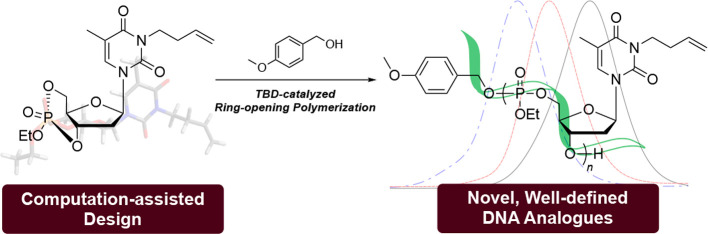
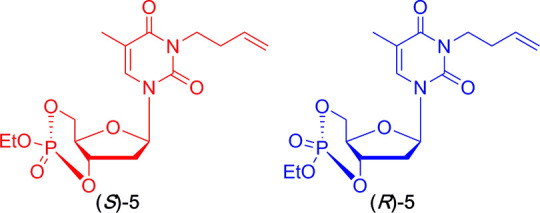
There are mistakes in the stereochemical structures for (R)-5 and (S)-5 in the original manuscript in the Abstract graphic, Scheme 1, Scheme 2, Figure 2, Figure 3, Table 2, and the Supporting Information (the chemical structures are shown here as original and corrected structures in Chart 1). These changes do not affect any of the conclusions. The corrected abstract graphic, figures, schemes, and tables are shown below. The corrected structures are provided in the revised Supporting Information. We apologize to the reader for any inconvenience.
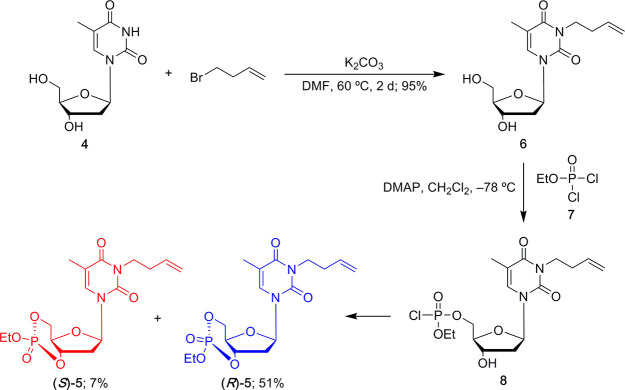
Scheme 1. Synthetic Route from Thymidine to Monomer 5.
Scheme 2. Polymerization of 5 with 4-Methoxybenzyl Alcohol as the Initiator and TBD as the Catalyst.
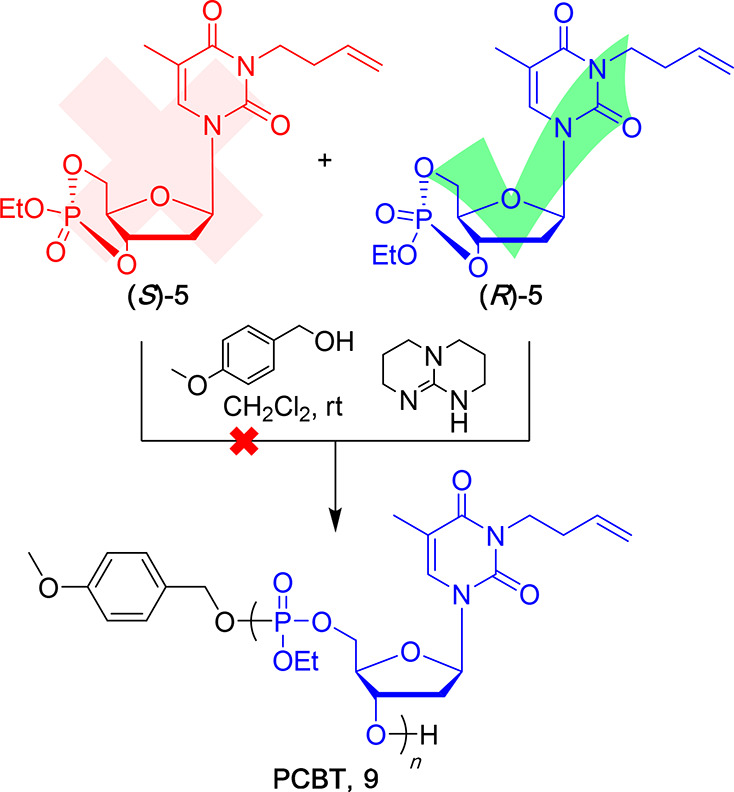
Although the polymer is illustrated with only one regiochemistry and no stereochemistry, 31P NMR spectra suggested that the polymers contained regioisomeric and diastereoisomeric repeat units.
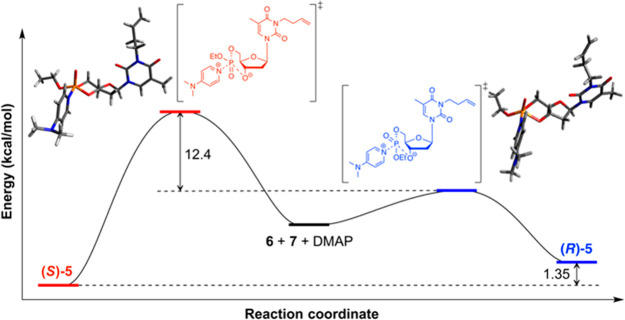
Figure 2.
Reaction coordinate diagram of using DMAP as activator to promote cyclization of 6 at the B3LYP/6-31+G* level of theory.
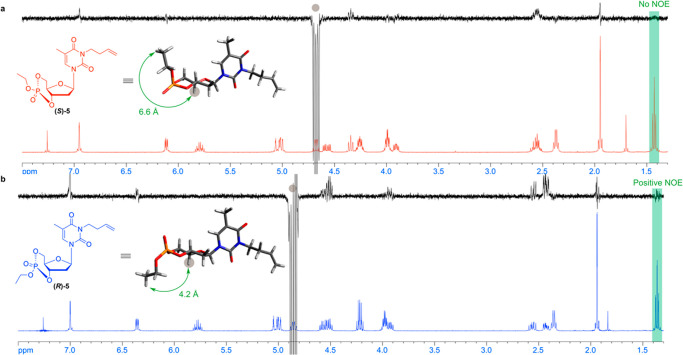
Figure 3.
Use of 1D-NOESY to identify the diastereomers (a) (S)-5 and (b) (R)-5, with atomic distances of 6.6 and 4.2 Å, respectively, calculated from DFT geometric optimization at the B3LYP/6-31+G* level of theory.
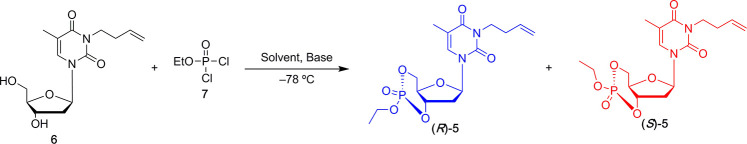
Table 2. Condition Screening for Cyclization of 6 with 7 Using Various Solvents and Bases at −78 °C.
| entry | solvent | base | result | yield (%) | (R)/(S)a |
|---|---|---|---|---|---|
| 1 | THF | Et3N | oligomerization | ||
| 2 | THF | pyridine | oligomerization | ||
| 3 | THF | DMAP | oligomerization | ||
| 4 | THF | DIPEA | oligomerization | ||
| 5 | DMF | Et3N | oligomerization | ||
| 6 | DMF | pyridine | oligomerization | ||
| 7 | DMF | DMAP | oligomerization | ||
| 8 | DMF | DIPEA | oligomerization | ||
| 9 | CH2Cl2 | Et3N | no reaction | ||
| 10 | CH2Cl2 | pyridine | no reaction | ||
| 11 | CH2Cl2 | DMAP | cyclization | 58 | 88:12 |
| 12 | CH2Cl2 | DIPEA | cyclization | 4 | 8:92 |
The (R)/(S) ratio was determined by 31P NMR of reaction crude.
Chart 1. Original and Corrected Chemical Structures of (R)-5 and (S)-5.
Acknowledgments
We thank Yelena Lipskerova of Chemical Abstracts Service for drawing our attention to the mistakes in the stereochemical assignments of the chemical structures.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b01661.
Experimental procedures, spectroscopic data for all new compounds including 1H, 13C, and 31P NMR spectra, TGA, DSC, and details of computational chemistry (PDF)
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.