Abstract
Cryptosporidiosis is a leading cause of moderate-to-severe diarrhea in low- and middle-income countries, responsible for high mortality in children younger than two years of age, and it is also strongly associated with childhood malnutrition and growth stunting. There is no vaccine for cryptosporidiosis and existing therapeutic options are suboptimal to prevent morbidity and mortality in young children. Recently, novel therapeutic agents have been discovered through high-throughput phenotypic and target-based screening strategies, repurposing malaria hits, etc., and these agents have a promising preclinical in vitro and in vivo anti-Cryptosporidium efficacy. One key step in bringing safe and effective new therapies to young vulnerable children is the establishment of some prospect of direct benefit before initiating pediatric clinical studies. A Cryptosporidium controlled human infection model (CHIM) in healthy adult volunteers can be a robust clinical proof of concept model for evaluating novel therapeutics. CHIM could potentially accelerate the development path to pediatric studies by establishing the safety of a proposed pediatric dosing regimen and documenting preliminary efficacy in adults. We present, here, perspectives regarding the opportunities and perceived challenges with the Cryptosporidium human challenge model.
Keywords: diarrhea, cryptosporidiosis, human-challenge model, drug discovery, Cryptosporidium, pediatric development, antiparasitic agent, CHIM
Cryptosporidiosis Medical Need
Cryptosporidium spp. are protozoan parasites responsible for acute enteritis with diarrhea as the primary clinical symptom. Cryptosporidiosis in humans is caused primarily by two species, Cryptosporidium parvum and Cryptosporidium hominis.1 Transmission typically occurs when feces containing Cryptosporidium oocysts from infected animals or humans contaminate food or water supplies, thereby infecting humans predominantly via the fecal oral route. Once ingested, the oocysts reach the small intestine, where motile, infectious sporozoites are released and infect intestinal epithelial cells. The organisms then goes through multiple cycles of asexual replication, followed by sexual reproduction, ultimately resulting in excretion of numerous mature oocysts in the feces.2 During a single infection period individuals may shed up to 108–109 oocysts.3
Cryptosporidiosis is a self-limiting infection in immunocompetent adults and can be successfully managed with supportive care and treatment. In the vulnerable patients population (young children and immunocompromised adults), Cryptosporidium infection is associated with prolonged (7–14 days) or persistent (>14 days) diarrhea.4 Indeed, Cryptosporidium spp. are a leading cause of pediatric diarrhea in low- and middle-income countries (LMICs) and represents one of the leading causes of diarrheal deaths in young children aged 0–24 months.5−8 Cryptosporidiosis is estimated to be responsible for 48 000–202 000 deaths annually in children younger than two years of age in South Asia and Sub-Saharan Africa and ∼7.6 million diarrhea cases annually are attributable to Cryptosporidium infection in these regions.5,9 In addition, evidence suggests that repeated Cryptosporidium infections in children are associated with long-term effects and debilitating growth-stunting.10,11
Nitazoxanide (Alinia) is the only drug approved by the U.S. Food and Drug Administration for the treatment of cryptosporidiosis in children aged 1 year of age and older and immunocompetent adults.12,13 It is a safe oral antiparasitic agent and significantly improves clinical response and reduces the duration of diarrhea and oocyst shedding in immunocompetent adults with cryptosporidiosis.14,15 As a parasitistatic agent,16 efficacy of nitazoxanide is largely dependent on host-immunity and is not effective for treating cryptosporidiosis in immunocompromised patients.17,18 In a study that enrolled HIV-negative, malnourished children, nitazoxanide treatment resulted in resolution of diarrhea in only 56% of children (23% in placebo group) and only 52% demonstrated oocyst clearance (14% in placebo group).19 The limited efficacy of nitazoxanide in malnourished children may be attributed to immunological alterations or intestinal dysbiosis associated with malnutrition in these children.20,21 Overall, there is a pressing, highly unmet therapeutic need to address enteric cryptosporidiosis in three major target patient populations: young children aged 0–24 months in LMICs, malnourished children under age five, and immunosuppressed individuals of any age.22
Anti-Cryptosporidium Drug Discovery and Development Efforts
Despite the substantial global disease burden and a clear need for effective antiparasitic treatments, cryptosporidiosis remains an under-appreciated global health concern. Earlier efforts to repurpose approved drugs, such as paromomycin, rifamycin, spiramycin, azithromycin, letrazuril, HIV protease inhibitors, or clofazimine, for the treatment of cryptosporidiosis in HIV-AIDS patients have been unsuccessful.1,23 Recently, significant progress has been made in identifying and optimizing diverse new chemical entities (NCEs) with promising in vitro activity and in vivo efficacy as defined in the proposed target product profile for cryptosporidiosis treatment.24,25 Some of the promising NCEs include Cryptosporidium calcium-dependent protein kinase 1 (CpCDPK1) inhibitors,26 phosphatidylinositol-4-OH kinase (PI(4)K) inhibitors,27 piperazine-based lead compound MMV665917,16 lysyl-tRNA synthetase (KRS) inhibitors,28 oxaboroles that are a cleavage and polyadenylation specificity factor3 inhibitors,29,30 bicyclic azetidines that are phenylalanyl-tRNA synthetase inhibitors,31 methionyl-tRNA synthetase inhibitors,32 a choline-based phospholipid VB-201,33 and multiple novel cell-active hits.34 Most of these NCEs have demonstrated antiparasitic activity against both C. parvum and C. hominis. Further, unlike nitazoxanide, many of these anti-Cryptosporidium NCEs are effective in reducing the fecal oocyst burden in immunocompromised mouse models. This rich and diverse pipeline of drug candidates is very encouraging and could also enable drug combinations to address the potential for drug resistance.32
To address the unmet medical need in the highly vulnerable young pediatric cryptosporidiosis patient population, the most critical aspects are an exceptional safety profile and robust efficacy demonstrated by rapid resolution of diarrhea to minimize the risk of dehydration. A few candidate molecules such as the CDPK inhibitor BKI-1369,26 PI(4)K inhibitor KDU731,27 MMV6659917,16 and 6-carboxamide benzoxaborole AN797329 have demonstrated promising activity in resolving diarrheal symptoms in neonatal calves, a preclinical model of cryptosporidiosis diarrhea which closely resemble pediatric infection and illness. Overall, in the past few years, substantial progress has been made in identifying diverse NCEs, and it is anticipated that some may soon start clinical development.
Challenges in Developing a Novel Antiparasitic Agent to Treat Pediatric Cryptosporidiosis
Cryptosporidiosis disproportionately affects young children, and the highest unmet medical need is in the malnourished who are at the greatest risk for severe disease and mortality.22 Drug development is expensive, takes considerable time, has a high attrition rate and the pediatric population in LMICs presents additional challenges. Four study populations to establish proof of concept (PoC) of new anti-Cryptosporidium compounds are possible: (i) adult immunocompromised patients in LMICs; (ii) adult patients during a sporadic outbreaks; (iii) malnourished pediatric patients in endemic regions; and (iv) a Cryptosporidium controlled human infection model (CHIM) in healthy adult volunteers. Typical drug development and regulatory pathways involve demonstrating the prospect of direct benefit in adult populations before initiating pediatric studies.35
Cryptosporidium infection is a common cause of chronic diarrhea in HIV/AIDS patients in LMICs.36 Currently, the best treatment is reconstitution of the immune response via antiretroviral therapy. HIV/AIDS patients are the only naturally occurring adult population of adequate size to facilitate early stage drug efficacy studies for cryptosporidiosis in LMICs. However, a recent controlled clinical trial to assess the safety and efficacy of clofazimine for the treatment of cryptosporidiosis in this population highlighted the significant challenges with this approach.23 In addition to the operational complexity of conducting early phase clinical trials in resource-poor settings,37 safety and efficacy evaluation in the HIV/AIDS cryptosporidiosis patient population is highly confounded by the severe immunocompromised state, presence of multiple diarrheal pathogens, other opportunistic coinfections, concurrent medications, failure of antiretroviral therapy, and high mortality.38 As a potential alternative to investigating NCEs in HIV/AIDS cryptosporidiosis coinfected patients, a Cryptosporidium CHIM in adult healthy volunteers is considered herein as it offers a scientifically robust path to PoC for novel antiparasitic agents. In CHIM, the infectious pathogen is administered to healthy adult volunteers with the intent to deliberately induce infection and clinical symptoms in a controlled setting. Novel therapies can then be evaluated in a randomized blinded treated cohort and compared to the clinical symptoms and disease duration in the untreated (placebo-treated) cohort. Across different infectious diseases, CHIM studies have played a very important role for the understanding of disease mechanisms and also for establishing PoC for drug and vaccine development.39−41 A sporadic outbreak of cryptosporidiosis is not a feasible option for structured drug development due to its anticipated protracted time period. Table 1 summarizes the pros and cons of the other three PoC human efficacy studies, namely, Cryptosporidium CHIM, HIV-positive adult cryptosporidiosis patients and pediatric cryptosporidiosis patient populations for testing novel anti-Cryptosporidium NCEs.
Table 1. Pros and Cons of Potential First in Human Proof of Concept Efficacy Studies for Testing a Novel Anti-Cryptosporidium NCEs.
| pros | cons | |
|---|---|---|
| Cryptosporidium controlled human infection model (CHIM) in healthy adults | · prospect of benefit in healthy adults with Cryptosporidium induced diarrhea | · C. parvum model utilized for technical reasons, although C. hominis more common human pathogen |
| · informs dose selection for studies in pediatric patients | · needs to be established and validated | |
| · clinical syndrome, parasitological and clinical end points under monoinfection condition | · limited viability period of GMP oocysts | |
| · conducted in healthy volunteers, mitigates safety confounders | · monoinfection state may not be clinically relevant to target pediatric patient population | |
| · phase 1 settings: faster recruitment and smaller sample size | · unknown translatability of efficacy to target population | |
| Adult HIV-positive cryptosporidiosis patients | · natural infection in potential secondary target population | · confounded safety and efficacy due to advanced immunocompromised state |
| · PK in context of high GI motility | · presence of other pathogens/coinfections and/or concurrent medications | |
| · high mortality | ||
| · operational complexity in the resource poor settings | ||
| Pediatric cryptosporidiosis patient population | · assessment of safety and efficacy in the target population | · prospect of clinical benefit will not have been previously established |
| · natural course of infection | · high-risk and vulnerable patient population | |
| · with relevant clinical strains | · uncertainty in predicted efficacious dose in the context of high GI motility | |
| · risk investment in juvenile toxicity study prior to phase I to avoid program delays | ||
| · operational complexity in the resource poor settings |
Establishing a Cryptosporidium CHIM to Enable Drug Discovery
More than 15 species of Cryptosporidium are known to cause human infection with two predominant clinical species, C. hominis (∼80%) and C. parvum (∼10%).9 The safety and feasibility of controlled human Cryptosporidium challenge studies are well documented in the literature, using C. parvum,42−48C. hominis,49C. meleagridis,50 and C. muris.51 These challenge studies focused on: identifying the minimum human infectious dose for C. parvum and C. hominis; comparing the clinical symptoms caused by different clinical isolates; understanding the impact of prior Cryptosporidium infection on rechallenge; assessing fecal inflammatory markers; and exploring mechanisms of pathogenesis (Table 2). To date, more than 200 healthy adult volunteers have been challenged with Cryptosporidium oocysts, of which ∼175 were infected with various isolates of C. parvum. Among them, C. parvum Iowa isolate from the University of Arizona was used in 5 of the 7 published studies.43,44,46−48 The C. parvum Iowa isolates used were not from a single oocyst stock, but were continuously propagated in calves, which could lead to mutations and/or genetic drift in oocysts over time. In these CHIM studies, a high percentage of infections with Cryptosporidium could be elicited and many of the infected individuals developed clinical symptoms after challenge (summarized in Table 2). In addition, no safety concerns (other than clinical symptoms of acute cryptosporidiosis) have been observed in any of the CHIM studies with doses up to 106 oocysts.
Table 2. Summary of the Published Cryptosporidium CHIM Studies.
| species isolate | study reference | dose | N | infectiona (%) | illnessa (%) | notes |
|---|---|---|---|---|---|---|
| C. parvum Iowa | DuPont, NEJM 1995 | 3 × 101–106 | 29 | 20–100 | 0–38 | · first Cryptosporidium human challenge study; ID50 established 132 oocysts |
| · oocyst purified from neonatal calves | ||||||
| · self-limited infection and illness observed; at dose ≥1000 oocysts: 100% infection, 71% enteric symptoms and 29% diarrheal illness observed | ||||||
| C. parvum Iowa | Okhuysen, Inf Imm 1998 | 5 × 102 | 19 | 84 | 58 | · rechallenge (extension of DuPont 1995) study in healthy adults |
| · fewer subjects shed oocysts after the second exposure (16%) than after the first exposure (63%) | ||||||
| · lower “intensity of diarrhea” with rechallenge | ||||||
| C. parvum Iowa | Chappell, AJTMH 1999 | 5 × 102–5 × 104 | 17 | 41 | 59 | · infectivity in pre-existing anti-C. parvum serum IgG |
| · ID50 is 1880 oocysts, 20× higher than in seronegative volunteers | ||||||
| · prior exposure provides protection from infection and illness at low oocyst doses | ||||||
| C. parvum Iowa | Okhuysen, JID 1999 | 3 × 101–105 | 29 | 40–100 | 52 | · virulence of 3 C. parvum isolates compared, Iowa and UCP, originally isolated from calves and passaged in calves; whereas TAMU isolated from a student who got infected from foal, also passaged in calves |
| C. parvum UCP | 5 × 102–104 | 17 | 10–100 | 59 | · ID50 for Iowa, UCP, and TAMU established as 87, 1042, and 9 oocysts, respectively, based on presumed infection | |
| C. parvum TAMU | 101–5 × 102 | 14 | 0–100 | 86 | · TAMU isolate induced higher diarrhea rate for a longer duration than Iowa or UCP isolates | |
| C. parvum Iowa/TAMU | Alcantara, AJTMH 2003 | 102–103 | 15 | 50–67 | 33–83 | · importance of intestinal inflammation in C. parvum challenge versus pediatric patients as measured by fecal IL8, lactoferrin, and TNFα |
| · significantly more inflammation in pediatric Cryptosporidium patients than adult volunteers | ||||||
| C. parvum UCP | Okhuysen, CID 1998 | 5 × 103–1 × 104 | 20 | 44–100 | 56–75 | · prophylactic effect of bovine hyperimmune anti-Cryptosporidium colostrum |
| hyperimmune colostrum not protective against infection | ||||||
| C. parvum Moredun | Okhuysen, JID 2002 | 102–3 × 103 | 16 | 33–75 | 60–75 | · oocysts originally isolated from red deer, passaged in sheep and later in calves |
| · ID50 300 oocysts; diarrheal illness was frequently associated with oocyst excretion | ||||||
| Healthy Volunteers Challenged with C. parvum: N = 176 | ||||||
| C. hominis TU502 | Chappell, AJTMH 2006 | 101–5 × 102 | 21 | 20–80 | 40–75 | · first C. hominis human challenge study, ID50 established 10–83 oocysts |
| · oocyst purified from gnotobiotic piglets | ||||||
| · infection and illness similar to C. parvum challenge studies | ||||||
| C. meleagridis | Chappell, AJTMH 2011 | 105 | 5 | 100 | 80 | · first C. meleagridis human high-dose challenge study |
| · oocyst purified from gnotobiotic piglets | ||||||
| · caused self-limited infection and mild diarrheal illness | ||||||
| C. muris | Chappell, AJTMH 2015 | 105 | 6 | 100 | 33 | · first C. muris human high-dose challenge study |
| · oocyst purified from Nu/Nu mouse | ||||||
| · caused persistent infection and self-limited diarrheal illness | ||||||
| · persistent shedders were treated with nitazoxanide, and the infection was resolved | ||||||
Note: In most studies, “illness” was defined as the passage of 3 unformed stools in 8 h or >3 unformed stools in 24 h accompanied by the presence of one or more enteric symptoms, including fever, nausea, vomiting, abdominal pain or cramps, and gas-related intestinal symptoms. “Infection” was defined as the excretion of oocysts in stool by a direct immunofluorescence assay (DFA) after a flow-through period of 36 h postchallenge.
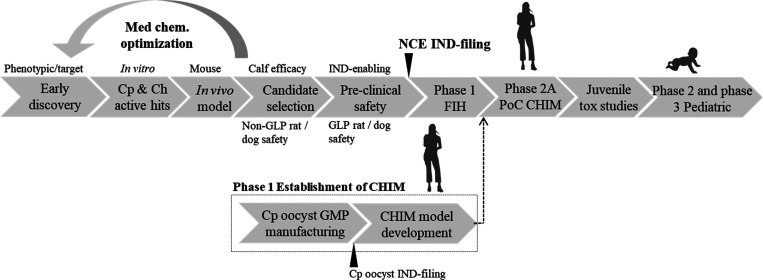
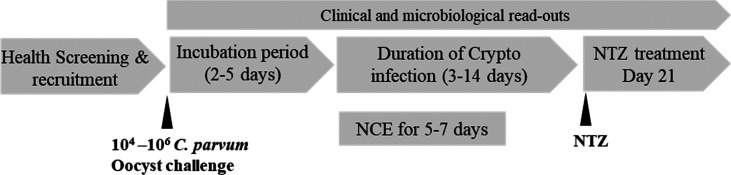
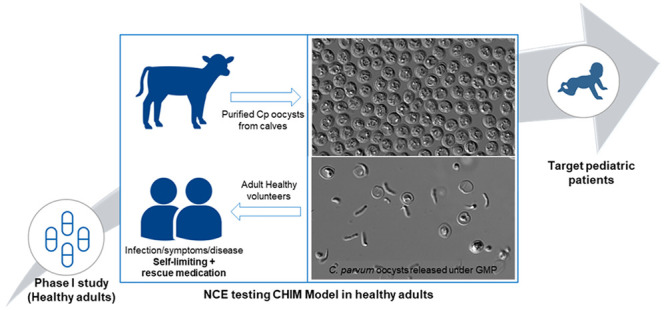
We propose establishing a C. parvum Iowa isolate high-dose oocyst human challenge model to enable future assessment of NCEs for the treatment of cryptosporidiosis. The proposed path for pediatric cryptosporidiosis drug discovery and development incorporates CHIM for establishing PoC for efficacy in adults (Figure 1). The synopsis of a CHIM study design is further outlined in Figure 2. Following Cryptosporidium challenge, healthy, immunocompetent individuals may experience profuse, watery, nonbloody diarrhea after an incubation period of 3–12 days. Without any treatment, symptoms are expected to resolve within 2–3 weeks or less (mean duration of 12.7 days) but could persist for up to a month. Thus, to minimize the risk of long-term asymptomatic shedding and/or recurrence, all subjects with elicited infection will receive nitazoxanide treatment, the standard of care, at the end of the 21-day study. Of note, as a precaution any subjects who remain asymptomatic postchallenge will also be treated to prevent any potential secondary transmission. Once CHIM is established, it could potentially be used for a NCE development after phase I studies. The following section highlights the opportunities and challenges with Cryptosporidium CHIM in healthy adult volunteers, a model designed for establishing efficacy with NCEs.
Figure 1.
Pediatric cryptosporidiosis drug discovery and development, a proposed path to registration. Development of C. parvum oocyst CHIM is shown above. Cp, C. parvum and Ch, C. hominis.
Figure 2.
Proposed controlled human C. parvum high-dose infection model for testing NCE with anticipated incubation period and duration of infection. NTZ, nitazoxanide.
Opportunities and Challenges in Cryptosporidium CHIM
Some of the major advantages of Cryptosporidium CHIM are as follows:
It enables the assessment of the prospect of benefit in adults before NCE is advanced into efficacy studies in the vulnerable pediatric patient population in the LMICs.
Subjects in a cryptosporidiosis CHIM will have a typical noninflammatory diarrhea because of a single pathogen, allowing for unconfounded interrogation of the effect of an investigational drug on clinical and parasitological end points.
It allows for careful and extensive analysis of the pharmacokinetic–pharmacodynamic (PK–PD) relationship of an investigational drug in the presence of diarrhea, providing valuable data that informs dose selection for future pediatric clinical trial designs.
Cryptosporidium challenges induce nonlife threatening, self-limiting infections in healthy adults with the option to use nitazoxanide as the rescue medication.
Finally, CHIM PoC efficacy studies can help prioritize NCEs for juvenile toxicity studies designed to understand potential adverse effects on postnatal growth and development, thereby hastening the pediatric clinical development.
Overall, Cryptosporidium CHIM may enable scientifically rigorous and rapid clinical development path for novel drug candidates to treat cryptosporidiosis in young children. However, there are some challenges in establishing and utilizing a Cryptosporidium CHIM for drug development. Some of these challenges and mitigation strategies are described below.
Challenge Organisms Are Regulated As Biological Products and Drugs in the US
According to a 2013 guidance from the US Food and Drug Administration (FDA), an Investigational New Drug Application (IND) is required for challenge studies in which a live organism is administered to subjects to study the pathogenesis of disease or the host response to the organism. While the challenge organism is not intended to have a therapeutic purpose, there is intent to affect the structure or function of the body. Consequently, the FDA considers the organism to be both a biological product and a drug and therefore subject to the corresponding regulatory requirements.52 As per the Federal Food, Drug, and Cosmetic Act, current good manufacturing practice (CGMP) must be in effect for the manufacture of investigational drug used during phase 1 clinical trials.53C. parvum human challenge studies (Table 2) were conducted in the US during 1990s and early 2000s with oocysts purified from experimentally infected neonatal calves. At the time, there was no requirement for an IND application for the challenge organism. Currently, there is no suitable robust manufacturing process available to produce Cryptosporidium oocysts ex vivo. A major barrier to producing large quantities of oocysts ex vivo has been the lack of a robust and reproducible in vitro culture system, although a hollow fiber continuous culture setup has been described with C. parvum.54 Our attempts to establish a robust hollow fiber C. parvum in vitro culture system were unsuccessful. The proposed alternate approach is by obtaining a purified C. parvum (Iowa isolate) oocysts from experimentally infected neonatal calves (a non-GLP source, Good Laboratory Practice). Oocysts are, then, surface sanitized, quality tested, and released by a GMP facility for use in the establishment of CHIM. We have also developed a surface sanitization protocol using peracetic acid to inactivate potential microbial and viral contaminants and demonstrated that this process is effective while having limited impact on C. parvum oocyst viability (Jumani et al., unpublished). Cryptosporidium oocysts can withstand peracetic acid treatment in contrast to other organisms.55,56 Peracetic acid is one of the most effective organic peroxide broad-spectrum biocide agents. It has been cleared by FDA as a sanitizer for direct food/food contact surfaces and recommended by CDC for the disinfection and sterilization of healthcare facilities and equipment, including reusable medical and dental devices. To confirm the effectiveness of the sanitization procedure, we have tested the oocysts treated with peracetic acid for the presence of microbial contaminants and shown that the sanitized oocysts do not contain any viable aerobic or anaerobic microorganisms as determined by regulatory guidelines. Furthermore, to confirm the effectiveness of the sanitization procedure on viral contaminants, purified oocysts were artificially contaminated with six different types of model viruses. The peracetic acid treatment reduced the infectivity of the spiked viruses to below the limit of detection. Currently, we are evaluating the logistics and feasibility of releasing C. parvum oocysts under GMP for CHIM studies.
C. parvum Oocysts Gradually Lose Viability with Storage
Despite being highly resistant to harsh disinfection conditions, reliable cryopreservation of Cryptosporidium oocysts has been a long-standing challenge. A cryopreservation method for C. parvum oocysts has been recently developed,57,58 but the scalability and impact of cryopreservation to elicit human infection has not been evaluated. Currently, the routinely used storage condition for C. parvum oocysts is in aqueous suspension at 2–8 °C for 4–6 months. As the oocyst suspension ages, the viability reproducibly decreases and thus, the potential to induce an infection also decreases. Consequently, to ensure consistent infectivity in the clinic, CHIM will require a fresh batch of GMP oocysts every few months and may need to adjust the oocyst dose for loss of viability over time.
Majority of Clinical Infections Are Caused by C. hominis and Anthroponotic C. parvum Strains
Epidemiologic studies have revealed that the majority of clinical infections in the endemic countries is caused by C. hominis (∼80%) and anthroponotic C. parvum (∼10%) isolates.59−61 The proposed high-dose oocyst human challenge model uses a C. parvum Iowa isolate, a zoonotic species which can cause a profuse watery diarrhea in both cattle and humans. Therefore, effectiveness of NCE in the CHIM may not directly reflect the efficacy against the most predominant clinical species. Though C. parvum and C. hominis share ∼96% nucleotide identity,62,63 it is critical to make sure the molecular target is conserved across Cryptosporidium species and determine the activity of NCE against C. hominis in early preclinical drug discovery stages. Several promising NCEs have been reported to have similar potency against C. parvum and C. homins in vitro suggesting the molecular target is conserved across these two species.16,26,27,29
No Clear Relationship between the C. parvum Oocyst Infective Dose and Clinical Illness in Healthy Adults Has Been Established
The C. parvum Iowa isolate human challenge studies described in the literature have demonstrated that this isolate is capable of inducing infection in up to 100% of healthy volunteers with adequate doses of oocysts, but not all infected volunteers will develop diarrhea or other gastrointestinal (GI) symptoms (Table 2).43,44,46−48 In one study, 100% of healthy volunteers (n = 7) receiving ≥1000 C. parvum oocysts, that is, approximately 10 times above ID50 (infective dose) developed infection as measured by fecal oocyst shedding; 71% had enteric symptoms, but of these only 29% had diarrheal illness.46 The absence of a clear relationship between infective dose and diarrheal illness in healthy adults poses a challenge for using CHIM to demonstrate efficacy in improving diarrheal syndrome. It is likely that the positive health status of CHIM participants contributes to this variability. Multiple factors contribute to the susceptibility of the host to clinical manifestations such as host immune status, gut health, GI microbiota, the virulence of the C. parvum isolate and prior exposure to Cryptosporidium. Our proposed strategy is to use a high oocyst dose to increase the probability of infection and clinical symptoms in CHIM participants. We anticipate that ideally robust parasitological infection will be observed. However, to test efficacy of NCE, sufficient and consistent clinical illness along with parasitological infection in a significant proportion of healthy adults may be needed.
Risk/Benefit Consideration for Participants
Aside from a long-term philanthropic contribution to the development of novel therapies for cryptosporidiosis, there is no direct benefit expected for healthy adults participating in a CHIM study. The risks to healthy participants may include GI cryptosporidiosis with mild to severe diarrhea, asymptomatic infections, persistent or recurrent illness, and possible secondary transmission. Extraintestinal manifestations in immunocompetent healthy adults have not been described in the published Cryptosporidium human challenge studies. Both symptomatic and asymptomatic infections may result in secondary transmission to household members and other contacts. Overall, the risks to participants and their contacts can be appropriately addressed in a clinical trial protocol for a CHIM study. In comparison, in longitudinal studies of adult outbreak-associated cryptosporidiosis, medium to long-term sequelae after resolution of the acute infection included diarrhea, abdominal pain, nausea, fatigue, headache, and joint pain.64,65 These long-term sequelae were more prevalent following infection with C. hominis than C. parvum.66,67 The impact of nitazoxanide treatment on long-term sequelae is unknown. In general, the interpretation of self-reported data from outbreak-associated cohorts requires caution given the potential for bias toward those most adversely affected and those who attributed postacute symptoms to acute cryptosporidiosis. Further, no such long-term sequelae have been described in the published C. parvum CHIM studies (Table 2). However, long-term follow up beyond 6–8 weeks was not conducted in most of these studies, but can be potentially monitored in future CHIM studies. Recently, an association between Cryptosporidium infections and GI cancers have been proposed, but a causal relationship has not been established.68,69 In a 2015, C. muris challenge study, two subjects with persistent oocyst shedding were successfully treated with nitazoxanide at 200 mg twice a day for 3 days, and the infection was resolved in both subjects, demonstrating the potential of nitazoxanide as a rescue drug.51 We propose to administer nitazoxanide to all participants in whom infection was elicited at the conclusion of the study or earlier in case of persistent or severe diarrhea to eliminate any remaining infection and decrease potential long-term risks.
Uncertain Translatability of NCE Efficacy in a CHIM to Pediatric Cryptosporidiosis Diarrhea
In immunocompetent adults, Cryptosporidium infection causes self-limiting GI illness and symptoms most often completely self-resolve within 1–2 weeks (Table 2). In contrast, Cryptosporidium infection in young children, especially the malnourished or otherwise immunocompromised, is associated with life-threatening diarrhea with severe morbidity and mortality.6−8 In this vulnerable patient population, Cryptosporidium infection is often associated with persistent diarrhea (>14 days) leading to a significant adverse effect on linear (height) growth and nutritional shortfalls.11,70 Young children with cryptosporidiosis have more severe inflammation as measured by fecal lactoferrin levels as compared to adult volunteers.48 This may be due to various factors, including more severe diarrheal illness in children than healthy adults, presence of other enteric pathogens, nutritional status, gut health, sensitivity to fluid loss, and also differences in the virulence of Cryptosporidium isolates. Overall, cryptosporidiosis induced experimentally in healthy adults is not the same as the disease observed in the pediatric patients especially with respect to host health status, severity of diarrheal illness and complexity of pathogenesis. However, since the human challenge model recapitulates logarithmic parasite replication in the GI tract leading to fecal oocyst shedding, acute watery diarrhea, and other GI symptoms similar to pediatric patients, the CHIM is a scientifically robust and efficient approach to assess promising antiparasitic agents.
Summary
Cryptosporidium is the second leading cause of diarrhea in young children and a major contributor for diarrheal deaths in LMICs. While cryptosporidiosis disproportionately affects young children, establishment of an adult CHIM is a scientifically robust and efficient way to assess novel antiparasitic agents with relatively less safety risk. Following a standard phase 1 with NCE in healthy adults, the Cryptosporidium CHIM would be a stepping stone to pediatric trials. It should help establishing a prospect of benefit for NCEs in healthy adults before advancing to the vulnerable pediatric population; derisking investment in juvenile toxicology studies; and providing PK/PD data to inform dose selection for pediatric trials.
Three key safety pillars of the proposed C. parvum CHIM studies protect participating healthy adults. First, a GMP-compliant oocyst manufacturing process to be established with sanitization, testing and batch release of oocysts as an investigational medical product. Second, the safety experience from several published CHIM studies.43,44,46−48 And finally, C. parvum infection in healthy adults causes a self-limiting illness and an effective rescue medication is available. A Cryptosporidium CHIM has the potential to accelerate the development of both new therapeutics and vaccines against cryptosporidiosis. The recent accomplishments in early drug discovery and availability of a Cryptosporidium controlled human infection model offer a compelling vision toward enabling a much-needed parasite-specific treatment for young children suffering from the debilitating effects of cryptosporidiosis.
Acknowledgments
We would like to thank Gu Feng, Jean Messina, Juanita Potts, Megan Osborn, Joe Young, Xiaojun Zhao, and other colleagues from Novartis for their support and Deborah Schaefer and Michael Riggs from the University of Arizona and Christopher Huston from the University of Vermont for advice. This work in part supported by Novartis and the Wellcome Trust (Project Number: 219639/Z/19/Z). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
† R.S.J. and J.B. contributed equally.
All views expressed are those of the authors and not necessarily of their affiliated institutions.
The authors declare the following competing financial interest(s): R.J., J.B., H.T., F.S., D.W., C.O., N.N., J.L., N.A., R.C., T.D., and U.M. are employees of Novartis. C.C. is officially retired from The University of Texas School of Public Health, Houston, and is a consultant to Novartis. W.C. was previously funded by the Bill and Melinda Gates Foundation to plan the establishment of Cryptosporidium CHIMs.
Author Status
‡ Retired.
References
- Checkley W.; White A. C.; Jaganath D.; Arrowood M. J.; Chalmers R. M.; Chen X. M.; Fayer R.; Griffiths J. K.; Guerrant R. L.; Hedstrom L.; Huston C. D.; Kotloff K. L.; Kang G.; Mead J. R.; Miller M.; Petri W. A.; Priest J. W.; Roos D. S.; Striepen B.; Thompson R. C.; Ward H. D.; Van Voorhis W. A.; Xiao L.; Zhu G.; Houpt E. R. (2015) A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis. 15 (1), 85–94. 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current W. L.; Reese N. C. (1986) A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J. Protozool. 33 (1), 98–108. 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- Chappell C. L.; Okhuysen P. C.; Sterling C. R.; DuPont H. L. (1996) Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J. Infect. Dis. 173 (1), 232–6. 10.1093/infdis/173.1.232. [DOI] [PubMed] [Google Scholar]
- Moore S. R.; Lima N. L.; Soares A. M.; Oria R. B.; Pinkerton R. C.; Barrett L. J.; Guerrant R. L.; Lima A. A. (2010) Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology 139 (4), 1156–64. 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil I. A.; Troeger C.; Rao P. C.; Blacker B. F.; Brown A.; Brewer T. G.; Colombara D. V.; De Hostos E. L.; Engmann C.; Guerrant R. L.; Haque R.; Houpt E. R.; Kang G.; Korpe P. S.; Kotloff K. L.; Lima A. A. M.; Petri W. A. Jr.; Platts-Mills J. A.; Shoultz D. A.; Forouzanfar M. H.; Hay S. I.; Reiner R. C. Jr.; Mokdad A. H. (2018) Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet. Global health 6 (7), e758–e768. 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K. L.; Nasrin D.; Blackwelder W. C.; Wu Y.; Farag T.; Panchalingham S.; Sow S. O.; Sur D.; Zaidi A. K. M.; Faruque A. S. G.; Saha D.; Alonso P. L.; Tamboura B.; Sanogo D.; Onwuchekwa U.; Manna B.; Ramamurthy T.; Kanungo S.; Ahmed S.; Qureshi S.; Quadri F.; Hossain A.; Das S. K.; Antonio M.; Hossain M. J.; Mandomando I.; Acacio S.; Biswas K.; Tennant S. M.; Verweij J. J.; Sommerfelt H.; Nataro J. P.; Robins-Browne R. M.; Levine M. M. (2019) The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet. Global health 7 (5), e568–e584. 10.1016/S2214-109X(19)30076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K. L.; Nataro J. P.; Blackwelder W. C.; Nasrin D.; Farag T. H.; Panchalingam S.; Wu Y.; Sow S. O.; Sur D.; Breiman R. F.; Faruque A. S.; Zaidi A. K.; Saha D.; Alonso P. L.; Tamboura B.; Sanogo D.; Onwuchekwa U.; Manna B.; Ramamurthy T.; Kanungo S.; Ochieng J. B.; Omore R.; Oundo J. O.; Hossain A.; Das S. K.; Ahmed S.; Qureshi S.; Quadri F.; Adegbola R. A.; Antonio M.; Hossain M. J.; Akinsola A.; Mandomando I.; Nhampossa T.; Acacio S.; Biswas K.; O’Reilly C. E.; Mintz E. D.; Berkeley L. Y.; Muhsen K.; Sommerfelt H.; Robins-Browne R. M.; Levine M. M. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382 (9888), 209–22. 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Platts-Mills J. A.; Babji S.; Bodhidatta L.; Gratz J.; Haque R.; Havt A.; McCormick B. J.; McGrath M.; Olortegui M. P.; Samie A.; Shakoor S.; Mondal D.; Lima I. F.; Hariraju D.; Rayamajhi B. B.; Qureshi S.; Kabir F.; Yori P. P.; Mufamadi B.; Amour C.; Carreon J. D.; Richard S. A.; Lang D.; Bessong P.; Mduma E.; Ahmed T.; Lima A. A.; Mason C. J.; Zaidi A. K.; Bhutta Z. A.; Kosek M.; Guerrant R. L.; Gottlieb M.; Miller M.; Kang G.; Houpt E. R.; (2015) Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet. Global health 3 (9), e564–75. 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow S. O.; Muhsen K.; Nasrin D.; Blackwelder W. C.; Wu Y.; Farag T. H.; Panchalingam S.; Sur D.; Zaidi A. K.; Faruque A. S.; Saha D.; Adegbola R.; Alonso P. L.; Breiman R. F.; Bassat Q.; Tamboura B.; Sanogo D.; Onwuchekwa U.; Manna B.; Ramamurthy T.; Kanungo S.; Ahmed S.; Qureshi S.; Quadri F.; Hossain A.; Das S. K.; Antonio M.; Hossain M. J.; Mandomando I.; Nhampossa T.; Acacio S.; Omore R.; Oundo J. O.; Ochieng J. B.; Mintz E. D.; O’Reilly C. E.; Berkeley L. Y.; Livio S.; Tennant S. M.; Sommerfelt H.; Nataro J. P.; Ziv-Baran T.; Robins-Browne R. M.; Mishcherkin V.; Zhang J.; Liu J.; Houpt E. R.; Kotloff K. L.; Levine M. M. (2016) The Burden of Cryptosporidium Diarrheal Disease among Children < 24 Months of Age in Moderate/High Mortality Regions of Sub-Saharan Africa and South Asia, Utilizing Data from the Global Enteric Multicenter Study (GEMS). PLoS Neglected Trop. Dis. 10 (5), e0004729. 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L.; Deboer M. D.; Moore S. R.; Scharf R. J.; Lima A. A. M. (2013) The impoverished gut: a triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 10, 220–9. 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W.; Epstein L. D.; Gilman R. H.; Black R. E.; Cabrera L.; Sterling C. R. (1998) Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 148 (5), 497–506. 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- (2003) New drug for parasitic infections in children. FDA Consumer 37 (3), 4. [PubMed] [Google Scholar]
- Fox L. M.; Saravolatz L. D. (2005) Nitazoxanide: a new thiazolide antiparasitic agent. Clin. Infect. Dis. 40 (8), 1173–80. 10.1086/428839. [DOI] [PubMed] [Google Scholar]
- Rossignol J. F.; Ayoub A.; Ayers M. S. (2001) Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J. Infect. Dis. 184 (1), 103–6. 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- Rossignol J. F.; Kabil S. M.; el-Gohary Y.; Younis A. M. (2006) Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin. Gastroenterol. Hepatol. 4 (3), 320–4. 10.1016/j.cgh.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Jumani R. S.; Bessoff K.; Love M. S.; Miller P.; Stebbins E. E.; Teixeira J. E.; Campbell M. A.; Meyers M. J.; Zambriski J. A.; Nunez V.; Woods A. K.; McNamara C. W.; Huston C. D. (2018) A Novel Piperazine-Based Drug Lead for Cryptosporidiosis from the Medicines for Malaria Venture Open-Access Malaria Box. Antimicrob. Agents Chemother. 62 (4), e01505-17. 10.1128/AAC.01505-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi B.; Mwiya M.; Sianongo S.; Payne L.; Watuka A.; Katubulushi M.; Kelly P. (2009) High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect. Dis. 9, 195. 10.1186/1471-2334-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J. F.; Hidalgo H.; Feregrino M.; Higuera F.; Gomez W. H.; Romero J. L.; Padierna J.; Geyne A.; Ayers M. S. (1998) A double-“blind” placebo-controlled study of nitazoxanide in the treatment of cryptosporidial diarrhoea in AIDS patients in Mexico. Trans. R. Soc. Trop. Med. Hyg. 92 (6), 663–6. 10.1016/S0035-9203(98)90804-5. [DOI] [PubMed] [Google Scholar]
- Amadi B.; Mwiya M.; Musuku J.; Watuka A.; Sianongo S.; Ayoub A.; Kelly P. (2002) Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360 (9343), 1375–80. 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- Amadi B.; Kelly P.; Mwiya M.; Mulwazi E.; Sianongo S.; Changwe F.; Thomson M.; Hachungula J.; Watuka A.; Walker-Smith J.; Chintu C. (2001) Intestinal and systemic infection, HIV, and mortality in Zambian children with persistent diarrhea and malnutrition. J. Pediatr. Gastroenterol. Nutr. 32 (5), 550–4. 10.1097/00005176-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Rytter M. J.; Kolte L.; Briend A.; Friis H.; Christensen V. B. (2014) The immune system in children with malnutrition--a systematic review. PLoS One 9 (8), e105017. 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashigbie P. G.; Shepherd S.; Steiner K. L.; Amadi B.; Aziz N.; Manjunatha U. H.; Spector J. M.; Diagana T. T.; Kelly P. (2021) Use-case scenarios for an anti-Cryptosporidium therapeutic. PLoS Neglected Trop. Dis. 15 (3), e0009057. 10.1371/journal.pntd.0009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iroh Tam P. Y.; Arnold S. L. M.; Barrett L. K.; Chen C. R.; Conrad T. M.; Douglas E.; Gordon M. A.; Hebert D.; Henrion M.; Hermann D.; Hollingsworth B.; Houpt E.; Jere K. C.; Lindblad R.; Love M. S.; Makhaza L.; McNamara C. W.; Nedi W.; Nyirenda J.; Operario D. J.; Phulusa J.; Quinnan G. V.; Sawyer L. A.; Thole H.; Toto N.; Winter A.; Van Voorhis W. C. (2020) Clofazimine for treatment of cryptosporidiosis in HIV-infected adults (CRYPTOFAZ): an experimental medicine, randomized, double-blind, placebo-controlled phase 2a trial. Clin. Infect. Dis. 10.1093/cid/ciaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston C. D.; Spangenberg T.; Burrows J.; Willis P.; Wells T. N.; van Voorhis W. (2015) A Proposed Target Product Profile and Developmental Cascade for New Cryptosporidiosis Treatments. PLoS Neglected Trop. Dis. 9 (10), e0003987. 10.1371/journal.pntd.0003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha U. H.; Chao A. T.; Leong F. J.; Diagana T. T. (2016) Cryptosporidiosis Drug Discovery: Opportunities and Challenges. ACS Infect. Dis. 2 (8), 530–7. 10.1021/acsinfecdis.6b00094. [DOI] [PubMed] [Google Scholar]
- Hulverson M. A.; Choi R.; Arnold S. L. M.; Schaefer D. A.; Hemphill A.; McCloskey M. C.; Betzer D. P.; Muller J.; Vidadala R. S. R.; Whitman G. R.; Rivas K. L.; Barrett L. K.; Hackman R. C.; Love M. S.; McNamara C. W.; Shaughnessy T. K.; Kondratiuk A.; Kurnick M.; Banfor P. N.; Lynch J. J.; Freiberg G. M.; Kempf D. J.; Maly D. J.; Riggs M. W.; Ojo K. K.; Van Voorhis W. C. (2017) Advances in bumped kinase inhibitors for human and animal therapy for cryptosporidiosis. Int. J. Parasitol. 47 (12), 753–763. 10.1016/j.ijpara.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha U. H.; Vinayak S.; Zambriski J. A.; Chao A. T.; Sy T.; Noble C. G.; Bonamy G. M. C.; Kondreddi R. R.; Zou B.; Gedeck P.; Brooks C. F.; Herbert G. T.; Sateriale A.; Tandel J.; Noh S.; Lakshminarayana S. B.; Lim S. H.; Goodman L. B.; Bodenreider C.; Feng G.; Zhang L.; Blasco F.; Wagner J.; Leong F. J.; Striepen B.; Diagana T. T. (2017) A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 546 (7658), 376–380. 10.1038/nature22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baragana B.; Forte B.; Choi R.; Nakazawa Hewitt S.; Bueren-Calabuig J. A.; Pisco J. P.; Peet C.; Dranow D. M.; Robinson D. A.; Jansen C.; Norcross N. R.; Vinayak S.; Anderson M.; Brooks C. F.; Cooper C. A.; Damerow S.; Delves M.; Dowers K.; Duffy J.; Edwards T. E.; Hallyburton I.; Horst B. G.; Hulverson M. A.; Ferguson L.; Jimenez-Diaz M. B.; Jumani R. S.; Lorimer D. D.; Love M. S.; Maher S.; Matthews H.; McNamara C. W.; Miller P.; O’Neill S.; Ojo K. K.; Osuna-Cabello M.; Pinto E.; Post J.; Riley J.; Rottmann M.; Sanz L. M.; Scullion P.; Sharma A.; Shepherd S. M.; Shishikura Y.; Simeons F. R. C.; Stebbins E. E.; Stojanovski L.; Straschil U.; Tamaki F. K.; Tamjar J.; Torrie L. S.; Vantaux A.; Witkowski B.; Wittlin S.; Yogavel M.; Zuccotto F.; Angulo-Barturen I.; Sinden R.; Baum J.; Gamo F. J.; Maser P.; Kyle D. E.; Winzeler E. A.; Myler P. J.; Wyatt P. G.; Floyd D.; Matthews D.; Sharma A.; Striepen B.; Huston C. D.; Gray D. W.; Fairlamb A. H.; Pisliakov A. V.; Walpole C.; Read K. D.; Van Voorhis W. C.; Gilbert I. H. (2019) Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proc. Natl. Acad. Sci. U. S. A. 116 (14), 7015–7020. 10.1073/pnas.1814685116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C. S.; Stebbins E. E.; Jumani R. S.; Hasan M. M.; Miller P.; Barlow J.; Freund Y. R.; Berry P.; Stefanakis R.; Gut J.; Rosenthal P. J.; Love M. S.; McNamara C. W.; Easom E.; Plattner J. J.; Jacobs R. T.; Huston C. D. (2019) Identification of a potent benzoxaborole drug candidate for treating cryptosporidiosis. Nat. Commun. 10 (1), 2816. 10.1038/s41467-019-10687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swale C.; Bougdour A.; Gnahoui-David A.; Tottey J.; Georgeault S.; Laurent F.; Palencia A.; Hakimi M. A. (2019) Metal-captured inhibition of pre-mRNA processing activity by CPSF3 controls Cryptosporidium infection. Sci. Transl. Med. 11 (517), eaax7161. 10.1126/scitranslmed.aax7161. [DOI] [PubMed] [Google Scholar]
- Vinayak S.; Jumani R. S.; Miller P.; Hasan M. M.; McLeod B. I.; Tandel J.; Stebbins E. E.; Teixeira J. E.; Borrel J.; Gonse A.; Zhang M.; Yu X.; Wernimont A.; Walpole C.; Eckley S.; Love M. S.; McNamara C. W.; Sharma M.; Sharma A.; Scherer C. A.; Kato N.; Schreiber S. L.; Melillo B.; Striepen B.; Huston C. D.; Comer E. (2020) Bicyclic azetidines kill the diarrheal pathogen Cryptosporidium in mice by inhibiting parasite phenylalanyl-tRNA synthetase. Sci. Transl. Med. 12 (563), eaba8412. 10.1126/scitranslmed.aba8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M. M., Stebbins E. E., Choy R. K. M., Gillespie J. R., de Hostos E. L., Miller P., Mushtaq A., Ranade R. M., Teixeira J. E., Verlinde C. L. M. J., Sateriale A., Zhang Z., Osbourn D. M., Griggs D. W., Fan E., Buckner F. S., and Huston C. D. (2021) Spontaneous selection of Cryptosporidium drug resistance in a calf model of infection. bioRxiv, 2021.01.04.425361. 10.1101/2021.01.04.425361. [DOI] [PMC free article] [PubMed]
- Janes J.; Young M. E.; Chen E.; Rogers N. H.; Burgstaller-Muehlbacher S.; Hughes L. D.; Love M. S.; Hull M. V.; Kuhen K. L.; Woods A. K.; Joseph S. B.; Petrassi H. M.; McNamara C. W.; Tremblay M. S.; Su A. I.; Schultz P. G.; Chatterjee A. K. (2018) The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc. Natl. Acad. Sci. U. S. A. 115 (42), 10750–10755. 10.1073/pnas.1810137115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. S.; McNamara C. W. (2021) Phenotypic screening techniques for Cryptosporidium drug discovery. Expert Opin. Drug Discovery 16, 59. 10.1080/17460441.2020.1812577. [DOI] [PubMed] [Google Scholar]
- Ward R. M.; Benjamin D. K. Jr.; Davis J. M.; Gorman R. L.; Kauffman R.; Kearns G. L.; Murphy M. D.; Sherwin C. M. T. (2018) The Need for Pediatric Drug Development. J. Pediatr. 192, 13–21. 10.1016/j.jpeds.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J.; Li J. Q.; Chen Y. C.; Zhang L. X.; Xiao L. H. (2018) Widespread occurrence of Cryptosporidium infections in patients with HIV/AIDS: Epidemiology, clinical feature, diagnosis, and therapy. Acta Trop. 187, 257–263. 10.1016/j.actatropica.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Toto N.; Douglas E.; Gmeiner M.; Barrett L. K.; Lindblad R.; Makhaza L.; Nedi W.; Phulusa J.; Quinnan G. V.; Sawyer L. A.; Thole H.; Van Voorhis W. C.; Iroh Tam P. Y. (2020) Conducting clinical trials in sub-Saharan Africa: challenges and lessons learned from the Malawi Cryptosporidium study. Trials 21 (1), 680. 10.1186/s13063-020-04620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston C. D. (2020) The Clofazimine for Treatment of Cryptosporidiosis in HIV-Infected Adults (CRYPTOFAZ) and Lessons Learned for Anticryptosporidial Drug Development. Clin. Infect. Dis. 10.1093/cid/ciaa425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton T. C.; Blohmke C. J.; Moorthy V. S.; Altmann D. M.; Hayden F. G.; Clutterbuck E. A.; Levine M. M.; Hill A. V.; Pollard A. J. (2015) Design, recruitment, and microbiological considerations in human challenge studies. Lancet Infect. Dis. 15 (7), 840–51. 10.1016/S1473-3099(15)00068-7. [DOI] [PubMed] [Google Scholar]
- Gordon S. B.; Rylance J.; Luck A.; Jambo K.; Ferreira D. M.; Manda-Taylor L.; Bejon P.; Ngwira B.; Littler K.; Seager Z.; Gibani M.; Gmeiner M.; Roestenberg M.; Mlombe Y.; Wellcome Trust C. w. p. (2017) A framework for Controlled Human Infection Model (CHIM) studies in Malawi: Report of a Wellcome Trust workshop on CHIM in Low Income Countries held in Blantyre, Malawi. Wellcome Open Res. 2 (70), 70. 10.12688/wellcomeopenres.12256.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard A. J.; Savulescu J.; Oxford J.; Hill A. V.; Levine M. M.; Lewis D. J.; Read R. C.; Graham D. Y.; Sun W.; Openshaw P.; Gordon S. B. (2012) Human microbial challenge: the ultimate animal model. Lancet Infect. Dis. 12 (12), 903–5. 10.1016/S1473-3099(12)70292-X. [DOI] [PubMed] [Google Scholar]
- Okhuysen P. C.; Rich S. M.; Chappell C. L.; Grimes K. A.; Widmer G.; Feng X.; Tzipori S. (2002) Infectivity of a Cryptosporidium parvum isolate of cervine origin for healthy adults and interferon-gamma knockout mice. J. Infect. Dis. 185 (9), 1320–5. 10.1086/340132. [DOI] [PubMed] [Google Scholar]
- Okhuysen P. C.; Chappell C. L.; Sterling C. R.; Jakubowski W.; DuPont H. L. (1998) Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 66 (2), 441–3. 10.1128/IAI.66.2.441-443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhuysen P. C.; Chappell C. L.; Crabb J. H.; Sterling C. R.; DuPont H. L. (1999) Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180 (4), 1275–81. 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- Okhuysen P. C.; Chappell C. L.; Crabb J.; Valdez L. M.; Douglass E. T.; DuPont H. L. (1998) Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin. Infect. Dis. 26 (6), 1324–9. 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- DuPont H. L.; Chappell C. L.; Sterling C. R.; Okhuysen P. C.; Rose J. B.; Jakubowski W. (1995) The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332 (13), 855–9. 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- Chappell C. L.; Okhuysen P. C.; Sterling C. R.; Wang C.; Jakubowski W.; Dupont H. L. (1999) Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am. J. Trop. Med. Hyg. 60 (1), 157–64. 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- Alcantara C. S.; Yang C. H.; Steiner T. S.; Barrett L. J.; Lima A. A.; Chappell C. L.; Okhuysen P. C.; White A. C. Jr.; Guerrant R. L. (2003) Interleukin-8, tumor necrosis factor-alpha, and lactoferrin in immunocompetent hosts with experimental and Brazilian children with acquired cryptosporidiosis. Am. J. Trop. Med. Hyg. 68 (3), 325–8. 10.4269/ajtmh.2003.68.325. [DOI] [PubMed] [Google Scholar]
- Chappell C. L.; Okhuysen P. C.; Langer-Curry R.; Widmer G.; Akiyoshi D. E.; Tanriverdi S.; Tzipori S. (2006) Cryptosporidium hominis: experimental challenge of healthy adults. Am. J. Trop. Med. Hyg. 75 (5), 851–7. 10.4269/ajtmh.2006.75.851. [DOI] [PubMed] [Google Scholar]
- Chappell C. L.; Okhuysen P. C.; Langer-Curry R. C.; Akiyoshi D. E.; Widmer G.; Tzipori S. (2011) Cryptosporidium meleagridis: infectivity in healthy adult volunteers. Am. J. Trop. Med. Hyg. 85 (2), 238–42. 10.4269/ajtmh.2011.10-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell C. L.; Okhuysen P. C.; Langer-Curry R. C.; Lupo P. J.; Widmer G.; Tzipori S. (2015) Cryptosporidium muris: infectivity and illness in healthy adult volunteers. Am. J. Trop. Med. Hyg. 92 (1), 50–5. 10.4269/ajtmh.14-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2013) Investigational New Drug Applications (INDs)—Determining Whether Human Research Studies Can Be Conducted Without an IND. FDA Guidance FDA-2010-D-0503. [Google Scholar]
- (2008) CGMP for Phase 1 Investigational Drugs. FDA Guidance FDA-2005-D-0157. [Google Scholar]
- Morada M.; Lee S.; Gunther-Cummins L.; Weiss L. M.; Widmer G.; Tzipori S.; Yarlett N. (2016) Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int. J. Parasitol. 46 (1), 21–9. 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Baldry M. G. (1983) The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 54 (3), 417–23. 10.1111/j.1365-2672.1983.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Barbee S. L.; Weber D. J.; Sobsey M. D.; Rutala W. A. (1999) Inactivation of Cryptosporidium parvum oocyst infectivity by disinfection and sterilization processes. Gastrointest Endosc 49 (5), 605–11. 10.1016/S0016-5107(99)70389-5. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz J. J.; Sevenler D.; Swei A. A.; Widmer G.; Toner M.; Tzipori S.; Sandlin R. D. (2020) Cryopreservation of infectious Cryptosporidium parvum oocysts achieved through vitrification using high aspect ratio specimen containers. Sci. Rep. 10 (1), 11711. 10.1038/s41598-020-68643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz J. J.; Sandlin R. D.; Swei A. A.; Widmer G.; Toner M.; Tzipori S. (2018) Cryopreservation of infectious Cryptosporidium parvum oocysts. Nat. Commun. 9 (1), 2883. 10.1038/s41467-018-05240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J. L.; Mathers T. C.; Ward B. J.; Pachebat J. A.; Swain M. T.; Robinson G.; Chalmers R. M.; Hunter P. R.; van Oosterhout C.; Tyler K. M. (2019) Evolutionary genomics of anthroponosis in Cryptosporidium. Nat. Microbiol 4 (5), 826–836. 10.1038/s41564-019-0377-x. [DOI] [PubMed] [Google Scholar]
- Morgan-Ryan U. M.; Fall A.; Ward L. A.; Hijjawi N.; Sulaiman I.; Payer R.; Thompson R. C.; Olson M.; Lal A.; Xiao L. (2002) Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J. Eukaryotic Microbiol. 49 (6), 433–40. 10.1111/j.1550-7408.2002.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Peng M. M.; Xiao L.; Freeman A. R.; Arrowood M. J.; Escalante A. A.; Weltman A. C.; Ong C. S.; Mac Kenzie W. R.; Lal A. A.; Beard C. B. (1997) Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerging Infect. Dis. 3 (4), 567–73. 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen M. S.; Templeton T. J.; Enomoto S.; Abrahante J. E.; Zhu G.; Lancto C. A.; Deng M.; Liu C.; Widmer G.; Tzipori S.; Buck G. A.; Xu P.; Bankier A. T.; Dear P. H.; Konfortov B. A.; Spriggs H. F.; Iyer L.; Anantharaman V.; Aravind L.; Kapur V. (2004) Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304 (5669), 441–5. 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- Xu P.; Widmer G.; Wang Y.; Ozaki L. S.; Alves J. M.; Serrano M. G.; Puiu D.; Manque P.; Akiyoshi D.; Mackey A. J.; Pearson W. R.; Dear P. H.; Bankier A. T.; Peterson D. L.; Abrahamsen M. S.; Kapur V.; Tzipori S.; Buck G. A. (2004) The genome of Cryptosporidium hominis. Nature 431 (7012), 1107–12. 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- Carter B. L.; Chalmers R. M.; Davies A. P. (2020) Health sequelae of human cryptosporidiosis in industrialised countries: a systematic review. Parasites Vectors 13 (1), 443. 10.1186/s13071-020-04308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff R. E.; Davies A. P.; Mason B. W.; Hutchings H. A.; Chalmers R. M. (2017) Long-term health effects after resolution of acute Cryptosporidium parvum infection: a 1-year follow-up of outbreak-associated cases. J. Med. Microbiol. 66 (11), 1607–1611. 10.1099/jmm.0.000609. [DOI] [PubMed] [Google Scholar]
- Carter B. L.; Stiff R. E.; Elwin K.; Hutchings H. A.; Mason B. W.; Davies A. P.; Chalmers R. M. (2019) Health sequelae of human cryptosporidiosis-a 12-month prospective follow-up study. Eur. J. Clin. Microbiol. Infect. Dis. 38 (9), 1709–1717. 10.1007/s10096-019-03603-1. [DOI] [PubMed] [Google Scholar]
- Hunter P. R.; Hughes S.; Woodhouse S.; Nicholas R.; Syed Q.; Chalmers R. M.; Verlander N. Q.; Goodacre J. (2004) Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin. Infect. Dis. 39 (4), 504–510. 10.1086/422649. [DOI] [PubMed] [Google Scholar]
- Sawant M.; Baydoun M.; Creusy C.; Chabe M.; Viscogliosi E.; Certad G.; Benamrouz-Vanneste S. (2020) Cryptosporidium and Colon Cancer: Cause or Consequence?. Microorganisms 8 (11), 1665. 10.3390/microorganisms8111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.; Yu X.; Zhang H.; Cui L.; Li X.; Zhang X.; Gong P.; Li J.; Li Z.; Wang X.; Li X.; Li T.; Liu X.; Yu Y.; Zhang X. (2020) Prevalence and Genotyping of Cryptosporidium parvum in Gastrointestinal Cancer Patients. J. Cancer 11 (11), 3334–3339. 10.7150/jca.42393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A. A.; Moore S. R.; Barboza M. S. Jr.; Soares A. M.; Schleupner M. A.; Newman R. D.; Sears C. L.; Nataro J. P.; Fedorko D. P.; Wuhib T.; Schorling J. B.; Guerrant R. L. (2000) Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J. Infect. Dis. 181 (5), 1643–51. 10.1086/315423. [DOI] [PubMed] [Google Scholar]