Abstract
Since December 2019, pneumonia caused by a novel coronavirus (SARS-CoV-2), namely 2019 novel coronavirus disease (COVID-19), has rapidly spread from Wuhan city to other cities across China. The present study was designed to describe the epidemiology, clinical characteristics, treatment, and prognosis of 74 hospitalized patients with COVID-19.
Clinical data of 74 COVID-19 patients were collected to analyze the epidemiological, demographic, laboratory, radiological, and treatment data. Thirty-two patients were followed up and tested for the presence of the viral nucleic acid and by pulmonary computed tomography (CT) scan at 7 and 14 days after they were discharged.
Among all COVID-19 patients, the median incubation period for patients and the median period from symptom onset to admission was all 6 days; the median length of hospitalization was 13 days. Fever symptoms were presented in 83.78% of the patients, and the second most common symptom was cough (74.32%), followed by fatigue and expectoration (27.03%). Inflammatory indicators, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) of the intensive care unit (ICU) patients were significantly higher than that of the non-ICU patients (P < .05). However, 50.00% of the ICU patients had their the ratio of T helper cells to cytotoxic T cells (CD4/CD8) ratio lower than 1.1, whose proportion is much higher than that in non-ICU patients (P < .01).
Compared with patients in Wuhan, COVID-19 patients in Anhui Province seemed to have milder symptoms of infection, suggesting that there may be some regional differences in the transmission of SARS-CoV-2 between different cities.
Keywords: 2019 novel coronavirus disease, epidemiological characteristics, pneumonia, prognosis, severe acute respiratory syndrome coronavirus 2, treatment
1. Introduction
In early December 2019, mysterious pneumonia was first discovered in Wuhan, Hubei Province. High-throughput sequencing revealed a novel coronavirus, initially named as 2019-nCoV,[1] and were subsequently named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by International Committee on Taxonomy of Virus. This novel pneumonia caused by SARS-CoV-2 were then named as coronavirus disease 2019 (COVID-19) by World Health Organization (WHO).[2] Accelerated by spring migration (also known as Chunyun in China), this contagious coronavirus becomes a pandemic rapidly.
Coronavirus induced mortalities could vary in different region. A study from the European Center for Disease Prevention and Control showed that Middle East respiratory syndrome coronavirus (MERS-CoV) infection had mortalities of 41.1% in the Middle East, 53.3% in Europe, 40.0% in Africa, and 19.2% in Asia, showing a significant regional difference.[3] This difference is difficult to explain with viral mutations.[4] As for COVID, there are differences in the mortality and cure rates between provinces and cities in China according to the official data released by the Chinese Center for Disease Control and Prevention.[5] A study of Huang et al[3] reported for the first time the clinical characteristics (including fever, dry cough, dyspnea, myalgia, fatigue, normal or decreased white blood cell count, and radiographic findings of pneumonia) and complications (29% respiratory distress syndrome, 12% heart injury, and 15% mortality) of 41 COVID-19 patients in Wuhan (28 discharged cases). Xu et al[6] conducted a retrospective analysis of clinical characteristics of 62 COVID-19 hospitalized patients in Zhejiang Province and found that most patients in Zhejiang Province had mild to moderate symptoms. In addition, only a small portion of the patients had dyspnea, and one of them developed respiratory distress syndrome who required intensive care unit (ICU) treatment. These researchers believe that the symptoms of patients diagnosed outside Wuhan are relatively mild. Anhui Province reported the first case of COVID-19 on January 21, 2020. By the end of February 25, 2020, the province had 989 confirmed cases, and no new cases were reported for next 4 consecutive days. Given the literature research findings that COVID-19 patients in Wuhan and cities outside Wuhan show inconsistencies, we conducted a retrospective analysis on the epidemiological and clinical characteristics of 74 COVID-19 patients in Hefei city, Anhui Province and compared the difference between ICU patients and non-ICU patients. This research presents the disease progression of COVID-19 in different places and helps in revealing the COVID-19 severity based on clinical characteristics.
2. Methods
As a provincial designated hospital, Anhui Provincial Hospital Infectious Disease Hospital (Hefei city, Anhui Province) is one of the 4 major bases for treating severe COVID-19 patients. This retrospective study included COVID-19 patients hospitalized in Anhui Provincial Hospital Infectious Disease Hospital from January 21, 2020 to February 25, 2020.
Selection criteria of exposed individuals were adopted from the Diagnosis and Treatment Protocol for the 2019 Novel Coronavirus Pneumonia (Sixth Trial Edition) published by the National Health Commission of China. Generally, a real-time reverse transcription-polymerase chain reaction was adopted as a diagnostic method of testing SARS-CoV-2 nucleic acids in nasopharyngeal swabs. Patients who had 2 positive tested results within 24 hours were confirmed with COVID-19.
Inclusion criteria of exposed individuals for ICU treatment were[7]: threatened airway; all respiratory arrests; respiratory rate ≥40 or ≤8 breaths/min; oxygen saturation <90% on ≥50% oxygen; all cardiac arrests; pulse rate <40 or >140 beats/min; systolic blood pressure <90 mm Hg; sudden fall in level of consciousness (fall in Glasgow coma score >2 points); repeated or prolonged seizures; rising arterial carbon dioxide tension with respiratory acidosis; any patient giving cause for concern.
The specific diagnosis and treatment processes of all patients in this study were carried out according to the guidelines of the WHO,[8] and the treatment plans for severe and critically ill patients were submitted to the provincial expert group for discussion and decision. Epidemiological characteristics, clinical symptoms and signs, laboratory test results, and treatment during hospitalization were extracted from the electronic medical records by 3 trained clinicians. Epidemiological investigation results were collected through a brief interview with each patient, and the patient's contact history for the 2 weeks before the disease onset was collected, including the date and time of close contact (meeting, gathering, or coworking) with people from Wuhan or patients confirmed with COVID-19. Laboratory assessments included complete blood count, T-cell differential counts, blood chemistry, coagulation tests, liver and kidney function, electrolytes, C-reaction protein, procalcitonin, lactate dehydrogenase, and creatine kinase tests. Radiological assessment was mainly a pulmonary computed tomography (CT) scan. Treatment measures included antiviral therapy, antibiotic therapy, corticosteroid therapy, interferon, gamma globulin pulse therapies, and high-flow oxygen therapy.
The endpoint of this study was discharge or death. Discharge criteria were normal body temperature for >3 days, significantly improved respiratory symptoms, imaging showing significant improvement in exudative changes, and 2 consecutive negative viral nucleic acid testing results in respiratory specimens (intervals >24 hours). Discharged patients were followed up at 7 and 14 days after discharge.
2.1. Statistical analysis
In this study, categorical variables were presented as percentages, and continuous variables were presented as the median (interquartile range [IQR]). The severity of admitted patients was evaluated according to the guidelines described in the literature.[9] Data of ICU and non-ICU patients were separated. SPSS 20.0 software (IBM Inc., Armonk, NY) was used for statistical analysis.
3. Results
3.1. Demographic and clinical characteristics
As of February 25, 2020, this study included data of clinical symptoms and outcomes of 74 COVID-19 patients. A total of 15 patients were discharged and completed the follow-up examinations, and all patients underwent viral nucleic acid testing and pulmonary CT scan.
81.08% of the COVID-19 patients in this study were young adults (19–65 years old), and the majority were men. Thirty six COVID-19 patients (48.65%) had a history of close contact with people from Wuhan 2 weeks before the disease onset and were mainly imported COVID-19 cases. Seventeen patients had history of contact with confirmed COVID-19 patients, accounting for 22.97%. In addition, 28.38% of the patients did not have a history of contact with people from Wuhan or with confirmed COVID-19 patients. Twenty one patients were in familial clusters of developing COVID-19. Among our patients, the median incubation period was 6 days, the median period from symptom onset to admission was 6 days, and the median length of hospital stay was 13 days. On admission, 83.78% of patients had fever, and the second most common symptom was dry cough (74.32%), followed by fatigue and expectoration (27.03%), headache (21.62%), and the less common symptoms, such as diarrhea, sore throat, and hemoptysis. The median age of COVID-19 patients in ICU was 56 years, and 55.56% of them had ≥2 complications (e.g., hypertension and cardiovascular disease). The median age of non-ICU patients was 43 years, and 12.50% of them had ≥2 complications. The median time from the onset of symptoms to hospital admission was 9.5 days of ICU patients, which was longer than that of non-ICU patients (5 days). The incubation period and contact history of ICU and non-ICU patients were similar (Table 1). Fifteen discharged patients had their viral nucleic acid testing and pulmonary CT scan repeated at the outpatient fever clinic or another local hospital, and 1 patient (6.67%) showed positive viral nucleic acid testing by the local center of disease control, followed by 2 consecutive negative results 3 days later. No sign of re-infection was found in that patient. The pulmonary CT results of the 15 patients in the follow-up showed further resolution and repairing of pulmonary lesions (see Fig. 1 for typical cases).
Table 1.
Clinical characteristics of 74 COVID-19 patients in this study.
| Cases (%) | ||||
| Clinical characteristics | Total (n = 74) | ICU patient (n = 18) | Non-ICU patient (n = 56) | P |
| Age, median age, y | 47 (35–56) | 56 (47–68) | 43 (29–52) | <.001 |
| Age group, y | .031 | |||
| ≤18 | 2 (2.70) | 0 (0.00) | 2 (3.70) | |
| 19–40 | 25 (33.78) | 2 (11.11) | 23 (41.07) | |
| 41–65 | 35 (47.30) | 10 (55.56) | 25 (44.62) | |
| ≥66 | 12 (16.22) | 6 (33.33) | 6 (11.11) | |
| Sex | .059 | |||
| Male | 48 (64.86) | 15 (83.33) | 33 (59.93) | |
| Female | 26 (35.14) | 3 (16.67) | 23 (41.07) | |
| Exposure within 2 weeks | .469 | |||
| People from Wuhan | 36 (48.65) | 8 (44.44) | 28 (50.00) | |
| Contact with confirmed COVID-19 patients | 17 (22.97) | 6 (33.33) | 11 (19.64) | |
| No | 21 (28.38) | 4 (22.22) | 17 (30.36) | |
| Familial clustering | 21 (28.37) | 9 (50.00) | 12 (21.43) | .019 |
| Incubation period, median, days | 6 (4–10) | 9.5 (6–13) | 5 (4–9) | .013 |
| Common signs and symptoms | ||||
| Fever | 62 (83.78) | 15 (83.33) | 47 (83.93) | .952 |
| Cough | 55 (74.32) | 10 (55.56) | 45 (80.36) | .036 |
| Fatigue | 20 (27.03) | 4 (22.22) | 16 (28.57) | .598 |
| Myalgia | 11 (14.86) | 8 (44.44) | 3 (5.36) | <.001 |
| Difficulty breathing | 5 (6.76) | 5 (27.78) | 0 (0.00) | <.001 |
| Sore throat | 7 (9.46) | 1 (5.56) | 6 (10.71) | .515 |
| Expectoration | 20 (27.03) | 3 (16.67) | 17 (30.36) | .255 |
| Diarrhea | 5 (6.76) | 3 (16.67) | 2 (3.57) | .054 |
| Headache | 16 (21.62) | 2 (11.11) | 14 (25.00) | .213 |
| Hemoptysis | 1 (1.35) | 1 (5.56) | 0 (0.00) | .076 |
| Time from symptom onset to admission, median, days | 6 (4–10) | 9.5 (6–12) | 5 (4–8.25) | .013 |
| Signs upon admission | ||||
| Body temperature, °C | .124 | |||
| ≤37.2 | 43 (58.11) | 9 (50.00) | 34 (60.71) | |
| 37.3–38.0 | 19 (25.68) | 5 (27.78) | 14 (25.00) | |
| 38.1–39.0 | 10 (13.51) | 3 (16.67) | 7 (12.50) | |
| ≥39.1 | 2 (2.70) | 1 (5.56) | 1 (1.79) | |
| Respiratory rate, median, breath/min | 20 (19–20) | 19.5 (17.25–20) | 20 (19–20) | .874 |
| Pulse, median, beats/min | 85 (79–90) | 81.5 (76.5–89.5) | 85 (80–90.5) | .168 |
| Mean arterial pressure, median, mmHg | 92 (86.7–97.3) | 93.65 (87.8–100.9) | 92 (86.7–97) | .211 |
| Oxygen saturation (without oxygen therapy), % | 98 (95–98.8) | 95 (91–97.8) | 98 (96–99) | .009 |
| Length of hospital stay | 13 (10.3–16) | 16 (3–18.8) | 12 (9.8–15) | .013 |
| ≥2 underlying diseases | 17 (22.97) | 10 (55.56) | 7 (12.50) | <.001 |
| Hypertension | 21 (28.38) | 8 (44.44) | 13 (23.21) | .082 |
| Cardiovascular disease | 18 (24.32) | 4 (22.22) | 14 (25.00) | .811 |
| Diabetes | 9 (12.16) | 1 (5.56) | 8 (14.29) | .324 |
| Chronic obstructive pulmonary disease (COPD) | 2 (2.70) | 1 (5.56) | 1 (1.79) | .391 |
| Chronic kidney disease | 5 (6.76) | 1 (5.56) | 4 (7.14) | .815 |
| Chronic liver disease | 8 (10.81) | 1 (5.56) | 7 (12.50) | .409 |
| Sequela of stroke | 4 (5.41) | 3 (16.67) | 1 (1.79) | .015 |
| Malignant tumor | 1 (1.35) | 1 (5.56) | 0 (0.00) | .391 |
Figure 1.
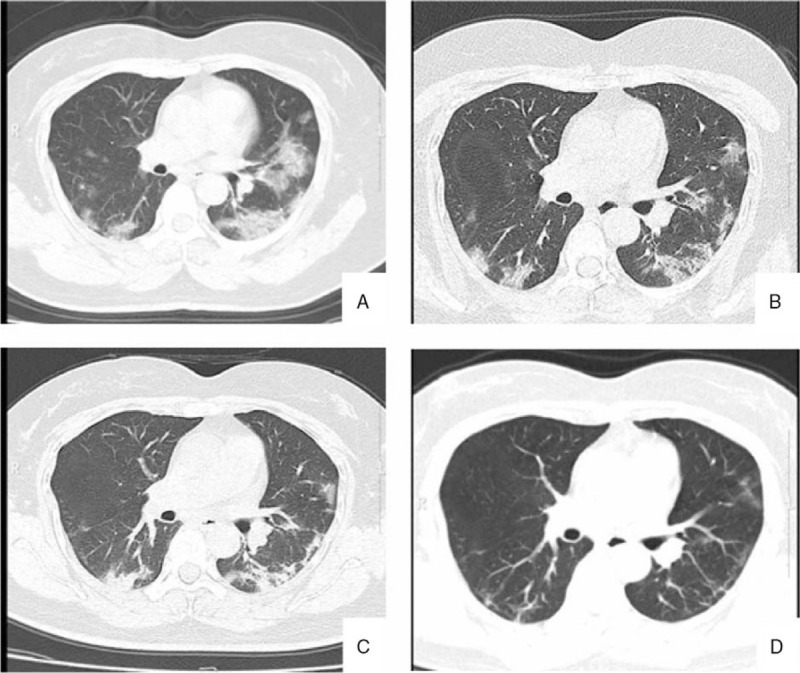
A typical case presentation. A 48 years old woman returned to Anhui from Wuhan within 2-days fever and cough. A: Ground glass opacities 2 days after onset; B: pulmonary CT findings on 6th day, bilateral lung consolidation began to absorb; C: pulmonary CT findings on 12th day, bilateral lung inflammation was gradually recovered; D: pulmonary CT findings on 30th day, bilateral lung inflammation was basically recovered.
3.2. Laboratory parameters and pulmonary CT scan
Laboratory examinations were performed at the time of admission and vast differences were observed between ICU patients and non-ICU patients (Table 2). 46% of all patients included in this study had lymphopenia, which was more common in ICU patients (61%) than the non-ICU patients. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), among other inflammatory indicators, were significantly higher in the ICU patients compared with the non-ICU patients (P < .05). 50.00% of ICU patients had their the ratio of T helper cells to cytotoxic T cells (CD4/CD8) ratio lower than 1.1, whose proportion is much higher than that in non-ICU patients. The creatine kinase, lactate dehydrogenase concentration, and activated partial thromboplastin time of the ICU patients were higher than those of the non-ICU patients (P < .01 and P < .05, respectively). However, albumin of ICU patients was lower than that of non-ICU patients while no statistical significance was observed.
Table 2.
Laboratory test results of the 74 COVID-19 patients.
| Median (IQR) | |||||
| Items | Total (n = 74) | ICU patient (n = 18) | Non-ICU patient (n = 56) | Normal range | P |
| White blood cell count, × 109/L | 5.9 (4.7–6.6) | 5.6 (4.2–6.6) | 5.1 (4.1–5.6) | 3.5–9.5 | .929 |
| Neutrophil count, ×109/L | 3.8 (2.5–4.8) | 4.1 (3.4–5.7) | 3.7 (2.7–4.3) | 1.8–6.3 | .77 |
| Lymphocyte count, × 109/L | 1.1 (0.6–1.5) | 0.8 (0.5–1.1) | 1.1 (0.7–1.8) | 1.1–3.2 | .022 |
| <1.1 × 109/L, (%) | 34 (45.95) | 11 (61.11) | 23 (41.07) | .138 | |
| Monocyte count, × 109/L | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) | 0.3 (0.4–0.5) | 0.1–0.6 | .127 |
| Platelet count, × 109/L | 166 (131–221) | 136 (107–168) | 200 (136–233) | 125.0–350.0 | <.001 |
| ESR, mm/h | 20 (10–33) | 31 (17–79) | 18 (8–31) | 0–20.0 | .011 |
| CRP, mg/L | 17.5 (4.5–36.2) | 43.3 (10.9–79.2) | 13.4 (4.0–26.9) | 0–8.0 | .015 |
| CD4/CD8 | 1.8 (1.6–2.2) | 1.9 (1.7–2.2) | 1.8 (1.6–2.2) | 1.1–1.7 | .532 |
| <1.1, (%) | 19 (25.68) | 9 (50.00) | 10 (17.86) | .007 | |
| Procalcitonin, ng/mL | 0.2 (0.1–0.4) | 0.3 (0.2–0.7) | 0.2 (0.1–0.4) | 0–0.5 | .056 |
| IL-6, pg/mL | 6.5 (5.6–7.3) | 7.1 (6.0–8.4) | 6.3 (5.0–7.0) | <7 | .742 |
| Creatine kinase, U/L | 90 (57–132) | 127 (58–266) | 89 (56–117) | 22–269 | .134 |
| Creatine kinase isoenzyme, U/L | 12 (10–17) | 11 (9–16) | 12 (10–18) | 0–225 | .321 |
| Lactate dehydrogenase, U/L | 228 (177–290) | 300 (242–292) | 201 (166–268) | 125–250 | <.001 |
| High-sensitivity troponin, pg/mL | 0.08 (0.06–0.12) | 0.10 (0.07–0.36) | 0.08 (0.06–0.11) | 0–0.3 | .181 |
| Alanine aminotransferase, U/L | 29 (17–44) | 29 (23–41) | 29 (16–45) | 9–50 | .483 |
| Aspartate aminotransferase, U/L | 31 (23–49) | 35 (28–42) | 29 (21–39) | 15–45 | .294 |
| Albumin, g/l | 43 (37–48) | 38 (34–44) | 45 (39–49) | 40–55 | .072 |
| Total bilirubin, mmol/L | 16.5 (11.2–23.5) | 13.9 (9.7–18.4) | 17.1 (12.9–23.8) | 3.4–21 | .377 |
| Creatinine, μmol/L | 68 (60–80) | 71 (61–81) | 68 (60–80) | 57–111 | .658 |
| Urea nitrogen, mmol/L | 4.3 (3.4–5.5) | 4.9 (2.9–6.5) | 4.2 (3.4–5.5) | 3.1–8.0 | .764 |
| Prothrombin time, s | 14.2 (13.6–15.1) | 14.2 (14.8–15.4) | 14 (13.1–14.6) | 9.5–14.5 | .072 |
| Activated partial thromboplastin time, s | 39.3 (34.4–42.3) | 37.2 (30.2–38.9) | 40.0 (35.8–42.3) | 20.0–40.0 | .028 |
| D-dimer, mg/L | 0.3 (0.1–0.5) | 0.5 (0.2–0.6) | 0.2 (0.1–0.4) | 0–1.1 | .152 |
In Table 3, the pulmonary imaging findings of the patients at admission showed that 6 out of 74 COVID-19 patients (8.11%) had no signs of pneumonia on pulmonary CT images, while 21.62% and 70.27% of the patients in this study had unilateral and bilateral pulmonary involvement, respectively. Pneumonia with lymphadenopathy or pleural effusion was rare in our patients. 62.50% of the non-ICU patients had ground-glass opacity, and 66.67% of the ICU patients had bilateral diffuse ground-glass opacities accompanied by partial pulmonary consolidation.
Table 3.
Pulmonary CT results of the 74 COVID-19 patients.
| Number of people (percentage) | ||||
| CT manifestation | Total(n = 74) | ICU patient (n = 18) | Non-ICU patient (n = 56) | P |
| Pneumonia | .018 | |||
| Negative | 6 (8.11) | 0 (0.00) | 6 (10.71) | |
| Unilateral ground-glass opacities | 16 (21.62) | 2 (11.11) | 14 (25.00) | |
| Bilateral ground-glass opacities | 25 (33.78) | 4 (22.22) | 21 (37.50) | |
| Bilateral diffuse ground-glass opacities accompanied by partial pulmonary consolidation | 27 (36.45) | 12 (66.67) | 15 (26.79) | |
| Comorbidities | ||||
| Mediastinal lymphadenopathy | 6 (8.11) | 4 (22.22) | 2 (3.57) | .001 |
| Pleural effusion | 5 (6.76) | 4 (22.22) | 1 (1.79) | .001 |
3.3. Treatment and prognosis
The treatment and prognosis of patients were analyzed and presented in Table 4. Among all COVID-19 patients, 98.65% of the patients received antiviral therapy (oral administration of lopinavir and ritonavir tablets; only a 4-year-old child did not receive this therapy), 81.08% had antibiotic prevention or treatment, 21.62% had interferon nebulization, and relatively few patients received steroid and gamma globulin pulse therapies. No patient received invasive ventilation, and 83.33% of the ICU patients received high-flow oxygen therapy. Four patients with acute respiratory distress syndrome received treatment in ICU. There was 1 case of death (1.35%), and this patient died of acute cerebral infarction accompanied by cerebral herniation and had ground-glass opacities in the pulmonary CT and positive viral nucleic acid testing during the hospitalization and neurological screening.
Table 4.
Complications and treatment of the 74 COVID-19 patients.
| Number of people (percentage) | P | |||
| Total (n = 74) | ICU patient(n = 18) | Non-ICU patient(n = 56) | ||
| Complications | ||||
| Shock | 1 (1.35) | 1 (5.56) | 0 (0.00) | .243 |
| Myocardial injury | 3 (4.05) | 2 (11.11) | 1 (1.79) | .145 |
| Arrhythmia | 1 (1.35) | 1 (5.56) | 0 (0.00) | .243 |
| Liver damage | 5 (6.76) | 3 (16.67) | 2 (3.57) | .089 |
| Renal impairment | 3 (4.05) | 2 (11.11) | 1 (1.79) | .145 |
| Acute respiratory distress syndrome | 4 (5.41) | 4 (22.22) | 0 (0.00) | .03 |
| Treatment | ||||
| Antiviral | 73 (97) | 18 (100.00) | 55 (98) | 1 |
| Antibiotic | 60 (81.08) | 18 (100.00) | 42 (75.00) | .018 |
| Glucocorticoid | 4 (5.41) | 2 (11.11) | 2 (3.57) | .247 |
| Interferon | 16 (21.62) | 12 (66.67) | 4 (7.14) | <.001 |
| Gamma globulin | 15 (20.27) | 11 (61.11) | 4 (7.14) | <.001 |
| Tocilizumab | 4 (5.41) | 4 (22.22) | 0 (0.00) | 1 |
| Oxygen | ||||
| High-flow | 15 (20.27) | 15 (83.33) | 0 (0.00) | <.001 |
| Nasal cannula | 38 (51.35) | 3 (16.67) | 35 (62.50) | .001 |
| Invasive ventilation | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Number of viral nucleic acid testing until getting 2 consecutive negative results | 4 (3–5) | 4 (3–5) | 4 (3–5) | .99 |
| Deaths | 1 (1.35) | 1 (5.56) | 0 (0.00) | .243 |
3.4. Clinical characteristics comparison of COVID-19 patients among Hefei, Wuhan, Zhejiang and whole China
Table 5 summarizes and compares the treatment data of COVID-19 patients in our province with the data of COVID-19 patients in Wuhan, Zhejiang, and the whole country. The minimum and maximum ages of the patients included in this study were 4 and 92 years old, respectively. 60% of our patients were young adults aged 19 to 65 years old, with the median age of 47 years, and 65% of our patients were men. These results were somewhat different from the findings reported earlier.[6] Male COVID-19 patients in Wuhan accounted for 73% of the total number of patients (30 men of 41 COVID-19 patients), with a median age of 49 years; whereas in Zhejiang, 58% of the COVID-19 patients were men (36 men of 62 COVID-19 patients), with a median age of 41 years.[3] Approximately 50% of the COVID-19 patients in this study had close contact with people from Wuhan 2 weeks before the onset. Although they were mainly imported cases, they had no history of contact with the Huanan Seafood Market in Wuhan. Familial clustering of COVID-19 occurred in 21 patients, which was consistent with the previous reports of human-to-human transmission.[6] The most common symptoms of patients in this study at the time of hospital admission were fever, dry cough, fatigue, and expectoration, while dyspnea symptoms accounted for only 7%, which was significantly different from the COVID-19 patients in Wuhan (53.66% of the patients had dyspnea).[3] Four of the 5 patients with dyspnea in this study had type I respiratory failure and were treated in the ICU. The median incubation period for COVID-19 in our patients was 6 days; while it was 4 and 8 days in Zhejiang Province and Wuhan city, respectively, which may be related to the disease evolution and the measures of prevention and control in different places. In this study, the incubation period of patients treated in the ICU was similar to that of non-ICU patients. However, the period from symptom onset to hospital admission was 4.5 days longer in ICU patients than in non-ICU patients, suggesting that the delay in treatment may be one of the reasons for the worsening of the disease condition. In addition, patients treated in the ICU were 13 years older than the non-ICU patients and were accompanied by >2 underlying diseases, suggesting that age and underlying diseases of the patients may be the causes of severe illness. Therefore, there were some differences in epidemiology and clinical symptoms between COVID-19 patients in Anhui Province, Wuhan, and Zhejiang Province (Table 5).
Table 5.
Patient demographics in Anhui Province, Wuhan city, Zhejiang Province, and the whole country.
| Item | Number of people (percentage) | |||
| Anhui(n = 74) | Wuhan(n = 41) | Zhejiang(n = 62) | Whole country(1099) | |
| Age, median, y | 47 (35–56) | 49 (41–58) | 41 (32–52) | 47 (35–58) |
| Sex, male (%) | 48 (64.86) | 30 (73.17) | 36 (58.06) | 637 (57.96) |
| Contact history | 53 (72) | – | 23 (37) | – |
| People from Wuhan or confirmed COVID-19 patient (s) | 53 (71.62) | – | 23 (37.10) | 676/1099 (61.51) |
| Wuhan seafood market or wildlife | 0 (0.00) | 27 (65.85) | 0 (0.00) | 687 (62.51) |
| No | 21 (28.38) | – | 39 (62.90) | 285 (25.93) |
| Familial clustering | 21 (28.38) | – | 21 (33.87) | – |
| Incubation period, median, d | 6 (4–10) | 8 (5–13) | 4 (3–5) | 4 (2–7) |
| Fever | 62 (83.78) | 40 (97.56) | 48 (77.42) | 975 (88.72) |
| Cough | 55 (74.32) | 31 (75.61) | 50 (80.65) | 745 (67.79) |
| Fatigue | 31 (41.89) | 18 (43.90) | 32 (51.61) | 419 (38.1320) |
| Expectoration | 20 (27.03) | 11 (26.83) | 35 (56.45) | 370 (33.67) |
| Difficulty breathing | 5 (6.76) | 22 (53.66) | 2 (3.23) | 205 (18.65) |
| Headache | 16 (21.62) | 3 (7.32) | 21 (33.87) | 150 (13.65) |
| Diarrhea | 5 (6.76) | 1 (2.44) | 3 (4.84) | 42 (3.82) |
| Hemoptysis | 1 (1.35) | 2 (4.88) | 2 (3.23) | 10 (0.91) |
| Symptom onset to admission, median, d | 6 (4–10) | 5 (1–8) | 2 (1–4) | 4 (2–7) |
| Any comorbidities | 34 (45.95) | 13 (31.71) | 20 (32.26) | 261 (23.75) |
| White blood cell count, median, × 109/L | 5.5 (4.4–6.6) | 6.2 (4.1–10.5) | 4.7 (3.5–5.9) | 4.7 (3.5–6.0) |
| Lymphocyte count, × 109/L | 1.0 (0.6–1.5) | 0.8 (0.6–1.1) | 1.0 (0.8–1.5) | 1.0 (0.7–1.3) |
| ESR, median, mm/h | 15 (10–18) | – | – | – |
| CRP, median, mg/L | 17.4 (4.2–49.3) | – | – | ≥10,481/793 |
| Procalcitonin, median, ng/mL | 0.3 (0.1–0.7) | 0.1 (0.1–0.1) | 0.04 (0.03–0.06) | ≥0.5, 35/633 |
| Creatine kinase, median, U/L | 91.4 (53.2–150.1) | 132 (62–139) | 65 (49–101) | ≥200, 90/657 |
| Lactate dehydrogenase, U/L | 269 (191–323) | 286 (242–408) | 205 (184–260) | ≥250, 277/675 |
| Alanine aminotransferase, median, U/L | 23.5 (16–36.5) | 32 (21–50) | 22 (14–34) | ≥40, 158/741 |
| Aspartate aminotransferase, median, U/L | 28.5 (21–38) | 34 (26–48) | 26 (20–32) | ≥40, 168/757 |
| Creatinine, μmol/L | 68 (59.5–80) | 139 (137–140) | 72 (61–84) | ≥133, 12/752 |
| D-dimer, median, mg/L | 0.3 (0.1–0.6) | 0.5 (0.3–1.3) | 0.2 (0.2–0.5) | ≥0.5, 260/560 |
| Pneumonia | 68 (91.89) | – | 61 (98.39) | 972 (88.44) |
| Bilateral involvement | 52 (70.27) | 40 (97.56) | 52 (83.87) | – |
| ICU treatment | 18 (24.32) | – | 1 (1.61) | 55 (5.00) |
| Antiviral | 72 (97.30) | 38 (92.68) | 55 (89) | 393 (36) |
| Antibiotic | 60 (81.08) | 41 (100.00) | 28 (45.16) | 638 (58.05) |
| Steroid | 4 (5.41) | 9 (21.95) | 16 (25.81) | 204 (18.56) |
| Gamma globulin | 15 (20.27) | – | – | 144 (13.10) |
| Interferon | 16 (21.62) | – | 28 (45.16) | – |
| Oxygen therapy | 53 (71.62) | 41 (100.00) | – | 454 (41.31) |
| Noninvasive ventilation | 53 (71.62) | 10 (24.39) | – | 56 (5.10) |
| Invasive ventilation | 0 (0.00) | 2 (4.88) | – | 25 (2.27) |
| ECO | 0 (0.00) | 2 (4.88) | – | 5 (0.45) |
| Length of hospital stay | 13 (10–16) | – | – | 12 (10–14) |
| Death | 1 (1.35) | 6 (14.63) | 0 (0.00) | 15 (1.36) |
| Discharged | 73 (98.65) | 28 (68.29) | 1 (1.61) | 55 (5.00) |
4. Discussion
Since December 2019, COVID-19 caused by SARS-CoV-2 has rapidly spread from Wuhan city (Hubei Province) to different provinces and cities across China. By the end of February 25, 2020, China has reported >70,000 confirmed cases of COVID-19, 989 of which have been reported in Anhui Province. As far as we know, this study is the retrospective report with the largest number of cured COVID-19 patients in Anhui Province so far, including a total of 74 COVID-19 patients discharged from hospital (2 critical, 25 severe, 41 moderate, and 6 mild cases).
Lymphocytes and CD4/CD8 are sensitive indicators for evaluating the immune function of the body. The proportion of COVID-19 patients with lymphopenia in Wuhan was 63%. In this study, lymphopenia occurred in 46% of hospitalized COVID-19 patients and was common in ICU patients with a significantly decreased CD4/CD8 ratio (61%). In addition, inflammatory indicators such as ESR and CRP were significantly higher in the ICU patients than in non-ICU patients. Taken with other published literatures, our results further suggesting that the patients’ body suffered immune damage in the early course of the disease and was at the peak of the inflammatory response.[10–12]
Among the COVID-19 patients in this study, pulmonary CT scanning was able to identify unique properties of COVID-19. Therefore, pulmonary CT scan can be utilized as a preliminary screening strategy for patients at the early stage of the disease if viral nucleic acid testing facilities were absent. However, 6 COVID-19 patients had negative findings in pulmonary CT at hospital admission. In addition, 1 confirmed COVID-19 case in this study showed substantial ground-glass opacities in the pulmonary CT. Interestingly, this patient had 4 consecutive negative results of viral nucleic acid testing of throat swabs before hospital admission. Therefore, epidemiological history, clinical symptoms, and imaging findings should be comprehensively analyzed for the diagnosis of COVID-19.
With the in-depth study of COVID-19, the results of laboratory parameters and pulmonary CT scan have also guided clinicians to continuously improve the treatment plan, and individualized treatment based on a patient's conditions is still the current consensus.[13] Of our 73 COVID-19 patients who were cured and discharged, except for 1 pediatric and 1 death cases, the rest of the patients were given oral antiviral therapy with lopinavir and ritonavir tablets. After determining whether the patients were accompanied by or had a lung infection, 81.08% of the COVID-19 patients were given antibiotic prophylaxis and treatment. Steroid pulse therapy was given to severe patients (approximately 5%) who showed rapid pulmonary imaging progression, and this was lower than those reported in Wuhan, Zhejiang Province, and the whole country. Four patients with extensive lung involvement and high levels of Interleukin 6 (IL-6) were treated with the immunosuppressive drug tocilizumab. These patients were enrolled in an ongoing registered clinical trial. The median length of hospitalization of 73 cured patients was 13 days, which was similar to the results reported by Guan et al[14] (12 days), suggesting that the time window for the immune system to recover in COVID-19 patients was approximately 2 weeks.
All patients in this study underwent approximately 6 viral nucleic acid tests (including 2 negative results). When the clinical signs and imaging results of the patients improved, the viral nucleic acids in the body were likely still in the eradication stage (data not shown). A study reported that 4 patients with COVID-19 who met the discharge or quarantine criteria were all positive for the viral nucleic acid testing after 5 to 13 days, suggesting that at least some of the patients who recovered from COVID-19 may still be carriers of the virus.[15] Out study showed that one of the COVID-19 patients had a transient positive result of the viral nucleic acid in the follow-up period and had no sign of viral transmission, suggesting that the viral nucleic acid fragments may be discharged instead of the virus particles.
Although the present study has revealed several intriguing findings, some limitations should be acknowledged. Firstly, biased observations may be existed as patients were all treated in a tertiary care teaching hospital with abundant medical resource. Particularly, customized treatment plans made by provincial expert group were given to severe and critically ill patients. Secondly, sample size included in current study was not larger enough. Finally, the severity of the infection stated in the study may reflect symptoms of the patients rather than the virus load. More studies were needed to confirm this observation.
5. Conclusions
COVID-19 may vary in different regions, and COVID-19 patients in cities outside Wuhan were relatively mild. Approximately half of the COVID-19 patients in our hospital had a history of close contact with people from Wuhan. Fever, cough, expectoration, and fatigue were the most common symptoms in our patients. Although greater than one-fifth of the hospitalized COVID-19 patients had >2 underlying diseases, individualized treatments achieved satisfactory clinical results in this study.
Author contributions
Conceptualization: Kerong Wu, Ziqin Yao, Shiyuan Fang.
Data curation: Jiazhao Yang, Kerong Wu, Ao Ding, Li Li, Hui Lu, Wanbo Zhu, Kai Xie, Ziqin Yao, Shiyuan Fang.
Formal analysis: Jiazhao Yang, Kerong Wu, Ao Ding, Li Li, Hui Lu, Wanbo Zhu, Kai Xie, Ziqin Yao, Shiyuan Fang.
Writing – original draft: Jiazhao Yang, Kerong Wu, Ao Ding, Ziqin Yao, Shiyuan Fang.
Writing – review & editing: Ziqin Yao, Shiyuan Fang.
Footnotes
Abbreviations: COVID-19 = 2019 novel coronavirus disease, ICU = intensive care unit, MERS-CoV = Middle East respiratory syndrome coronavirus, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, WHO = World Health Organization.
How to cite this article: Yang J, Wu K, Ding A, Li L, Lu H, Zhu W, Xie K, Yao Z, Fang S. Clinical characteristics, treatment, and prognosis of 74 COVID-19 patients in Hefei: a single-center retrospective study. Medicine. 2021;100:21(e25645).
JY and KW have contributed equally to this paper.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Availability of data and materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate: This study was approved by the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China. Since the private information of patients in this research were excluded, the signing of written informed consent by the patients was waived.
Consent for publication: Not applicable.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
COVID-19 = 2019 novel coronavirus disease.
COVID-19 = 2019 novel coronavirus disease.
COVID-19 = 2019 novel coronavirus disease; ICU = intensive care unit.
COVID-19 = 2019 novel coronavirus disease; ICU = intensive care unit.
References
- [1].Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol 2020;92:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. WHO Interim guidance 28 January 2020. Clinical management of severe acute respiratory infection when novelcoronavirus (2019-nCoV) infection is suspected. (WHO/nCoV/Clinical/2020.2). [Google Scholar]
- [3].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:P497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hui DS, Perlman S, Zumla A. Spread of MERS to South Korea and China. Lancet Respir Med 2015;3:509–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Available at: http://2019ncov.chinacdc.cn/2019-nCoV/global.html. [Google Scholar]
- [6].Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606.https://pubmed.ncbi.nlm.nih.gov/32075786/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith G, Nielsen M. ABC of intensive care. Criteria for admission. BMJ 1999;318:1544–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. World Health Organization. Novel Coronavirus (2019-nCoV). Situation report-5; 2020. Available at: www.who.int/docs/default-source/coronaviruse/situation-reports/20200125-sitrep-5-2019-ncov. pdf?sfvrsn=429b143d_4. [Google Scholar]
- [9].Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med 2019;200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Słomka A, Kowalewski M, Żekanowska E. Coronavirus Disease 2019 (COVID-19): a short review on hematological manifestations. Pathogens 2020;9:493.https://pubmed.ncbi.nlm.nih.gov/32085846/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rabi FA, Al Zoubi MS, Kasasbeh GA, et al. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens 2020;9:231.https://pubmed.ncbi.nlm.nih.gov/32575786/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gencer S, Lacy M, Atzler D, et al. Immunoinflammatory, thrombohaemostatic, and cardiovascular mechanisms in COVID-19. Thromb Haemost 2020;160:1629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lan L, Xu D, Ye G, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020;323:1502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


