio: 1:3) and ZnCl2-chloline chloride (molar ratio 1:1), with formation of [PtClx]y−.111
Although ILs are considered as environmentally benign reagents and although they can efficiently dissolve various metals under mild conditions, they are in general quite expensive, so that they cannot be used in an economically way to dissolve base metals. However, the high cost of ILs can be justified for the extraction or recovery of precious metals from mineral ores or secondary resources.
5.2. Deep-Eutectic Solvents
Deep-eutectic solvents (DESs) are considered as ionic-liquid-analogues in the literature, because DESs and ILs have quite similar properties. For example, they both have low vapor pressure, have a relatively wide liquid-range, and are often nonflammable. However, they are two different types of solvent. ILs are pure compounds that are composed of one type of cation and only one type of anion, whereas DESs which are formed from a eutectic mixture of Lewis or Brønsted acids and bases contain a variety of anions and cations. DESs can be formed by combination of an organic salt (e.g., ammonium halide and imidazolium halide) with a metal salt or hydrogen-bond donor. The majority of studies in the literature have focused on the system comprising choline chloride (hydroxyethyltrimethylammonium chloride), because choline chloride is a nontoxic and cheap compound. More detailed information on DESs and their applications can be found in a review paper in this journal.112
Generally, DESs have no oxidizing power themselves, so they are often used as solvents for metal dissolution either with the aid of an oxidizing agent or as an electrolyte for electrooxidation of metals (electrolytic dissolution). The behavior of metals (for example the redox potential of ion couples) in DESs and ILs is different from that in aqueous solutions. Iodine in DESs (e.g., in Ethaline which is a mixture of choline chloride and ethylene glycol in a 1:2 molar ratio) shows strong oxidizing power which can be used for leaching of gold from gold-bearing ores and from SEM samples where gold had been coated on the surface of the sample to minimize charging effects.17,113 In this case, iodine in DESs used for metal dissolution is similar to the halogens in organic solvents systems, since DESs and organic solvents are both used as solvent for halogen and the formed metal salts. The difference is that most DESs have components that can act as ligands, whereas most organic solvents are much less coordinating. For example, the chloride ions in Ethaline can complex with gold to form [AuCl4]−, and this complex can dissolve in DESs easily by interacting with the countercation choline.
DESs can be used as an electrolyte for dissolution of metals via an electrochemical process, such as anodic dissolution and electropolishing of metals.114,115 DESs as electrolytes have several advantages (e.g., nonacidic formulation, improved surface finish on cast pieces, and improved current efficiency) compared to conventional electrolytes such as concentrated phosphoric acid and sulfuric acid mixtures. Metal polishing is to dissolve a metal surface controllably to reduce the surface roughness. Electropolishing of stainless steel was realized in Ethaline.114 Differently from conventional electropolishing in aqueous solutions, the metal dissolved in DESs without prior passivation and no gas was observed at the anode surface. A pilot-scale unit for electropolishing of Series 300 stainless steel and other nickel-containing alloys was built and operated by the IONMET consortium.116
6. Other Oxidizing Agents
6.1. Oxygen Gas
Gold can be effectively oxidized by oxygen gas in the presence of cetyltrimethylammonium bromide to form [AuBr4]− complex, with the aid of ultrasound.117 The ligand is crucial as it assists the oxidation process and stabilizes the formed Au(III) cation. Sonication is a must for this process because it can continuously provide oxygen to the solution. In the absence of sonication, no observable reaction occurred, even after several days. Barnartt et al. investigated the reactivity of 23 metals with acetylacetone in the presence or absence of oxygen gas.118 Acetylacetone is the conjugated acid of the acetylacetonate anion, a bidentate ligand that forms chelate complexes with many metals. It was found that 10 metals have an appreciable reaction rate with acetylacetone in the presence of oxygen gas, namely Pb, Mn, Zn, Mg, Cd, Fe, Cu, Co, In, and Sn, whereas another 13 metals show no reactions (Ti, Zr, V, Nb, Ta, Cr, Mo, W, Ni Al, Si, Ge, and Sb). Besides the oxygen-absorption reaction, Mg, Mn, and Zn react with acetylacetone in the absence of oxygen gas, with evolution of hydrogen gas. The reactions of metals with acetylacetone in the presence or absence of oxygen gas are
| 19 |
| 20 |
where HA is acetylacetone and x and y are the valence of the metal.
It is evident that pure acetylacetone can react or etch metal surfaces. However, side reactions were observed in the presence of oxygen gas, with the evolution of CO2 and CO, probably due to the metal-catalyzed oxidation of acetylacetone by O2 gas.118
Oxygen gas can fully oxidize gold in the presence of 4-pyridinethiol (4-PS) in methanol or ethanol solutions.119 No dissolution of gold was observed in an argon atmosphere, indicating that oxygen is required as oxidizing agent in this oxidative dissolution process. The dissolution rate increases noticeably with increasing 4-PS concentration, whereas the oxygen pressure had only a marginal effect. Increased temperature (80 °C) only increased the dissolution rate at the very beginning of the reaction. Afterward, the gold concentration in the solution decreased rapidly and finally dropped to zero. This is due to the thermal instability of the soluble gold(I) complex which decomposes to 4,4′-dipyridyl sulfide, elemental sulfur, and metallic gold. Moreover, 4-PS decomposes at room temperature under the reaction conditions and this decomposition is enhanced at elevated temperatures, which can be a reason for the low gold concentration in the solution. Furthermore, Cu(II) ions accelerate the dissolution of gold as well.
Other organic compounds which have similar structure to 4-pyridinethiol cannot dissolve gold in ethanol solutions, for example, pyridine, thiophenol, and 4,4′-pyridyl disulfide (4,4′-PSSP) (Scheme 14).119 2-PS can dissolve gold, but with a significantly reduced rate. Therefore, the pyridinethiol structural unit is required for dissolution, and the relative positions of the N and S atoms on the heterocycle affect the efficiency of the dissolution process. The same amount of gold can be quantitatively dissolved by 4-PS within 2 days, while with 2-PS the dissolution takes several weeks to occur. In addition, silver and copper can be dissolved in 4-PS–ethanol solutions, whereas palladium, platinum, and titanium did not react. This selectivity might be related to an oxidation state +1 for group 11 elements.
Scheme 14. Structure of 4-Pyridinethiol (4-PS) That Was Effective for Dissolution of Gold and Other Organic Components with Similar Structures to 4-Pyridinethiol.
A dissolution mechanism was proposed by the authors (Figure 11). Dissolution of gold proceeds through several steps: (1) isomerization of 4-pyridinethiol to pyridine-4-thione, (2) coordination with gold(0) from the corners of gold thin films, and (3) 4-pyridinethiol-assisted oxidation of gold(0) by loss of a hydrogen atom from one of the coordinated thiols with oxygen gas as oxidizing agent, and finally, (4) alcohols, although weak Brønsted acids, provide a proton, whereas a negatively charged alkoxide moiety ensures a charge balance. Note that the thermally instable gold(I) complex decomposes and forms 4,4′-dipyridyl sulfide, elemental sulfur, and metallic gold when kept at 80 °C for several hours.
Figure 11.
Proposed reaction mechanism for the oxidation of gold with 4-pyrindinethiol (4-PS) in alcohol solutions in open air. Reproduced with permission from ref (119). Copyright 2007 American Chemical Society.
Similar work was conducted by the same research group with dissolution of gold based on the same ligand 4-PS, but with hydrogen peroxide as oxidizing agent dissolved in DMF.120 Due to the strong oxidizing capability of hydrogen peroxide, the dissolution rate in this new solvent system was 1600 times faster than in the previously reported 4-PS-EtOH-O2 system.119 Moreover, the latter system decomposes 4-pyridinethiol to elemental sulfur, whereas hydrogen peroxide can further oxidize elemental sulfur to the sulfate anion (SO42–), which can act as counterion of the gold(I) complex bearing two 4-PS ligands ([Au(4-PS)2]2[SO4]). The other ligands 4-PS, 2-PS, 4,4′-PSSP, and 2-mercaptobenzimidazole (2-MBIH) shown in Scheme 14 with hydrogen peroxide in DMF could dissolve gold and followed the order: 4-PS d(84%) > 2-PSH = 4,4′-PSSP (11%) > 2-MBIH (8%). The dissolution rate is related to the formation energy of the bis-ligand intermediate. The bis-ligand intermediates can lower the oxidation potential of gold significantly because of the delocalization of the 6s1 electron on the ligands, thus allowing a one-electron oxidation of the ligand instead of the metal. 4-PS with hydrogen peroxide could dissolve copper and silver with high efficiencies, but platinum and palladium did not react, as their dissolution would involve a two-electron oxidation reaction.
Oxidative dissolution of various metals (Cu, Sn, Bi, In, Fe, Sm, Mg, and Ca) was observed using oxygen gas as oxidizing agent in the presence of triflic acid (HOTf) in DMSO, with formation of metallic triflates.121 Similarly, several metals (Cu, Sn, Bi, and Mg) reacted with triflimidic acid (HNTf2) in O2/DMSO to form metallic triflimides. The metallic salts formed were solvated by DMSO. The reaction mechanism is shown in eq 21 using the oxidative dissolution of Bi in HOTf as an example.
| 21 |
Alternatively, the metal triflates and triflimides salts could be prepared under ultrasonic conditions in the presence of the corresponding protic acid in organic polar solvents.122
Organometallic compounds can be synthesized by direct oxidation of metals in organic solvents (= direct synthesis).123−125 Since direct synthesis is a vast research area, we give in this review only a few representative examples and the reader is referred to the reviews in refs (125−127) for more detailed information. Direct synthesis of nickel complexes was achieved by interaction of nickel metal with ammonium chloride or thiocyanate in the presence of triethylenediamine (Ten) in organic solvents such as DMF, DMSO, MeOH, and ACN. Examples of formed complexes were Ni(HTen)(Ten)Cl3, Ni(Ten)2(NH3)2(NCS)2·4DMSO, and Ni(HTen)(NCS)3·3DMF.124 The solvents DMF and DMSO were better than MeOH and ACN for dissolution of metals, because insoluble compounds were formed in MeOH and acetonitrile and prevented the completion of dissolution. By reaction of manganese metal and cadmium halide (chloride or iodide) with Schiff base ligand N,N’-ethylene-bis-salicylideneaminato (salen) in nonaqueous solutions of DMF or methanol, mixed-metal complexes were formed, namely [Mn(salen)(dmf)2]3[Mn(salen)(dmf)(H2O)][CdCl4]2·H2O and [Mn(salen)(CH3OH)]2[Mn(salen)(CH3OH)2]2[CdI4]2.128 Direct synthesis of polynuclear and mixed-valent metal complexes from elemental metals was reviewed in nonaqueous solutions of the proton-donating reagents ammonium or alkylammonium salts (NH4X).125 Oxygen was involved in the reaction as oxidizing agent. Various metal complexes were obtained through this synthetic method, such as the mixed-valent copper complex [Cu(II)(DMSO)4(H2O)2][Cu(I)4(SCN)2(CN)3]2 and the mixed-metal complex [CuPbBr2(Me2Ea)2DMSO]2 (Ea is 2-aminoethanol). Examples of the reaction mechanism are presented in eq 22–25), where M, NH4X, and Ea represent metal, ammonium halides, and 2-aminoethanol, respectively.
| 22 |
| 23 |
| 24 |
| 25 |
6.2. Nitrogen Dioxide
We mentioned in section 2.1 that uranium can be oxidized by chlorine in the presence of TBP in TCE, but it can also be oxidized by nitrogen dioxide (NO2) in TBP-TCE at moderate temperatures (30–70 °C).129 The reaction rate increases with an increase in TBP and NO2 concentrations. The reaction mechanism is proposed as shown in eq 26, with NO2 acting as oxidizing agent and TBP as donor solvent.
| 26 |
The dissolution rate of uranium increased in the presence of water and reached the maximum at a water content of 1.0–2.0 vol %, which is similar to the system of TBP–TCE–Cl2.30 The possible reason for this increase is the formation of HNO3 from the reaction between NO2 and water. The organic solvent mixture TBP–TCE (30:70 v/v) can be replaced by DMF, DMSO, acetonitrile, nitromethane, diethylformamide, or hexamethylphosphoramide (HMPA).129 In all systems, uranium metal did react with NO2 at an appreciable rate, but HMPA reacted vigorously with NO2, and its nitration rate is considerably higher than the uranium dissolution rate.
6.3. Copper(II) Bromide
Another oxidizing agent that can be used for metal dissolution is copper(II) bromide, CuBr2.130,131 Gold can be dissolved oxidatively in DMSO or propylene carbonate (PC) in the presence of CuBr2, where copper(II) acts as an oxidizing agent and bromide as complexing agent that can significantly lower the reduction potential of gold.130 Addition of KBr can enhance the total dissolution amount of gold which can be further improved with increasing concentration of CuBr2 and KBr. When only CuBr2 is used in DMSO, oxidation of gold(0) to gold(III) is the dominant process, whereas upon addition of KBr, oxidation of gold(0) to gold(I) takes place as well. The reaction mechanisms are shown in eqs 27 and 28:
| 27 |
| 28 |
The metals Pd, Cu, Sn, Co, Ni, and Zn can be dissolved at a relatively fast rate in CuBr2–PC, while Ag, Ta, Ti, and W cannot, probably due to the presence of a passivating oxide film on the metal surface of Ag, Ta, Ti, and W.
6.4. Organic Compounds
Supercritical carbon dioxide (scCO2) has been investigated as leaching agent for etching of metal layers such as copper and zinc on silicon substrates in the microelectronic industry, to avoid the technical and environmental drawbacks of aqueous-based processes and organic solvents-based processes.132−135 Copper was etched by a homogeneous solution in scCO2 that contained an oxidizing agent and a β-diketone chelating agent with formation of copper(II) complexes. The oxidizing agents were ethyl peroxydicarbonate (EPDC) and tert-butyl peracetate (t-BuPA). Various chelating agents were investigated, namely1,1,1-trifluoro-2,4-pentanedione (Htfac), hexafluoroacetylacetone (Hhfac), 2,2,6,6-tetramethyl-3,5-heptanedione (Htmhd), substituted bis(acetylacetonate)ethylenediimine (R4BAE, where R = CH3 or CF3), and lithium or sodium dialkyldithiocarbamate (M+(R2DTC–), where M+ = Li+ or Na+ and R = ethyl, n-propyl, n-butyl, or 1,1,1-trifluoroethyl) ligands. The structures of the oxidizing agents and the chelating agents are shown in Scheme 15.
Scheme 15. Chemical Structures of Oxidizing Agents and Chelating Agents for Dissolution of Copper in Supercritical CO2.
The efficiencies of copper removal by the mixtures of various oxidizing agents and chelating agents in scCO2 are listed in Table 5. It is noteworthy to mention that the solution of t-BuPA should be treated as potentially explosive and should be handled with care. Moreover, the leaching efficiency of different leaching solutions presented in different references cannot be compared, because the dimensions of the copper metal samples and the experimental conditions were different. The efficiency of copper removal using EPDC as oxidizing agent followed the order: Htmhd ≈ Htfac < Hhfac. This trend is in agreement with the solubility of the formed product in scCO2. By dissolution of metals, tert-butyl peracetate was decomposed to tert-butyl alcohol and acetic acid.133 The oxidative dissolution rate of copper was increased as the alkyl chain length in R2DTC– increased or as fluorine substitution was introduced for both the R4BAE and R2DTC– ligands. The Li(R2DTC) salts were more effective in copper removal than Na(R2DTC). The kinetics of oxidation and removal of copper were independent of the concentration of oxidizing agents but varied with the concentration of chelating agents.135
Table 5. Efficiency of copper Removal in scCO2 in the Presence of Oxidizing Agents and Chelating Agents.
| Oxidizing agent | Chelating agent | Cu removal (%) | Conditions | Reference |
|---|---|---|---|---|
| EPDC | Hhfac | 34 | 40 °C and 214 bar for 20 h | (132) |
| EPDC | Htfac | 12 | 40 °C and 214 bar for 20 h | (132) |
| EPDC | Htmhd | 11 | 40 °C and 214 bar for 20 h | (132) |
| t-BuPA | Hhfac | 14 | 40 °C and 214 bar for 20 h | (133) |
| t-BuPA | Htfac | 1 | 40 °C and 214 bar for 20 h | (133) |
| t-BuPA | Htmhd | 4 | 40 °C and 214 bar for 20 h | (133) |
| t-BuPA | Me4BAE | 28 | 40 °C and 207 bar for 18 h | (134) |
| t-BuPA | Me2(CF3)2BAE | 22 | 40 °C and 207 bar for 18 h | (134) |
| t-BuPA | (CF3)4BAE | 44 | 40 °C and 207 bar for 18 h | (134) |
| t-BuPA | Li(Et2DTC) | 24 | 40 °C and 172 bar for 4 h | (134) |
| t-BuPA | Li(Pr2DTC) | 27 | 40 °C and 172 bar for 4 h | (134) |
| t-BuPA | Li(Bu2DTC) | 28 | 40 °C and 172 bar for 4 h | (134) |
| t-BuPA | Li(CF3CH2)2DTC) | 36 | 40 °C and 172 bar for 4 h | (134) |
| t-BuPA | Na(Et2DTC) | 20 | 40 °C and 172 bar for 4 h | (134) |
| t-BuPA | Na(Pr2DTC) | 20 | 40 °C and 172 bar for 4 h | (134) |
| t-BuPA | Na(Bu2DTC) | 23 | 40 °C and 172 bar for 4 h | (134) |
It was found that Hhfac in scCO2 without added oxidizing agent can also etch metal films (e.g., Fe, Cu, Ni, and Co) at relatively high temperature (100–250 °C) at 100 bar.136 The reaction mechanism might consist of two steps (eqs 29 and 30), regardless of the stoichiometry:
| 29 |
| 30 |
Without any oxidizing agent, the etching could be initiated by the reaction between Hhfac and a native metal oxide layer on the metal surface, with formation of M(hfac)2 and water. Water then acted as an oxide source to react with metal to make the etching reaction proceed further.
Electrochemical synthesis of metallic bis(trifluoromethanesulfonyl)imide salts (NTf2–) was reported using a sacrificial metal anode and the acid bis(trifluoromethanesulfonyl)imide as electrolyte in a polar organic solvent such as DMF or nitromethane at room temperature.137 The metal was oxidized at the anode, and the acid HNTf2 was reduced at the cathode to form the NTf2– anion and hydrogen gas. Electrochemical dissolution of a sacrificial metal anode in nonaqueous solvents could also direct synthesis of the organometallic compound.127 Silver was electrochemically dissolved in benzene with crown-ether-complexed sodium tetraphenylborate as electrolyte. However, copper and zinc could not be oxidized under same conditions.138 Furthermore, ultrasonic treatment could increase the oxidative dissolution rate of copper in nonaqueous solutions, because the product layer was removed from the metal surface to enhance the further dissolution of metals.139
7. Conclusions and Outlook
Dissolution of metals in organic solvents has been studied for more than half a century. Various leaching systems containing different oxidizing agents have been developed for dissolution of metals and for application in various fields, such as extraction of metals from ores, chemical etching of semiconductors, separation of metals and metal oxides, and metal recovery from end-of-life products. Some leaching systems such as halogens in organic solvents were studied for dissolution of various metals including both base metals and noble metals. Other leaching systems were studied with a focus on certain types of metals. For example, the dihalogen or interhalogen adducts were used most often for dissolution of noble metals. Although these leaching systems can oxidize metals, the volatility of halogens and organic solvents makes processes involving these leaching systems less attractive. Moreover, some of these leaching systems are toxic, such as SO2 and SOCl2. Polyhalide ILs have attracted interest for dissolution of metals due to their unique properties. On one hand, they are strongly oxidizing agents, and on the other hand, they can safely store halogens, which make them easily to handle. Solvent systems containing ILs and DESs have several advantages compared to those that are based on molecular organic solvents: they have low volatility and a wide liquid range. They can be used as electrolytes in electrochemical processes for dissolution of metals such as electropolishing, because of their ionic characteristics. Moreover, the reduction potential of metals can be lowered in ILs and DESs, which makes the oxidation of these metals easier. However, the prices of DES and especially of ILs are usually much higher than those of conventional organic solvents. To design an economically feasible process, the dissolution efficiency needs to be improved and meanwhile the recycling of solvents is a must. This review gives an overview of the state of the art of technologies for dissolution of metals, and it is hoped that it might inspire other researcher to carry out further work in this field.
Acknowledgments
The research leading to these results received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for critical metals (SOLCRIMET). The research was also funded by a postdoctoral grant of the Research Foundation Flanders (FWO) to X. Li (12ZA520N).
Glossary
Abbreviations
- (PhHN)2DTM
N,N’-diphenyl dithiomalonamide
- (PhHN)2DTL
3,5-bis(phenylamino)-1,2-dithiolylium
- 2-MBIH
2-mercaptobenzimidazole
- 2-MBPy
1-butyl-2-methylpyridinium
- 4-MBPy
1-butyl-4-methylpyridinium
- 4-PS
4-pyridinethiol
- 4,4′-PSSP
4,4′-pyridyl disulfide
- ACN
acetonitrile
- Bmim
1-butyl-3-methylimidazolium
- BMPip
1-butyl-1-methylpiperidinium
- BMPyrr
1-butyl-1-methylpyrrolidinium
- BPy
1-butylpyridinium
- BuOAc
butyl acetate
- CAN
acetonitrile
- CPB
cetylpyridinium bromide
- CPI
cetylpyridinium iodide
- DESs
deep eutectic solvents
- DMAA
dimethylacetamide
- DMF
N,N-dimethylformamide
- Dmim
1-decyl-3-methylimidazolium
- DMSO
dimethyl sulfoxide
- Ea
2-aminoethanol
- EG
ethylene glycol
- EPDC
peroxydicarbonate
- Et4TDS
tetraethylthiuram disulfide
- EtOAc
ethyl acetate
- EtOH
ethanol
- GEOBROM 3114
1-chloro-3-bromo-5,5-dimethylhydrantoin
- GEOBROM 5500
1,3-dibromo-5,5-dimethylhydantoin
- Hhfac
hexafluoroacetylacetone
- HMPA
hexamethylphosphoramide
- HNTF2
triflimidic acid
- HOTf
triflic acid
- HPy
1-hexylpyridinium
- Htfac
1,1,1-trifluoro-2,4-pentanedione
- Htmhd
2,2,6,6-tetramethyl-3,5-heptanedione
- HX
halide acid
- ILs
ionic liquids
- mbit
1-methyl-1H-benzimidazole-2(3H)-thione
- mbtt
3-methyl-benzothiazole-2-thione
- Me2dazdt
N,N’-dimethylperhydrodiazepine-2,3-dithione
- Me2Pipdt
N,N’-dimethylpiperazinium-2,3-dithione
- MeOAc
methyl acetate
- MeOH
methanol
- Mo2DTL
3,5-bis(morpholino)-1,2-dithiolylium
- Mo2DTM
dimorpholyldithiomalonamide
- N1888
Methyltrioctylammonium
- N4444
tetrabutylammonium
- NBS
N-bromosuccinimide
- Nd–Fe–B
neodymium–iron–boron
- NH4X
ammonium halides
- NTf2–
bis(trifluoromethane)sulfonamide
- P44410
tributyldecylphosphonium
- P444,14
tributyl(tetradecyl)phosphonium
- P4444
tetrabutylphosphonium
- P666,14
trihexyl(tetradecyl)phosphonium
- PGMs
platinum-group metals
- Py
pyridine
- R2DTC
dialkyldithiocarbamate
- REEs
rare-earth elements
- salen
N,N’-ethylene-bis-salicylideneaminato
- scCO2
supercritical carbon dioxide
- SEM
scanning electron microscopy
- Sm–Co
samarioum-cobalt
- t-BuPA
tert-butyl peracetate
- TBP
tri-n-butylphosphate
- TCE
tetrachloroethylene
- Ten
triethylenediamine
Biographies
Xiaohua Li was born in Anyang, China. She obtained her master degree from Chinese Academy of Sciences, Institute of Process Engineering under the supervision of Prof. Suojiang Zhang and obtained her doctor degree under the supervision of Prof. Sascha R.A. Kersten and Prof. Boelo Schuur from University of Twente in The Netherlands in February 2017. From November 2016 to the present, she has worked as a postdoc researcher with Prof. Koen Binnemans at KU Leuven in Belgium. In October 2019, she received an individual postdoc fellowship from the Research Foundation Flanders (FWO) and continued to work in the same group. Her main research interests are ionic liquids, oxidative dissolution of metals in nonaqueous solutions, metal recovery, solvent extraction, separation technology, and aqueous biphasic systems.
Koen Binnemans was born in Geel, Belgium. He obtained his M.Sc. (1992) and Ph.D. (1996) degrees in Chemistry at the University of Leuven (KU Leuven). In the period 1999–2005, he was a postdoctoral fellow of the Research Foundation Flanders. From 2005 until 2010 he was a professor, and presently he is full professor of Chemistry at the University of Leuven. He has published over 500 papers in international scientific journals. His main research interests are metallurgical chemistry, solvometallurgy, solvent extraction, and ionic liquids. As an ERC Advanced Grant holder (SOLCRIMET), he is cofounder of the SOLVOMET Industrial Service Centre for Solvometallurgy. He is an elected member of the Royal Flemish Academy of Belgium for Science and the Arts (KVAB).
The authors declare no competing financial interest.
References
- Yue C.; Sun H.; Liu W. J.; Guan B.; Deng X.; Zhang X.; Yang P. Environmentally Benign, Rapid, and Selective Extraction of Gold from Ores and Waste Electronic Materials. Angew. Chem., Int. Ed. 2017, 56, 9331–9335. 10.1002/anie.201703412. [DOI] [PubMed] [Google Scholar]
- Brunzie G. F.; Johnson T. R.; Steunenberg R. K. Selective Dissolution of Uranium from Uranium— Uranium Oxide Mixtures by Bromine—Ethyl Acetate. Anal. Chem. 1961, 33, 1005–1006. 10.1021/ac60176a043. [DOI] [Google Scholar]
- Strubbe K.; Gomes W. P. Bromine-Methanol as an Etchant for Semiconductors: A Fundamental Study on GaP: II. Interaction Between Chemical and Anodic Etching of P-Type. J. Electrochem. Soc. 1993, 140, 3301–3305. 10.1149/1.2221027. [DOI] [Google Scholar]
- Nakao Y. One-Step Syntheses of Polyhalogenometal Complexes by Direct Dissolution of the Metals in Halogen-Halide-Acetonitrile Systems. Chem. Lett. 1999, 28, 433–434. 10.1246/cl.1999.433. [DOI] [Google Scholar]
- Palden T.; Onghena B.; Regadío M.; Binnemans K. Methanesulfonic Acid: A Sustainable Acidic Solvent for Recovering Metals from the Jarosite Residue of the Zinc Industry. Green Chem. 2019, 21, 5394–5404. 10.1039/C9GC02238D. [DOI] [Google Scholar]
- Rodriguez Rodriguez N.; Machiels L.; Onghena B.; Spooren J.; Binnemans K. Selective Recovery of Zinc from Goethite Residue in the Zinc Industry Using Deep-Eutectic Solvents. RSC Adv. 2020, 10, 7328–7335. 10.1039/D0RA00277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . Communication from the Commission to the European Parliament, the Council, the Eurpean Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU. 2017.
- Ellis L. US Department of the Interior - Office of the Secretary: Final List of Critical Minerals 2018. Fed. Regist. 2018, 83, 23295–23296. [Google Scholar]
- Sheng P. P.; Etsell T. H. Recovery of Gold from Computer Circuit Board Scrap Using Aqua Regia. Waste Manage. Res. 2007, 25, 380–383. 10.1177/0734242X07076946. [DOI] [PubMed] [Google Scholar]
- Gökelma M.; Birich A.; Stopic S.; Friedrich B. A Review on Alternative Gold Recovery Re-Agents to Cyanide. J. Mater. Sci. Chem. Eng. 2016, 04, 8–17. 10.4236/msce.2016.48002. [DOI] [Google Scholar]
- Binnemans K.; Jones P. T. Solvometallurgy: An Emerging Branch of Extractive Metallurgy. J. Sustain. Metall. 2017, 3, 570–600. 10.1007/s40831-017-0128-2. [DOI] [Google Scholar]
- Li X.; Monnens W.; Li Z.; Fransaer J.; Binnemans K. Solvometallurgical Process for Extraction of Copper from Chalcopyrite and Other Sulfidic Ore Minerals. Green Chem. 2020, 22, 417–426. 10.1039/C9GC02983D. [DOI] [Google Scholar]
- Li Z.; Zhang Z.; Smolders S.; Li X.; Raiguel S.; Nies E.; De Vos D. E.; Binnemans K. Enhancing Metal Separations by Liquid–Liquid Extraction Using Polar Solvents. Chem. - Eur. J. 2019, 25, 9197–9201. 10.1002/chem.201901800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Mercken J.; Li X.; Riaño S.; Binnemans K. Efficient and Sustainable Removal of Magnesium from Brines for Lithium/Magnesium Separation Using Binary Extractants. ACS Sustainable Chem. Eng. 2019, 7, 19225–19234. 10.1021/acssuschemeng.9b05436. [DOI] [Google Scholar]
- Li X.; Li Z.; Orefice M.; Binnemans K. Metal Recovery from Spent Samarium-Cobalt Magnets Using a Trichloride Ionic Liquid. ACS Sustainable Chem. Eng. 2019, 7, 2578–2584. 10.1021/acssuschemeng.8b05604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deferm C.; Malaquias J. C.; Onghena B.; Banerjee D.; Luyten J.; Oosterhof H.; Fransaer J.; Binnemans K. Electrodeposition of Indium from the Ionic Liquid Trihexyl(Tetradecyl)Phosphonium Chloride. Green Chem. 2019, 21, 1517–1530. 10.1039/C8GC03389G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott A. P.; Frisch G.; Gurman S. J.; Hillman A. R.; Hartley J.; Holyoak F.; Ryder K. S. Ionometallurgy: Designer Redox Properties for Metal Processing. Chem. Commun. 2011, 47, 10031–10033. 10.1039/c1cc13616j. [DOI] [PubMed] [Google Scholar]
- Larsen R. P. Dissolution of Uranium Metal and Its Alloys. Anal. Chem. 1959, 31, 545–549. 10.1021/ac50164a026. [DOI] [Google Scholar]
- Eberle A. R.; Lerner M. W. Determination of Boron in Beryllium, Zirconium, Thorium, and Uranium Dissolution in Bromine-Methanol. Anal. Chem. 1960, 32, 146–149. 10.1021/ac60158a001. [DOI] [Google Scholar]
- Beeghly H. F. Determination of Aluminum Nitride Nitrogen in Steel. Anal. Chem. 1949, 21, 1513–1519. 10.1021/ac60036a024. [DOI] [Google Scholar]
- Busheina I. S.; Headridge J. B. Studies in Chemical Phase Analysis Part I. Determination of the Solubilities of Elements in Certain Organic Solvent - Bromine Mixtures. Analyst 1980, 105, 600–604. 10.1039/an9800500600. [DOI] [Google Scholar]
- Abou Zeid G. T.; Headridge J. B. Studies in Chemical Phase Analysis Part III.*Determination of the Solubilities of Certain Elements and Compounds Pertinent to Steels in Organic Solvent - Halogen Mixtures with Particular Emphasis on Manganese Silicon Nitride. Analyst 1982, 107, 200–205. 10.1039/an9820700200. [DOI] [Google Scholar]
- Busheina I. S.; Headridge J. B. Studies in Chemical Phase Analysis Part II. Determination of the Solubilities of Carbides, Nitrides, Oxides and Sulphides in Certain Organic Solvent - Bromine Mixtures. Analyst 1981, 106, 221–226. 10.1039/an9810600221. [DOI] [Google Scholar]
- Strubbe K.; Gomes W. P. Bromine-Methanol as an Etchant for Semiconductors: A Fundamental Study on GaP: I. Etching Behavior of N- and P-Type. J. Electrochem. Soc. 1993, 140, 3294–3300. 10.1149/1.2221026. [DOI] [Google Scholar]
- Sullivan M. V.; Kolb G. A. The Chemical Polishing of Gallium Arsenide in Bromine-Methanol. J. Electrochem. Soc. 1963, 110, 585. 10.1149/1.2425820. [DOI] [Google Scholar]
- Drobot N. F.; Noskova O. A.; Ovchinnikova N. A.; Zvereva G. A.; Larin G. M.; Krenev V. A.; Trifonova E. N.; Drobot D. V. Complex Formation during Molybdenum Chlorination in DMF Medium. Russ. J. Coord. Chem. 2003, 29, 474–477. 10.1023/A:1024774829021. [DOI] [Google Scholar]
- Drobot N. F.; Trifonova E. N.; Krenev V. A.; Drobot D. V. Oxidative Dissolution of Refractory Metals by Chlorination in Aqueous Organic Media. Russ. J. Coord. Chem. 2005, 31, 243–246. 10.1007/s11173-005-0084-4. [DOI] [Google Scholar]
- Drobot N. F.; Kupriyanova T. A.; Krenev V. A.; Filippov M. N. Rhenium and Platinum Recovery from Platinum and Rhenium Catalysts Used. Theor. Found. Chem. Eng. 2009, 43, 539–543. 10.1134/S0040579509040319. [DOI] [Google Scholar]
- Chekmarev A. M.; Buchikhin E. P.; Sidorov D. S.; Koshcheev A. M. Zirconium Dissolution by Low-Temperature Chlorination in Dimethylformamide. Theor. Found. Chem. Eng. 2007, 41, 752–754. 10.1134/S0040579507050521. [DOI] [Google Scholar]
- Buchikhin E. P.; Kuznetsov A. Y.; Vidanov V. L.; Shatalov V. V.; Chekmarev A. M. Nonaqueous Chlorination of Uranium Metal in Tributyl Phosphate. Radiochemistry 2005, 47, 263–265. 10.1007/s11137-005-0084-8. [DOI] [Google Scholar]
- Park T. H.; Cho Y. H.; Kang B.; Kim J. G.; Suh K.; Kim J.; Bae S. E.; Kim J. Y.; Giglio J. J.; Jones M. M. Constituent Analysis of Metal and Metal Oxide in Reduced SIMFuel Using Bromine-Ethyl Acetate. J. Radioanal. Nucl. Chem. 2018, 316, 1253–1259. 10.1007/s10967-018-5841-1. [DOI] [Google Scholar]
- Cosstick R. J.; Nancarrow P. C. Estimation of Metallic Iron in Rusted Sponge-Iron: Dissolution of Iron Oxides by Bromine/Methanol. Talanta 1978, 25, 486–488. 10.1016/0039-9140(78)80030-7. [DOI] [PubMed] [Google Scholar]
- Violante E. J. Phase Separation and Analysis of Sintered Titanium Carbide-Nickel Cermets Using Alcoholic Bromine. Anal. Chem. 1961, 33, 1600–1602. 10.1021/ac60179a042. [DOI] [Google Scholar]
- Orefice M.; Eldosouky A.; Škulj I.; Binnemans K. Removal of Metallic Coatings from Rare-Earth Permanent Magnets by Solutions of Bromine in Organic Solvents. RSC Adv. 2019, 9, 14910–14915. 10.1039/C9RA01696A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon F.; Jerusalem I.. Process for Extracting Noble Metals. U.S. Patent 4,997,532, 1991.
- Bowman P. T.; Ko E. I.; Sides P. J. A Potential Hazard in Preparing Bromine-Methanol Solutions. J. Electrochem. Soc. 1990, 137, 1309–1311. 10.1149/1.2086655. [DOI] [Google Scholar]
- Nakao Y. Dissolution of Metals in Halogen-Cetylpyridinium Halide-Benzene Systems. J. Chem. Res., Synop. 1991, 228–229. [Google Scholar]
- Nakao Y. Dissolution of Noble Metals in Halogen-Halide-Polar Organic Solvent Systems. J. Chem. Soc., Chem. Commun. 1992, 426–427. 10.1039/C39920000426. [DOI] [Google Scholar]
- Nakao Y. Three Procedures of Reversible Dissolution/Deposition of Gold Using Halogen-Containing Organic Systems. Chem. Commun. 1997, 1765–1766. 10.1039/a704171c. [DOI] [Google Scholar]
- Nakao Y.; Sone K. Reversible Dissolution/Deposition of Gold in Iodine-Iodide-Acetonitrile Systems. Chem. Commun. 1996, 897–898. 10.1039/CC9960000897. [DOI] [Google Scholar]
- Nakao Y.; Kaeriyama K. 5139752 Method for Extraction of Gold and Silver from Ore with a Solution Containing a Halogen, Halogenated Salt and Organic Solvent. Miner. Eng. 1993, 6, 438. 10.1016/0892-6875(93)90031-H. [DOI] [Google Scholar]
- Nakao Y.; Kaeriyama K.. Quaternary Ammonium Trihalide and Method for Dissolution of Metal with Liquid Containing the Compound. U.S. Patent 5,264,191, 1993.
- Nakao Y.Method for the Recovery of Gold Value. U.S. Patent 5,389,124, 1995.
- Bricklebank N.; Godfrey S. M.; McAuliffe C. A.; Pritchard R. G. Facile One-Step Synthesis of the Cobalt(III) and Nickel(III) Tertiary Arsine Complexes [MI3(AsMe3)2] (M = Co or Ni) Directly from the Powdered Elemental Metals. J. Chem. Soc., Dalton Trans. 1996, 157–160. 10.1039/dt9960000157. [DOI] [Google Scholar]
- Godfrey S. M.; McAuliffe C. A.; Pritchard R. G. Inorganic Grignard Analogues. Reaction of Nickel Powder with Dihalogenotriorganophosphorus Compounds to Form Nickel-(II) and -(III) Phosphine Complexes; Isolation of Planar [Ni(PPh3)I3]− and the Crystal Structure of [Ni(PPhMe2)2Br2]. J. Chem. Soc., Dalton Trans. 1993, 2875–2881. 10.1039/dt9930002875. [DOI] [Google Scholar]
- Godfrey S. M.; McAuliffe C. A.; Pritchard R. G. The Reaction of Bromo- and Iodo-Phosphoranes with Unactivated Coarse Grain Manganese Metal Powder to Yield [MnI2(Phosphine)2] and [{MnX2(Phosphine)}n] (X = Br or I) by Insertion of Mn into the P-X Bond. The Crystal Structure of [MnI2(PPh3)2]. J. Chem. Soc., Dalton Trans. 1993, 371–375. 10.1039/DT9930000371. [DOI] [Google Scholar]
- Godfrey S. M.; McAuliffe C. A.; Pritchard R. G. Extreme Symbiosis: The Facile One-Step Synthesis of the Paramagnetic Cobalt(III) Complex of Triphenylantimony, Col3(SbPh3)2, from the Reaction of Triphenylantimonydiiodine with Unactivated Coarse Grain Cobalt Metal Powder. J. Chem. Soc., Chem. Commun. 1994, 45–46. 10.1039/c39940000045. [DOI] [Google Scholar]
- Bricklebank N.; Godfrey S. M.; McAuliffe C. A.; MacKie A. G.; Pritchard R. G. The X-Ray Crystal Structure of [Zn(PEt3)I2]2, the First 1:1 Zinc(II) Complex of a Tertiary Phosphine of Low Steric Requirements, Prepared by the Reaction of Unactivated Zinc Metal with Diiodotriethylphosphorane. J. Chem. Soc., Chem. Commun. 1992, 944–945. 10.1039/c39920000944. [DOI] [Google Scholar]
- Godfrey S. M.; Ho N.; McAuliffe C. A.; Pritchard R. G. The Oxidation of Gold Powder by Me3EI2 (E = P, As) under Ambient Conditions; Structures of [AuI3(PMe3)2], [AuI3(AsMe3)I, and I(Me3PO)2H][AuI2]. Angew. Chem., Int. Ed. Engl. 1996, 35, 2344–2346. 10.1002/anie.199623441. [DOI] [Google Scholar]
- Bigoli F.; Deplano P.; Mercuri M. L.; Pellinghelli M. A.; Pintus G.; Serpe A.; Trogu E. F. A Powerful New Oxidation Agent towards Metallic Gold Powder: N,N′-Dimethylperhydrodiàzepine-2,3-Dithione (D) Bis(Diiodine). Synthesis and X-Ray Structure of [AuDI2]I3. Chem. Commun. 1998, 2351–2352. 10.1039/a806158k. [DOI] [Google Scholar]
- Bigoli F.; Deplano P.; Mercuri M. L.; Pellinghelli M. A.; Pintus G.; Serpe A.; Trogu E. F. N,N′-Dimethylpiperazinium-2,3-Dithione Triiodide, [Me2pipdt]I3, as a Powerful New Oxidation Agent toward Metallic Platinum. Synthesis and x-Ray Structures of the Reagent and the Product [Pt(Me2pipdt)2](I3)2. J. Am. Chem. Soc. 2001, 123, 1788–1789. 10.1021/ja0056015. [DOI] [PubMed] [Google Scholar]
- Vanzi M.; Bonfiglio A.; Salaris P.; Deplano P.; Trogu E. F.; Serpe A.; Salmini G.; De Palo R. Gold Removal in Failure Analysis of GaAs-Based Laser Diodes. Microelectron. Reliab. 1999, 39, 1043–1047. 10.1016/S0026-2714(99)00144-4. [DOI] [Google Scholar]
- Mercuri M. L.; Serpe A.; Marchiò L.; Artizzu F.; Espa D.; Deplano P. Effective One-Step Removal-Inertization of Hazardous Metals (Cd and Hg) by Environmental Friendly Reagents. Inorg. Chem. Commun. 2014, 39, 47–50. 10.1016/j.inoche.2013.10.045. [DOI] [Google Scholar]
- Cau L.; Deplano P.; Marchiò L.; Mercuri M. L.; Pilia L.; Serpe A.; Trogu E. F. New Powerful Reagents Based on Dihalogen/N,N′- Dimethylperhydrodiazepine-2,3-Dithione Adducts for Gold Dissolution: The IBr Case. Dalt. Trans. 2003, 1969–1974. 10.1039/B210281A. [DOI] [Google Scholar]
- Cuscusa M.; Rigoldi A.; Artizzu F.; Cammi R.; Fornasiero P.; Deplano P.; Marchiò L.; Serpe A. Ionic Couple-Driven Palladium Leaching by Organic Triiodide Solutions. ACS Sustainable Chem. Eng. 2017, 5, 4359–4370. 10.1021/acssuschemeng.7b00410. [DOI] [Google Scholar]
- Isaia F.; Aragoni M. C.; Arca M.; Caltagirone C.; Demartin F.; Garau A.; Lippolis V. Gold Oxidative Dissolution by (Thioamide)-I2 Adducts. Dalt. Trans. 2013, 42, 492–498. 10.1039/C2DT31855E. [DOI] [PubMed] [Google Scholar]
- Serpe A.; Artizzu F.; Mercuri M. L.; Pilia L.; Deplano P. Charge Transfer Complexes of Dithioxamides with Dihalogens as Powerful Reagents in the Dissolution of Noble Metals. Coord. Chem. Rev. 2008, 252, 1200–1212. 10.1016/j.ccr.2008.01.024. [DOI] [Google Scholar]
- Serpe A.; Marchiò L.; Artizzu F.; Mercuri M. L.; Deplano P. Effective One-Step Gold Dissolution Using Environmentally Friendly Low-Cost Reagents. Chem. - Eur. J. 2013, 19, 10111–10114. 10.1002/chem.201300940. [DOI] [PubMed] [Google Scholar]
- Jantan K. A.; Kwok C. Y.; Chan K. W.; Marchiò L.; White A. J. P.; Deplano P.; Serpe A.; Wilton-Ely J. D. E. T. From Recovered Metal Waste to High-Performance Palladium Catalysts. Green Chem. 2017, 19, 5846–5853. 10.1039/C7GC02678A. [DOI] [Google Scholar]
- Serpe A.; Artizzu F.; Espa D.; Rigoldi A.; Mercuri M. L.; Deplano P. From Trash to Resource: A Green Approach to Noble-Metals Dissolution and Recovery. Green Process. Synth. 2014, 3, 141–146. 10.1515/gps-2014-0004. [DOI] [Google Scholar]
- Serpe A.; Bigoli F.; Cabras M. C.; Fornasiero P.; Graziani M.; Mercuri M. L.; Montini T.; Pilia L.; Trogu E. F.; Deplano P. Pd-Dissolution through a Mild and Effective One-Step Reaction and Its Application for Pd-Recovery from Spent Catalytic Converters. Chem. Commun. 2005, 1040–1042. 10.1039/b415799k. [DOI] [PubMed] [Google Scholar]
- Cechin C. N.; Razera G. F.; Tirloni B.; Piquini P. C.; de Carvalho L. M.; Abram U.; Lang E. S. Oxidation of Crude Palladium Powder by a Diiodine Adduct of (2-PyTe)2 to Obtain the Novel PdII Complex [PdI(TePy-2)(I2TePy-2)2]. Inorg. Chem. Commun. 2020, 118, 107966. 10.1016/j.inoche.2020.107966. [DOI] [Google Scholar]
- Holthoff J. M.; Engelage E.; Kowsari A. B.; Huber S. M.; Weiss R. Noble Metal Corrosion: Halogen Bonded Iodocarbenium Iodides Dissolve Elemental Gold—Direct Access to Gold–Carbene Complexes. Chem. - Eur. J. 2019, 25, 7480–7484. 10.1002/chem.201901583. [DOI] [PubMed] [Google Scholar]
- Tezuka Y.; Miya M.; Hashimoto A.; Imai K. Dissolution of Copper Metal in a Dimethyl Sulfoxide-Carbon Tetrachloride Mixture. J. Chem. Soc., Chem. Commun. 1987, 1642–1643. 10.1039/C39870001642. [DOI] [Google Scholar]
- Egorov A. M.; Matyukhova S. A.; Anisimov A. V. Kinetics and Mechanism of the Reaction of Carbon Tetrachloride with Copper in Dimethylacetamide. Kinet. Catal. 2003, 44, 471–475. 10.1023/A:1025129731071. [DOI] [Google Scholar]
- Egorov A. M.; Matyukhova S. A.; Uvarova N. V.; Anisimov A. V. Kinetics and Mechanism of the Reaction of Benzyl Bromide with Titanium in Dimethylformamide. Russ. J. Gen. Chem. 2005, 75, 1445–1451. 10.1007/s11176-005-0443-3. [DOI] [Google Scholar]
- Yanagihara N.; Nakayama M.; Tai H. A Potent Solvent for Dissolution of Metallic Copper. Chem. Lett. 2003, 32, 640–641. 10.1246/cl.2003.640. [DOI] [Google Scholar]
- Jackson N. R. C.; Harrison W. D.; Goodall D. C. New Reactions of Precious Metals and Their Binary Compounds in Solvents Containing Carbon Halides. J. Chem. Soc., Chem. Commun. 1988, 729–730. 10.1039/c39880000729. [DOI] [Google Scholar]
- van Meersbergen M. T.; Lorenzen L.; van Deventer J. S. J. The Electrochemical Dissolution of Gold in Bromine Medium. Miner. Eng. 1993, 6, 1067–1079. 10.1016/0892-6875(93)90075-X. [DOI] [Google Scholar]
- Kokoreva S. G.; Shirshova L. V.; Kir’yakov N. V.; Shul’ga Y. U. M.; Lavrent’ev I. P. Specific Features of the Interaction of Components in the DMSO-HBr-Pd0 System. Russ. Chem. Bull. 2000, 49, 1722–1725. 10.1007/BF02496341. [DOI] [Google Scholar]
- Shirshova L. V.; Lavrent’ev I. P. Synthesis of Silver Complexes in DMSO-HX and DMSO-HX-Ketone Systems. Russ. J. Coord. Chem. 2001, 27, 511–515. 10.1023/A:1011397801630. [DOI] [Google Scholar]
- Shirshova L. V.; Kokoreva S. G.; Lavrentév I. P. Dissolution of Noble Metals and Copper in the DMSO-HBraq System. Russ. Chem. Bull. 2008, 57, 2447–2451. 10.1007/s11172-008-0351-x. [DOI] [Google Scholar]
- Shirshova L. V.; Lavrent’ev I. P.; Kokoreva S. G. The Study of Copper Dissolution in Aqueous and Nonaqueous Systems DMSO-HXsolv (Solv = H2O, X = Br, Cl; Solv = MeCN, PhNO2; X = Cl) by Resistometric Method. Russ. Chem. Bull. 2010, 59, 1692–1697. 10.1007/s11172-010-0298-6. [DOI] [Google Scholar]
- Shirshova L. V.; Lavrentiev I. P. Dissolution of Copper and Gold in the Donor-Acceptor Systems DMSO−NH4Xaq (X = Cl, Br, and I) and NH4IAq−O2. Russ. Chem. Bull. 2012, 61, 1063–1068. 10.1007/s11172-012-0144-0. [DOI] [Google Scholar]
- Harrison W. D.; Gill J. B.; Goodall D. C. Direct Reaction of Metals with the Mixed Non-Aqueous System Dimethyl Sulfoxide-Sulphur Dioxide. J. Chem. Soc., Chem. Commun. 1976, 540–541. 10.1039/C39760000540. [DOI] [Google Scholar]
- Jeffreys B.; Gill J. B.; Goodall D. C. Reactions in Mixed Non-Aqueous Systems Containing Sulphur Dioxide. Part 6. The Reaction of Metal Oxides with Dimethyl Sulfoxide-Sulphur Dioxide. J. Chem. Soc., Dalton Trans. 1985, 99–100. 10.1039/DT9850000099. [DOI] [Google Scholar]
- Graham N. K.; Gill J. B.; Goodall D. C. Reactions in Mixed Non-Aqueous Systems Containing Sulphur Dioxide. Part 3. The Electrolytic Dissolution of Metals. J. Chem. Soc., Dalton Trans. 1983, 1363–1365. 10.1039/dt9830001363. [DOI] [Google Scholar]
- Harrison W. D.; Gill J. B.; Goodall D. C. Reactions in Mixed Non-Aqueous Systems Containing Sulphur Dioxide. Part 1. The Dissolution of Main-Group Metals in the Binary Mixture Dimethyl Sulfoxide-Sulphur Dioxide. J. Chem. Soc., Dalton Trans. 1978, 1431–1433. 10.1039/DT9780001431. [DOI] [Google Scholar]
- Harrison W. D.; Gill J. B.; Goodall D. C. Reactions in Mixed Non-Aqueous Systems Containing Sulphur Dioxide. Part 2. The Dissolution of Transition Metals in the Binary Mixture Dimethyl Sulfoxide-Sulphur Dioxide, and Ion-Pair Formation Involving the Sulphoxylate Radical Ion in Mixed Solvents Cont. J. Chem. Soc., Dalton Trans. 1979, 847–850. 10.1039/DT9790000847. [DOI] [Google Scholar]
- Graham N. K.; Gill J. B.; Goodall D. C. Reactions in Mixed Non-Aqueous Systems Containing Sulfur Dioxide. V The Formation of Metal Sulfur Oxyanion Compounds by the Electrolytic Solution of Metals. Aust. J. Chem. 1983, 36, 1991–1995. 10.1071/CH9831991. [DOI] [Google Scholar]
- Gill J. B.; Goodall D. C.; Harrison W. D. Reactions in Mixed Non-Aqueous Solution Containing Sulphur Dioxide. Part 9. Mechanisms of Dissolution of Metals, Oxides, and Sulphites. J. Chem. Soc., Dalton Trans. 1987, 2995–2997. 10.1039/dt9870002995. [DOI] [Google Scholar]
- Gill J. B.; Goodall D. C.; Harrison W. D. The Solution of Metals in Dimethyl Sulfoxide and Other Non-Aqueous Solvents Containing Sulphur Dioxide. Hydrometallurgy 1981, 6, 347–351. 10.1016/0304-386X(81)90051-7. [DOI] [Google Scholar]
- Gill J. B.; Goodall D. C.; Jeffreys B. Reactions in Mixed Non-Aqueous Solutions Containing Sulphur Dioxide. Part 8. Phase Studies of Sulphur Dioxide-Dimethyl Sulfoxide and Sulphur Dioxide-Dimethylformamide Mixtures. J. Chem. Soc., Dalton Trans. 1986, 2603–2605. 10.1039/DT9860002603. [DOI] [Google Scholar]
- Lin W.; Zhang R. W.; Jang S. S.; Wong C. P.; Hong J. Il. Organic Aqua Regia-Powerful Liquids for Dissolving Noble Metals. Angew. Chem., Int. Ed. 2010, 49, 7929–7932. 10.1002/anie.201001244. [DOI] [PubMed] [Google Scholar]
- Lin W.Organic Aqua Regia: Discovery, Fundamentals, and Potential Applications. In Noble Metals; Su Y.-H., Ed.; InTech, 2012; pp 335–352. [Google Scholar]
- Lin W. Recovery of High-Purity Pt from Pt-Au Bimetallic Nanoparticles Using Organic Aqua Regia. Rare Met. 2012, 31, 92–95. 10.1007/s12598-012-0469-8. [DOI] [Google Scholar]
- Zhao J.; Wang B.; Xu X.; Yu Y.; Di S.; Xu H.; Zhai Y.; He H.; Guo L.; Pan Z.; Li X. Alternative Solvent to Aqua Regia to Activate Au/AC Catalysts for the Hydrochlorination of Acetylene. J. Catal. 2017, 350, 149–158. 10.1016/j.jcat.2017.02.027. [DOI] [Google Scholar]
- Binnemans K. Lanthanides and Actinides in Ionic Liquids. Chem. Rev. 2007, 107, 2592–2614. 10.1021/cr050979c. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Sun J.; Zhang X.; Xin J.; Miao Q.; Wang J. Ionic Liquid-Based Green Processes for Energy Production. Chem. Soc. Rev. 2014, 43, 7838–7869. 10.1039/C3CS60409H. [DOI] [PubMed] [Google Scholar]
- Schuur B.; Brouwer T.; Smink D.; Sprakel L. M. J. Green Solvents for Sustainable Separation Processes. Curr. Opin. Green Sustain. Chem. 2019, 18, 57–65. 10.1016/j.cogsc.2018.12.009. [DOI] [Google Scholar]
- Rogers R. D.; Seddon K. R. Ionic Liquids - Solvents of the Future?. Science (Washington, DC, U. S.) 2003, 302, 792–793. 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- Li X.; Kersten S. R. A.; Schuur B. Efficiency and Mechanism of Demulsification of Oil-in-Water Emulsions Using Ionic Liquids. Energy Fuels 2016, 30, 7622–7628. 10.1021/acs.energyfuels.6b01415. [DOI] [Google Scholar]
- Li X.; Kersten S. R. A.; Schuur B. Extraction of Guaiacol from Model Pyrolytic Sugar Stream with Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55, 4703–4710. 10.1021/acs.iecr.6b00100. [DOI] [Google Scholar]
- Li X.; Van Den Bossche A.; Vander Hoogerstraete T.; Binnemans K. Ionic Liquids with Trichloride Anions for Oxidative Dissolution of Metals and Alloys. Chem. Commun. 2018, 54, 475–478. 10.1039/C7CC08645H. [DOI] [PubMed] [Google Scholar]
- Van Den Bossche A.; De Witte E.; Dehaen W.; Binnemans K. Trihalide Ionic Liquids as Non-Volatile Oxidizing Solvents for Metals. Green Chem. 2018, 20, 3327–3338. 10.1039/C8GC01061G. [DOI] [Google Scholar]
- Schmidt B.; Sonnenberg K.; Beckers H.; Steinhauer S.; Riedel S. Synthesis and Characterization of Nonclassical Interhalides Based on Bromine Monochloride. Angew. Chem., Int. Ed. 2018, 57, 9141–9145. 10.1002/anie.201803705. [DOI] [PubMed] [Google Scholar]
- Haller H.; Riedel S. Recent Discoveries of Polyhalogen Anions - From Bromine to Fluorine. Z. Anorg. Allg. Chem. 2014, 640, 1281–1291. 10.1002/zaac.201400085. [DOI] [Google Scholar]
- Sonnenberg K.; Mann L.; Redeker F. A.; Schmidt B.; Riedel S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem., Int. Ed. 2020, 59, 5464–5493. 10.1002/anie.201903197. [DOI] [PubMed] [Google Scholar]
- Bortolini O.; Bottai M.; Chiappe C.; Conte V.; Pieraccini D. Trihalide-Based Ionic Liquids. Reagent-Solvents for Stereoselective Iodination of Alkenes and Alkynes. Green Chem. 2002, 4, 621–627. 10.1039/b209436n. [DOI] [Google Scholar]
- Chiappe C.; Leandri E.; Pieraccini D. Highly Efficient Bromination of Aromatic Compounds Using 3-Methylimidazolium Tribromide as Reagent/Solvent. Chem. Commun. 2004, 2536–2537. 10.1039/b410796a. [DOI] [PubMed] [Google Scholar]
- Cristiano R.; Ma K.; Pottanat G.; Weiss R. G. Tetraalkylphosphonium Trihalides. Room Temperature Ionic Liquids As Halogenation Reagents. J. Org. Chem. 2009, 74, 9027–9033. 10.1021/jo901735h. [DOI] [PubMed] [Google Scholar]
- Rogers R. D.; Holbrey J.. Ionic Liquid Solvents of Perhalide Type for Metals and Metal Compounds. WO 2010116167A1, 2010.
- Van Den Bossche A.; Vereycken W.; Vander Hoogerstraete T.; Dehaen W.; Binnemans K. Recovery of Gallium, Indium, and Arsenic from Semiconductors Using Tribromide Ionic Liquids. ACS Sustainable Chem. Eng. 2019, 7, 14451–14459. 10.1021/acssuschemeng.9b01724. [DOI] [Google Scholar]
- May B.; Lexow M.; Taccardi N.; Steinrück H. P.; Maier F. Reactions of a Polyhalide Ionic Liquid with Copper, Silver, and Gold. ChemistryOpen 2019, 8, 15–22. 10.1002/open.201800149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B.; Schröder B.; Sonnenberg K.; Steinhauer S.; Riedel S. From Polyhalides to Polypseudohalides: Chemistry Based on Cyanogen Bromide. Angew. Chem., Int. Ed. 2019, 58, 10340–10344. 10.1002/anie.201903539. [DOI] [PubMed] [Google Scholar]
- Whitehead J. A.; Lawrance G. A.; McCluskey A. Green” Leaching: Recyclable and Selective Leaching of Gold-Bearing Ore in an Ionic Liquid. Green Chem. 2004, 6, 313–315. 10.1039/B406148A. [DOI] [Google Scholar]
- Whitehead J. A.; Zhang J.; McCluskey A.; Lawrance G. A. Comparative Leaching of a Sulfidic Gold Ore in Ionic Liquid and Aqueous Acid with Thiourea and Halides Using Fe(III) or HSO5− Oxidant. Hydrometallurgy 2009, 98, 276–280. 10.1016/j.hydromet.2009.05.012. [DOI] [Google Scholar]
- Whitehead J. A.; Zhang J.; Pereira N.; McCluskey A.; Lawrance G. A. Application of 1-Alkyl-3-Methyl-Imidazolium Ionic Liquids in the Oxidative Leaching of Sulphidic Copper, Gold and Silver Ores. Hydrometallurgy 2007, 88, 109–120. 10.1016/j.hydromet.2007.03.009. [DOI] [Google Scholar]
- Bentley C. L.; Bond A. M.; Hollenkamp A. F.; Mahon P. J.; Zhang J. Unexpected Complexity in the Electro-Oxidation of Iodide on Gold in the Ionic Liquid 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide. Anal. Chem. 2013, 85, 11319–11325. 10.1021/ac402150y. [DOI] [PubMed] [Google Scholar]
- Balva M.; Legeai S.; Leclerc N.; Billy E.; Meux E. Environmentally Friendly Recycling of Fuel-Cell Membrane Electrode Assemblies by Using Ionic Liquids. ChemSusChem 2017, 10, 2922–2935. 10.1002/cssc.201700456. [DOI] [PubMed] [Google Scholar]
- Deferm C.; Hulsegge J.; Möller C.; Thijs B. Electrochemical Dissolution of Metallic Platinum in Ionic Liquids. J. Appl. Electrochem. 2013, 43, 789–796. 10.1007/s10800-013-0541-6. [DOI] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Jenkin G. R. T.; Al-Bassam A. Z. M.; Harris R. C.; Abbott A. P.; Smith D. J.; Holwell D. A.; Chapman R. J.; Stanley C. J. The Application of Deep Eutectic Solvent Ionic Liquids for Environmentally-Friendly Dissolution and Recovery of Precious Metals. Miner. Eng. 2016, 87, 18–24. 10.1016/j.mineng.2015.09.026. [DOI] [Google Scholar]
- Abbott A. P.; Capper G.; Swain B. G.; Wheeler D. A. Electropolishing of Stainless Steel in an Ionic Liquid. Trans. Inst. Met. Finish. 2005, 83, 51–53. 10.1179/002029605X17657. [DOI] [Google Scholar]
- Abbott A. P.; Frisch G.; Hartley J.; Karim W. O.; Ryder K. S. Anodic Dissolution of Metals in Ionic Liquids. Prog. Nat. Sci. 2015, 25, 595–602. 10.1016/j.pnsc.2015.11.005. [DOI] [Google Scholar]
- Smith E. L.; Fullarton C.; Harris R. C.; Saleem S.; Abbott A. P.; Ryder K. S. Metal Finishing with Ionic Liquids: Scale-up and Pilot Plants from IONMET Consortium. Trans. Inst. Met. Finish. 2010, 88, 285–291. 10.1179/174591910X12856686485734. [DOI] [Google Scholar]
- Mortier T.; Persoons A.; Verbiest T. Oxidation of Solid Gold in Chloroform Solutions of Cetyltrimethylammonium Bromide. Inorg. Chem. Commun. 2005, 8, 1075–1077. 10.1016/j.inoche.2005.08.015. [DOI] [Google Scholar]
- Barnartt S.; Charles R. G.; Littau L. W. Reactions of Liquid Acetylacetone with Metal Surfaces. J. Phys. Chem. 1958, 62, 763–766. 10.1021/j150564a036. [DOI] [Google Scholar]
- Räisänen M. T.; Kemell M.; Leskelä M.; Repo T. Oxidation of Elemental Gold in Alcohol Solutions. Inorg. Chem. 2007, 46, 3251–3256. 10.1021/ic0624468. [DOI] [PubMed] [Google Scholar]
- Räisänen M.; Heliövaara E.; Al-Qaisi F.; Muuronen M.; Eronen A.; Liljeqvist H.; Nieger M.; Kemell M.; Moslova K.; Hämäläinen J.; Lagerblom K.; Repo T. Pyridinethiol-Assisted Dissolution of Elemental Gold in Organic Solutions. Angew. Chem., Int. Ed. 2018, 57, 17104–17109. 10.1002/anie.201810447. [DOI] [PubMed] [Google Scholar]
- Antoniotti S.; Duñach E. Facile Preparation of Metallic Triflates and Triflimidates by Oxidative Dissolution of Metal Powders. Chem. Commun. 2008, 993–995. 10.1039/b717689a. [DOI] [PubMed] [Google Scholar]
- Legrave N.; Couhert A.; Olivero S.; Desmurs J. R.; Duñach E. Efficient Preparation of Anhydrous Metallic Triflates and Triflimides under Ultrasonic Activation. Eur. J. Org. Chem. 2012, 2012, 901–904. 10.1002/ejoc.201101686. [DOI] [Google Scholar]
- Panteleev S. V.; Maslennikov S. V.; Egorochkin A. N.; Vakulenko V. Y. Solvent Effect on Metal Oxidation Rates in Aprotic Media. Russ. J. Gen. Chem. 2007, 77, 1004–1007. 10.1134/S1070363207060084. [DOI] [Google Scholar]
- Petrusenko S. R.; Sieler J.; Kokozay V. N. Direct Synthesis of Zinc and Nickel(II) Complexes with 1,4-Diazabicyclo[2.2.2]Octane. Z. Naturforsch., B: J. Chem. Sci. 1997, 52, 331–336. 10.1515/znb-1997-0305. [DOI] [Google Scholar]
- Kokozay V. N.; Vassilyeva O. Y.; Weinberger P.; Wasinger M.; Linert W. Direct Synthesis of Polynuclear Complexes. Rev. Inorg. Chem. 2000, 20, 255–282. 10.1515/REVIC.2000.20.4.255. [DOI] [Google Scholar]
- Abbott J. K. C.; Dougan B. A.; Xue Z. L.. Synthesis of Organometallic Compounds. In Modern Inorganic Synthetic Chemistry; Xu R., Pang W., Huo Q., Eds.; Elsevier: Amsterdam, 2011; pp 269–293. [Google Scholar]
- Chakravorti M. C.; Subrahmanyam G. V. B. Electrosynthesis of Coordination Compounds by the Dissolution of Sacrificial Metal Anodes. Coord. Chem. Rev. 1994, 135–136, 65–92. 10.1016/0010-8545(94)80065-0. [DOI] [Google Scholar]
- Chygorin E. M.; Makhankova V. G.; Ischenko M. V.; Kokozay V. N.; Zubatyuk R. I.; Shishkin O. V.; Jezierska J. Direct Synthesis and Properties of Monomeric and Dimeric Mn III-Salen Complexes Tuned by Tetrahalocadmate Anions. Inorg. Chem. Commun. 2012, 20, 282–285. 10.1016/j.inoche.2012.03.029. [DOI] [Google Scholar]
- Buchikhin E. P.; Kuznetsov A. Y.; Vidanov V. L.; Shatalov V. V. Reaction of Metallic Uranium with NO2 in TBP. Radiochemistry 2006, 48, 459–461. 10.1134/S1066362206050080. [DOI] [Google Scholar]
- Yoshimura A.; Takai M.; Matsuno Y. Novel Process for Recycling Gold from Secondary Sources: Leaching of Gold by Dimethyl Sulfoxide Solutions Containing Copper Bromide and Precipitation with Water. Hydrometallurgy 2014, 149, 177–182. 10.1016/j.hydromet.2014.08.003. [DOI] [Google Scholar]
- Umehara K.; Matsuno Y. Fundamental Studies on a Recycling System for Precious and Rare Metals Using a Propylene Carbonate Solvent Containing CuBr2 and KBr. Mater. Trans. 2015, 56, 1579–1584. 10.2320/matertrans.M2015202. [DOI] [Google Scholar]
- Bessel C. A.; Denison G. M.; DeSimone J. M.; DeYoung J.; Gross S.; Schauer C. K.; Visintin P. M. Etchant Solutions for the Removal of Cu(0) in a Supercritical CO2-Based “Dry” Chemical Mechanical Planarization Process for Device Fabrication. J. Am. Chem. Soc. 2003, 125, 4980–4981. 10.1021/ja034091m. [DOI] [PubMed] [Google Scholar]
- Visintin P. M.; Bessel C. A.; White P. S.; Schauer C. K.; DeSimone J. M. Oxidative Dissolution of Copper and Zinc Metal in Carbon Dioxide with Tert-Butyl Peracetate and a β-Diketone Chelating Agent. Inorg. Chem. 2005, 44, 316–324. 10.1021/ic049765o. [DOI] [PubMed] [Google Scholar]
- Dunbar A.; Omiatek D. M.; Thai S. D.; Kendrex C. E.; Grotzinger L. L.; Boyko W. J.; Weinstein R. D.; Skaf D. W.; Bessel C. A.; Denison G. M.; DeSimone J. M. Use of Substituted Bis(Acetylacetone)Ethylenediimine and Dialkyldithiocarbamate Ligands for Copper Chelation in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2006, 45, 8779–8787. 10.1021/ie060947v. [DOI] [Google Scholar]
- Weinstein R. D.; Richard J. G.; Bessel C. A.; Hanlon W. H.; Skaf D. W. The Removal of Copper with Dialkyldithiocarbamate Ligands in Condensed Carbon Dioxide. Chem. Eng. Technol. 2008, 31, 575–581. 10.1002/ceat.200700444. [DOI] [Google Scholar]
- Rasadujjaman M.; Nakamura Y.; Watanabe M.; Kondoh E.; Baklanov M. R. Supercritical Carbon Dioxide Etching of Transition Metal (Cu, Ni, Co, Fe) Thin Films. Microelectron. Eng. 2016, 153, 5–10. 10.1016/j.mee.2015.12.018. [DOI] [Google Scholar]
- Favier I.; Duñach E. Novel Electrosynthesis of Metallic Bis(Trifluoromethanesulfonyl) Imides. Tetrahedron Lett. 2003, 44, 2031–2032. 10.1016/S0040-4039(03)00214-4. [DOI] [Google Scholar]
- Abbott A. Electrochemistry in Media of Low Dielectric Constant. Chem. Soc. Rev. 1993, 22, 435–440. 10.1039/cs9932200435. [DOI] [Google Scholar]
- Kharisov B. I.; Blanco L. M.; Salinas M. V.; Garnovskii A. D. Direct Synthesis of Copper Dimethyldithiocarbamate Derivatives: An Optimization Using Ultrasonic Treatment. J. Coord. Chem. 1999, 47, 135–143. 10.1080/00958979908024548. [DOI] [Google Scholar]